Abstract
Although radiation pneumonitis is usually confined to irradiated areas, some studies have reported that radiation-induced lymphocytic alveolitis can also spread to the non-irradiated lung. However, there have been few reports of radiation-induced eosinophilic alveolitis. We report the case of a 27-year-old female with radiation pneumonitis, occurring 4 months after radiation therapy for cancer of the left breast. Clinical and radiological relapse followed withdrawal of corticosteroids. Examination of bronchoalveolar lavage (BAL) in patchy airspace consolidations revealed increased eosinophil counts. Finally, clinical and radiological signs resolved rapidly after reintroduction of corticosteroids. Eosinophilic alveolitis may be promoted by radiation therapy. In the present case report, possible mechanisms for radiation-induced eosinophilic alveolitis are also reviewed.
Keywords: Bronchoalveolar lavage (BAL), eosinophilic alveolitis, migration, radiation therapy
Introduction
The lung is one of the most radiosensitive organs, yet is frequently irradiated during treatment for thoracic malignancy. Radiation-induced lung injury often results in radiation pneumonitis and radiation fibrosis. Radiation pneumonitis becomes apparent at about 1 to 6 months after radiotherapy and fibrosis after at least 6 to 12 months. The association between pneumonitis and the development of fibrosis is still uncertain (1-4). Classically, the pathological and radiological changes caused by radiation are confined to the irradiated area. However, several reports have described an intense lymphocytic alveolitis not only in the irradiated lung but also in the non-irradiated lung. These changes have been demonstrated by diffusely increased gallium uptake, lymphocytosis in bronchoalveolar lavage (BAL) from non-irradiated lung, and a histologic pattern of organizing pneumonia from the migratory opacities (3,5-11).
The term “alveolitis” was proposed to define the inflammatory involvement of the lung parenchyma on the basis of cellular pattern obtained by BAL. Studies of the cellular constituents from BAL suggested that alveolitis may be categorized according to the cell type relevant to the pathological process. Therefore, eosinophilic alveolitis characterizes chronic eosinophilic pneumonia (CEP) (12). Recently, CEP was reported among the spectrum of radiation-induced lung injury in patients with a history of asthma and/or allergy (13,14). Generally, alveolitis with eosinophilic BAL caused by radiation is rare. In the present study, we report a case of migratory eosinophilic alveolitis in radiation pneumonitis, occurring 4 months after radiation therapy in a breast cancer patient without history of asthma or allergy.
Case report
A 27-year-old female with invasive ductal carcinoma of the left breast underwent a partial mastectomy and axillary lymph node dissection followed by four cycles of chemotherapy, then radiotherapy (5,940 cGy in 33 fractions). The information of radiotherapy planning (Figure 1) were ipsilateral lung mean dose of 724.9 cGy (V5: 25.1%, V20: 11.4%), contralateral lung mean dose of 5.6 cGy (V5: 0%, V20: 0%) and total lung mean dose of 337.0 cGy (V5: 11.5%, V20: 5.2%). Four months after completion of radiotherapy, the patient developed a nonproductive cough, and chest X-ray revealed an air bronchogram in the left lung (Figure 2A). She had no history of smoking, asthma, allergy, drug, other lung diseases, significant occupational exposures, or recent travel or consumption of raw food. She had inspiratory crackle in left lung, and the remainder of her physical examination was normal. Chest computed tomography (CT) showed airspace consolidation involving the lingula of left upper lobe (Figure 2B). Laboratory data included a white blood cell (WBC) count of 5,670/mm3 (6.6% eosinophils: normal, 0-8), an erythrocyte sedimentation rate (ESR) of 38 mm/h (normal, 0-22) and a C-reactive protein (CRP) level of 2.10 mg/dL (normal, 0-0.3). Serology for Mycoplasma pneumonia, Legionella pneumophila, Chlamydia pneumonia, and Aspergillus were negative, as were sputum stains for Mycobacterium tuberculosis. Bronchoscopy revealed no endobronchial abnormalities. BAL of the left lingular inferosegment revealed differential cell counts of 41% lymphocytes, 17% eosinophils, 24% neutrophils, 8% macrophages and 10% monocytes. Methenamine silver stains revealed no fungi or Pneumocystis jiroveci, and cultures for bacteria, fungi, mycobacteria, and viruses were negative.
