Abstract
The p53 tumor suppressor gene plays a critical role in regulation of proliferation, cell death and differentiation. The MDM2 oncoprotein is a major negative regulator for p53 by binding to and targeting p53 for proteasome-mediated degradation. The small molecule inhibitor, nutlin-3, disrupts MDM2-p53 interaction resulting in stabilization and activation of p53 protein. We have previously shown that nutlin-3 activates p53, leading to MDM2 accumulation as concomitant of reduced retinoblastoma (Rb) protein stability. It is well known that Rb is important in muscle development and myoblast differentiation and that rhabdomyosarcoma (RMS), or cancer of the skeletal muscle, typically harbors MDM2 amplification. In this study, we show that nutlin-3 inhibited myoblast proliferation and effectively prevented myoblast differentiation, as evidenced by lack of expression of muscle differentiation markers including myogenin and myosin heavy chain (MyHC), as well as a failure to form multinucleated myotubes, which were associated with dramatic increases in MDM2 expression and decrease in Rb protein levels. These results indicate that nutlin-3 can effectively inhibit muscle cell differentiation.
Keywords: MDM2, Rb, muscle differentiation, Nutlin
Introduction
The tumor suppressor p53 plays a crucial role in protection from carcinogenesis by regulating numerous cellular processes, including cell cycle progression, apoptosis, metabolic homeostasis, antioxidant defense, DNA repair and senescence. Under physiological condition, p53 protein levels are maintained low by MDM2-meadiated protein degradation. Upon cellular stresses, however, the p53-MDM2 interaction is inhibited, leading to p53 accumulation and activation of downstream responses. Importantly, p53 can potently up-regulate MDM2 gene expression, forming a negative feedback regulatory loop that restrains p53 activity [1].
The specificity of the p53-MDM2 interaction has prompted the development of a family of small molecule inhibitors termed nutlins, which fit in the hydrophobic p53-binding pocket in the MDM2 N-terminal domain and disrupt p53-MDM2 interaction [2]. Treatment of cancer cells with nutlins has been shown to activate the p53 pathway and to promote cell cycle arrest, premature cellular senescence and apoptosis [3,4]. Therefore, nutlins hold good promise for the development of targeted cancer therapies against cancers harboring wild type p53 and MDM2 amplification. One of these types of cancer is rhabdomyosarcoma (RMS), a primarily pediatric malignancy of the skeletal muscle [5]. Indeed, MDM2 amplification and wild type p53 retention has been found in numerous RMS cases exhibiting a non-differentiated phenotype [6,7,8,9,10].
In addition to its role in regulating p53 levels, MDM2 has been shown to possess oncogenic functions that are independent of p53. Notably, MDM2 directly binds to the Retinoblastoma protein (Rb) via its central acidic domain, thus inhibiting Rb growth suppressive function and targeting Rb to proteasomal degradation [11,12]. Rb is an important tumor suppressor that plays pivotal roles in a number of biological processes, including cell cycle control, DNA damage response, senescence and apoptosis [13]. A key function of Rb is to bind and inhibit E2F transcription factors, which would otherwise induce expression of genes that enhance cell cycle progression [14]. In addition, Rb plays a major role in development and muscle cell differentiation, as evidenced from the severe deficiencies in skeletal muscle development exhibited in Rb-null mice [15,16,17]. Rb promotes muscle differentiation at multiple stages of myogenesis by a variety of mechanisms, as shown by studies in which Rb deficiency results in inhibition of myoblast differentiation [18,19,20]. For instance, negative regulation of E2F-mediated gene expression by Rb induces permanent cell cycle withdrawal, which is required for terminal differentiation [21,22]. Moreover, Rb has been shown to promote activation of myogenic regulatory factors (MRFs), including MyoD, a basic helix-loop-helix (bHLH) transcription factor, and Myocite Enhancer Factor 2 (MEF2), thereby inducing expression of muscle-specific genes, such as Myosin Heavy Chain (MyHC) [23,24].
Since histological analyses of RMS cases usually reveal undifferentiated myoblastic phenotypes [25], we investigated the effects of nutlin treatment on muscle cell differentiation. We found that nutlin treatment led to significant up-regulation of MDM2 and down-regulation of Rb protein levels, and a blockage of myoblast proliferation and differentiation.
