Abstract
Background
This study aims to explore the feasibility and safety of video-assisted thoracic surgery (VATS) double sleeve lobectomy in patients with non-small lung cell cancer (NSCLC).
Methods
Between June 2012 and August 2014, 13 NSCLC patients underwent thoracoscopic double sleeve lobectomy and mediastinal lymphadenectomy at three institutions. A retrospective analysis of clinical characteristics, operative data, postoperative events and follow-up was performed.
Results
Thirteen NSCLC patients (median age, 60 years; range, 43-67 years) underwent thoracoscopic double sleeve lobectomy. There were no conversions to thoracotomy. Left upper lobectomy was most frequently performed (eleven patients). Median operative time was 263 minutes (range, 218-330 minutes), and median blood loss was 224 mL (range, 60-400 mL). The learning curve revealed reductions in both operative times and blood loss of ten cases from one center. Median data were duration of blocking pulmonary artery (PA) 72 minutes (range, 44-143 minutes), resected lymph nodes 24 (range, 10-46), stations of retrieved lymph nodes 6 (range, 5-9), thoracic drainage 1,042 mL (range, 500-1,700 mL), duration of thoracic drainage 5 days (range, 3-8 days), postoperative hospital stay 10 days (range, 7-20 days), and ICU stay 1 day (range, 1-2 days). One patient (1/13, 7.70%) suffered from pneumonia after surgery. There were no deaths at 30 days. Median duration of follow-up was 6 months (range, 1-26 months). And no local recurrences or distant metastasis were reported.
Conclusions
Thoracoscopic double sleeve lobectomy is a technically challenging, but feasible procedure for NSCLC patients and it should be restricted to skilled VATS surgeons.
Keywords: Non-small lung cell cancer (NSCLC), video-assisted thoracic surgery (VATS), sleeve lobectomy, learning curve
Introduction
Cao and his colleagues (1) reported that video-assisted thoracic surgery (VATS) lobectomy for non-small lung cell cancer (NSCLC) can yield similar long-term survival outcomes with conventional open lobectomy. With the widely application of VATS technique, the indication of this procedure has been greatly broadened, and the technical barriers have been constantly broken (2,3). As an less invasive alternative procedure to total pneumonectomy in patients with locally advanced tumors involving the pulmonary artery (PA) and bronchus (4), sleeve lobectomy by conventional thoracotomy, especially double sleeve lobectomy (vascular and bronchial) is still the preferred approach due to high difficulty in operation and potentially undesirable complications, even when performed by skilled VATS surgeons.
Since the first reported VATS bronchial sleeve lobectomy was published (5), more and more technical challenges have become reality, which would be VATS angioplasty (6-8), uniportal VATS bronchial sleeve lobectomy (9,10), and even thoracoscopic double sleeve lobectomy (11-13). However, all of these reports were single center experiences, and most series were less than five patients. Hence, the thoracic society urged for a multi-center data of thoracoscopic double sleeve lobectomy to addresses the feasibility and safety of this operation. In this study, we present the first multi-center experiences of thoracoscopic double sleeve lobectomy.
Patients and methods
The medical ethics board of all participating hospitals approved the study. Between June 2012 and August 2014, 13 patients underwent a thoracoscopic double sleeve lobectomy including mediastinal lymphadenectomy for primary NSCLC at the First Affiliated Hospital of Guangzhou Medical University (Guangzhou, China), Coruña University hospital (Coruña, Spain) and Tyumen Regional Cancer Center (Tyumen, Russia). Clinical records of the patients were retrospectively analyzed.
All patients were diagnosed with NSCLC by bronchoscopy. Preoperative staging was determined mainly by enhanced thoracic computerized tomography, brain magnetic resonance or computed tomography (CT), and bone scintigraphy, except that one patient received positron emission tomography/CT (PET-CT). Physical examination, standard laboratory tests, electrocardiograms, and lung function tests were performed in all patients.
There were two patients with clinic N2 disease, and both received induction chemotherapy. One patient with squamous cell carcinoma had four cycles of paclitaxel + cisplatin before surgery (case 6), the other patient with adenocarcinoma had six cycles of pemetrexed + cisplatin (case 11). Two patients rejected to receive adjuvant chemotherapy.
