Abstract
Aims
Many epidemiological studies have shown that low bone mineral density (BMD) and atherosclerosis appear to be related. However, their precise correlation is not completely understood after full adjustment the shared confounders of atherosclerosis and bone metabolism. The aim of this cross-sectional study was to investigate the relationship between BMD and subclinical atherosclerosis in a healthy Chinese population and the difference in gender.
Methods
The study population consisted of 2,487 subjects (1,467 men, 1,020 women) who participated in health check-up programs and were selected to be free of major diseases which might affect atherosclerosis and bone metabolism. Bone status was assessed by BMD in lumbar spine. The brachial-ankle PWV (baPWV) was assessed as a functional marker of atherosclerosis. The ankle-brachial index (ABI), carotid artery intima-media thickness (CIMT), estimated glomerular filtration rate (eGRF) and microalbuminuria were evaluated as indexes of structural markers of atherosclerosis.
Results
After adjustment for risk factors, significant association was shown between baPWV and BMD in both genders (male: r=−0.084, P=0.035; female: r=−0.088, P=0.014). The correlation was stronger in females than in males, and in females, the correlation was stronger after menopause. Similarly, mean baPWV differed significantly according to the decreased BMD (normal BMD, Osteopenia, Osteoporosis). In contrast, no significant differences were observed for ABI, CIMT, eGFR or microalbuminuria with BMD.
Conclusions
Independent of confounding factors, low BMD is associated with the functional marker of subclinical atherosclerosis (increased baPWV), but not with structural markers (ABI, CIMT, eGFR or microalbuminuria) among healthy females and males.
Keywords: Low bone mineral density (BMD), atherosclerosis, health check-up, arterial stiffness
Introduction
Osteoporosis is a substantial worldwide public health issue (1). As a metabolic disorder, osteoporosis characterized low bone mass and bone microarchitectural deterioration. Atherosclerosis is a chronic disease characterized by thickening and losses of elasticity in the walls of arteries and exhibits a high rate of morbidity and mortality due to strokes, cardiovascular and peripheral vascular diseases (2). Recently, compelling evidence has suggested a direct, age-independent association between these two degenerative disorders (3-5). Lower bone mass and rapid bone loss are associated with an increased risk of developing atherosclerosis.
There are several measures of assessing subclinical atherosclerosis. The brachial-ankle PWV (baPWV) is a simple and non-invasive method to assess arterial stiffness and is known to be an independent predictor for cardiovascular events (6). The ankle-brachial index (ABI) is initially a simple measure of the severity of lower-extremity peripheral arterial disease (PAD). Later, it was shown that the ABI is an indicator of atherosclerosis at other vascular sites (7). Carotid artery intima-media thickness (CIMT) is a surrogate marker of generalized atherosclerosis and correlated with coronary artery disease and stroke (8). Renal dysfunction and albuminuria could be as new cardiovascular risk factors. Both estimated glomerular filtration rate (eGFR) and microalbuminuria share a common association with atherosclerosis, which may be explained in part by inflammation, metabolic abnormality of calcium and phosphorus, endothelial damage or dysfunction (9-11).
Several studies have evaluated the relationship between low bone mineral density (BMD) and some of the measures of subclinical atherosclerosis. However, most of the studies examined the relationship without adjusting risk factors of atherosclerosis and bone metabolism. And the study population are most of postmenopausal women with hypertension, diabetes or other cardiovascular diseases (12-14). So the apparent relationship may be distorted by the effects of these confounders. In addition, only a few studies have explored this association in the Asian population, and even they have reported conflicting results (15-17).
In the present study, we sought to improve upon these previous investigations in two ways. First, we examined the relationship between low BMD and atherosclerosis in healthy Chinese people from health check-up programs. The study subjects included both men and female, and also both premenopausal and postmenopausal women. Second, we extended the previous studies by examining baPWV, ABI, CIMT, eGRF and microalbuminuria; this expanded investigation allowed us to evaluated the overall measures of subclinical atherosclerosis with BMD.