Materials and Methods
Cell Culture and drug treatment
C2C12 mouse myoblasts were maintained in growth media Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin G and streptomycin sulfate. Rat L6 myoblasts were cultured in DMEM supplemented with 10% FBS and 1% penicillin G and streptomycin sulfate. Cells were maintained at 37 °C under 5% CO2 in a humidified incubator. For induction of differentiation, 4.2 × 105 C2C12 cells or 10.8 × 105 L6 cells were plated in 100 mm dishes and grown in normal growth media for 24 hours. Then, the media were replaced with differentiation media, which were supplemented with 2% horse serum (C2C12) or 2% calf serum (L6) instead of FBS. Cells were treated with 10 µM nutlin-3 or vehicle (DMSO), as indicated. Media were replaced with fresh media with or without nutlin-3 every 48 hours.
Western blot analysis
Whole cell lysates were prepared using EBC250 lysis buffer (50 mM Tris-HCl pH 8.0, 250 mM NaCl, 0.5% Nonidet P-40, 1 mM PMSF, 2 µg/mL aprotinin, 40 mM NaF, and 0.5 mM NaVO4). Equal amounts of total protein were separated by SDS-PAGE and then transferred onto PVDF membranes and hybridized to a specific primary antibody and HRP-conjugated secondary antibody for subsequent detection by ECL (Pierce). Primary antibodies and dilutions used: Rb 1:500 (G3-245, BD Bioscience); myogenin 1:400 (F5D, Santa Cruz); actin 1:1000 (C-11, Santa Cruz); murine MDM2 (mixture of 2A10, 4B2, and 4B11; 1.0 mL each in 20 mL), and MyHC 1:400 (F-20, DSHB). Secondary antibodies were purchased from Santa Cruz Biotechnology and used at 1:3000 dilutions, including goat anti-mouse IgG-HRP (sc-2005), goat anti-rabbit IgG-HRP (sc-2004), and donkey anti-goat IgG-HRP (sc-2020).
Immunofluorescence
For immunofluoresence, 7.0 × 104 C2C12 cells or 1.8 × 105 L6 cells were grown on glass microscope coverslips in 35 mm plates, each containing 3 coverslips. Twenty-four hours after plating, cells were fixed with cold methanol and acetone (1:1) for ten minutes and air-dried overnight. The samples were blocked for one hour at room temperature with PBS containing 5% bovine serum albumin (BSA, Fisher), incubated for one hour at 37 °C with antibody specific for Myosin Heavy Chain (1:200 dilution), washed three times with PBS, and then incubated with a secondary antibody (anti-mouse IgG-Alexa488, Invitrogen) at 1:200 dilution for one hour at room temperature. The cells were washed with PBS, counterstained with propidium iodide (500 nM in water) for one minute and then washed three more times with PBS. Glass coverslips were mounted onto microscope slides with Prolong Antifade (Invitrogen). Fluorescent images were captured using an inverted Axiovert 200M microscope (Carl Zeiss) with a CCD ORCA-ER camera (Hamamatsu).