Surgical technique
All patients received a combination of epidural and general anesthesia before the operation. The patients were placed in a lateral decubitus position. All 13 procedures were performed via 3-4 ports, or uniportal. The detailed port design for different methods was described in Table 1.
Table 1. The ports design of thoracoscopic double sleeve lobectomy.
| Method | Case | Ports | Camera port | Operative port | VBF port 1 | VBF port 2 |
|---|---|---|---|---|---|---|
| A | Case 1-2 | 3 | Midaxillary line/7th ICS/10 mm | Preaxilary line/4th ICS/3.5 cm | Postaxillary line/7th ICS/10 mm | – |
| B | Case 3 | 3 | Postaxillary line/7th ICS/10 mm | Preaxilary line/4th ICS/3.5 cm | Midaxillary line/7th ICS/10 mm | – |
| C | Case 4-10 | 4 | Midaxillary line/7th ICS/10 mm | Preaxilary line/3th ICS/3.5 cm | Postaxillary line/7th ICS/10 mm | Anterior chest/PA level/5 mm |
| D | Case 11-13 | 1 | Preaxilary line/4th or 5th ICS/4-5cm | |||
VBF, vascular blocking forcep; ICS, intercostal space; PA, pulmonary artery.
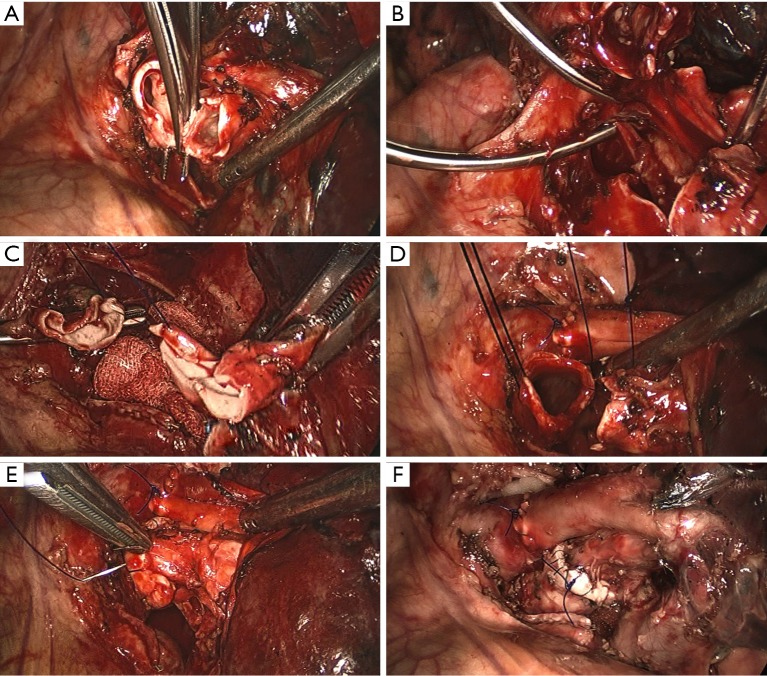
Before dissection, the mediastinal pleura were inspected to assess the mobility of the tumor and its invasion into surrounding structures. Once radical surgery (Figure 1) was guaranteed, the superior or inferior pulmonary vein would be dissected and then transected with endostapler (Ethicon Endo-Surgery, Johnson & Johnson, Cincinnati, OH, USA). The main bronchus and distal bronchus were transected with a long handle blade and scissor (Figure 1A).
Figure 1.
Surgical technique of thoracoscopic double sleeve lobectomy. (A) Transecting the main bronchus; (B) sleeve resecting the blocked PA; (C) sleeve reconstructing the blocked PA; (D) completed vascular reconstruction and starting sleeve bronchial reconstruction; (E) sleeve reconstructing the bronchus; (F) over view of double sleeve reconstruction. PA, pulmonary artery.
