Abstract
Context:
Sugar overconsumption and chronic stress are growing health concerns because they both may increase the risk for obesity and its related diseases. Rodent studies suggest that sugar consumption may activate a glucocorticoid-metabolic-brain-negative feedback pathway, which may turn off the stress response and thereby reinforce habitual sugar overconsumption.
Objective:
The objective of the study was to test our hypothesized glucocorticoid-metabolic-brain model in women consuming beverages sweetened with either aspartame of sucrose.
Design:
This was a parallel-arm, double-masked diet intervention study.
Setting:
The study was conducted at the University of California, Davis, Clinical and Translational Science Center's Clinical Research Center and the University of California, Davis, Medical Center Imaging Research Center.
Participants:
Nineteen women (age range 18–40 y) with a body mass index (range 20–34 kg/m2) who were a subgroup from a National Institutes of Health-funded investigation of 188 participants assigned to eight experimental groups.
Intervention:
The intervention consisted of sucrose- or aspartame-sweetened beverage consumption three times per day for 2 weeks.
Main Outcome Measures:
Salivary cortisol and regional brain responses to the Montreal Imaging Stress Task were measured.
Results:
Compared with aspartame, sucrose consumption was associated with significantly higher activity in the left hippocampus (P = .001). Sucrose, but not aspartame, consumption associated with reduced (P = .024) stress-induced cortisol. The sucrose group also had a lower reactivity to naltrexone, significantly (P = .041) lower nausea, and a trend (P = .080) toward lower cortisol.
Conclusion:
These experimental findings support a metabolic-brain-negative feedback pathway that is affected by sugar and may make some people under stress more hooked on sugar and possibly more vulnerable to obesity and its related conditions.
Chronic stress and overconsumption of sugar are growing health concerns. Chronic stress, along with easy access to foods high in sugar, may elevate the risk for habitual sugar overconsumption and metabolic disease. Stress can increase selection and consumption of palatable (comfort) foods, which are typically high in sugar and fat (1). Although approximately 40% of people report eating more in response to stress, an estimated 80% report that they eat more sweets per calorie, regardless of whether they report eating more or less in response to stress (2). Consuming sugar to cope with stress is likely a difficult habit to break and one that may increase risk for chronic overeating, obesity, and related conditions (3).
Eating to relieve stress is a widely appreciated phenomenon, but the physiological basis for this behavior is unknown. Studies in rodent models suggest that consuming sugar switches off activity in brain stress networks which mediate stress-induced hypothalamic-pituitary-adrenal (HPA), autonomic nervous system, and emotional reactivity (4–6). In the brain, one target candidate through which the effects of sucrose consumption act to down-regulate stress-associated HPA reactivity and dampen the distressing, affective aspects of stress is corticotropin-releasing factor (CRF). CRF stimulates HPA and autonomic nervous system activity and elevates fear (7). In rodents, drinking sucrose beverages were shown to inhibit stress-induced CRF mRNA and peptide expression in the brain (8–11). These inhibitory effects of sugar on CRF and HPA reactivity may be linked to sugar-induced increases in central opioidergic activity. Sugar consumption promotes elevated opioidergic tone in the brain (12), and opioids inhibit synthesis and release of CRF and stress-induced HPA reactivity (13).
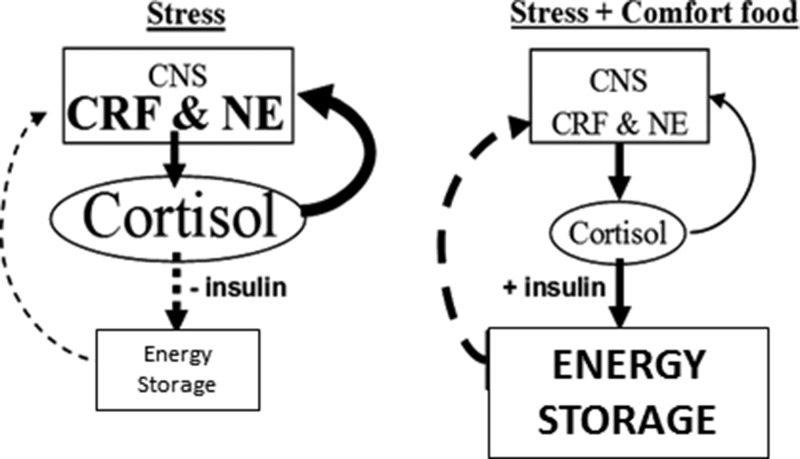
As a mechanism for explaining how sugar consumption inhibits brain stress reactivity, animal studies suggest that, beyond its sweet taste, sugar acts in the periphery to activate a metabolic-brain feedback pathway (see Figure 1 describing the metabolic-brain feedback model) (14). We previously showed that ingestion of sucrose, but not artificially sweetened, beverages dampens adrenalectomy- and stress-induced activation of brain CRF and the HPA axis (14). Compared with sucrose, nonnutritive saccharin was less effective in dampening the stress response (10) and ineffective in normalizing CRF and HPA activity in adrenalectomized rats (8). Furthermore, consumption of sucrose, but not saccharin, inhibits the catabolic effects of adrenalectomy (8). Together these findings imply that the metabolically restorative effects of sucrose may, in part, mediate sucrose-induced inhibition of central stress reactivity. However, human evidence for a metabolic-brain feedback pathway is lacking.
