Abstract
Background
Obstructive sleep apnoea (OSA) is the most common form of sleep-disordered breathing. The standard treatment, continuous positive airway pressure (CPAP), has limited long-term compliance. Alternative treatment options are required and new methods, including hypoglossal nerve stimulation (HNS) and continuous transcutaneous electrical stimulation (CTES), are currently emerging. We report on patients’ preference for different treatments of OSA.
Methods
We recorded patients’ age, gender, body mass index (BMI), Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire with 10 questions (FOSQ10), severity of OSA, and current treatment. We showed pictures of existing [CPAP, mandibular advancement device (MAD)] and emerging treatments (HNS and CTES). We then asked (I) whether participants were interested in further information about HNS/CTES; (II) if they would be willing to try HNS/CTES; and (III) if they were to choose only one of the four listed treatments, which one would they prefer to use every night.
Results
One hundred sixty-two patients completed the survey {81 males, mean age 52 [12] years, BMI 34 [7] kg/m2, ESS 10.2 (6.0) points, FOSQ10 28.5 (8.1) points}. The majority of the respondents (89.5%) had been diagnosed with OSA. A total of 91.3% of the respondents were interested in more information and were willing to try HNS/CTES. Most respondents preferred the potential use of CTES (56.7%), while 21.7% chose HNS, 17.8% CPAP, and 3.8% the MAD. There were no differences in the characteristics of the patients who preferred CTES compared to those who preferred other treatments, but a regression analysis revealed that a low ESS score was an independent predictor of patients choosing CTES (P<0.05).
Conclusions
More than 9 out of 10 of the respondents were interested in trying emerging technologies to treat OSA, most preferring CTES. Less sleepy patients were more likely to choose less invasive treatments. These findings will likely impact on future research and development of therapies for sleep-disordered breathing.
Keywords: Sleep apnoea, continuous positive airway pressure (CPAP), sleepiness, electrical stimulation
Introduction
Obstructive sleep apnoea (OSA) is the most common form of sleep-disordered breathing, affecting up to 10% of middle-aged men and 3% of middle-aged women (1). It is characterised by intermittent and repeated episodes of upper airway obstruction during sleep and can result in irregular breathing at night and excessive sleepiness during the day (2). OSA is also associated with several co-morbidities, including hypertension (3), ischaemic heart disease (4), stroke (5), congestive heart failure (6), obesity (7), and diabetes (8).
Permanent tracheostomy was the first effective therapy for OSA, and it was the most common treatment used for OSA in the 1970’s and early 1980’s (9). The management of OSA was revolutionized in 1981 by the introduction of continuous positive airway pressure (CPAP), which can completely prevent upper airway occlusion during sleep in patients with OSA (10). In a subsequent study done 2 years later, Sullivan et al. also showed that CPAP is an effective treatment for patients with severe obesity-hypoventilation syndrome and can lead to remission of the underlying disordered breathing during sleep (11). Since its development in the 1980’s, CPAP has been shown to reduce the symptoms of OSA, improve the risk for associated co-morbidities, especially arterial hypertension, and potentially impact on mortality in patients with severe OSA (12,13).
According to current guidelines, CPAP therapy is the standard treatment in patients with moderate to severe OSA (14). The effectiveness of CPAP therapy, however, is dependent on patients’ adherence and symptomatic response to the treatment (15). At the least, one third of all patients initiated on CPAP therapy do not use the treatment at 5 years. Long-term CPAP use can be predicted by the uptake within the first 3 months and is dependent on disease severity and daytime sleepiness (16).
Because of the limited long-term compliance to CPAP, alternative treatments for OSA are required for those patients that fail to comply or respond to CPAP therapy. Mandibular advancement devices (MADs) have been developed to reposition the jaw and keep the upper airway patent during sleep. These devices have been shown to reduce the severity of OSA and improve daytime sleepiness. MADs are currently recommended for the treatment of mild OSA (17).
Since 2011, electrical stimulation of the upper airway dilator muscles has emerged as a potential alternative to CPAP, as upper airway patency is correlated with the electromyographic activity of the genioglossus muscle. Strollo et al. have shown that unilateral stimulation of the hypoglossal nerve by a surgically implanted neuro-stimulator device significantly reduces the severity of OSA, daytime sleepiness, and improves quality of life (18). Consequently, hypoglossal nerve stimulation (HNS) has recently been approved by the US Food and Drug Administration (FDA) to treat moderate to severe OSA (19).