Materials and methods
Study population
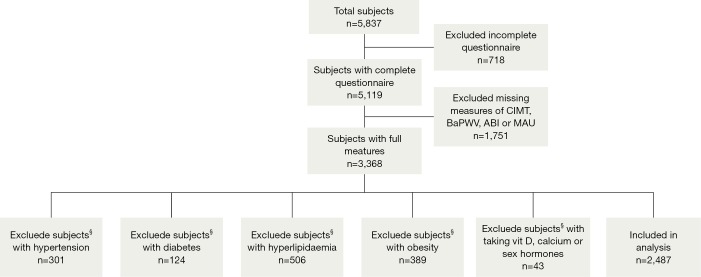
Study subjects comprised 5,837 consecutive individuals who visited the Third Xiangya Hospital of Central South University for health check-up programs including BMD measurement between 2012 and 2013. Subjects consisted of inhabitants of a mixed urban/rural area. Exclusion criteria are the participants who are known to be suffering from any major diseases which might affect atherosclerosis and bone metabolism, such as hypertension, diabetes mellitus, hyperlipidaemia, obesity, osteomalacia, history of osteoporotic fractures and so on. And taking medications with established effects on bone turnover (including vit D, calcium, corticosteroids, bisphosphonates and sex hormones) were also excluded. Consequently, 2,487 healthy participants were included in the analysis. The subjects selection diagram is described in Figure 1. Informed written consent was obtained from all of the participants and the study protocol was approved by the Ethics Guidelines Committee of the Central South University.
Figure 1.
Flow chat describing the subjects selection procedure. §, the subjects have a certain degree of overlap.
Hypertension was defined to be present in subjects with a history of hypertension who were taking anti-hypertensive drugs. Hyperlipidaemia was diagnosed in subjects with serum total cholesterol (TC) levels ≥6.22 mmol/L and/or triglyceride (TG) level ≥2.26 mmol/L and/or low-density lipoprotein cholesterol (LDL-C) level ≥4.14 mmol/L, or in subjects with history of cholesterol lowering therapy. Diabetic state was diagnosed in patients with fasting plasma glucose level ≥7.1 mmol/L and/or random plasma glucose level ≥11.1 mmol/L, or history of anti-diabetic therapy. In addition, the presence of obesity is regarded as body mass index (BMI) ≥28 kg/m2.
Clinical measurements
All participants underwent a routine clinical examination and a detailed questionnaire. Age, sex, height, weight, current medications from pill bottles, previous medical diagnoses, exercise, smoking history and alcohol consumption were recorded.
The term smokers included subjects who smoking >1 cigarette per day and last over 6 months. The alcohol consumption was defined as those who take beer, wine (including Chinese wine) or liquor at least two days per week and last over 12 months. The standard for exercise was physical activity for 30 min or longer and more than 3 times per week. BMI was calculated as body weight in kilograms divided by the square of body height in meters. Blood pressure (BP) was measured on the right upper arm in the sitting position after 10-15 min of seated rest using a validated digital automatic BP monitor.
Measurement of lumber spine BMD
The lumbar spine BMD was measured at the lumbar spine L1-L4 by dual-energy X-ray absorptiometry (DXA) with a Lunar prodigy densitometer (Discovery A, Hologic, USA). The results were interpreted in terms of BMD (g/cm2). According to the World Health Organization’s criteria, the participants were categorized into the normal BMD (T-score at –1.0 and above), osteopenia (T-score between –1.0 and –2.5), or osteoporosis (T-score at or below –2.5) groups (18). Daily quality control was carried out by the measurement of a Lunar phantom. To avoid inter-observer variability, all DXA measurements were performed by a well-trained technician who was blinded to subjects’ characteristics. The correlation coefficient of intraobserver reproducibility was 96.4%.