Figure 1.
Metabolic-brain feedback model. Solid lines are stimulatory; dashed lines are inhibitory. During stress, the feed-forward actions of cortisol on brain stress pathways [eg, CRF, norepinephrine (NE)] promote palatable feeding, increase anxiety and fear, and stimulate activity in the sympathetic nervous system and HPA axis (6). Reduced feeding during stress decreases insulin, rendering cortisol, along with increased sympathetic nervous system outflow, catabolic in the periphery. Decreased energy storage disinhibits metabolic feedback and perpetuates the feed-forward actions of cortisol. However, with the ingestion of highly energetic comfort foods, increased cortisol stimulates insulin and energy storage, which feeds back to inhibit activity in the HPA axis and reduces cortisol output and its feed-forward effects, temporarily providing relief from stress. If the source of stress is not removed, continued self-medication in this fashion might lead to central obesity. Consistent with the concept that chronic stress increases allostatic load (23), the relative impact to brain stress pathways of the metabolic-feedback signal (ie, energy reserve or net anabolic activity) may decrease with chronic stress, thereby increasing the signal magnitude needed to impart its feedback effects; ie, tolerance might be developed. [Reproduced from M. Dallman et al: Chronic stress and obesity: a new view of “comfort food.” Proc Natl Acad Sci USA. 2003;100(20):11696–11701 (14), with permission. ©The Endocrine Society]
Therefore, we conducted a study in young women to test our hypothesis that relative to the effects of aspartame consumption, sucrose consumption would inhibit the Montreal Imaging Stress Task (MIST)-induced cortisol response and parallel responses in brain regions known to mediate HPA/cortisol responses to stressors. Furthermore, given that sucrose promotes opioidergic tone in the brain, we tested whether sugar consumption would dampen the inductive effects of opioid blockade on HPA activity.
Materials and Methods
Participants
Nineteen women [aged 26.9 (mean) ± 6.5 (SD) y; range 18–40 y: body mass index (BMI) 25.7 ± 3.3 kg/m2; range 20–34 kg/m2] who were a subgroup from a National Institutes of Health-funded investigation in which a total of 188 participants assigned to eight experimental groups were studied. Results from the first 48 participants studied who consumed beverages sweetened with fructose, glucose, or high-fructose corn syrup are reported elsewhere (15). None of these 48 participants were in the group of participants in the current study. Participants were recruited through http://sacramento.craigslist.org/, completed telephone and in-person interviews, and provided written informed consent. Inclusion criteria included age 18–40 years, BMI 18–35 kg/m2, and absence of disease (15). Experimental groups of this substudy were matched for BMI. The University of California, Davis (Davis, California) Institutional Review Board approved the experimental protocol for this study.
General study design
This was a parallel-arm, double-masked diet intervention study with three phases: 1) 3.5-day inpatient preintervention period during which participants resided at the University of California, Davis, Clinical and Translational Science Center's Clinical Research Center, consumed a standardized, low-sugar, baseline diet, and participated in experimental procedures; 2) 12-day outpatient intervention period during which participants consumed their assigned sweetened beverages providing 0% (aspartame sweetened; n = 8) or 25% (sucrose sweetened; n = 11) of the energy requirement along with their usual ad libitum diets; and 3) 3.5-day inpatient intervention period during which participants resided at the Clinical and Translational Science Center's Clinical Research Center and consumed standardized intervention diets that included the sweetened beverages. Inpatient intervention meals were as identical as possible with preintervention meals (15) except for the inclusion of the aspartame-sweetened beverage and/or the substitution of the sucrose-sweetened beverage in place of isocaloric amounts of complex carbohydrate.
During the 12-day outpatient phase, participants received three servings of study beverages for each day and instructed to drink one serving with each meal, consume their usual diet, and not consume other sugar-sweetened beverages, including fruit juices. We used the Mifflin equation (16) with a 1.5 adjustment for activity to calculate the energy content of the 25% sucrose beverages for each participant. The sucrose-sweetened beverages were flavored with unsweetened Kool-Aid (Kraft), and the aspartame-sweetened beverages were prepared from fruit-flavored aspartame drink mix (Market Pantry). Study beverages contained riboflavin that was measured fluorimetrically in urine samples collected at the times of the beverage pickup. These measurements indicated comparable compliance in the experimental groups.
During the second day of each preintervention inpatient period and after the 12-day outpatient intervention, participants completed a functional magnetic resonance imaging (fMRI) task (MIST; see below). Participants arrived at the University of California, Davis, Imaging Research Center at 2:00 pm, received magnetic resonance imaging (MRI) safety screening and instructions about the imaging task, and started the imaging stress task at 3:00 pm. To further probe the ability of sucrose consumption to inhibit the stress response, participants performed a naltrexone-induced opioid blockade experiment at home (see below) 3 days prior to each MIST visit. For the MIST experiments, there were 14 days of exposure to the sweetened beverages between each of the two MIST visits. There were 11 days of exposure to the sweetened beverages between each of the two naltrexone sessions.
