Abstract
This study was conducted to enhance genetic variability in peppers (Capsicum annuum, cv B12) using ethyl methanesulphonate (EMS). Exposure to an EMS concentration of 0.6%, v/v for 12 h was used to mutagenize 2000 seeds for the first generation (M1). It was observed that the growth behaviors including plant height, flowering date, and number of seeds per first fruit were different in the M1 generation than in wild type (WT) plants. In addition one phenotypic mutation (leaf shape and plant architecture) was observed during the M1 generation. During the seedling stage in the M2 generation, the observed changes were in the form of slow growth or chlorophyll defect (e.g., albino, pale green, and yellow seedlings). At maturity, there were three kinds of phenotypic mutations observed in three different families of the mutant population. The first observed change was a plant with yellow leaf color, and the leaves of this mutant plant contained 62.19% less chlorophyll a and 64.06% less chlorophyll b as compared to the wild-type. The second mutation resulted in one dwarf plant with a very short stature (6 cm), compact internodes and the leaves and stem were rough and thick. The third type of mutation occurred in four plants and resulted in the leaves of these plants being very thick and longer than those of WT plants. Furthermore, anatomical observations of the leaf blade section of this mutant plant type contained more xylem and collenchyma tissue in the leaf midrib of the mutant plant than WT. In addition, its leaf blade contained thicker palisade and spongy tissue than the WT.
Keywords: pepper, Capsicum annuum, EMS, mutations, M1 generation, M2 generation
Introduction
Peppers (Capsicum annuum L.) are one of the most cultivated vegetable crops in tropical and subtropical regions. Inducing mutations in peppers is important for pepper breeding and crop improvement (Bosland and Votava, 2012). EMS, as a chemical mutagen, can be used as a supplementary approach to improve desired identifiable characters such as yield related characters (Botticella et al., 2011). It produces random point mutations in genetic material. Mutation frequency, detected using various techniques, displays a wide range of variation according the EMS treatment conditions. Mutation frequency per gene differs from one crop to another starting from one mutation per Mb in barley (Caldwell et al., 2004), to one mutation per 170 kb in Arabidopsis (Greene et al., 2003), one mutation per 50 kb in pepper (Pathirana, 2012; Just et al., 2013), and one mutation per 25 kb in the polyploid (hexaploid) wheat (Slade et al., 2004). Lethal dose 50 (LD50) can be defined as the EMS concentration and conditions that contributes to 50% lethality (out of the total number of seeds). Determination of LD50 is necessary to produce a high frequency of desirable mutations (Hohmann et al., 2005; Arisha et al., 2014).
In the M1 generation, only mutations of dominant characters can be identified, and it is not possible to identify mutations in a recessive character. Moreover, in the M1 generation, there are some signals for mutagen efficiency, for example: pollen sterility, reduction in plant height, late or early flowering, or curled leaves (Arisha et al., 2014). In the M2 generation, the mutation will segregate to create homozygotes for recessive or dominant alleles (Page and Grossniklaus, 2002). At that time visual screening is the most effective way to identify the phenotypic mutation. Visual screening can be used as a primary indicator to select plants that have desired characters, for example: disease resistance, flowering earliness, plant height, fruit color changes, or growth period (Østergaard and Yanofsky, 2004).
Presence of chlorophyll mutations in the mutant population is the most dependable index for evaluating the genetic effects of mutagenic treatments. In addition, chlorophyll mutations are important for identifying gene function and elucidation of chlorophyll metabolism and its regulation (Wu et al., 2007). Chlorophyll development seems to be controlled by many genes located on several chromosomes, which could be adjacent to centromeres or on proximal segments of chromosomes (Swaminathan, 1964). Another possible use for mutants is to detect dwarf mutants (Daskalov, 1986), which have been previously identified in peppers (Lippert et al., 1964; Alcantara et al., 1996; Jabeen and Mirza, 2004). In addition, induction of mutations affecting pepper plant architecture and leaf shape can provide us with information about the genetic control of plant architecture, since these characters have only been studied in detail in a limited number of model species such as Arabidopsis (Angenent et al., 2005), Lycopersicon esculentum (Prusinkiewicz et al., 2007), and Petunia hybrida (Quinet and Kinet, 2007).
Induced mutations can rapidly create variability in quantitatively and qualitatively inherited traits in crops. Therefore, the objective of this study is to obtain new germplasm to use in the improvement of the B12 pepper cultivar.
Materials and Methods
Plant Material
B12, a sweet pepper cultivar (C. annuum L.), was used in this experiment. Pepper seeds were provided by the Capsicum Research Group from the College of Horticulture at Northwest A&F University, China.
Experimental Procedures
In our previous study we standardized the EMS treatment in (C. annuum cv B12) by conducting a kill curve analysis (Arisha et al., 2014). Therefore, the concentration of 0.6%, V/V EMS for 12 h was chosen to mutagenize 2000 seeds. EMS solution was prepared in a 0.1 M phosphate buffer at pH 7.0 to avoid rapid hydrolysis (Bosland, 2002).
Seeds (2000) were presoaked in water for 6 h then treated with the above-mentioned concentration of EMS at 20°C with orbital shaking (110 rpm), while 50 seeds were used as a control (untreated). Seeds were then thoroughly washed under running water for 3 h and transferred to petri dishes containing wet filter paper and kept in a growth chamber at 28°C in the dark until germination (at 10 days after the treatment). Control seeds (untreated) were exposed to the same conditions except for the EMS treatment.
All germinated and non-germinated seeds were sown into pots that were placed in a greenhouse and standard cultural practices were carried-out thereafter. When four to six true leaves had grown, seedlings were transferred to soil under high tunnels to grow until maturity. Phenotypic data during the M1 generation were recorded. At the fruit ripening stage, the mature seeds of the first fruit from each individual was collected, packed, labeled, air dried, and stored in a freezer at -20°C (Arisha et al., 2014).