Figure 1.
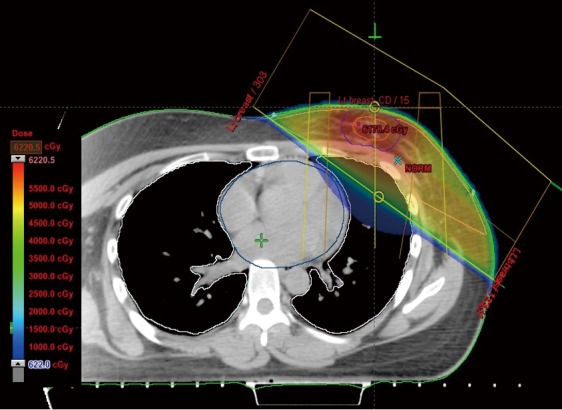
Axial planning image showing the partial mastectomy bed, lung, heart and isodose curves.
Figure 2.
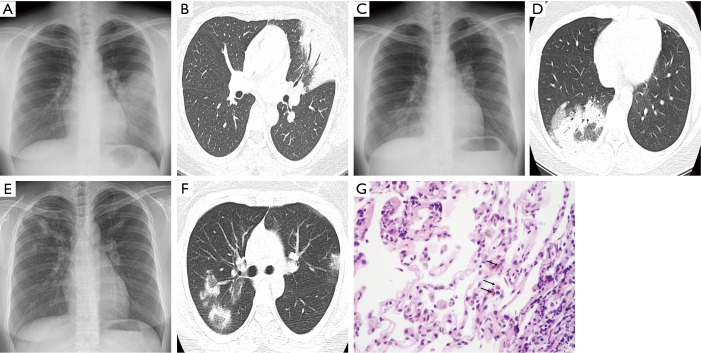
Chest X-ray (A) and computed tomography (CT) (B) during the first episode of alveolitis (4 months after completion of radiotherapy), showing airspace consolidation in the lingular segment of the left upper lobe (LUL). Chest X-ray (C) and CT (D) during the second episode (7.5 months after radiotherapy) showing patchy airspace infiltrates and consolidation in the lateral and posterior segments of the right lower lobe (RLL) and clearance of the previous LUL lesion. Chest X-ray (E) and CT (F) during the third episode (11 months after radiotherapy) showing multiple airspace consolidations in both upper lobes and clearance of the previous RLL lesion. Transbronchial lung biopsy specimen (G) showing chronic inflammation with several eosinophils (arrows) (H&E stain, ×400).
The patient was diagnosed with radiation pneumonitis and, because of the mildness of her symptoms, treated with inhaled steroids for 3 months. Two months later, she was asymptomatic, and chest X-ray confirmed that the left lung process had cleared. At 15 days after discontinuation of inhaled steroid treatment (7.5 months after irradiation), however, her chest discomfort and cough returned. Chest X-ray and CT indicated patchy airspace infiltrates and consolidation involving the right lower lobe (Figure 2C,D). At that time, laboratory data included a WBC of 6,380/mm3 (5.5% eosinophils), an immunoglobulin (IgE) of 658.19 IU/mL (normal, 0.00-183.00), an ESR of 39 mm/h and a CRP level of 1.95 mg/dL. Tests for antinuclear antibodies, rheumatoid factors and anti-neutrophil cytoplasmic antibody were negative. BAL of the lateral segment of the right lower lobe, yielded differential cell counts of 40% lymphocytes, 25% eosinophils, 2% neutrophils, 15% macrophages and 18% monocytes. As earlier, culture of BAL fluid revealed no organisms. Corticosteroid (prednisolone 0.5 mg/kg/day) was administered for 2 weeks, and consequent subjective and radiographic improvements were dramatic. The corticosteroid was then tapered and discontinued at 6 weeks.