Measurement of baPWV and ABI
The baPWV and ABI were simultaneously measured with an automatic waveform analyser (Colin Co., Komaki, Japan). Briefly, after having taken at least a 5-min rest in the supine position, the subjects attached cuffs around their upper arms and ankles. The baPWVs were recorded and calculated using the formula (La–Lb)/△Tba, in which La is the distance from the heart to the ankle; Lb the distance from the heart to the brachium; and △Tba the transmission time between the brachial and posterior tibial artery waveforms. ABI was measured as the ratio of the ankle systolic BP divided by the arm systolic BP. The average baPWV and ABI from the left and right sides were used for the analysis. Two trained technicians performed the measurements of baPWV and ABI, and the inter-observer and intra-observer coefficients of variation were 4.9% and 7.8%, respectively.
Measurement of CIMT
Measurements of CIMT were performed on a common carotid artery and evaluated using a Siemens Acuson Sequoia™ 512 Ultrasound System (Mountain View, CA, USA) with a 10 MHz linear array transducer with the subjects in the supine position. The CIMT was identified as the distance between the leading edge of the lumen-intima echo and the leading edge of the media-adventitia echo, which was measured at three different sites on the left and right sides (the far wall of the common carotid artery, approximately 1.5 cm to proximal to the carotid bulb, as well as at sites 0.5 cm upstream and downstream, free from plaques) on the longitudinal views. We calculated the bilateral mean and then the average of both sides, using a total of six measurements per subject. Two experienced ultrasonography technicians conducted all of the ultrasound examinations. The correlation coefficients for inter- and intra-observer variability were 7.9% and 9.6%, respectively.
Laboratory measurements
Morning blood and urine samples were collected after at least 12 h of overnight fasting. Several chemical parameters such as fasting blood glucose (FBG), TC, TG, high-density lipoprotein cholesterol (HDL-C), LDL-C, serum creatinine (Scr), blood urea nitrogen (BUN) and serum uric acid (SUA) levels were obtained by standard enzymatic methods. Glomerular filtration rate (GFR) was estimated using the modified MDRD equation for the Chinese population (19). Microalbuminuria concentrations in our reference range (0-30 mg/dL) were evaluated by the immuneturbidimetry method.
Statistical analysis
There was a large gender difference in the BMD; therefore all analyses were stratified by sex. Statistical analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). Percent and means with standard deviations of variables were calculated for descriptive statistics. Multivariable linear regression models were applied to evaluate the association between BMD and variables of atherosclerosis, using BMD as the dependent variable and the ABI, baPWV, CIMT, eGFR and microalbuminuria as independent variables, respectively. Associations were adjusted for sets of covariates in two models. Model 1 adjusted for age. Model 2 adjusted additionally for body mass index, systolic BP, diastolic BP, TG, HDL-C, LDL-C, FBG, cigarette smoking (former + current), alcohol consumption (former + current), exercise and menopausal status (female only). Because the formula of eGFR referred to age and SCr, the parameter of age not included in the multivariate linear regression analysis to avoid confusing linearity within the regression model when eGFR was the independent variable. Multi-collinearity was not detected during screening for tolerance values of less than 0.15 for all variables in the fully adjusted models.
Pearson’s correlation coefficient analyses were used to examine the relationship between the values of BMD and the values of baPWV. Mean baPWV by BMD stratified (normal BMD, osteopenia and osteoporosis) were tested by analysis of covariance first after adjustment for age and then after full adjustments. Values of P<0.05 were considered statistically significant.
Results
Clinical characteristics of the study participants
The sex-specific characteristics of 1,467 male and 1,020 female participants are shown in Table 1. The average age of men was 45.72 years, and the average age of women was 44.71 years. For both men and women, the means of BMI, SBP, DBP, TG, TC, LDL-C, HDL-C and FBG were within normal range. All other variables were significantly lower in women than in men except exercise, HDL-C, eGFR and BMD is higher (P<0.05).