After the main PA was dissected, there were four methods with different port design to clamp the PA: (method A) two patients (case 1-2) underwent three ports thoracoscopic double sleeve lobectomy (Table 1). One pair of vascular blocking forceps was placed through the operative port (3.5 cm) on the proximal PA, and the other pair of forceps was placed through the left port (10 mm) on the distal PA (Figure 2); (method B) one patients (case 3) underwent three ports thoracoscopic double sleeve lobectomy (Table 1). One pair of vascular blocking forceps was placed through the operative port (3.5 cm) on the proximal PA. Different with method A, the other pair of forceps was placed through the camera port (10 mm) on the distal PA (Figure 3); (method C) seven patients (case 4-10) underwent four ports thoracoscopic double sleeve lobectomy (Table 1). One pair of vascular blocking forceps was placed through a 5-mm port located in anterior chest wall at the level of the proximal PA, and the other pair of forceps was placed through the posterior axillary line port (10 mm) on the distal PA (Figure 4); (method D) three patients (case 11-13) underwent uniportal thoracoscopic double sleeve lobectomy (Table 1). A bulldog clamp was used for the distal PA while the vascular blocking forceps were used to clamp the proximal PA (Figure 5). After the PA clamp was completed, the invasive part of main PA was resected (Figure 1B). The surgical technique for PA circumferential sleeve resection is similar to previous reports (11,14). The wedge anastomosis for uniportal approach would only be applied if the tumor invasion was less than 1/3 of the circumference and 2 cm width of the basilar part. After confirming the resected margin of PA, the PA was reconstructed with a primary closure using 4-0 Prolene (Ethicon, Somerville, NJ, USA) (Figure 1C). A standard needle holder and a pair of forceps were inserted to complete running suture through the 3.5-5 cm operative port (Table 1). After the bronchial margins were confirmed as negative by intraoperative frozen section, the bronchial sleeve reconstruction was performed by using a 3-0 Prolene (Ethicon, Somerville, NJ, USA) for cartilaginous and membranous portions (Figure 1D,E). The residual lobe was inflated and no air leakage was detected underwater. Then, the distal clamp was removed before tying the arterial sutures to remove the intravascular air. The proximal clamp was finally removed to ensure hemostasis of the sewn PA (Figure 1F).
Figure 2.
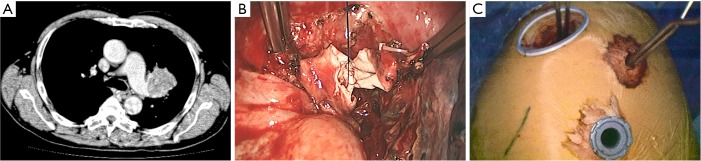
Method A: ports design of thoracoscopic double sleeve lobectomy.
Figure 3.
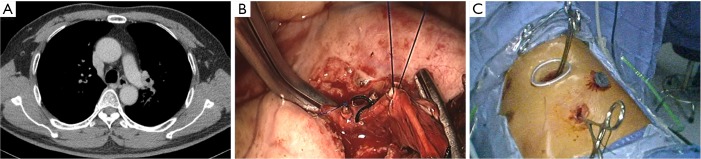
Method B: ports design of thoracoscopic double sleeve lobectomy.
Figure 4.
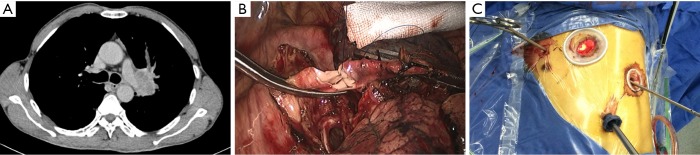
Method C: ports design of thoracoscopic double sleeve lobectomy.
Figure 5.
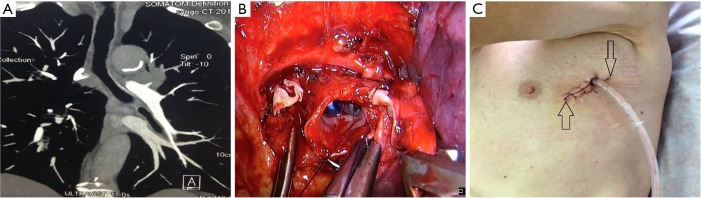
Method D: ports design of thoracoscopic double sleeve lobectomy.