Again, relapse occurred within 6 weeks after withdrawal of corticosteroid (11 months after irradiation), characterized by cough and new multi-airspace consolidations in both upper lobes (Figure 2E,F) and associated with a WBC of 6,950/mm3 (2.8% eosinophils), an ESR of 10 mm/h and a CRP level of 0.35 mg/dL. BAL and transbronchial lung biopsy at the posterosegment of right upper lobe indentified differential cell counts of 38% lymphocytes, 21% eosinophils, 2% neutrophils, 25% macrophages and 14% monocytes. Transbronchial biopsy demonstrated mild chronic inflammation with several eosinophils (Figure 2G). Histology and BAL fluid provided no evidence of infections or vasculitis. Corticosteroid (prednisolone 0.5 mg/kg/day) was reintroduced, and rapidly improved clinical signs and radiological opacities. The corticosteroid dose was gradually tapered and discontinued again with normalization of laboratory data after 4 months, and the patient did not experience symptom recurrence during 18 months of follow-up (Figure 3).
Figure 3.
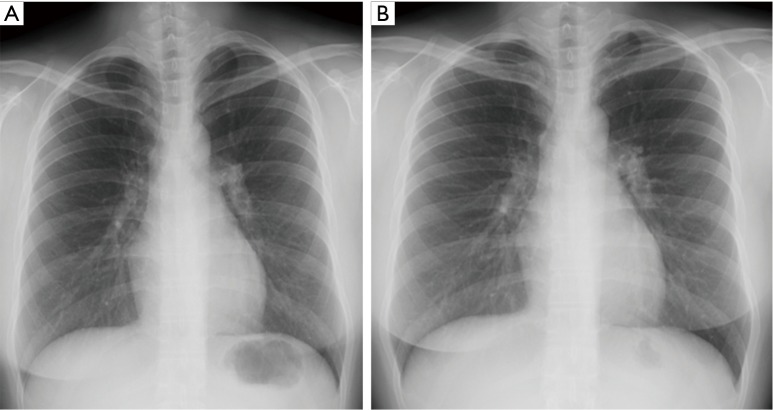
Chest X-ray upon discontinuation of corticosteroid therapy (A) and 18 months after (B) show complete clearing of pulmonary infiltrates.
Discussion
The use of thoracic radiotherapy to treat cancer inevitably exposes normal lung tissues to radiation. The pathological processes of radiation injury begin immediately after radiation exposure, but the clinical and histological features may not become apparent for weeks, months, or even years after treatment. The effects of radiation injury are commonly classified as acute, consequential, or late, according to the time between exposure and appearance of symptoms. Acute effects are those that are observed during the course of treatment or within a few weeks after treatment. Consequential effects appear later, and are caused by persistent acute damage. Late effects emerge months to years after radiation exposure (1,2,4). Early literature reported the rate of symptomatic radiation pneumonitis ranges from 1% to 34%, whereas the rate of radiological changes ranges from 13% to 100% (15).
Radiation-induced lung injury is a type of inflammatory reaction of the lung tissue in response to radiation exposure (1,15,16). Marked lymphocytic alveolitis has been demonstrated not only in irradiated but also in non-irradiated lungs of radiation pneumonitis patients using BAL, used widely to investigate inflammatory reactions in the lung interstitium (4). Accumulation of lymphocytes, which consist predominantly of CD4+ T cells, is more pronounced in patients who develop clinical pneumonitis. These findings suggest that radiation may cause a generalized lymphocyte-mediated hypersensitivity reaction (3-5,7). Furthermore, characteristic organizing pneumonia or CEP with/without migratory infiltrates is often reported to follow radiation lung injury (8-10,13,14,17,18).