Thirty seeds from each M1 individual were germinated in petri dishes at 28°C. All germinated and non-germinated seeds were sown in one pot and the germination ratio was recorded. M2 seedlings with four to six true leaves were transferred to soil under high tunnels and grow there to maturity stage. The M2 population was screened for morphological mutants (from germination until maturity) and the survival percentage was recorded.
Parameters Studied
M1 generation
During the M1 generation, the germination percentage at 7 days after germination and the survival percentage were recorded (at the age of four to six true leaves). Moreover, a comparison between mutant and wild type (WT) populations for growth parameters [e.g., plant height (cm) at 100 DAS, days to flowering and number of seeds/first fruit) was conducted. Growth abnormalities were observed and recorded for the M1 generation compared to the WT plants.
M2 generation
Germination percentage at 7 days after starting germination and survival ratio (at maturity) for each family of M2 population was recorded. To identify morphological mutant’s growth abnormalities from the seedling stage until fruit harvesting were recorded. In addition, the frequency and segregation ratio of the mutant plants out of the total number of individuals in the same M2 family was calculated.
Photosynthetic pigment analysis
Chlorophyll a and b and carotenoids were extracted from 0.2 g of fresh leaves (obtained from the upper third of the leaf) of yellow green mutants and WT plants at the age of 100 DAS using ice-cold acetone. Extracts were centrifuged at 15000 rpm for 10 min at 10°C. The supernatant volume was diluted with 80% acetone. The chlorophyll contents were quantified spectrophotometrically following the method of (Porra et al., 1989).
Leaf anatomy
In the M2 generation, at 100 DAS, specimens were taken from the lower third of the leaf blade. The upper third of the leaves from the mutant and WT plants were taken for anatomical measurements. These specimens (1 cm long) were killed and fixed for 72 h in FAA (formalin, acetic acid, and ethanol) that was made using the following formula: 50 mL ethyl alcohol (95%), 5 mL glacial acetic acid, 10 mL formaldehyde (37–40%), and 35 mL distilled water. The selected materials were stained with both safranin and light green, and cleared in xylene (Berlyn and Miksche, 1976). The stained specimens were then washed in 50% ethyl alcohol, dehydrated in a normal butyl alcohol series, and embedded in paraffin wax with a melting point of 52–54°C. Sections were prepared using a rotary microtome at 12 microns, and selected sections were examined and photographed using a system Microscope (BX53). The dimensions of leaf blade sections were measured using the same system Microscope.
Statistical analysis
All obtained data from the M1 and M2 generations were subjected to analysis of variance (ANOVA) using SPSS software (Corp, 2010). The analyzed data were presented as means ± SE of two replicates in all measured parameters except for chlorophyll, which was done using three replicates.
Results
M1 Generation Observations
Out of the total number of seeds, 1009 seeds germinated (50.4%) showing 49.6% lethality during the seedling stage. After transplantation, further mortality occurred, with 939 plants (93.06%) reaching maturity (Table 1).
Table 1.
The germination and survival percentage during the first generation (M1).
| Treatments | Total No of seeds | No. of germinated seeds | Seed germination (%) | No. of survived seedlings | Survival (%) out of germinated seeds |
|---|---|---|---|---|---|
| EMS population | 2000 | 1009 | 50.40 | 939.0 | 93.06 |
| Wild type (WT) | 50.00 | 47.00 | 94.00 | 47.00 | 100.0 |
As shown in Table 2, no significant change was observed in plant height in 80.05% of the M1 population as compared to the WT population, while 0.21% of the M1 plants were dwarf plants, 9.01% were short plants, 10.52% were long plants, and 0.21% were very long plants. The plant height in 68.08% of WT plants was 41–80 cm, 29.79% were 20–40 cm, and 2.12% of plants were 81–100 cm. The time to flowering in 0.97% of the mutant population was earlier than 70 DAS, 19.31% of plants flowered at 70–80 DAS, 50% flowered at 81–100 DAS, 28.65% flowered at 101–120 DAS, and 1.07% of plants flowered very late (after 120 DAS). On the other hand, in the WT population the flowering time was limited to just three categories: before 70 DAS, 70–80 DAS and 81–100 DAS, and the distribution of the WT plants in these categories was 12.77, 31.91, and 55.32%, respectively (Table 2).
Table 2.
Categories of the growth pattern changes observed in the M1 generation as compared to the wild type (WT) population.
| Categories | M1 population |
WT population |
||
|---|---|---|---|---|
| Number of plants ± SE | % | Number of plants ± SE | % | |
| Plant height (cm) | ||||
| Dwarf (<20 cm) | 01.00 ± 01.00 | 00.21 | 00.00 ± 00.00 | 00.00 |
| Short (20–40 cm) | 42.00 ± 03.00 | 09.01 | 07.00 ± 02.00 | 29.79 |
| Normal (41–80 cm) | 373.0 ± 75.00 | 80.05 | 16.00 ± 02.00 | 68.08 |
| Long (81–100 cm) | 49.00 ± 08.00 | 10.52 | 00.50 ± 00.50 | 02.12 |
| Very long (>100 cm) | 01.00 ± 00.00 | 00.21 | 00.00 ± 00.00 | 00.00 |
| Number of days to flowering | ||||
| Very early (<70 DAS) | 04.50 ± 00.50 | 00.97 | 03.00 ± 01.00 | 12.77 |
| Early (70–80 DAS) | 90.00 ± 13.00 | 19.31 | 07.50 ± 01.50 | 31.91 |
| Normal (81–100 DAS) | 233.0 ± 13.00 | 50.00 | 13.00 ± 02.00 | 55.32 |
| Late (101–120 DAS) | 133.5 ± 07.50 | 28.65 | 00.00 ± 00.00 | 00.00 |
| Very late (>120 DAS) | 05.00 ± 01.00 | 01.07 | 00.00 ± 00.00 | 00.00 |
| Number of seeds/first fruit | ||||
| Number of seeds (0) | 20.50 ± 01.50 | 04.40 | 00.00 ± 00.00 | 00.00 |
| Lower number (<50) | 32.00 ± 09.00 | 06.87 | 02.00 ± 00.00 | 08.51 |
| Normal (50–199) | 407.0 ± 17.00 | 87.34 | 21.50 ± 00.50 | 91.48 |
| High number (200–300) | 05.00 ± 01.00 | 01.07 | 00.00 ± 00.00 | 00.00 |
| Very much (>300) | 01.50 ± 00.50 | 00.32 | 00.00 ± 00.00 | 00.00 |
| Leaf architecture | ||||
| Aberrant | 1 | 00.21 | 0 | 0 |
DAS, days after sowing; all measurements for the mutant population were calculated as the average of 466 surviving plants; for the WT population all measurements were based on the average of 23.5 surviving plants (average of two replicates). The bolded values are the standard error of the means.