Montreal Imaging Stress Task
fMRI is useful for mapping neuroanatomical regions associated with psychological stress. In the present study, we used an adapted paradigm from the Trier Social Stress Test to assess the effects of consuming sweetened beverages on the brain and neuroendocrine stress responses. This paradigm (MIST) is an induced failure task combining mental arithmetic challenges with social evaluative threat. We administered the MIST according to Pruessner et al (17), who showed the MIST to induce a physiological stress response as indicated by elevated cortisol levels and widespread altered activity within the limbic system. The MIST comprised a block design with two replicate runs. Each run lasted approximately 10 minutes with random presentation of rest, control, and experimental conditions (Figure 2). The experimental condition induced stress using a timed mental arithmetic task and negative feedback. The control condition was the same challenging arithmetic task but was untimed. In the rest condition, participants viewed the same screen without math problems.
Figure 2.

Montreal Imaging Stress Task experimental paradigm. The MIST paradigm was performed according to Pruessner et al (17) and consisted of a block design with two replicate runs. Each run lasted approximately 10 minutes and consisted of a rest, control, and experimental condition. Saliva samples for the examination of circulating free cortisol concentrations were taken upon arrival to the Imaging Research Center, 15 minutes later, immediately (time 0) prior to the first MIST run, and 15, 30, and 60 minutes (peak response) after the start of the first MIST run.
MRI acquisition, processing and analysis
A Siemens Tim Trio 3T magnet located within the Imaging Research Center at the University of California, Davis, Medical Center acquired MRI structural and functional data. Each MRI session included a single-plane localizer scan, a true sagittal calibration scan, a high-resolution anatomical scan, and two fMRI scans of the MIST. Foam inserts restricted head motion in a 32-channel head coil. Functional images were acquired in an interleaved format using a single-shot T2*-weighted EPI pulse sequence with an echo time of 25 milliseconds, repetition time of 2 seconds, and a 90° flip angle for 36 slices 3.4 mm thick.
Data preprocessing was completed using the SPM8 software package (Wellcome Department Imaging Neuroscience). Functional MRI data were slice time corrected and realigned to the first image volume collected. Data were normalized into standard Montreal Neurological Institute space and smoothed using an 8-mm full width at half maximum Gaussian filter. Inclusionary criteria for data were such that movement before correction was less than half of the voxel length in translational movement and less than 1.5° in rotational movement.
MIST-induced brain activity changes were determined by calculating the contrasts of (experimental > control), (experimental > rest), and (control > rest) conditions, and computing t maps for these contrasts. After analysis was completed for each subject, we performed a higher-order analysis for the whole group using SPM8 and GLMflex (Harvard Aging Brain Study, Martinos Center, MGH, http://nmr.mgh.harvard.edu/harvardagingbrain/People/AaronSchultz/Aarons_Scripts). To enhance the statistical power, a region of interest (ROI) approach was implemented. A single anatomical mask was generated from the WFU Pickatlas and the AAL and Talairach Daemon atlases (18–20). ROIs within the mask included the anterior cingulate cortex (ACC), hippocampus, hypothalamus, and medioorbitalfrontal cortex; striatum; caudate/putamen; occipital cortex; and premotor areas. We used a repeated-measures ANOVA to assess the group-wise differences in the first-level acquisition t maps between the sucrose and aspartame groups. Statistical significance for these ROIs were assessed at P < .01. We used a false discovery rate correction (P < .05) to correct for multiple comparisons.
Naltrexone-induced opioid blockade
Blocking of the opioid receptor causes increased cortisol release into the blood (12). Opioid blockade by naltrexone can be used to probe the level of opioid tone, with stronger cortisol and nausea responses indicating weaker opioidergic tone (12, 21). Participants were instructed to take oral naltrexone (50 mg) after lunch (1:00 pm). For cortisol examination, participants collected saliva samples before 1:00 pm, at 2:00, 3:00, 4:00, and 5:00 pm, and before bedtime. Participants completed a study log with their actual times of naltrexone ingestion and saliva collection and postdose symptoms, with the most common one being nausea.