Table 1. Characteristics of participants distributed by gender.
| Characteristics | Male (n=1,467) | Female (n=1,020) | P value |
|---|---|---|---|
| Age (years) | 45.72±8.38 | 44.71±8.51 | 0.004 |
| BMI (kg/m2) | 23.94±2.38 | 22.04±2.37 | <0.001 |
| Systolic BP (mmHg) | 121.68±10.98 | 112.72±12.81 | <0.001 |
| Diastolic BP (mmHg) | 77.19±8.48 | 71.42±8.91 | <0.001 |
| TC (mmol/L) | 4.74±0.71 | 4.66±0.71 | <0.001 |
| LDL cholesterol (mmol/L) | 2.67±0.64 | 2.40±0.63 | 0.005 |
| TGs (mmol/L) | 1.37±0.44 | 1.04±0.40 | <0.001 |
| HDL cholesterol (mmol/L) | 1.45±0.32 | 1.78±0.37 | <0.001 |
| Fasting glucose (mmol/L) | 5.01±0.59 | 4.92±0.51 | <0.001 |
| eGFR (mL/min/1.73 m2) | 128.70±23.46 | 141.07±27.06 | <0.001 |
| Microalbuminuria (mg/L) | 20.41±38.60 | 17.00±35.16 | 0.025 |
| BaPWV (cm/s) | 1,321.10±175.88 | 1,226.11±200.35 | <0.001 |
| ABI | 1.12±0.07 | 1.08±0.07 | <0.001 |
| CIMT (mm) | 0.69±0.07 | 0.65±0.07 | <0.001 |
| BMD (L1-L4) (g/cm2) | 1.07±0.14 | 1.09±0.15 | <0.001 |
| No. of subjects with: (%) | |||
| Cigarette smoking | 832 (56.71) | 37 (3.63) | <0.001 |
| Alcohol consumption | 803 (54.73) | 253 (24.80) | <0.001 |
| Exercise | 327 (22.29) | 273 (26.76) | 0.010 |
| Menopausal status | 221 (21.67) |
BMI, body mass index; BP, blood pressure; TC, total cholesterol; LDL, low-density lipoprotein; TG, triglyceride; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; baPWV, brachial-ankle pulse wave velocity; ABI, ankle-brachial index; BMD, bone mineral density; CIMT, carotid intima-media thickness.
Associations between BMD and variables of subclinical atherosclerosis
Table 2 displays the results of multiple linear regression analysis of variables of subclinical atherosclerosis, used to evaluate their association with the BMD. The baPWV was significantly associated with BMD in both male and female after adjusting for age only (model 1) (male: β=−0.079, P=0.006; female: β=−0.121, P<0.001). When adjusted for other covariates (model 2), significantly associations persisted in both sexes (male: β=−0.064, P=0.035; female: β=−0.088, P=0.014). The other variables of subclinical atherosclerosis (ABI, CIMT, eGFR or microalbuminuria) failed to a reach statistical significance with the BMD.
Table 2. Multiple linear regression models to test variables with Lumbar spine BMD.
| Independent variable | Adjustment model | Male |
Female |
|||
|---|---|---|---|---|---|---|
| Standardised coefficients (β) | P value | Standardised coefficients (β) | P value | |||
| baPWV (cm/s) | Model 1 | −0.079 | 0.006 | −0.121 | <0.001 | |
| Model 2 | −0.064 | 0.035 | −0.088 | 0.014 | ||
| ABI | Model 1 | 0.050 | 0.061 | 0.03 | 0.304 | |
| Model 2 | 0.030 | 0.251 | 0.019 | 0.498 | ||
| CIMT (mm) | Model 1 | 0.015 | 0.606 | −0.032 | 0.295 | |
| Model 2 | −0.012 | 0.675 | −0.041 | 0.154 | ||
| eGFR (mL/min/1.73 m2) | Model 1 | −0.006 | 0.826 | −0.037 | 0.199 | |
| Model 2 | −0.001 | 0.979 | −0.013 | 0.646 | ||
| Microalbuminuria (mg/L) | Model 1 | −0.040 | 0.119 | 0.021 | 0.436 | |
| Model 2 | −0.027 | 0.296 | 0.026 | 0.322 | ||