Regarding the number of seeds per first fruit, 4.4% of plants did not produce any seeds in the first fruit, 6.87% of plants had less than 50 seeds in first fruit, 87.34% had 50–199 seeds, 1.07% of plants have 200–300 seeds, and 0.32% of plants contained more than 300 seeds in the first fruit (Table 2). In the WT plants 91.48% of plants contained 50–199 seeds in the first fruit and 8.51% contained less than 50 seeds per first fruit. It was also observed during the M1 generation that there was one abnormal plant that had different leaf architecture (Figure 1; Table 2). In almost all of the studied characters, the growth performance significantly varied in the M1 generation compared to the WT population, which establishes a reasonable ground for EMS efficiency.
FIGURE 1.
Abnormal plant (leaf architecture and growth habit) observed during the first (M1) generation as compared to the wild type (WT). (A) WT plant, (B) mutant plant.
M2 Generation Observations
Germination and Survival Percentages in M2 Generation
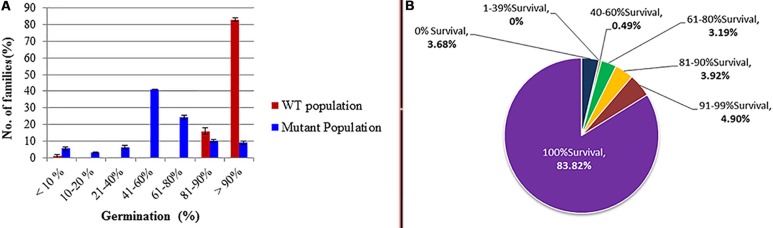
Data analysis of seed germination during the M2 generation (Figure 2A) showed that the effect of the mutagen on seed germination did not cease in the M1 but continued to affect the M2 generation. Further reduction was observed in the percentage of germinated seeds in different mutant families as compared to the WT. The germination ratio ranged from 41 to 60% in more than 40% of M2 families. Moreover, about 25% of families showed 61 to 80% germination, while the germination percentage in the WT families was about 80%. The survival ratios in the mutagenized and WT populations were recorded as the number of surviving seedlings out of the germinated seeds in all families (Figure 2B). About 83% of M2 families produced 100% viable seedlings, while 3.68% of families produced no viable seedlings, and the seedlings from other families showed survival ratio between 40–99%. On the other hand, the survival ratio in the WT population was 100%.
FIGURE 2.
Germination and survival percentages as affected by the ethyl methanesulphonate (EMS) treatment in the M2 generation wild type. (A) Germination ratio; (B) survival ratio: it calculated out of the total number of germinated seeds (in the diagram the upper values indicates survival percentage categories and the lower value (bold) indicates the percent of survived families out of the total number of germinated families.
Screening of Chlorophyll Mutations During the Seedling Stage in M2 Generation
The appearance of chlorophyll mutants during seedling stage was a good indicator for EMS efficiency. As shown in Table 3, it was observed that among 500 families there were about 14 families had chlorophyll mutants, i.e., Albino, yellow, pale green seedlings (Figure 3). Furthermore, all the albino seedlings (totally white) were died, while some of yellow or pale green seedlings were able to survive till maturity stage.
Table 3.
Ethyl methanesulphonate (EMS) induced chlorophyll mutations during seedling stage of the M2 generation.
| Family No. | Total No. of plants in the family | Albino seedlings |
Yellow seedlings |
Pale green seedlings |
Green (normal) seedlings |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| 602 | 20.00 | 4.00 | 20.00 | 0.00 | 00.00 | 0.00 | 0.00 | 16 | 80.00 |
| 963 | 30.00 | 0.00 | 0.00 | 7.00 | 23.33 | 9.00 | 30.00 | 14 | 46.67 |
| 574 | 30.00 | 7.00 | 23.33 | 0.00 | 00.00 | 0.00 | 0.00 | 23 | 76.67 |
| 711 | 30.00 | 2.00 | 06.67 | 1.00 | 03.33 | 0.00 | 0.00 | 27 | 90.00 |
| 608 | 30.00 | 8.00 | 26.67 | 0.00 | 00.00 | 0.00 | 0.00 | 22 | 73.33 |
| 576 | 30.00 | 0.00 | 00.00 | 3.00 | 10.00 | 0.00 | 0.00 | 27 | 90.00 |
| 765 | 30.00 | 0.00 | 00.00 | 2.00 | 06.67 | 0.00 | 0.00 | 28 | 93.33 |
| 911 | 35.00 | 0.00 | 00.00 | 1.00 | 02.86 | 0.00 | 0.00 | 34 | 97.14 |
| 827 | 30.00 | 0.00 | 00.00 | 1.00 | 03.33 | 0.00 | 0.00 | 29 | 96.67 |
| 616 | 35.00 | 1.00 | 02.86 | 0.00 | 00.00 | 0.00 | 0.00 | 34 | 97.14 |
| 702 | 30.00 | 0.00 | 00.00 | 2.00 | 06.67 | 0.00 | 0.00 | 28 | 93.33 |
| 779 | 40.00 | 0.00 | 00.00 | 1.00 | 02.50 | 0.00 | 0.00 | 39 | 97.50 |
| 768 | 40.00 | 0.00 | 00.00 | 2.00 | 05.00 | 0.00 | 0.00 | 38 | 95.00 |
| 890 | 20.00 | 0.00 | 00.00 | 3.00 | 15.00 | 0.00 | 0.00 | 17 | 85.00 |
FIGURE 3.