Cortisol sampling and analysis
For the fMRI experiment, saliva samples for examining circulating free cortisol concentrations were collected using Salimetrics oral swabs upon arrival to the Imaging Research Center, 15 minutes later, immediately before the first MIST run (time 0), and 15, 30, and 60 minutes (peak response) after the start of the first MIST run (Figure 2). Saliva samples were placed on ice, centrifuged for 5 minutes at 1000 × g, and stored at −70°C until analyzed for cortisol using Salimetrics an expanded-range, high-sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics). Change from 0 to 60 minutes was used to assess peak cortisol response (reactivity). We also examined the total cortisol output as an index of both basal (possibly anticipatory) and reactive (to the MIST task) cortisol during each of the fMRI visits. Total cortisol output was estimated by calculating the area under the curve (AUC; nanomoles per liter minutes) for cortisol concentrations from arrival (−30 min) to the end (60 min). Analyses of covariance (ANCOVAs), adjusted for repeated measures, were computed using SAS version 9.3 (SAS Institute Inc) to test for the differences in cortisol reactivity and total cortisol output. For the naltrexone response, we defined cortisol response for each visit as the difference in salivary cortisol levels, nausea ratings, and AUC between prenaltrexone (1:00 pm) and peak postnaltrexone response (4:00 pm). We applied natural logarithmic transformations to the cortisol data prior to analysis due to the abnormal distribution. For all analyses, we used a Bonferroni correction when assessing post hoc differences between groups at pre- and postintervention visits. The Satterthwaite method was used to calculate the degrees of freedom for all tests.
Results
At baseline (before the intervention), the two experimental groups did not significantly differ in age, BMI, chronic stress score, and cortisol and nausea responses (Table 1).
Table 1.
Mean ± SE of preintervention age, BMI, Wheaton Chronic Stress score, MIST-induced cortisol response, total cortisol output (AUC) during the MIST visit, and naltrexone-induced cortisol and nausea response for the sucrose and aspartame intervention groups
| Preintervention measure | Sucrose (n = 11) | Aspartame (n = 8) | P value |
|---|---|---|---|
| Age, y | 27.2 ± 2.0 | 26.5 ± 2.4 | .829 |
| BMI, kg/m2 | 25.7 ± 1.0 | 25.8 ± 1.2 | .932 |
| Wheaton Chronic Stress Score | 17.7 ± 2.3 | 16.8 ± 2.7 | .784 |
| MIST-induced cortisol response, nmol/L Δa | 2.3 ± 1.6 | 1.0 ± 1.9 | .971 |
| Total cortisol output (AUC) during the MIST visit, nmol/Lmin | 121.4 ± 13.2 | 130.0 ± 15.4 | .660 |
| Naltrexone-induced cortisol response, nmol/L Δa | 0.15 ± 0.13 | 0.10 ± 0.31 | .878 |
| Naltrexone-induced nausea response ratinga | 0.50 ± 0.31 | 1.17 ± 0.40 | .207 |
For testing differences in Δ cortisol and nausea, the pretask (ie, MIST; naltrexone) value was included as an independent variable in the statistical model.
Sugar effects on neural responses
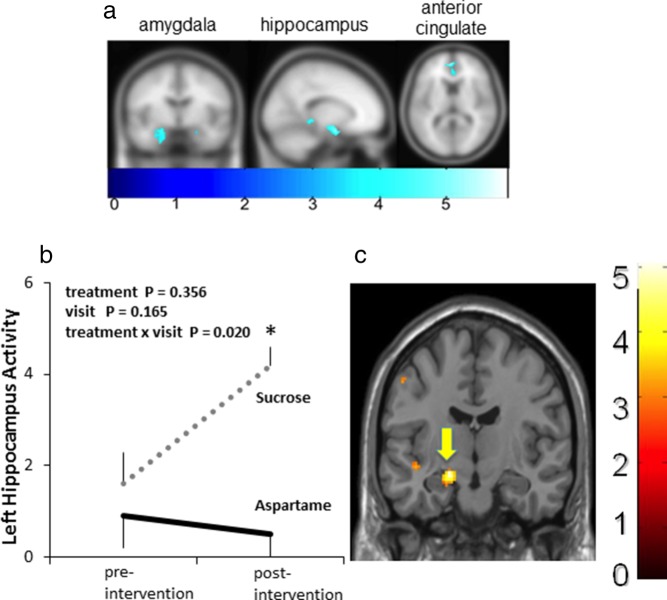
Consistent with previous reports of MIST effects (17), the MIST before dietary intervention led to significant (P < .010) unilateral (left) deactivation in the parahippocampal gyrus, anterior cingulate cortex (BA10), hippocampus, and amygdala (Table 2 and Figure 3A). The purpose of presenting the pooled preintervention imaging data was to provide evidence of the MIST paradigm's consistency with regard to eliciting responses in specific regions of the brain as previously reported (eg, Pruessner). In a repeated-measures ANCOVA, which included treatment group and visit as independent variables, we found a significant treatment group by visit interaction in the left hippocampus [F (1, 15) = 6.8, P = .020; effect size (η2p) = 0.15]. We observed no group differences [t (17) = 0.76, P = .4556] for the preintervention visit. However, after 2 weeks of sugar consumption (relative to aspartame), sucrose consumption was associated with significantly [t (13) = 4.2, P = .001] higher stress (MIST)-induced activity in the left hippocampus (Figure 3, B and C).
Table 2.