HNS, however, involves the surgical implantation of a neuro-stimulator and is highly expensive. A non-invasive form of electrical stimulation could be advantageous in that it offers to assess patients with limited risk and at low costs. Continuous transcutaneous electrical stimulation (CTES) involves low-current stimulation of the genioglossus muscle. CTES has been shown to reduce ventilatory load and neural drive in patients with OSA to a similar degree as HNS (20). The efficacy of CTES as a treatment option for OSA, however, is still being evaluated. The current published studies on the non-invasive use of electrical stimulation have revealed varying results, which is likely to reflect the lack of standardisation of the approach (21).
The low compliance remains the significant limitation of CPAP as a long-term treatment for OSA. It is crucial that patients can tolerate any long-term treatment for OSA, and in this overview we present data on treatment preferences of patients with OSA and discuss the implications.
Methods
We performed face-to-face interviews in outpatient and inpatient clinics in the Sleep Disorders Center and Lane Fox Respiratory Unit at Guy’s and St. Thomas’ NHS Foundation, London, UK in addition to using a web platform supported by a patients’ group to distribute a survey (local registration number 2014-4621).
In the survey, we recorded patients’ age, gender, body mass index (BMI), Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire with 10 questions (FOSQ10), diagnosis of OSA, and the current treatment.
We showed pictures of existing treatments (CPAP, MAD) and emerging treatments for OSA (HNS and CTES). The survey concluded by asking:
Whether patients would like to receive more information about HNS and CTES;
If the participants would be willing to try out HNS or CTES;
If they had to choose one of the four listed treatments (CPAP, MAD, HNS, CTES) to use every night to treat their OSA, which would they prefer to use.
Participants were given the one page survey along with the pictures of the CPAP, MAD, HNS, and CTES. The online survey contained the same questions and pictures as the survey that was distributed in person. We did not answer questions about the survey or treatments in order to standardise the approach between face-to-face interviews and online participants. A link to the online survey was distributed by one of our expert patients at Guy’s and St. Thomas’ NHS Foundation Trust.
Statistical analysis
Data were analysed using GraphPad Prism (Version 5.02, GraphPad Software Inc., San Diego, California, USA) and SPSS statistics 20 (IBM, New York, USA). Data are presented as mean (standard deviation, SD) unless otherwise indicated. When we compared the data of the two subgroups of patients who underwent a face-to-face or an online interview, we used t-tests for continuous variables and Fisher’s exact test for categorical variables. We divided our sample of patients according to treatment preferences, and data were further analysed using a one-way ANOVA with a Kruskal-Wallis test and Dunn’s correction for multiple test comparisons. Lastly, logistic regression was employed to identify correlations between independent variables (gender, age, BMI, FOSQ10, ESS, OSA severity) and treatment preference for CTES as the dependent variable. A level of significance was defined as P<0.05.
Results
A total of 136 patients filled out the online survey {64 males, mean age 51 [11] years, BMI 34.4 (7.3) kg/m2, ESS 10.1 (6.0) points, FOSQ10 27.9 (8.3) points} and 26 patients completed the survey in a face-to-face interview {17 males, mean age 58 [14] years, BMI 30.7 (7.1) kg/m2, ESS 10.6 (5.6) points, FOSQ10 31.5 (5.8) points}. The online group and face-to-face interview group were similar in terms of gender, BMI, daytime sleepiness, and quality of life related to OSA; however, patients in the face-to-face interview group were older than the patients in the online group (Table 1).
Table 1. Main characteristics of the subgroups of interviewed patients.
| Items | All patients (n=162) | Online survey (n=136) | Face-to-face interview (n=26) | P value |
|---|---|---|---|---|
| Gender (male/female) | 81/81 | 64/72 | 9/17 | 0.133 |
| Age (years) | 52 [12] | 51 [11] | 58 [14] | 0.011 |
| Height (m) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 0.100 |
| Weight (kg) | 98.8 (23.2) | 99.8 (22.7) | 92.8 (26.2) | 0.135 |
| BMI (kg/m2) | 33.9 (7.3) | 34.4 (7.3) | 30.7 (7.1) | 0.056 |
| ESS (points) | 10.1 (5.9) | 10.1 (6.0) | 10.6 (5.6) | 0.830 |
| FOSQ10 (points) | 28.5 (8.1) | 27.9 (8.3) | 31.5 (5.8) | 0.097 |
BMI, body mass index; ESS, Epworth Sleepiness Scale; FOSQ10, Functional Outcomes of Sleep Questionnaire with 10 questions.