Different types of chlorophyll mutants during the seedling stage in the M2 generation. (A) Albino seedlings (totally white), (B) yellow seedlings, (C) pale green seedlings.
Characterization of Specific M2 Mutants
The phenotypic mutations were identified by visual evaluation during the M2 generation. Among 500 families (15000 plants), three types of phenotypic mutations, e.g., leaf color, dwarf plants, leaf architecture, and growth habit, were clearly observed in three different families (Figures 4–6).
FIGURE 4.
Leaf color changes in the M2 generation as compared to WT plants. (A) The mutant plant as compared to the WT, (B) the mutant plant, (C) near view for petiole and blade of the wild type leaf, (D) near view for the petiole (purple color) and blade (pale green color) of the mutant plant leaf, and (E) WT plant; WT, Wild type, M, Mutant.
FIGURE 6.

Leaf architecture and growth habit mutation as compared to the WT in the M2 generation. (A) Photo for all the mutant plants as compared to the WT, (B–E) mutant plants, (C1–E1) WT plants, (C2–E2) horizontal view for the mutant plants, (G1–G3, E3, D3, F) lateral view for the mutant plants.
Leaf color mutation
During the M2 generation, a single family (No. 576) produced a distinct mutant phenotype with yellow leaves in one plant (No. 576–18). In this family there were three yellow plants out of the total number of plants in this family (30 plants) with a 10% frequency and segregation ratio of 1:10, which is not consistent with the classic Mendelian model (Tables 3–5). At the seedling stage, the leaf color of these mutant plants was yellow, which was very clear when compared to WT plants (Figure 3B). One plant of the three mutants survived until maturity, while the other two plants died. At maturity, the surviving plant also exhibited differences in leaf color, distinguishing it from the WT (Figure 4). In addition, the petioles of this mutant plant were dark purple, while the petioles of WT leaves were green (Figures 4C,D).
Table 5.
Phenotypic characters for the observed mutations as compared to the WT in the M2 generation.
| Mutant No. | Plant height (cm) | No. of leaves/plant | Leaves color | No. of nodes/plant (main stem) | No. of flowers/plant | No. of fruits/plant | Final fruit color | No. seeds/first fruit |
|---|---|---|---|---|---|---|---|---|
| WT | 45.0 | 84 | Normal | 15 | 26 | 3 | Red | 65 |
| Leaf architecture and growth habit mutation | ||||||||
| 891-1 | 30.0 | 58 | Normal | 5 | 0 | 0 | – | – |
| 891-2 | 13.5 | 19 | Normal | 7 | 0 | 0 | – | – |
| 891-3 | 19.0 | 16 | Normal | 5 | 0 | 0 | – | – |
| 891-4 | 14.0 | 42 | Normal | 9 | 1 | 1 | – | – |
| Dwarf mutation | ||||||||
| 690-1 | 06.0 | 7 | Normal | 4 | 7 | 0 | – | – |
| Leaf colored mutation | ||||||||
| 576-18 | 29.0 | 30 | Yellow | 7 | 4 | 2 | Bright red | 10 |
| 576-5 | 59.0 | 62 | Dark green | 15 | 15 | 3 | Red | 18 |
Numbers 891, 690, and 576 indicate the family number during the second generation and the second number (1, 2, 3, 4, 5, and 18) after the family number indicates the mutant plant number in the family.
The chlorophyll content of leaves in all plants of this family as well as WT plants were determined to characterize the nature of the yellow leaf phenotype (Table 6). The results indicated that the leaves of one plant (No. 576-18) from the family contained 62.19% less chlorophyll a and 64.06% less chlorophyll b than those from the WT. The growth parameters recorded for this yellow plant included: plant height (29 cm), number of leaves (30 leaves), number of internodes (seven), number of flowers (four), number of fruits (two), and number of seeds per first fruit (10), while the WT plants had: 45, 84, 15, 26, 3, and 65 for the above-mentioned traits, respectively (Table 5).
Table 6.
Chlorophyll a and b and carotenoids (mg/g FW) in the assayed leaf colored mutants as compared to the WT in the M2 generation.
| Plant No. | Chlorophyll A ± SE | Chlorophyll B ± SE | Carotenoids ± SE | Total Chlorophyll ± SE | Chlorophyll A/B ± SE |
|---|---|---|---|---|---|
| WT | 1.64 ± 0.009 | 0.64 ± 0.009 | 0.87 ± 0.011 | 2.27 ± 0.017 | 2.57 ± 0.029 |
| 576-18 | 1.02 ± 0.048 | 0.41 ± 0.008 | 0.77 ± 0.042 | 1.43 ± 0.036 | 2.48 ± 0.027 |
SE, standard error; FW, fresh weight.
Dwarf mutation
The dwarf mutant that was isolated was categorized as less than 10 cm in height with short internodes and compact nodes. Among the total numbers of plants in this family (30 plants) 23 plants survived and one of them was a dwarf mutant (No. 690-1). The dwarf mutant had a frequency of 4.34% and a segregation ratio 23:1, which was not consistent with the classic Mendelian model as shown in Tables 4 and 5. At 100 DAS, this dwarf plant had thick, rough and fibrous leaves, and a ribbed, rough, and thick stem, while the WT plants had smooth green leaves and stems (Figure 5). The mutant achieved a height of 6 cm and produced seven sterile flowers as compared to the WT, which had an average height of 45 cm and 26 fertile flowers.
Table 4.
Observed mutations during the second generation; its frequency (%) and segregation ratio.
| Observed mutations | Total No. of plants/family | No. of mutant plants | Mutants’ frequency (%) | Segregation ratio |
|---|---|---|---|---|
| Growth habit and leaf shape mutation | 17 | 4 | 23.52 | 4:1 |
| Dwarf mutation | 23 | 1 | 04.34 | 23:1 |
| Leaf color mutation | 30 | 3 | 10.00 | 10:1 |
FIGURE 5.