Results
Nutlin-3 blocks myoblast cell differentiation
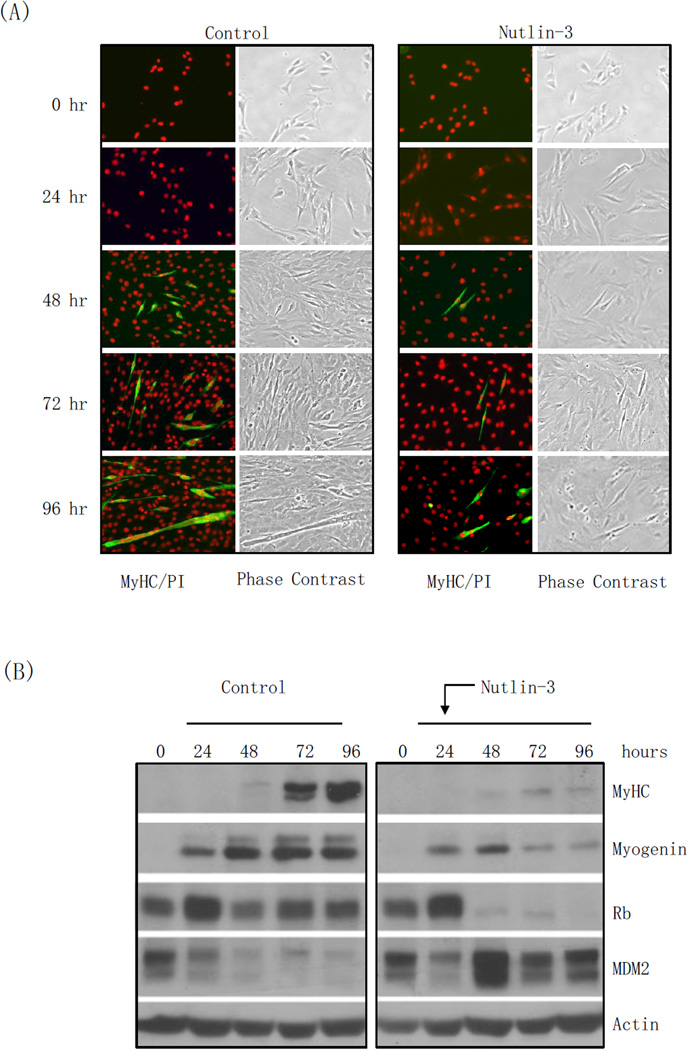
The small molecule nutlin-3 is a potent inhibitor of the MDM2-p53 interaction. Since p53 promotes MDM2 expression as part of a negative-feedback regulatory loop, and MDM2 has been shown to induce Rb degradation, which is known to play an important role in muscle differentiation, we investigated the effects of nutlin-3 on muscle cell differentiation. To this end, we used rat myoblast L6 cells and induced differentiation into myotubes using differentiation medium (DM) in the presence or absence of nutlin-3. Myoblast differentiation into myotubes is characterized by strong expression of myogenin, an early differentiation marker, and myosin heavy chain (MyHC), a late differentiation marker, and it culminates with cell fusion to form multi-nucleated myotubes [26]. As shown in Figure 1A, control L6 cells remained proliferative within the first 48 hours in differentiation medium, as evidenced from the cell density gradually reaching confluency. Cell differentiation first became evident after 24 hours, at which point a number of single cells stained positive for MyHC. After 48 hours, a large portion of cells exhibited phenotypic characteristics of myotubes, including MyHC expression and multiple nuclei. By 72 hours, the majority of the cells had terminally differentiated into myotubes, which were usually large with a dramatic increase in the number of nuclei. On the other hand, nutlin-3 treatment notoriously down-regulated cell proliferation, and dramatically inhibited myoblast differentiation. Indeed, only a minimal number of cells displayed MyHC staining after 72 hours of exposure to DM, and there was virtually no morphological evidence of myotube formation. Moreover, while control cells exhibited marked up-regulation of Myogenin expression as early as 24 hours post exposure to DM that was maintained through 72 hours, cells treated with nutlin-3 exhibited only modest Myogenin up-regulation that declined back to basal levels through the course of the treatment. Similarly, MyHC expression was markedly down-regulated in cells treated with nutlin-3, compared to control cells. Notably, MDM2 protein levels in control cells were not affected by differentiation, and remained low throughout the course of the experiment. By sharp contrast, nutlin-3 treatment led to a dramatic MDM2 up-regulation within 24 hours that was maintained through the course of the experiment, likely due to increased transcription of the MDM2 gene by up-regulated p53 (Figure 1B).
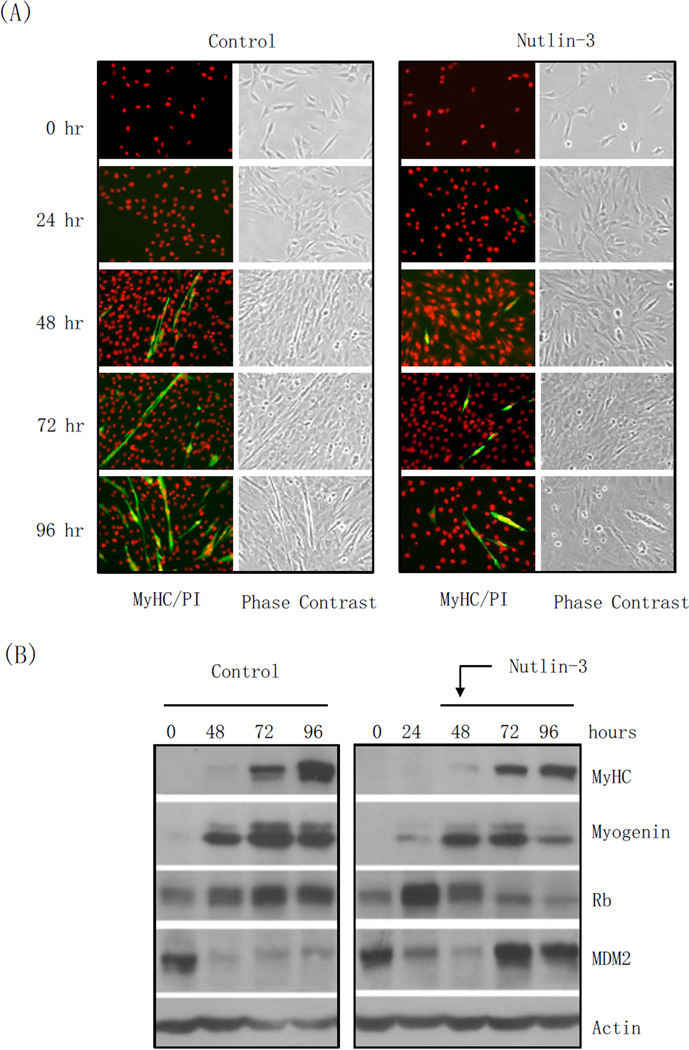
Figure 1. Nutlin-3 inhibits L6 and C2C12 cells proliferation and differentiation, correlated with down-regulation of Rb.