The originality of our case lies in the identification of eosinophilic BAL (17%, 25% and 21%, respectively) in migratory radiological opacities of a patient with radiation pneumonitis without history of asthma or allergy. Though a few previous cases have suggested that eosinophilic BAL may be present in radiation pneumonitis, its significance has not been elucidated (4,6,8,9,13,19). These cases suggest that radiation-induced lung injury may promote eosinophilic alveolitis, similar to its ability to promote lymphocytic alveolitis (3-9,11). We believe that in our case, eosinophilic alveolitis was related to irradiation. First, lung infiltrates were detected initially in the irradiated area, with a temporal relationship that fits with the usually reported delay between radiation exposure and acute lung disease (1,2). Second, other possible causes of eosinophilic pneumonia, such as drugs, toxic agents, infection, vascular collagen disease, or allergy, were excluded. Moreover, no histological evidence for other causes of eosinophilic pneumonia was found (20).
Generally, eosinophilic BAL in patients with radiation pneumonitis is rare. Although eosinophilic BAL has been reported in a few irradiated patients, the mechanism by which radiation induces eosinophilic alveolitis is unknown (4,6,10). A recent study has suggested that eosinophilic pneumonia may be primed by radiation, and then triggered by antigenic stimulation several months later (13). Recent progress in molecular pathology and radiobiology has improved the mechanistic understanding of radiation injury. The early phases of fibrogenesis after irradiation can be seen as a wound-healing response characterized by an almost immediate upregulation of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukins 1 and 6 and many growth factors in the irradiated tissue. Especially transforming growth factor-β (TGF-β) plays an important role in radiation fibrogenesis (16). TNF-α and TGF-β are multifunctional cytokines, and chemoattractants for eosinophils (21,22). This function suggests that TNF-α and TGF-β could be part of the molecular mechanism linking radiation to eosinophilic alveolitis. Further investigation of this mechanism is warranted.
Eosinophils are toxic to airway epithelial cells, and are associated with lower response to treatment and steeper declines in pulmonary function in idiopathic pulmonary fibrosis, and with a poor outcome in lung transplant patients (6,23,24). However, several cases of radiation pneumonitis (6,8,9,13), including that presented here, have shown that corticosteroid therapy rapidly eliminates the clinical and radiological signs of eosinophilic BAL, even after relapse. These findings suggest that radiation-induced eosinophilic alveolitis may be a predictable clinical marker of the good response to corticosteroid therapy and responsible for the recurrent migratory radiological changes in radiation pneumonitis.
In summary, this case illustrates several distinctive aspects of the care of patients with radiation pneumonitis. First, eosinophilic alveolitis may be promoted by radiation therapy. Second, radiation-induced eosinophilic alveolitis could serve as a predictable clinical marker of the good response to corticosteroid therapy and could underlie recurrent radiological migration. Third, radiation pneumonitis with eosinophilic alveolitis may require long-term corticosteroid treatment to prevent relapse.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 2003;4:529-36. [DOI] [PubMed] [Google Scholar]
- 2.Davis SD, Yankelevitz DF, Henschke CI. Radiation effects on the lung: clinical features, pathology, and imaging findings. AJR Am J Roentgenol 1992;159:1157-64. [DOI] [PubMed] [Google Scholar]
- 3.Arbetter KR, Prakash UB, Tazelaar HD, et al. Radiation-induced pneumonitis in the “nonirradiated” lung. Mayo Clin Proc 1999;74:27-36. [DOI] [PubMed] [Google Scholar]