We combined the online and face-to-face interview groups to analyse the remaining data. The majority of the respondents (89.5%) had been diagnosed with OSA, and 46.9% of the patients had severe OSA. A total of 91.7% of the patients diagnosed with OSA were on treatment, while 8.3% were not receiving any treatment. A total of 95.4% of the respondents who received treatment were using CPAP, while 1.5% used MAD, and 3.1% used an alternative form of treatment. A total of 91.3% of all respondents were interested in more information and were willing to try emerging treatments, HNS or CTES. Most respondents (56.7%) preferred the potential use of CTES as a treatment for OSA, while 21.7% chose HNS, 17.8% preferred CPAP, and 3.8% the MAD (Figure 1). There were no differences between the patients who preferred different treatments in terms of age, BMI, gender, quality of life, and OSA severity (Table 2). A regression analysis included age, gender, OSA severity, quality of life, and daytime sleepiness as possible predictors of CTES preference. The analysis showed that a low ESS score was an independent predictor of patients preferring CTES (P<0.05, Table 3).
Figure 1.
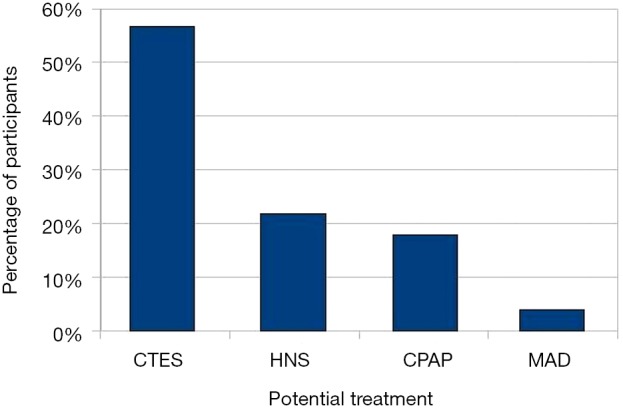
Patients’ preferences for potentially available treatments for OSA. CTES, continuous transcutaneous electrical stimulation; HNS, hypoglossal nerve stimulation; CPAP, continuous positive airway pressure; MAD, mandibular advancement device; OSA, obstructive sleep apnoea.
Table 2. Characteristics according to preferred treatments.
| Items | CTES (n=75) | HNS (n=32) | CPAP (n=20) | P value |
|---|---|---|---|---|
| Gender (M/F) | 37/38 | 15/17 | 9/11 | 0.932 |
| Age (years) | 53 [12] | 51 [9] | 48 [11] | 0.225 |
| BMI (kg/m2) | 34.3 (7.7) | 34.5 (6.5) | 35.3 (7.8) | 0.896 |
| ESS (points) | 9.1 (6.0) | 11.6 (5.4) | 11.2 (6.4) | 0.056 |
| FOSQ10 (points) | 28.5 (8.7) | 26.9 (7.8) | 27.9 (7.8) | 0.576 |
| OSA severity | ||||
| Mild OSA (%) | 12.6 | 13.3 | 11.1 | 0.975 |
| Moderate OSA (%) | 28.5 | 30.0 | 38.8 | 0.702 |
| Severe OSA (%) | 58.7 | 56.6 | 50.0 | 0.805 |
CTES, continuous transcutaneous electrical stimulation; HNS, hypoglossal nerve stimulation; CPAP, continuous positive airway pressure; M, male; F, female; BMI, body mass index; ESS, Epworth Sleepiness Scale; FOSQ10, Functional Outcomes of Sleep Questionnaire with 10 questions; OSA, obstructive sleep apnoea.
Table 3. Multiple regression analysis to determine patient’s treatment preferences.
| Items | Coefficientsa |
t | Sig. | |||
|---|---|---|---|---|---|---|
| Unstandardized coefficients |
Standardized coefficients |
|||||
| B | Std. error | Beta | ||||
| Constant | 0.663 | 0.371 | 1.787 | 0.077 | ||
| Age | 0.008 | 0.004 | 0.173 | 1.805 | 0.074 | |
| BMI | 8.87E-05 | 0.004 | 0.002 | 0.023 | 0.981 | |
| Gender | −0.010 | 0.095 | −0.010 | −0.107 | 0.915 | |
| ESS | −0.021 | 0.010 | −0.250 | −2.132 | 0.035 | |
| OSA severity | −0.003 | 0.051 | −0.005 | −0.057 | 0.954 | |
| FOSQ10 | −0.009 | 0.007 | −0.157 | −1.267 | 0.208 | |
a, dependent variable is CTES preference, R2, 0.59. BMI, body mass index; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnoea; FOSQ10, Functional Outcomes of Sleep Questionnaire with 10 questions.
Discussion
Over 90% of patients, most of them treated with CPAP, were interested in more information and were willing to try emerging treatments for OSA. Non-invasive electrical stimulation was the most preferred treatment compared with invasive electrical stimulation, and followed by CPAP and MADs. Less than one fifth of the respondents preferred to use CPAP, the current standard treatment for OSA. Less symptomatic patients, as demonstrated by a lower Epworth score, were more likely to choose a less invasive treatment option.