Effects of the stress task (MIST) at the preintervention examination, collapsed across all subjects from both intervention groups
| Region | Cluster Size | t | Coordinates |
|---|---|---|---|
| L parahippocampal gyrus | 119 | 5.87 | −26, −38, −12 |
| L ACC (BA10) | 84 | 5.70 | −4, 52, 12 |
| L hippocampus | 168 | 5.15 | −22, −16, −22 |
| L amygdala | 231 | 5.04 | −20, −4, −16 |
| L hippocampus (BA27) | 57 | 4.67 | −16, −34, −4 |
| L ACC (BA10) | 23 | 3.97 | −4, 52, −2 |
Abbreviations: BA, Brodmann area; L, left hemisphere. The stress task led to significant (P < .01) unilateral (left) deactivation in the parahippocampal gyrus, anterior cingulate cortex (BA10), hippocampus, and amygdala. Regions of interest are displayed as right or left hemisphere, Brodmann areas, t values for the statistical contrast, and Montreal Neurological Institute coordinates.
Figure 3.
Effects of consuming sucrose and aspartame sweetened beverages on regional brain activity. A, Preintervention brain images showing effects of the stress task (MIST) at the preintervention visit, collapsed across all subjects from both intervention groups. The stress task led to significant (P < .01) unilateral (left panel) deactivation in the amygdala (−20, −4, −16), hippocampus (−22, −16, −22), and anterior cingulate cortex (BA10; −4, 52, 12). B, Repeated-measures ANCOVA showed a significant treatment group by visit interaction [F (1, 15) = 6.8, P = .020; effect size (η2p) = 0.15]. In contrast to the preintervention visit, significantly greater MIST-induced hippocampal activity was observed in the sucrose, but not aspartame, group at the postintervention visit. The brain image in panel C shows the net effect of sucrose consumption to increase above preintervention and the aspartame group hippocampal activity in response to the stress task (MIST). A higher number on the color scale indicates greater activity. *, P = .001 for statistical difference in hippocampal activity between sucrose and aspartame groups at the postintervention visit.
Effects of sugar on MIST-related salivary cortisol responses
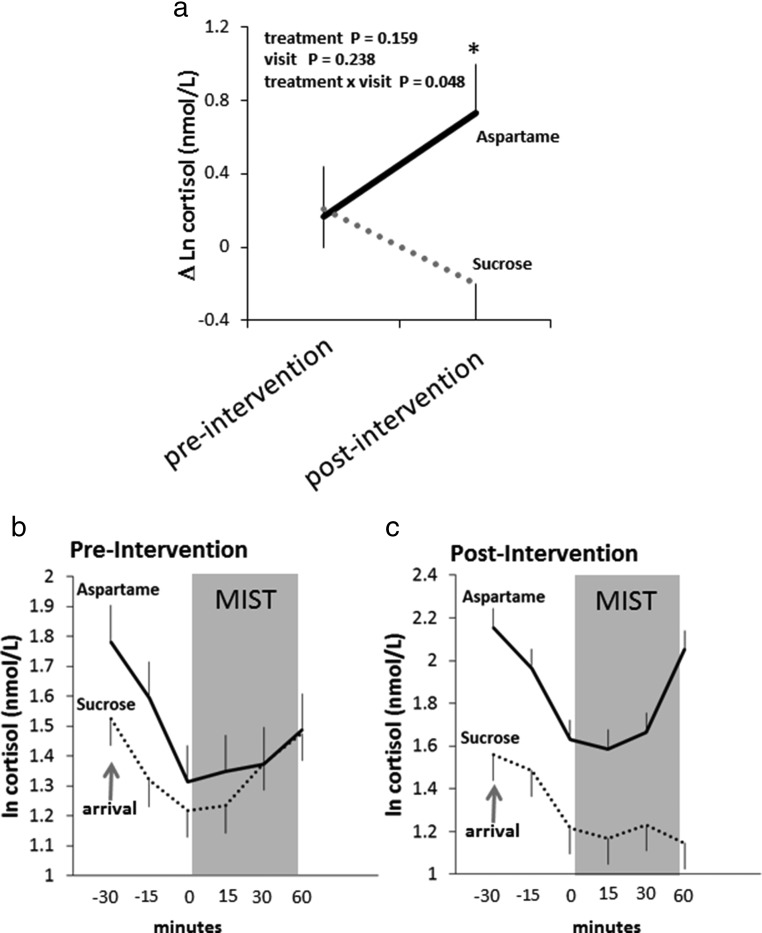
We found a significant group by visit interaction for the MIST-induced cortisol response [F (1, 15) = 4.7, P = .048; effect size (η2p) = 0.36]. The cortisol response to the MIST was diminished after 2 weeks of consuming sucrose but elevated after 2 weeks of consuming aspartame (Figure 4, A–C). The postintervention cortisol response was significantly different (P = .024) between the aspartame (mean ± SE, 6.5 ± 2.0 nmol/L Δ) and sucrose (mean ± SE, −0.7 ± 1.6 Δ) groups. We also found a significant (Pgroup × visit = .043) group × visit interaction for total cortisol output, as estimated by the AUC across the entire visit. Total cortisol output during the preintervention visit did not differ significantly [t (24) = 0.42, P = .6602] between the aspartame and sucrose groups (Table 1). However, during the postintervention visit, total cortisol output was significantly higher [t (24) = 2.4, P = .0130] in the aspartame (mean ± SE, 161.6 ± 12.7 nmol/L min) group compared with the sucrose group (mean ± SE, 114.9 ± 10.8 nmol/L min).