Model 1, adjusted for age; Model 2, adjusted for the following: age, sex, cigarette smoking, alcohol consumption, exercise, menopausal status (female), BMI, systolic blood pressure, diastolic blood pressure, TG, HDL-C, LDL-C, fasting glucose. Categorical variables (sex, cigarette smoking, alcohol consumption, exercise, menopausal status) were used as dummy variable, therefore assigned values of 1 (yes) and 0 (no), respectively. BMI, body mass index; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Correlations of BMD with baPWV
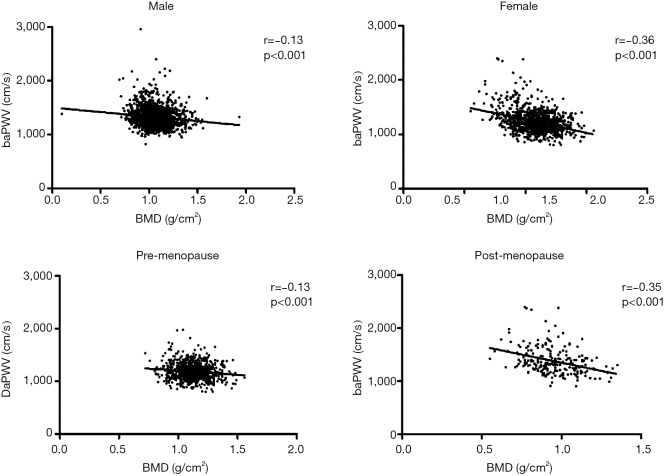
We detected that baPWV was an independent factor significantly with BMD. Pearson’s correlation was used to identify the BMD associated with the change in baPWV. Figure 2 shows baPWV significantly correlated with BMD in both genders. The correlation was stronger in females than in males (male: r=−0.013, P<0.001; female: r=−0.36, P<0.001). In females, the correlation was stronger in post-menopause than pre-menopause (pre-menopause: r=−0.13, P<0.001; post-menopause: r =−0.35, P<0.001).
Figure 2.
Relationship between baPWV and BMD in male, female, pre- and post-menopausal women. baPWV, brachial-ankle PWV; BMD, bone mineral density.
Comparison of mean baPWV according to the decrease in BMD
Table 3 lists the mean values (± SE) for baPWV according to the BMD classified as different groups (normal BMD, Osteopenia, Osteoporosis). The mean baPWV values differed significantly by the decrease in BMD and these differences remained significant after we adjusted for other covariates in both sexes (male: 1,310.85, 1,320.18, and 1,342.93 cm/s, respectively (P=0.029); female: 1,216.34, 1,231.57, and 1,269.11 cm/s, respectively (P=0.039).
Table 3. Comparison means of baPWV according to sex-specific of normal BDM, osteopenia, osteoporosis.
| BMD | Mean baPWV (mean ± SD) (cm/s) |
||||
|---|---|---|---|---|---|
| Male |
Female |
||||
| Model 1 | Model 2 | Model 1 | Model 2 | ||
| Normal | 1,307.87±7.45 | 1,310.85±7.03 | 1,212.00±6.92 | 1,216.34±6.64 | |
| Osteopenia | 1,319.27±5.64 | 1,320.18±5.35 | 1,237.15±8.88 | 1,231.57±8.37 | |
| Osteoporosis | 1,348.43±9.43 | 1,342.93±9.68 | 1,276.6±19.11 | 1,269.11±18.90 | |
| P | 0.009 | 0.029 | 0.004 | 0.039 | |
Model 1 and Model 2 adjusted for same confounders listed in Table 2. BMD, bone mineral density.
Discussion
The present study has several strengths. First, the study population is represented by a comparatively large sample size and was highly selected to exclude hypertension, diabetes mellitus, hyperlipidaemia, and cardiovascular disease as confounders to interfere with the credibility of the results. Second, cardiovascular risk factors as exercise, cigarette smoking, alcohol consumption and menopausal status are well characterized. Third, we explored the comprehensive measurements of subclinical atherosclerosis with BMD. To our knowledge, this is the first study to evaluate at the same time baPWV as a functional marker (sclerosis), ABI, CIMT, eGRF and microalbuminuria as structural markers (atherosis) in relation to BMD in a healthy population. Additionally, our study extends these findings to men and also the middle-aged group.