During uniportal thoracoscopic double sleeve lobectomy, bronchial sleeve reconstruction was completed before angioplasty to avoid traction on the arterial suture. In three cases (case 1, case 3 and case 6), pericardium, pleura and other tissue were used to separate the PA and bronchus to prevent bronchial artery fistula.
Thoracic surgery was completed by placement of one or two intercostal drainage tubes and closure of the thoracic incisions. Postoperative bronchoscopy is then performed to clear the airways of blood and secretions before extubation.
Statistical analysis
Clinical information was recorded in Microsoft EXCEL (Microsoft Corp, Redmond, WA, USA) for further processing. Enumeration data were presented with frequencies and percentages. Measurement data were presented with median and range.
Results
The clinical characteristics of all patients were summarized in Table 2. All thirteen patients were males. Median age was 60 years (range, 43-67years). Twelve patients (12/13, 92.3%) had a smoking history. Ten patients (10/13, 76.9%) were diagnosed as squamous carcinoma, while two patients (2/13, 15.4%) were adenocarcinoma, and one patient (1/13, 7.7%) was adenosquamous carcinoma. The location of the tumors was as follow: eleven left upper lobe (LUL) (11/13, 84.6%), one right upper lobe (RUL) (1/13, 7.7%), and one left lower lobe (LLL) (1/13, 7.7%). There were ten invasion of main PA (10/13, 76.9%), and two invasion of branch PA (2/13, 15.4%).
Table 2. Patient characteristics.
| Character | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 60 | 67 | 58 | 57 | 54 | 63 | 62 | 67 | 60 | 43 | 65 | 66 | 52 |
| Gender | Male | Male | Male | Male | Male | Male | Male | Male | Male | Male | Male | Male | Male |
| Smoking history | 40 y, 40/d | 30 y, 20/d | 35 y, 20/d | 35 y, 60/d | 25 y, 60/d | 50 y, 60/d | 40 y, 20/d | 30 y, 20/d | 40 y, 40/d | No | 31 y, 40/d | 40 y, 40/d | 32 y, 40/d |
| Histology | Sqa | Ad-Sqa | Sqa | Sqa | Sqa | Sqa | Sqa | Sqa | Sqa | Ade | Ade | Sqa | Sqa |
| Tumor size | 4.5×3.4 | 5.7×5.3 | 3.0×2.2 | 5.8×4.8 | 4.3×3.2 | 3.8×2.2 | 4.8×4.3 | 3.6×3.5 | 1.6×1.1 | 7.6×7.4 | 7.5×7.5 | 5 | 5 |
| Pathological stage | T3N1M0 | T3N1M0 | T3N0M0 | T3N0M0 | T3N1M0 | T3N1M0 | T3N2M0 | T3N0M0 | T3N2M0 | T3N1M0 | T3N0M0 | T3N1M0 | T2aN0M0 |
| Location | LUL | LUL | LUL | LUL | LUL | LUL | LLL | LUL | RUL | LUL | LUL | LUL | LUL |
| PA invasion | Main | Main | Branch | Main | Main | Main | Main | Main | Branch | Main | Main | Main | – |
| Pulmonary function | |||||||||||||
| FEV1 | 2.71 | 2.58 | 2.67 | 2.98 | 2.73 | 1.79 | 2.48 | 2.32 | 1.86 | 3.35 | NA | NA | NA |
| FEV1% | 93.33 | 87.61 | 88.73 | 89.17 | 74 | 63.15 | 87.52 | 92.97 | 69.58 | 92.53 | 109 | NA | NA |
| MVV | 120.6 | 88.86 | 78.43 | 109.4 | 100.7 | 80.9 | 102.58 | 96.3 | 99.51 | 125.9 | NA | NA | NA |
Sqa, squamous carcinoma; Ad-Sqa, adenosquamous carcinoma; Ade, adenocarcinoma; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; PA, pulmonary artery; NA, no available; FEV1, forced expiratory volume in first second; MVV, maximal ventilatory volume; y, years.