- 4.Toma CL, Serbescu A, Alexe M, et al. The bronchoalveolar lavage pattern in radiation pneumonitis secondary to radiotherapy for breast cancer. Maedica (Buchar) 2010;5:250-7. [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson PG, Bryant DH, Morgan GW, et al. Radiation-induced lung injury: a hypersensitivity pneumonitis? Ann Intern Med 1988;109:288-91. [DOI] [PubMed] [Google Scholar]
- 6.Bjermer L, Franzén L, Littbrand B, et al. Effects of smoking and irradiated volume on inflammatory response in the lung of irradiated breast cancer patients evaluated with bronchoalveolar lavage. Cancer Res 1990;50:2027-30. [PubMed] [Google Scholar]
- 7.Roberts CM, Foulcher E, Zaunders JJ, et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med 1993;118:696-700. [DOI] [PubMed] [Google Scholar]
- 8.Bayle JY, Nesme P, Béjui-Thivolet F, et al. Migratory organizing pneumonitis “primed” by radiation therapy. Eur Respir J 1995;8:322-6. [DOI] [PubMed] [Google Scholar]
- 9.Crestani B, Kambouchner M, Soler P, et al. Migratory bronchiolitis obliterans organizing pneumonia after unilateral radiation therapy for breast carcinoma. Eur Respir J 1995;8:318-21. [DOI] [PubMed] [Google Scholar]
- 10.Crestani B, Valeyre D, Roden S, et al. Bronchiolitis obliterans organizing pneumonia syndrome primed by radiation therapy to the breast. The Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires (GERM“O”P). Am J Respir Crit Care Med 1998;158:1929-35. [DOI] [PubMed] [Google Scholar]
- 11.Martín C, Romero S, Sánchez-Payá J, et al. Bilateral lymphocytic alveolitis: a common reaction after unilateral thoracic irradiation. Eur Respir J 1999;13:727-32. [DOI] [PubMed] [Google Scholar]
- 12.Olivieri D, Pesci A, Bertorelli G. Eosinophilic alveolitis in immunologic interstitial lung disorders. Lung 1990;168 Suppl:964-73. [DOI] [PubMed] [Google Scholar]
- 13.Cottin V, Frognier R, Monnot H, et al. Chronic eosinophilic pneumonia after radiation therapy for breast cancer. Eur Respir J 2004;23:9-13. [DOI] [PubMed] [Google Scholar]
- 14.Chaaban S, Salloum V. Chronic eosinophilic pneumonia in a breast cancer patient post-radiation therapy: a case report. Respir Care 2014;59:e81-3. [DOI] [PubMed] [Google Scholar]
- 15.Movsas B, Raffin TA, Epstein AH, et al. Pulmonary radiation injury. Chest 1997;111:1061-76. [DOI] [PubMed] [Google Scholar]
- 16.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006;6:702-13. [DOI] [PubMed] [Google Scholar]
- 17.Cornelissen R, Senan S, Antonisse IE, et al. Bronchiolitis obliterans organizing pneumonia (BOOP) after thoracic radiotherapy for breast carcinoma. Radiat Oncol 2007;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akita K, Ikawa A, Shimizu S, et al. Cryptogenic organizing pneumonia after radiotherapy for breast cancer. Breast Cancer 2005;12:243-7. [DOI] [PubMed] [Google Scholar]
- 19.Kawai S, Baba K, Tanaka H, et al. A case of radiation pneumonitis with eosinophilia in bronchoalveolar lavage fluid. Nihon Kokyuki Gakkai Zasshi 2008;46:44-9. [PubMed] [Google Scholar]
- 20.Cottin V, Cordier JF. Eosinophilic pneumonias. Allergy 2005;60:841-57. [DOI] [PubMed] [Google Scholar]
- 21.Nam HS, Lee SY, Kim SJ, et al. The soluble tumor necrosis factor-alpha receptor suppresses airway inflammation in a murine model of acute asthma. Yonsei Med J 2009;50:569-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luttmann W, Franz P, Matthys H, et al. Effects of TGF-beta on eosinophil chemotaxis. Scand J Immunol 1998;47:127-30. [DOI] [PubMed] [Google Scholar]
- 23.Verleden SE, Ruttens D, Vandermeulen E, et al. Elevated bronchoalveolar lavage eosinophilia correlates with poor outcome after lung transplantation. Transplantation 2014;97:83-9. [DOI] [PubMed] [Google Scholar]
- 24.Peterson MW, Monick M, Hunninghake GW. Prognostic role of eosinophils in pulmonary fibrosis. Chest 1987;92:51-6. [DOI] [PubMed] [Google Scholar]