Figure 4.
Effects of consuming sucrose- and aspartame-sweetened beverages on MIST-induced cortisol. A, The mean ± SE stress task (MIST) induced plasma cortisol concentration change before (preintervention) and 2 weeks after (postintervention) daily (three times per day) consumption of sucrose or aspartame-sweetened beverages. Δ-Cortisol was calculated as the difference between the cortisol values before (0 min) induction of the first MIST run and after the second MIST run was completed (60 min). We included the prestress cortisol concentration and prestress cortisol concentration × visit day terms in the repeated-measures statistical model. As supported by a significant treatment group × visit interaction [F (1, 15) = 4.7, P = .048; effect size (η2p) = 0.36], the cortisol response to the stress task (MIST) was diminished after 2 weeks of consuming sucrose but not aspartame. Log-transformed cortisol concentrations at each sample time are shown for the preintervention (B) and postintervention (C) visits. *, P = .024 for statistical difference in δ-cortisol between sucrose and aspartame groups at the postintervention visit.
Effects of sugar on naltrexone-induced nausea and cortisol responses
The sugar and aspartame groups did not differ significantly in nausea [t (14) = 1.32, P = .2070] or cortisol reactivity [t (18) = 0.16, P = .8780] at the preintervention period (Table 1). However, postintervention nausea response (delta) was significantly lower [t (13) = 2.24, P = .0410] in the sugar group (mean ± SE, 1.67 ± 0.49 δ) relative to the aspartame group (mean ± SE, 0.56 ± 0.24 Δ). Although the postintervention naltrexone-induced cortisol response (delta) was not significantly different (P = .53) between the sucrose (mean ± SE, 0.09 ± 0.13 Δ) and aspartame (mean ± SE, 0.29 ± 0.28 δ) groups, when examining the individual cortisol values, sugar consumption evidenced a statistical trend [t (15) = 1.91, P = .0800] toward reducing cortisol concentrations at 2:00 and 5:00 pm.
Discussion
Results presented here are among the first evidence that consumption of beverages sweetened with sugar, but not the artificial sweetener aspartame, inhibits stress-induced cortisol secretion in humans. Furthermore, sugar consumption, but not aspartame consumption, associated with greater activation of the hippocampus, which is typically inhibited during acute stress (22), as it was at the preintervention visit. That is, sugar inhibited stress-induced deactivation in the hippocampus. This offers a new clue to how sugar may work at positively reinforcing comfort food intake. Besides increasing opioids (1), it suppresses HPA axis stress reactivity, and this may be due in part to activation of the hippocampus during stress.
The hippocampus plays a primary role in glucocorticoid negative feedback, and diminished hippocampal structure and function is linked to exaggerated HPA responsiveness (23). Specifically, MIST-induced increases in circulating cortisol were previously shown to be associated with greater deactivation in the hippocampus, suggesting a facilitation of the HPA axis through the inhibition of the negative feedback loop during acute stress (17). Furthermore, reduced activity in the hippocampus has been observed in mice bred for exaggerated stress-induced HPA reactivity (24). Our results during the preintervention MIST (Table 2 and Figure 4A) are consistent with those findings and further suggest that sugar consumption inhibits activity in the HPA axis via some unknown, possibly metabolic-related (14) signal to the hippocampus.
We can only speculate as to the meaning or significance of the observed unilateral activity in the hippocampus. It is possible that the association or interactions between sugar consumption, stress responsiveness, and regulation in the HPA axis may bias or be linked to the net activity in one side of this brain region. However, other functional imaging studies using different paradigms of stress have elicited unilateral activity in the hippocampus as well (eg, 25, 26).
This study provides novel evidence for the glucocorticoid-metabolic-brain feedback pathway in humans. Previous findings suggested that anabolic (or anticatabolic) effects of consuming highly palatable and calorically dense foods or beverages signal the brain to turn off the HPA stress response (14). Teleologically, this makes sense because, in anticipation of or during stress, elevated concentrations of glucocorticoids such as cortisol stimulate catabolism to ensure fuel for the brain and the fight or flight response (27). During recovery from stress, these steroid hormones promote energy recovery by motivating energy intake and stimulating lipogenesis and glycogen synthesis (28). Unlike artificial sweeteners, sugar may provide the fuel needed to meet the energetic demands of stress, which may reduce the need for glucocorticoid-driven energy catabolism and mobilization of the body's energy stores. Consistent with this notion, rodent data have shown that sucrose consumption prevents body catabolism and the activation in the HPA axis (8, 10, 29). The results we present here show that humans' ingestion of sucrose, but not artificially, sweetened beverages reduced stress-induced increases in circulating cortisol.