The operative data of all patients were shown in Table 3. There were no conversions to thoracotomy. The median operative time was 264 minutes (range, 218-330 minutes). The median blood loss was 224 mL (range, 60-400 mL). There were reductions in both operative times and blood loss of ten cases from one center, which were from 298.5 to 253 minutes, and 300 to 150 mL separately (Figure 6). The median duration of blocking PA was 72 minutes (range, 44-143 minutes); the median duration for PA anastomosis time was 45 minutes (range, 26-75 minutes); the median duration for bronchial anastomosis was 31 minutes (range, 15-50 minutes); the median length of resected PA was 2 cm (range, 1-3 cm); the median length of resected bronchus was 2 cm (range, 1.5-3 cm). The median numbers of resected lymph nodes were 24 (range, 10-46), and the median stations of retrieved lymph nodes were 6 (range, 5-9).
Table 3. The operative data.
| Character | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood loss (mL) | 200 | 400 | 400 | 60 | 70 | 400 | 200 | 300 | 100 | 200 | 80 | 300 | 200 |
| Operative time (min) | 274 | 323 | 253 | 218 | 222 | 274 | 230 | 256 | 230 | 276 | 260 | 330 | 280 |
| Duration of blocking PA (min) | 68 | 108 | 76 | 44 | 45 | 55 | 50 | 60 | 60 | 143 | 110 | 70 | 50 |
| Duration of angioplasty (min) | 44 | 60 | 30 | 26 | 35 | 40 | 45 | 41 | 35 | 75 | 60 | 60 | 40 |
| Duration of bronchialplasty (min) | 35 | 32 | 42 | 30 | 25 | 15 | 30 | 23 | 30 | 24 | 40 | 50 | 30 |
| Length of resected PA (cm) | 2 | 3 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 2 | 3 | 3 | 3 |
| Length of resected bronchus (cm) | 2 | 2 | 2 | 1.5 | 2 | 1.5 | 1.5 | 2 | 1.5 | 1.5 | 1.5 | 3 | 3 |
| Numbers of resected LN | 37 | 26 | 20 | 46 | 27 | 21 | 19 | 20 | 34 | 24 | 10 | 12 | 13 |
| Stations of retrieved LN | 7 | 7 | 6 | 7 | 9 | 5 | 6 | 7 | 6 | 6 | 5 | 5 | 5 |
PA, pulmonary artery; LN, lymph nodes.
Figure 6.
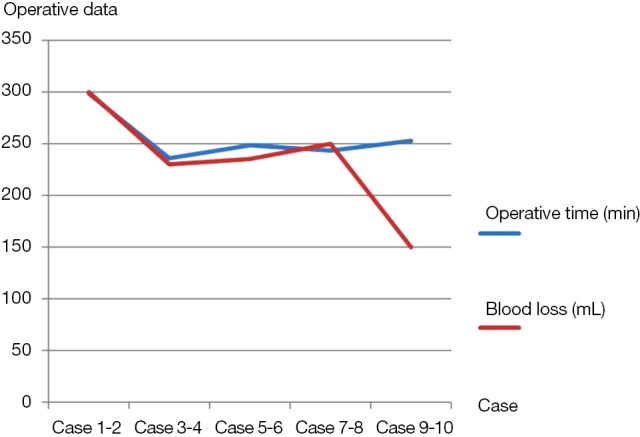
Promising reductions in operative times and blood loss of ten cases from one center
Postoperative events were summarized in Table 4. One patient suffered from pneumonia after surgery, and no patients died at 30 days. The median postoperative hospital stay was 10 days (range, 7-20 days). The median ICU stay was 1 day (range, 1-2 days). The median duration of thoracic drainage was 5 days (range, 3-8 days); the median thoracic drainage was 1,042 mL (range, 500-1,700 mL). The median duration of follow-up was 6 months (range, <1-26 months). Eight patients had completed four cycles platinum-based adjuvant chemotherapy, the chemotherapy treatment of the remaining three patients is still ongoing. Two patients received at least four cycles of neoadjuvant chemotherapy, and so, did not receive adjuvant chemotherapy. To date, no local recurrences or distant metastasis were reported.