Dwarf plant as compared to the WT plants during the M2 generation. (A) WT plant (WT) and dwarf plant (M), (B) near view for the mutant (dwarf plant).
Leaf architecture and growth habit mutation
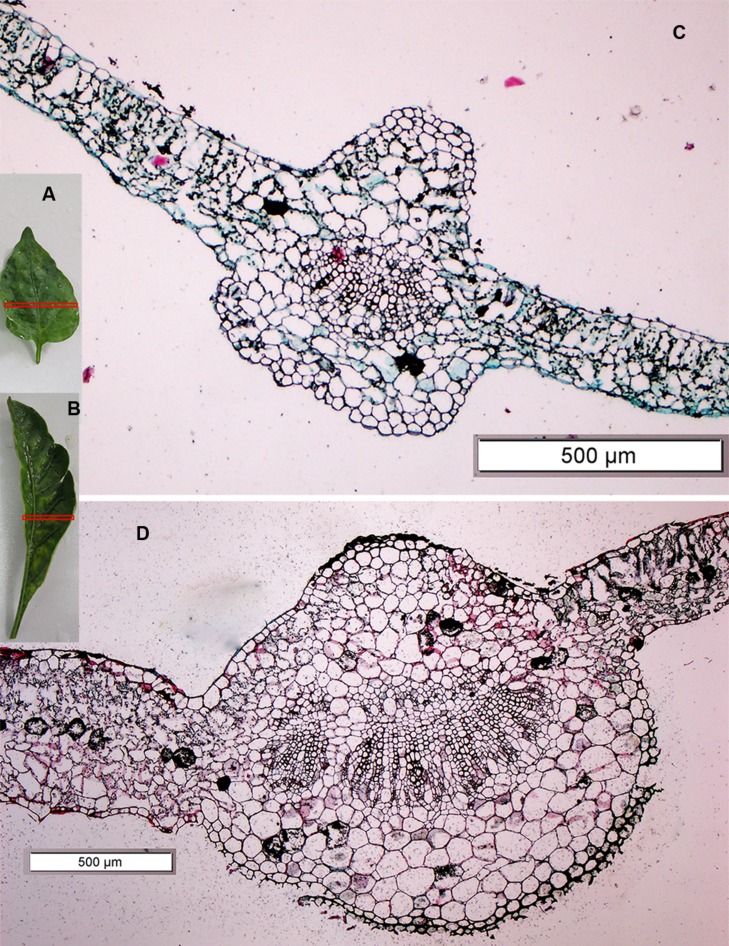
Four mutants with an altered growth habit were identified in the M2 population (Figure 6), although, these mutations did not appear in M1 generation. These four mutant plants were found in one family (No. 891) and had a frequency of 23.52% and a segregation ratio 4:1, which is consistent with the classic Mendelian model as shown in Table 4. Leaf blades of these four mutant plants were thicker and longer than those of the WT (Figure 7). In addition, all of these mutant plants lacked branches during their growth stages.
FIGURE 7.
A leaf cross section of the leaf architecture and altered growth habit mutant plant as compared to the WT. (A,B) The third leaf of the WT plant and mutant plant, respectively, Red rectangle on the leaves (A,B) shows the position of cross section where taken, (C,D) the cross section under microscope of the WT plant and mutant plant, respectively.
The growth habit of the four mutant plants was different when compared with the WT. In the WT B12 cultivar, growth was determinate; the shoot apical meristem produced approximately eight true leaves on the main stem and then terminated in a flower. Further growth continued from the uppermost axillary meristems creating branches and so on. The observed mutations caused a dramatic change in plant architecture. In one mutant plant (No. 891-1), many leaves were produced from the third node and then growth continued by producing one leaf for each node until the sixth node, where many leaves were produced at the same node (sixth) without branching (Figures 6C1,C2). In the other three mutants (No. 891-2, 891-3, and 891-4), plants started to produce leaves at the fifth node without branching. Furthermore, one of the mutant plants (No. 891-4) induced one lateral fruit at the second node (Figures 6E1,E2). The results shown in Table 5 indicated that the growth habit of all mutant plants (plant height, number of leaves, and number of nodes) were different than those of the WT plants.
Comparing the third leaf cross section of the mutant and WT using a scanning microscope (Figure 7), we found that the right and left sides of the leaf blade in the mutant plant were very thick (551.55 and 530.00 μm, respectively) as compared to the WT (139.24 and 211.55 μm, respectively). In addition, the leaf blade of the mutant contained thicker palisade and spongy tissue than the WT. A cross section of the main midrib also showed clear differences in the xylem and collenchyma tissue, as it was very thick in the mutant plant compared to the WT. Xylem and collenchyma tissue thickness in the mutant plant was 242.42 and 530.34 μm, while in the WT leaf it was 61.85 and 240.53 μm, respectively (Table 7). These results indicated that the leaves of the mutant plants are thicker than the WT (Figure 7).
Table 7.
Leaf sections of the mutant and WT plants using a scanning microscope.
| Parameters | Thickness (μm) |
|
|---|---|---|
| Mutant | WT | |
| Leaf blade (right side) | 0551.55 | 139.24 |
| Leaf blade (left side) | 0530.00 | 211.55 |
| Midrib | 1474.57 | 639.96 |
| Xylem tissues | 0242.42 | 061.85 |
| Collenchyma tissues | 0530.34 | 240.53 |
Discussion
In mutation breeding programs, the selection of an effective and efficient mutagen concentration and growth condition is essential to produce a high frequency of desirable mutations (Wani, 2009; Arisha et al., 2014). Arisha et al. (2014) studied the LD50 in the same pepper cultivar and found that 0.6% V/V EMS was the concentration that produced about 50% lethality. The reduction in germination may be due to the seeds absorbing the mutagen, which subsequently reaches the meristemic region and affects the germ cell (Serrat et al., 2014). Also, a reduction in germination may be because of the damage of cell constituents (Kumar et al., 2013), alteration of enzyme activity or delay or inhibition of physiological and biological processes (Talebi et al., 2012).