Compared with women consuming sugar, cortisol concentrations across the MIST visit were elevated in the aspartame-consuming group. Although it is possible that daily aspartame consumption stimulated cortisol output, there lacks evidence for any inductive effects of aspartame consumption on cortisol concentrations. It is more likely that repeating the MIST task led to greater anticipation (higher basal cortisol) and a facilitated stress response (higher peak) and that any such sensitization was blocked in the sugar condition. Repeated stress can lead to enhanced HPA responsiveness (6). Rodent data indicate that sucrose consumption inhibited the stress-induced facilitated HPA response (9).
Major depression of the melancholic type (30), type 2 diabetes (31), and metabolic syndrome (32) have been associated with exaggerated cortisol responsiveness. Cortisol hyporesponsiveness has been linked to atypical depression, posttraumatic stress disorder, fibromyalgia, and chronic fatigue syndrome (33). We know little about the factors that determine cortisol hypo- and hyperresponsiveness, however, we believe that diet may play a role. A cross-sectional study found that stress-induced cortisol hyporesponsiveness was associated with greater self-reports of eating after facing a stressful situation (34). In a parallel cross-sectional study of women, Tryon et al (35) found an association between greater consumption of high-fat and high-sweet foods from a voluntary snack food buffet and a blunted cortisol response to the Trier Social Stress Test. The experimental findings we present here show that the consumption of an artificial sweetener and sugar may differentially influence cortisol responsiveness.
The current study used a relatively small sample. However, the consistency of our findings with previous results from rodent studies comparing sugar and nonnutritive sweetener consumption strongly suggests that sucrose consumption may have stress dampening effects in humans. We studied only the effects of sweet beverages and do not know whether other palatable foods (eg, fat) exert similar effects. High-fat consumption in rodents has been shown to enhance (36) and blunt (37) glucocorticoid activity. Further examination of the effects of different macro- and micronutrients on stress-system responsiveness is warranted. Our experimental design does not allow us to determine whether the observed outcomes resulted from acute or long-term sugar consumption. The neural and endocrine effects of acute vs long-term sugar consumption should be examined in future studies. We also cannot, with certainty, conclude that the actions of sucrose were completely independent from taste effects and strictly resulted as a consequence of postingestive mechanisms. Furthermore, we did not monitor preexperimental or out-patient use of the sweeteners. Therefore, it cannot be completely ruled out as to whether consumption patterns during the out-patient period influenced the results. Finally, we studied only women in this study and therefore cannot determine whether our results extend to men. Overall, our results underscore the importance of future studies that should probe the glucocorticoid-metabolic-brain feedback pathway in humans and both explore and test its possible clinical implications.
In conclusion, both dysfunctional stress system responsiveness and overconsumption of sugar are growing health concerns. Each has been linked to obesity, cardiovascular diseases, and type 2 diabetes. Our results provide new evidence in humans for a physiological link between sugar consumption and cortisol reactivity to stress. We speculate that the stress-dampening effects of sugar may promote the behaviorally entrenched daily sugar consumption that may increase risk for obesity and may explain differences in disease subtypes, such as major depression.
Acknowledgments
We thank Rashel DeCant (Obesity and Metabolism Research Unit/Western Human Nutrition Research Center, Agricultural Research Services, US Department of Agriculture, Davis, California) for her help in conducting the cortisol assays and functional magnetic resonance imaging experiments. We also thank Vivien Lee, Hazel Lam, Marinelle Nunez, Guoxia Chen, and James Graham, B.S. (University of California, Davis) for their excellent clinical and technical support, Dr Tissa Kappagoda, MD, (University of California, Davis) for physician support, and the nursing staff at the University of California, Davis, Clinical and Translational Science Center's Clinical Research Center for their dedicated nursing support.
Author contributions include the following: K.D.L. had full access to the reported data in this study and accepts responsibility for the integrity of the data and the accuracy of their analysis. K.D.L., P.J.H., K.L.S., and E.S.E. conceived of the study concept and design. M.S.T., K.D.L., and K.L.S. were involved in the acquisition of the data. M.S.T., K.D.L., A.E.M., and R.B. performed the statistical analysis. K.D.L. and M.S.T. drafted the manuscript, with the critical revision of the manuscript for important intellectual content by K.D.L., M.S.T., E.S.E., K.L.S., P.J.H., and A.E.M. K.D.L., M.S.T., E.S.E., K.L.S., and V.M. provided the administrative, technical, or material support, and M.S.T., K.D.L., and K.L.S. supervised the study.
The contents of this manuscript are solely the responsibility of the authors and do not represent the official view of the US Department of Agriculture, which is an equal opportunity provider and employer.
This study is registered (clinicaltrials.gov) with the identifying number of NCT01103921.