Our data demonstrate that, decreased BMD is significantly associated with baPWV in both genders, but not with ABI, CIMT, eGRF and microalbuminuria. After adjustment was made for several confounding factors, the relationship is weakened, but remained significant. The results indicated that the association was both age- and traditional cardiovascular risk-independent. As in this study, previous studies showed an association between BMD and baPWV mainly in women (16,17,20,21). Two Japanese studies found that lumbar spine BMD was associated with baPWV in postmenopausal women (16,20). A Korean study found an association between lumbar spine BMD and baPWV only in women, and no association between femur BMD and baPWV in both genders (21). Another Korean study found an association between total hip BMD and baPWV in women (17). However, there are some controversial results (22,23). In a study of 385 Chinese individuals, Liang et al. detected no association between total hip BMD and lumbar spine BMD with baPWV in both women and men (22). The above studies may have been limited by the sample size or the inability to adjust for many proposed confounding factors.
Although the correlation between BMD and baPWV was significant in both genders, it was weaker in men, raising the possibility of a sex-differential association. In women, the correlation was stronger after menopause. This may explain why estrogen might play a unique role in regulating the calcification of the bones and atherosclerosis. Bones and arteries are all target tissues for estrogen. Estrogen receptors have been identified on osteoclasts, osteoblasts, endothelial and smooth muscle cells in the arterial wall, indicating that estrogen has a direct effect on the bones and arteries (24). Estrogen also down-regulates the pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α. This increased state of pro-inflammatory cytokine activity is able to accelerate postmenopausal bone mineral loss and to change vascular homeostasis to favour the development of atherosclerosis (25). In addition, the lack of estrogens induces a decrease in osteoprotegerin (OPG), which may play an important role in the mechanisms of bone loss and atherogenesis (26). The changes in the action of estrogen deficiency would make the relationship between baPWV and BMD more conspicuous in females, especially in postmenopausal women.
The previous investigations gave little evidence for a correlation between ABI and BMD (15,22). But several studies have reported that a low bone density is associated with peripheral artery disease (PAD) (27,28). The patients with PAD were more likely to have osteoporosis or increased fracture risk. Therefore, the association between ABI and BMD could probably be observed in the stage of clinical atherosclerosis, rather than in any preclinical status, especially in healthy people free of cardiovascular diseases in our study.
Few studies have found an association between CIMT and BMD (22,23,29). Shaffer et al. reported decreased BMD is correlated with increased CIMT in older (>60 years of age) (30). de Almeida Pereira Coutinho et al. found a significant association between CIMT and BMD in men with type 2 Diabetes (31). In addition, some researchers detected that the presence of carotid plaque was associated with bone mineral loss (12,29). These findings indicated that the association between CIMT and BMD may be age- and cardiovascular risk factor-dependent or that it might be seen in relatively late-stage atherosclerosis, such as carotid plaque formation.
To our knowledge, this is the first study used eGRF and microalbuminuria as measures of subclinical atherosclerosis to investigate the association with BMD. The eGRF and microalbuminuria are surrogate markers that assess the cardiovascular subclinical organ damage. Recently, several reports have been noted that decreased eGFR and increased microalbuminuria were associated with atherosclerosis (10,11). The atherosclerotic process, especially in small vessels within the glomerular basement membrane may modify glomerular barrier permeability, thus leading to the excretion of albumin into the urine and the decreased eGFR. In our study, microalbuminuria and eGRF were not associated with BMD in both men and women. This result may be explained by the healthy subjects who were enrolled in our study, as their eGRF and microalbuminuria values were in the normal range, making the correlation not significant. The patients with chronic renal dysfunction (eGRF <60 mL/min per 1.73 m2) may disturb extracellular calcium and phosphorous metabolic to the bones, resulting in bone loss and extensive vascular calcification.