These results are important as new therapies for OSA are being developed. The biggest limitation of CPAP as a treatment for OSA is a relatively low long-term compliance, as demonstrated by McArdle et al., who found that more than one-third of patients started on CPAP do not use it at all after 5 years (16). The benefits of controlled OSA include a reduction in daytime sleepiness and, in addition, less complications due to associated co-morbidities, such as hypertension, ischaemic heart disease, and stroke. These benefits are not seen, however, if patients cannot tolerate and comply with long-term treatment.
It is crucial, therefore, that alternative treatments to CPAP therapy are developed and tested. While these therapies are being developed, we need to take patients’ preferences into consideration in order to achieve a good long-term compliance to OSA treatment, as it is likely that new treatments will not provide cure from the condition.
There are limitations to this study, however, with a limited dataset of only 162 patients filling out the survey. This was a pre-selected cohort of mainly patients on CPAP, and the results require cautious interpretation due to a potential reporting bias. Nonetheless, the survey can be used as a guidance of public and patient involvement for future research interests aimed at developing alternative treatments for OSA.
Conclusions
Compliance remains the most significant limitation of CPAP as a long-term treatment for OSA. Due to significant short- and long-term complications of untreated OSA, the development of alternative treatments to CPAP is crucial to guarantee patients’ adherence. The majority of patients that we tested preferred the potential use of emerging technologies and would potentially be available to test these methods in clinical trials. The preferred option was non-invasive electrical stimulation (CTES), followed by the invasive electrical stimulation (HNS), CPAP, and MADs. These findings are important in that they can be used to support future research applications into non-CPAP treatment methods for OSA.
Acknowledgements
We are grateful for the support of Richard Brown, Adam Birdseye, and Karen Bacon, who helped distribute our survey. This work was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Authors’ contributions: MF Pengo and J Steier designed overall study. T Campbell, MF Pengo, and J Steier designed survey, and T Campbell distributed the survey, collected and compiled results, and wrote the paper. MF Pengo analysed the results for significance, and MF Pengo and J Steier edited the paper.
Disclosure: The authors declare no conflict of interest.
References
- 1.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strollo PJ, Jr, Rogers RM. Obstructive sleep apnea. N Engl J Med 1996;334:99-104. [DOI] [PubMed] [Google Scholar]
- 3.Narkiewicz K, Somers VK. Obstructive sleep apnea as a cause of neurogenic hypertension. Curr Hypertens Rep 1999;1:268-73. [DOI] [PubMed] [Google Scholar]
- 4.Martinez D, Klein C, Rahmeier L, et al. Sleep apnea is a stronger predictor for coronary heart disease than traditional risk factors. Sleep Breath 2012;16:695-701. [DOI] [PubMed] [Google Scholar]
- 5.Palomäki H, Partinen M, Juvela S, et al. Snoring as a risk factor for sleep-related brain infarction. Stroke 1989;20:1311-5. [DOI] [PubMed] [Google Scholar]
- 6.Bradley TD, Rutherford R, Grossman RF, et al. Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis 1985;131:835-9. [DOI] [PubMed] [Google Scholar]
- 7.Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J 2009;34:243-60. [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Sorkin JD, Katzel LI, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 2002;165:677-82. [DOI] [PubMed] [Google Scholar]
- 9.Mehra P, Wolford LM. Surgical management of obstructive sleep apnea. Proc (Bayl Univ Med Cent) 2000;13:338-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983;128:177-81. [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. [DOI] [PubMed] [Google Scholar]
- 13.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [DOI] [PubMed] [Google Scholar]
- 14.Resource & Cost Impact Report for Scotland and by NHS Board. Continuous Positive Airway Pressure (CPAP) for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Available online: http://www.healthcareimprovementscotland.org/idoc.ashx?docid=18137349-a5bd-42ee-992a-7d954474856e&version=-1
- 15.Sawyer AM, Gooneratne NS, Marcus CL, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 2011;15:343-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:1108-14. [DOI] [PubMed] [Google Scholar]
- 17.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J 2011;37:1000-28. [DOI] [PubMed] [Google Scholar]
- 18.Strollo PJ, Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370:139-49. [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. Inspire Upper Airway Stimulation - P130008. Available online: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm398321.htm
- 20.Steier J, Seymour J, Rafferty GF, et al. Continuous transcutaneous submental electrical stimulation in obstructive sleep apnea: a feasibility study. Chest 2011;140:998-1007. [DOI] [PubMed] [Google Scholar]
- 21.Pengo MF, Steier J. Emerging technology: electrical stimulation in obstructive sleep apnoea. J Thorac Dis 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