This work was supported by a multicampus grant from the University of California, Office of the President (Award 142691; principal investigator E.S.E.) and US Department of Agriculture's Agricultural Research Service intramural Project Number 5306–51530-019–00 (principal investigator K.D.L.). The parent study was supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute Grant 1R01 HL09133 (principal investigator P.J.H.) and Grant 1R01 HL107256 (principal investigator P.J.H.); National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research Grant UL1 RR024146 (principal investigator L. Berglund). K.L.S. is supported by a Building Interdisciplinary Research Careers in Women's Health award (Grant K12 HD051958; principal investigator E. Gold) supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Office of Research on Women's Health, Office of Dietary Supplements, and the National Institute of Aging.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACC
- anterior cingulate cortex
- ANCOVA
- analysis of covariance
- AUC
- area under the curve
- BMI
- body mass index
- CRF
- corticotropin-releasing factor
- fMRI
- functional MRI
- HPA
- hypothalamic-pituitary-adrenal
- MIST
- Montreal Imaging Stress Task
- MRI
- magnetic resonance imaging
- ROI
- region of interest.
References
- 1. Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. [DOI] [PubMed] [Google Scholar]
- 2. Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89(1):53–61. [DOI] [PubMed] [Google Scholar]
- 3. Tryon MS, Carter CS, DeCant R, Laugero KD. Chronic stress exposure may affect the brain's response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav. 2013;120:233–242. [DOI] [PubMed] [Google Scholar]
- 4. Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46(9):1167–1180. [DOI] [PubMed] [Google Scholar]
- 5. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67(1):259–284. [DOI] [PubMed] [Google Scholar]
- 6. Dallman MF, Akana SF, Strack AM, et al. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann NY Acad Sci. 2004;1018(1):141–150. [DOI] [PubMed] [Google Scholar]
- 7. Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44(1):525–557. [DOI] [PubMed] [Google Scholar]
- 8. Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001;142(7):2796–2804. [DOI] [PubMed] [Google Scholar]
- 9. Laugero KD, Gomez F, Manalo S, Dallman MF. Corticosterone infused intracerebroventricularly inhibits energy storage and stimulates the hypothalamo-pituitary axis in adrenalectomized rats drinking sucrose. Endocrinology. 2002;143(12):4552–4562. [DOI] [PubMed] [Google Scholar]
- 10. Ulrich-Lai YM, Ostrander MM, Thomas IM, et al. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148(4):1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foster MT, Warne JP, Ginsberg AB, et al. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150(5):2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daubenmier J, Lustig RH, Hecht FM, et al. A new biomarker of hedonic eating? A preliminary investigation of cortisol and nausea responses to acute opioid blockade. Appetite. 2014;74:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(4):729–741. [DOI] [PubMed] [Google Scholar]
- 14. Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food.” Proc Natl Acad Sci USA. 2003;100(20):11696–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanhope KL, Bremer AA, Medici V, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96(10):E1596–E1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. [DOI] [PubMed] [Google Scholar]
- 17. Pruessner JC, Dedovic K, Khalili-Mahani N, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63(2):234–240. [DOI] [PubMed] [Google Scholar]
- 18. Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. [DOI] [PubMed] [Google Scholar]
- 20. Lancaster JL, Summerlin JL, Rainey L, Freitas CS, Fox PT. Technical demonstrations. NeuroImage 1997;5(4 Part 2):S631–S636. [Google Scholar]
- 21. Roche DJO, Childs E, Epstein AM, King AC. Acute HPA axis response to naltrexone differs in female vs. male smokers. Psychoneuroendocrinology. 2010;35(4):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149(11):5482–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(suppl 2):17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knapman A, Kaltwasser SF, Martins-de-Souza D, et al. Increased stress reactivity is associated with reduced hippocampal activity and neuronal integrity along with changes in energy metabolism. Eur J Neurosci. 2012;35(3):412–422. [DOI] [PubMed] [Google Scholar]
- 25. Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry. 2002;52(11):1113–1119. [DOI] [PubMed] [Google Scholar]
- 26. Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann NY Acad Sci. 2004;1032(1):254–257. [DOI] [PubMed] [Google Scholar]
- 27. Peters A, Schweiger U, Pellerin L, et al. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28(2):143–180. [DOI] [PubMed] [Google Scholar]
- 28. Dallman MF, Pecoraro NC, La Fleur SE, et al. Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75–105. [DOI] [PubMed] [Google Scholar]
- 29. Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. [DOI] [PubMed] [Google Scholar]
- 30. Gold PW, Chrousos GP. Melancholic and atypical subtypes of depression represent distinct pathophysiological entities: CRH, neural circuits, and the diathesis for anxiety and depression. Mol Psychiatry. 2013;18(6):632–634. [DOI] [PubMed] [Google Scholar]
- 31. Chiodini I, Adda G, Scillitani A, et al. Cortisol secretion in patients with type 2 diabetes relationship with chronic complications. Diabetes Care. 2007;30(1):83–88. [DOI] [PubMed] [Google Scholar]
- 32. Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann NY Acad Sci. 2006;1083(1):111–128. [DOI] [PubMed] [Google Scholar]
- 33. Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. [DOI] [PubMed] [Google Scholar]
- 34. Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36(10):1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tryon MS, DeCant R, Laugero KD. Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol Behav. 2013;114–115:32–37. [DOI] [PubMed] [Google Scholar]
- 36. Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol. 1997;273(6 Pt 1):E1168–E1177. [DOI] [PubMed] [Google Scholar]
- 37. La Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146(5):2193–2199. [DOI] [PubMed] [Google Scholar]