The exact mechanism behind the association between BMD and arterial stiffness is not completely understood. There are several potential mechanisms to explain this link. Both osteoporosis and atherosclerosis share similar or common risk factors. Bone-associated matrix proteins, homocysteine, high levels of OPG, inflammatory mediators, estrogen and vitamin D deficiency all play an important role both in bone metabolism and in the development of atherosclerosis (32). In addition, osteoporosis and cardiovascular diseases may share common genetic bases (33). Still, this claim needs to be clarified in the future research.
Atherosclerosis consists two distinguished process: one is structural process (atherosis) caused by lipid infiltration in cells and extracellularly, and the other is functional process (sclerosis) caused by connective tissue deposition and by functional disturbance of the endothelium, leading to reduced arterial compliance (34). In our study, the relationship exists between functional marker (baPWV) and BMD. Of note, however, is that no significant association was observed between structural markers (ABI, CIMT, eGRF or Microalbuminuria) and BMD. Therefore, we suppose that the association between atherosclerosis and BMD would be more prone to be conducted in the process of functional disturbance.
This study possesses some limitations. First, the BMD measured in the study was only at the lumbar spine, a single bone site. It was possible that degenerative conditions (osteophytosis and endplate sclerosis) contributed to increased lumbar spine BMD, potentially weakening the relationship between BMD and atherosclerosis. Second, in order to represent of healthy subjects, we used the factitious exclusion method to select the study population, which could introduce bias. Third, we did not measure bone metabolism regulators, such as OPG, osteopontin (OPN) and matrix Gla protein (MGP) levels, which could present possible mechanisms for the link between BMD and atherosclerosis. Finally, this is a cross-sectional design of this study. Therefore, no conclusions on causes or consequences can be made.
Conclusions
In conclusion, our results showed that decreased BMD is correlated with increased baPWV among healthy females and males, independent from any confounding factors. We suggest that lower BMD may be associated with arterial stiffness and a higher risk of cardiovascular disease. Therefore, we need to pay adequate attention to populations with lower BMD, to provide effective non-pharmacological and pharmacological interventions and to conduct further evaluations on vascular disease, especially in post-menopausal women.
Acknowledgements
Funding: Supported by Foundation of Hunan Provincial Science & Technology Department (2014SK3062); Supported by Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan of China (2013BAI04B01).
Disclosure: The authors declare no conflict of interest.
References
- 1.Franchitto A, Onori P, Renzi A, et al. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med 2013;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chivian E. Global Environmental Threats: why they are hard to see and how a medical model may contribute to their understanding. Cardiovasc Diagn Ther 2013;3:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farhat GN, Strotmeyer ES, Newman AB, et al. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcif Tissue Int 2006;79:102-11. [DOI] [PubMed] [Google Scholar]
- 4.Farhat GN, Newman AB, Sutton-Tyrrell K, et al. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int 2007;18:999-1008. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen L, Engstad T, Jacobsen BK. Bone mineral density in acute stroke patients: low bone mineral density may predict first stroke in women. Stroke 2001;32:47-51. [DOI] [PubMed] [Google Scholar]
- 6.Tomiyama H, Koji Y, Yambe M, et al. Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J 2005;69:815-22. [DOI] [PubMed] [Google Scholar]
- 7.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890-909. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14-22. [DOI] [PubMed] [Google Scholar]
- 9.Knight EL, Rimm EB, Pai JK, et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol 2004;15:1897-903. [DOI] [PubMed] [Google Scholar]
- 10.Choi SW, Kim HY, Lee YH, et al. eGFR is associated with subclinical atherosclerosis independent of albuminuria: the Dong-gu Study. Atherosclerosis 2010;212:661-7. [DOI] [PubMed] [Google Scholar]