Table 4. Postoperative events.
| Character | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case7 | Case 8 | Case 9 | Case 10 | Case11 | Case12 | Case 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morbidity | – | – | – | – | – | – | – | – | – | – | Pneumonia | – | – |
| Postoperative hospital stay (d) | 7 | 10 | 12 | 8 | 7 | 15 | 8 | 9 | 7 | 13 | 20 | 12 | 9 |
| ICU stay (d) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Duration of thoracic drainage (d) | 3 | 6 | 6 | 5 | 3 | 8 | 5 | 4 | 5 | 6 | 5 | 4 | 2 |
| Thoracic drainage (mL) | 700 | 1500 | 1,500 | 1,330 | 835 | 1,700 | 950 | 1,100 | 850 | 680 | 700 | NA | NA |
| Adjuvant chemotherapy | TXT/TO | TXT/LOP | PAX/.LOP | PAX/DDP | PAX/LOP | – | PAX/DDP | PAX/DDP | – | – | – | VP-16/DDP | – |
| Chemotherapy cycles | 4 | 4 | 4 | 4 | 4 | – | 4 | 4 | On | On | – | 4 | On |
| Follow-up duration (month) | 26 | 15 | 9 | 7 | 4 | 3 | 3 | 3 | 2 | <1 | 3 | 6 | 1 |
| Status | Live | Live | Live | Live | Live | Live | Live | Live | Live | Live | Live | Live | Live |
TXT, docetaxe; TO, oxaliplatin; LOP, lobaplatin; PAX, paclitaxel; DDP, cisplatin; VP-16, etoposide; NA, no available.
Discussion
In this retrospective multi-center series report, thoracoscopic double sleeve lobectomy was successfully performed to thirteen NSCLC patients. There were no conversions to thoracotomy. The median operative time was 263 minutes. The median blood loss was 224 mL. The reductions in operative times and blood loss of ten cases from one center were promising. The median numbers of resected lymph nodes were 24. The median postoperative hospital stay was 10 days. The median duration of follow-up was 6 months. To date, no local recurrences or distant metastasis were reported.
Although VATS lobectomy has been widely applied (15), double sleeve lobectomy is still a contraindication to VATS in most medical centers (16). To offer potential benefits of VATS to more NSCLC patients, progressively technical innovations have been made, and several institutes have reported their initial experiences of thoracoscopic double sleeve lobectomy (11-13). However, all of these recent reports were same series, or even single patients from one medical center, and they mainly focused on technical feasibility instead of general safety.
Technically, though there were separate methods of clamping the PA for uniportal or multiport procedures, each method was equally effective in blocking PA. In the uniportal procedure, a bulldog clamp is placed inside the chest cavity to clamp the distal artery, which allowed surgeons more operative space. Additionally, once the proximal PA was cut, the exposure and reconstruction of the bronchus could be more convenient through the 4-5 cm operative port, based on the relative anatomical position of the PA and bronchus. For bronchial anastomosis eased subsequent PA reconstruction and reduced vascular tension at the same time. In multiport procedure, an additional 5 mm incision greatly eased the surgical performance, which has already been reported in major thoracic pulmonary resection (17) and minimally invasive cardiac surgery (18,19). Although the choice involving the numbers of port during the procedure is simply based on the surgeons’ preference and experience, it is not a key issue when it comes to the success of thoracoscopic double sleeve lobectomy.
As for surgical trauma, the median operative time and blood loss of this study were 264 minutes and 224 mL, which were consistent with previous reports of thoracoscopic double sleeve lobectomy (11,13,14). And the Figure 6 also revealed promising reductions in both operative times and blood loss of ten cases from one center, which were from 298.5 to 253 minutes, and 300 to 150 mL separately. Hence, it indicated that thoracoscopic double sleeve lobectomy could be easily done by skilled VATS surgeons with progressive accumulation of surgical experience.