In the present investigation the growth behaviors (plant height, number of days to flowering, and number of seeds per first fruit) of the mutant population exhibited changes in the above-mentioned characters as compared to the WT population. This might be due to the biological damage of the embryo as induced by EMS, and this in turn reflected on the plant growth behavior until maturity (Arisha et al., 2014). Genetically, during the M1 generation the probability of the occurrence of phenotypic mutation is extremely low and only dominant mutations can be identified (Roychowdhury and Tah, 2013). In the current study, during the M1 generation, among 2000 mutagenized seeds, only one phenotypic mutation was observed. This mutant had different leaf shapes and a different growth habit (Figure 1). In concordance with the current results, Honda et al. (2006) surveyed mutagenized pepper plants for mutants during the M1 generation and found two dwarf plants and one plant with a yellow fruit pericarp.
The harmful effects of EMS did not stop at the M1 generation, but also affected the M2 generation. In the M2 generation, data analysis showed a significant reduction in seed germination in most families as compared to the WT population. The survival ratio was slightly affected and about 83% of the mutant population survived until maturity, which was similar to the rate of the WT population. Furthermore, during the M2 generation a chlorophyll defect appeared. It was found that EMS reduced seed germination and survival ratio during the M1 generation and that effect continued in the second generation in peppers (Alcantara et al., 1996; Jabeen and Mirza, 2002), tomatoes (Saba and Mirza, 2002), and Dianthus (Roychowdhury et al., 2012). This might be due to the effect of EMS on M0 seeds, which negatively affected the embryo, germination and this negative effect reflected on the plant growth and fruit formation of M1 plants (including fruits and formed seeds inside the fruit), which in turn produced bad quality of seeds (Abdul-Salam, 2007). In agreement with our results, previous studies found that the changes in the M2 generation appeared during the seedling stage in the formation of chlorophyll or slow growth and also affected the growth until maturity (Pawar et al., 2010).
During the M2 generation, the chance for identifying visible changes or phenotypic mutations should be higher and mostly due to genetics. Therefore observed mutations in the M2 generation are considered more stable (Parry et al., 2009). In the present work, during the M2 generation, among 500 families, there were three types of mutations in three families that could be visibly confirmed. One type of visible mutation was observed in one plant that had a uniform yellow–green color, characterizing chlorophyll deficiency. The plant with this mutation was able to survive until maturity. In agreement with our results, previous investigations found that a significant change in chlorophyll always resulted in the variation of leaf color (Hooda et al., 2004; Chen et al., 2007; Pawar et al., 2010). Previous studies reported that chlorophyll development seems to be controlled by many genes that are located on different chromosomes (Larkin and Scowcroft, 1981; Wang et al., 2013). Mutations affecting the production of chlorophyll are important for identifying gene function and the elucidation of chlorophyll metabolism and its regulation (Wu et al., 2007). Identifying a gene that regulates leaf color can be considered a critical issue because it can offer an alternative color to pepper breeders. Therefore, we are currently conducting further molecular analyses to detect the changes in genes that are responsible for chlorina in mutant peppers.
Dwarf mutants in peppers are crucial to understanding the regulatory mechanisms for plant growth and development (Ashikari et al., 1999). Furthermore, dwarfism is a favorable character for breeding programs. In the present investigation, the second type of mutation observed was dwarfism, and we recorded a plant with a height less than 10 cm with short internodes and thick, rough, fibrous, green leaves. Dwarf mutants were also reported earlier in peppers (Lippert et al., 1964; Alcantara et al., 1996; Jabeen and Mirza, 2004), which might be due to the inhibition of the elongation of epidermal cells, or a defect in GA biosynthesis (Fridborg et al., 1999). Discovery of recessive, monogenic mutations causing dwarfism is very important to allow for easier identification of the genes that control pepper growth (Wang and Bosland, 2006).
Another mutation that we observed affected the plant architecture and leaf texture. In plants with this mutation, the changes appeared in more than one character. In support of our results, mutations affecting branching pattern were previously observed in peppers (Mao et al., 2000). The mutant identified in the present study could be considered promising for establishing a reasonable ground for the genes controlling pepper architecture. The multiple phenotypes could be caused by one mutation with pleiotropic effects, or multiple mutations. The genetic control of plant architecture has been studied mostly in limited model plants such as arabidopsis, rice, maize, tomato, and petunia (Izawa, 2007; Wang and Li, 2008; Castel et al., 2010). In addition, these mutants also produced very thick leaves as compared to the WT.
Finally, we observed that two types of detected mutations in the present study are not consistent with the classic Mendelian model. The unusual segregation ratio in the dwarf (10:1) and leaf color mutants (23:1) might be due to the fact that changed characters are controlled by more than one gene and different genes interact to give this observed mutation (Hartl and Clark, 1997). In addition, unusual segregation ratios may also be due to using a fewer number of individuals in the mutant population, which may not have been enough to result in the usual segregation ratios (Hartl and Clark, 1997). Therefore, we are currently conducting further molecular study on this observed mutation to detect the genes responsible for inducing these changes, which can ultimately be used to improve the B12 pepper cultivar.
Conclusion
In the present study, EMS was used to induce genetic variability in the B12 cultivar of C. annuum. During the M1 generation there was a clear difference between the mutant and the WT populations. In the M1 we observed one visible mutation in which the plant had a change in leaf shape and growth habit. During the M2 generation a chlorophyll defect was observed at the seedling stage. Three phenotypic mutations were recognized during the M2 generation: yellow leaf color, dwarf stature, and a mutation in plant architecture and leaf shape. The yellow leaf color mutation can be used to understand the gene(s) that regulate chlorophyll metabolism and function. The dwarf mutation may allow for identification of the genes controlling pepper growth, which is a commercially desirable trait, as dwarf plants are easy to maintain and can grow in limited space. The identified mutants in plant architecture and leaf shape can provide an understanding of the genes controlling pepper architecture. Further work is needed to analyze these mutants and determine the genetic reasons underlying the visible changes in order to genetically improve C. annuum cultivars.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31272163), the Shaanxi Provincial Science and Technology Coordinating Innovative Engineering Project (2012KTCL02-09), and Cyrus Tang Seed Development Fund (Northwest A&F University). We thank MogoEdit Company (Xi’an, China) for providing language revision service.