- 11.Kong X, Jia X, Wei Y, et al. Association between microalbuminuria and subclinical atherosclerosis evaluated by carotid artery intima-media in elderly patients with normal renal function. BMC Nephrol 2012;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennisi P, Russo E, Gaudio A, et al. The association between carotid or femoral atherosclerosis and low bone mass in postmenopausal women referred for osteoporosis screening. Does osteoprotegerin play a role? Maturitas 2010;67:358-62. [DOI] [PubMed] [Google Scholar]
- 13.Mikumo M, Okano H, Yoshikata R, et al. Association between lumbar bone mineral density and vascular stiffness as assessed by pulse wave velocity in postmenopausal women. J Bone Miner Metab 2009;27:89-94. [DOI] [PubMed] [Google Scholar]
- 14.McFarlane SI, Qureshi G, Singh G, et al. Bone Mineral Density as a Predictor of Atherosclerosis and Arterial Wall Stiffness in Obese African-American Women. Cardiorenal Med 2012;2:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong SY, Kwok T, Woo J, et al. Bone mineral density and the risk of peripheral arterial disease in men and women: results from Mr. and Ms Os, Hong Kong. Osteoporos Int 2005;16:1933-8. [DOI] [PubMed] [Google Scholar]
- 16.Sumino H, Ichikawa S, Kasama S, et al. Elevated arterial stiffness in postmenopausal women with osteoporosis. Maturitas 2006;55:212-8. [DOI] [PubMed] [Google Scholar]
- 17.Seo SK, Cho S, Kim HY, et al. Bone mineral density, arterial stiffness, and coronary atherosclerosis in healthy postmenopausal women. Menopause 2009;16:937-43. [DOI] [PubMed] [Google Scholar]
- 18.Kanis JA, Melton LJ, 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137-41. [DOI] [PubMed] [Google Scholar]
- 19.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937-44. [DOI] [PubMed] [Google Scholar]
- 20.Mikumo M, Okano H, Yoshikata R, et al. Association between lumbar bone mineral density and vascular stiffness as assessed by pulse wave velocity in postmenopausal women. J Bone Miner Metab 2009;27:89-94. [DOI] [PubMed] [Google Scholar]
- 21.Kim NL, Jang HM, Kim SK, et al. Association of arterial stiffness and osteoporosis in healthy men undergoing screening medical examination. J Bone Metab 2014;21:133-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang DK, Bai XJ, Wu B, et al. Associations between bone mineral density and subclinical atherosclerosis: a cross-sectional study of a Chinese population. J Clin Endocrinol Metab 2014;99:469-77. [DOI] [PubMed] [Google Scholar]
- 23.Yamada S, Inaba M, Goto H, et al. Associations between physical activity, peripheral atherosclerosis and bone status in healthy Japanese women. Atherosclerosis 2006;188:196-202. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999;340:1801-11. [DOI] [PubMed] [Google Scholar]
- 25.Pfeilschifter J, Köditz R, Pfohl M, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002;23:90-119. [DOI] [PubMed] [Google Scholar]
- 26.Min H, Morony S, Sarosi I, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med 2000;192:463-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis 2014;6:1120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins TC, Ewing SK, Diem SJ, et al. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation 2009;119:2305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyder JA, Allison MA, Barrett-Connor E, et al. Bone mineral density and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis, Abdominal Aortic Calcium Study. Atherosclerosis 2010;209:283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaffer JR, Kammerer CM, Rainwater DL, et al. Decreased bone mineral density is correlated with increased subclinical atherosclerosis in older, but not younger, Mexican American women and men: the San Antonio Family Osteoporosis Study. Calcif Tissue Int 2007;81:430-41. [DOI] [PubMed] [Google Scholar]
- 31.de Almeida Pereira Coutinho M, Bandeira E, de Almeida JM, et al. Low Bone Mass is Associated with Increased Carotid Intima Media Thickness in Men with Type 2 Diabetes Mellitus. Clin Med Insights Endocrinol Diabetes 2013;6:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldini V, Mastropasqua M, Francucci CM, et al. Cardiovascular disease and osteoporosis. J Endocrinol Invest 2005;28:69-72. [PubMed] [Google Scholar]
- 33.Marini F, Brandi ML. Genetic determinants of osteoporosis: common bases to cardiovascular diseases? Int J Hypertens 2010;2010. [DOI] [PMC free article] [PubMed]
- 34.Blankenhorn DH, Kramsch DM. Reversal of atherosis and sclerosis. The two components of atherosclerosis. Circulation 1989;79:1-7. [DOI] [PubMed] [Google Scholar]