In this series, the median duration of blocking PA was 60 minutes (range, 44-110 minutes) and no complications associated with clamp of the PA occurred. The longest duration of blocking PA among these patients was 143 min, postoperative recovery was uneventful, and no reperfusion injury or thrombosis occurred. Jiang and his colleagues (20) reported a pulmonary vessel blocking model in rabbits that underwent a block of the PA and veins compared to block of the PA alone and found that it might be safe to block the pulmonary vessels up to one hour during pulmonary surgery. In our experience, with satisfactory blocking PA, the arterial reconstruction would be safer and easier during the operation. Since there were no surgical reports concerning this issue, we appeal for further research to determine the proper time for this procedure.
With regard to postoperative complications, only one significant complication was observed and was attributed to effects from the second line of treatment: pneumonia was diagnosed in a patient who received six cycles of neoadjuvant chemotherapy. This suggests that neoadjuvant chemotherapy patients can successfully undergo the operation, but postoperative management needs more attention, especially in regards to anti infection, and nutrition. There were no significant complications observed in the remaining 12 patients. However, there are also several limitations to our study. First, there were only 13 patients in this series, and most tumors of these patients were located in LUL. This might contribute to the surgeons’ preference and experience. Second, the median duration of follow-up was only 6 months, and there were still three patients on chemotherapy. The potential long-term benefit of this operation is still unclear. Hence, further experience of both short-term and long-term benefit of thoracoscopic double sleeve lobectomy is needed to be accumulated.
Conclusions
Thoracoscopic double sleeve lobectomy is safe and feasible when performed by a skilled VATS surgeon, although further investigations are needed to confirm this conclusion.
Acknowledgements
Funding: This work was supported by the Civil Science Major Foundation of Science and Information Bureau of Guangzhou (No. 2011Y2-00024).
Disclosure: The authors declare no conflict of interest.
References
- 1.Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [DOI] [PubMed] [Google Scholar]
- 2.Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda N, Saji H, Hagiwara M, et al. Recent advances in video-assisted thoracoscopic surgery for lung cancer. Asian J Endosc Surg 2013;6:9-13. [DOI] [PubMed] [Google Scholar]
- 4.Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [DOI] [PubMed] [Google Scholar]
- 5.Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [DOI] [PubMed] [Google Scholar]
- 6.Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [DOI] [PubMed] [Google Scholar]
- 7.Mahtabifard A, Fuller CB, McKenna RJ, Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [DOI] [PubMed] [Google Scholar]
- 8.Yu DP, Han Y, Zhao QY, et al. Pulmonary lobectomy combined with pulmonary arterioplasty by complete video-assisted thoracic surgery in patients with lung cancer. Asian Pac J Cancer Prev 2013;14:6061-4. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Rivas D, Delgado M, Fieira E, et al. Left lower sleeve lobectomy by uniportal video-assisted thoracoscopic approach. Interact Cardiovasc Thorac Surg 2014;18:237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Mei J, Pu Q, et al. Thoracoscopic bronchovascular double sleeve lobectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;46:493-5. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Rivas D, Fieira E, de la Torre M, et al. Bronchovascular right upper lobe reconstruction by uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2014;6:861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerfolio RJ, Bryant AS. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg 2007;83:1971-6; discussion 1976-7. [DOI] [PubMed]
- 15.Yan TD. Video-assisted thoracoscopic lobectomy-from an experimental therapy to the standard of care. J Thorac Dis 2013;5 Suppl 3:S175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Shao W, Cao C, et al. Long-term outcome of hybrid surgical approach of video-assisted minithoracotomy sleeve lobectomy for non-small-cell lung cancer. Surg Endosc 2011;25:2509-15. [DOI] [PubMed] [Google Scholar]
- 18.Aybek T, Dogan S, Wimmer-Greinecker G, et al. The micro-mitral operation comparing the Port-Access technique and the transthoracic clamp technique. J Card Surg 2000;15:76-81. [DOI] [PubMed] [Google Scholar]
- 19.Sansone F, Ceresa F, Patanè F. Transcutaneous insertion of the Chitwood® clamp in case of minimally invasive cardiac surgery. Personal experience. G Chir 2013;34:278-9. [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang F, Xu L, Yuan F, et al. Carinal resection and reconstruction in surgical treatment of bronchogenic carcinoma with carinal involvement. J Thorac Oncol 2009;4:1375-9 [DOI] [PubMed] [Google Scholar]