Abbreviations
- DAS
days after sowing
- EMS
ethyl methanesulphonate
- GA
Gibberellin.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00399/abstract
References
- Abdul-Salam C. M. (2007). Studies on Chemical Mutagenesis in Varieties Ujwala and Co 1 of Capsicum annuum L. Ph.D. thesis, University of Calicut, Malappuram. [Google Scholar]
- Alcantara T., Bosland P., Smith D. (1996). Ethyl methanesulfonate-induced seed mutagenesis of Capsicum annuum. J. Hered. 87 239–241. 10.1093/oxfordjournals.jhered.a022992 [DOI] [Google Scholar]
- Angenent G. C., Stuurman J., Snowden K. C., Koes R. (2005). Use of petunia to unravel plant meristem functioning. Trends Plant Sci. 10 243–250. 10.1016/j.tplants.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Arisha M. H., Liang B.-K., Shah S. N. M., Gong Z.-H., Li. D.-W. (2014). Kill curve analysis and response of first generation Capsicum annuum L. B12 cultivar to ethyl methane sulfonate. Genet. Mol. Res. 13 10049–10061. 10.4238/2014.November.28.9 [DOI] [PubMed] [Google Scholar]
- Ashikari M., Wu J., Yano M., Sasaki T., Yoshimura A. (1999). Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. U.S.A. 96 10284–10289. 10.1073/pnas.96.18.10284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn G. P., Miksche J. P. (1976). Botanical microtechnique and cytochemistry. New Phytol. 78 245–255. 10.2307/2418781 [DOI] [Google Scholar]
- Bosland P. (2002). Inheritance of a novel flaccid mutant in Capsicum annuum. J. Hered. 93 380–382. 10.1093/jhered/93.5.380 [DOI] [PubMed] [Google Scholar]
- Bosland P. W., Votava E. J. (2012). Peppers: Vegetable and Spice Capsicums. Cambridge, MA: CABI; 10.1079/9781845938253.0000 [DOI] [Google Scholar]
- Botticella E., Sestili F., Hernandez-Lopez A., Phillips A., Lafiandra D. (2011). High resolution melting analysis for the detection of EMS induced mutations in wheat SbeIIa genes. BMC Plant Biol. 11:156 10.1186/1471-2229-11-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. G., Mccallum N., Shaw P., Muehlbauer G. J., Marshall D. F., Waugh R. (2004). A structured mutant population for forward and reverse genetics in Barley (Hordeum vulgare L.). Plant J. 40 143–150. 10.1111/j.1365-313X.2004.02190.x [DOI] [PubMed] [Google Scholar]
- Castel R., Kusters E., Koes R. (2010). Inflorescence development in petunia: through the maze of botanical terminology. J. Exp. Bot. 61 2235–2246. 10.1093/jxb/erq061 [DOI] [PubMed] [Google Scholar]
- Chen T., Zhang Y., Zhao L., Zhu Z., Lin J., Zhang S., et al. (2007). Physiological character and gene mapping in a new green-revertible albino mutant in rice. J. Genet. Genomic. 34 331–338. 10.1016/S1673-8527(07)60035-6 [DOI] [PubMed] [Google Scholar]
- Corp I. (2010). “IBM SPSS statistics for Windows, Version 19.0.” Armonk, NY: IBM Corporation. [Google Scholar]
- Daskalov S. (1986). Mutation breeding in pepper. Mutat. Breed. Rev. 4 1–26. [Google Scholar]
- Fridborg I., Kuusk S., Moritz T., Sundberg E. (1999). The Arabidopsis dwarf mutant shi exhibits reduced gibberellin responses conferred by overexpression of a new putative zinc finger protein. Plant Cell 11 1019–1031. 10.1105/tpc.11.6.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E. A., Codomo C. A., Taylor N. E., Henikoff J. G., Till B. J., Reynolds S. H., et al. (2003). Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics 164 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L., Clark A. G. (1997). Principles of Population Genetics. Sunderland: Sinauer Associates. [Google Scholar]
- Hohmann U., Jacobs G., Jung C. (2005). An EMS mutagenesis protocol for sugar beet and isolation of non-bolting mutants. Plant Breed. 124 317–321. 10.1111/j.1439-0523.2005.01126.x [DOI] [Google Scholar]
- Honda I., Kikuchi K., Matsuo S., Fukuda M., Saito H., Ryuto H., et al. (2006). Heavy-ion-induced mutants in sweet pepper isolated by M1 plant selection. Euphytica 152 61–66. 10.1007/s10681-006-9177-5 [DOI] [Google Scholar]
- Hooda M., Dhillon R., Bangarwa K. (2004). Albinism in jojoba (Simmondsia chinensis). Natl. J. Plant Improv. 1 69–70. [Google Scholar]
- Izawa T. (2007). Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J. Exp. Bot. 58 3091–3097. 10.1093/jxb/erm159 [DOI] [PubMed] [Google Scholar]
- Jabeen N., Mirza B. (2002). Ethyl methane sulfonate enhances genetic variability in Capsicum annuum. Asian J. Plant Sci. 1 425–428. 10.3923/ajps.2002.425.428 [DOI] [Google Scholar]
- Jabeen N., Mirza B. (2004). Ethyl methane sulfonate induces morphological mutations in Capsicum annuum. Int. J. Agric. Biol. 6 340–345. [Google Scholar]
- Just D., Garcia V., Fernandez L., Bres C., Mauxion J.-P., Petit J., et al. (2013). Micro-Tom mutants for functional analysis of target genes and discovery of new alleles in tomato. Plant Biotechnol. 30 225–231. 10.5511/plantbiotechnology.13.0622a [DOI] [Google Scholar]
- Kumar A. P., Boualem A., Bhattacharya A., Parikh S., Desai N., Zambelli A., et al. (2013). SMART–Sunflower mutant population and reverse genetic tool for crop improvement. BMC Plant Biol. 13:38–46. 10.1186/1471-2229-13-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin P. J., Scowcroft W. (1981). Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60 197–214. 10.1007/BF02342540 [DOI] [PubMed] [Google Scholar]
- Lippert L., Bergh B., Cook A. (1964). Three variegated seedling mutants in the pepper multiple allelism indicated in crossing studies. J. Hered. 55 79–83. [Google Scholar]
- Mao L., Begum D., Chuang H.-W., Budiman M. A., Szymkowiak E. J., Irish E. E., et al. (2000). JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature 406 910–913. 10.1038/35022611 [DOI] [PubMed] [Google Scholar]
- Østergaard L., Yanofsky M. F. (2004). Establishing gene function by mutagenesis in Arabidopsis thaliana. Plant J. 39 682–696. 10.1111/j.1365-313X.2004.02149.x [DOI] [PubMed] [Google Scholar]
- Page D. R., Grossniklaus U. (2002). The art and design of genetic screens: Arabidopsis thaliana. Nat. Rev. Genet. 3 124–136. 10.1038/nrg730 [DOI] [PubMed] [Google Scholar]
- Parry M. A., Madgwick P. J., Bayon C., Tearall K., Hernandez-Lopez A., Baudo M., et al. (2009). Mutation discovery for crop improvement. J. Exp. Bot. 60 2817–2825. 10.1093/jxb/erp189 [DOI] [PubMed] [Google Scholar]
- Pathirana R. (2012). “Plant mutation breeding in agriculture,” in Plant Sciences Reviews 2011. CAB Rev. 107. [Google Scholar]
- Pawar N., Pai S., Nimbalkar M., Kolar F., Dixit G. (2010). Induction of chlorophyll mutants in Zingiber officinale Roscoe by gamma rays and EMS. Emir. J. Food. Agric. 22 406–411. 10.9755/ejfa.v22i5.4828 [DOI] [Google Scholar]
- Porra R., Thompson W., Kriedemann P. (1989). Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 975 384–394. 10.1016/S0005-2728(89)80347-0 [DOI] [Google Scholar]
- Prusinkiewicz P., Erasmus Y., Lane B., Harder L. D., Coen E. (2007). Evolution and development of inflorescence architectures. Science 316 1452–1456. 10.1126/science.1140429 [DOI] [PubMed] [Google Scholar]
- Quinet M., Kinet J.-M. (2007). Transition to flowering and morphogenesis of reproductive structures in tomato. Int. J. Plant Dev. Biol. 1 64–74. [Google Scholar]
- Roychowdhury R., Alam M. J. F., Bishnu S., Dalal T., Tah J. (2012). Comparative study for effects of chemical mutagenesis on seed germination, survivability and pollen sterility in M1 and M2 generations of Dianthus. Plant Breed. Seed Sci. 65 29–38. [Google Scholar]
- Roychowdhury R., Tah J. (2013). “Mutagenesis-a potential approach for crop improvement,” in Crop Improvement: New Approaches and Modern Techniques, 1st Edn, eds Hakeem K. R., Ahmad P., Ozturk M. (New York, NY: Springer Science+Business Media; ), 149–187. [Google Scholar]
- Saba N., Mirza B. (2002). Ethyl methane sulfonate induced genetic variability in Lycopersicon esculentum. Int. J. Agric. Biol. 4 89–92. 10.1186/1746-4811-10-5 [DOI] [Google Scholar]
- Serrat X., Esteban R., Guibourt N., Moysset L., Nogués S., Lalanne E. (2014). EMS mutagenesis in mature seed-derived rice calli as a new method for rapidly obtaining TILLING mutant populations. Plant Methods 10:5 10.1038/nbt1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade A. J., Fuerstenberg S. I., Loeffler D., Steine M. N., Facciotti D. (2004). A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat. Biotechnol. 23 75–81. [DOI] [PubMed] [Google Scholar]
- Swaminathan M. (1964). A comparison of mutation induction in diploids and polyploids. Rad. Bot. (Suppl.) 5 619–641. 10.4236/ajps.2012.312202 [DOI] [Google Scholar]
- Talebi A. B., Talebi A. B., Shahrokhifar B. (2012). Ethyl methane sulphonate (EMS) induced mutagenesis in Malaysian rice (cv. MR219) for lethal dose determination. Am. J. Plant Sci. 3 1661–1665. [Google Scholar]
- Wang D., Bosland P. W. (2006). The genes of Capsicum. HortScience 41 1169–1187. 10.1146/annurev.arplant.59.032607.092902 [DOI] [Google Scholar]
- Wang Y., Li J. (2008). Molecular basis of plant architecture. Annu. Rev. Plant Biol. 59 253–279. 10.1007/s13258-013-0094-4 [DOI] [PubMed] [Google Scholar]
- Wang Z.-K., Huang Y.-X., Miao Z.-D., Hu Z.-Y., Song X.-Z., Liu L. (2013). Identification and characterization of BGL11 (t), a novel gene regulating leaf-color mutation in rice (Oryza sativa L.). Genes Genomics 35 491–499. 10.3923/ajps.2009.318.321 [DOI] [Google Scholar]
- Wani A. A. (2009). Mutagenic effectiveness and efficiency of gamma rays, ethyl methane sulphonate and their combination treatments in chickpea (Cicer arietinum L.). Asian J. Plant Sci. 8 318–322. 10.1104/pp.107.100321 [DOI] [Google Scholar]
- Wu Z., Zhang X., He B., Diao L., Sheng S., Wang J., et al. (2007). A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol. 145 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.