Rat myoblast L6 and mouse myoblast C2C12 cells were grown in DMEM containing 10% FBS. L6 cells were induced to differentiation in DMEM containing 2% calf serum in the presence or absence of 10 µM nutlin-3 for 24, 48 or 72 hours. C2C12 cells were induced to differentiate in DMEM containing 2% horse serum in the presence or absence of 10 µM nutlin-3 for 24, 48, 72, 96 hours or 7 days. DMSO was used as a vehicle control. (A, C) L6 (A) and C2C12 (C) cells were immunostained for myosin heavy chain (MyHC) and counterstained with propidium iodide (PI). Images were captured by fluorescence or phase contrast microscopy. (B, D) Whole-cell lysates were subjected to western blot analyses, as indicated. S = Short exposure; L = Long exposure.
Next, we explored whether the effect of nutlin-3 on myoblast differentiation is cell type specific. Thus, we induced differentiation in the presence or absence of nutlin-3 in C2C12 mouse myoblasts and observed for an extended period of seven days. Consistent with our previous results, cells treated with DM continued to proliferate, exhibited MyHC expression, and fused to form elongated, multi-nucleated myotubes. Conversely, in the presence of nutlin-3, cell proliferation was greatly inhibited and MyHC was not detectable by immunostaining even after seven days (Figure 1C). As shown in Figure 1D, western blot analyses revealed that induction of Myogenin and MyHC expression was greatly down-regulated in nutlin-3-treated cells, compared to control cells, while MDM2 expression was strongly up-regulated. Moreover, Rb protein levels in control cells were maintained at a near constant level. On the other hand, nutlin-3 treatment resulted in dramatically reduced Rb protein levels. These data indicate that nutlin-3 not only inhibits cell proliferation, but also effectively blocks myoblast cell differentiation.
Short-term nutlin-3 treatment blocks proliferation and differentiation
Since prolonged nutlin-3 treatment dramatically blocks muscle cell differentiation, we were interested in determining the effects of short-term treatment with nutlin-3. To this effect, we examined the ability of myoblasts to recover from acute exposure to nutlin-3 treatment. We subjected C2C12 cells to differentiation conditions with or without nutlin-3 for 24 hours, after which time we removed nutlin-3 and added fresh DM back to the cells. As shown in Figure 2, acute nutlin-3 treatment significantly delayed proliferation and differentiation, compared to control cells. However, removal of nutlin-3 from the DM allowed differentiation to proceed. These results suggest that nutlin-3 inhibits myoblast commitment to differentiation, and indicate that this inhibition is not irreversible, since continued exposure is required to prevent differentiation into myotubes.
Figure 2. Short-term treatment with nutlin-3 blocks C2C12 proliferation and differentiation.
C2C12 cells were induced to differentiate in DMEM containing 2% horse serum in the presence or absence of 10 µM nutlin-3 for 24 hours. DMSO was used as a vehicle control. Cells were washed twice with PBS and were then maintained in differentiation media for the indicated times. Cells were immunostained for myosin heavy chain (MyHC) and counterstained with propidium iodide (PI). Images were captured by fluorescence or phase contrast microscopy.
Nutlin-3 blocks the progression of muscle differentiation
Thus far, we have shown that nutlin-3 inhibits the initiation of differentiation by treating myoblasts simultaneously with DM and nutlin-3. Next, we examined the effect of nutlin-3 on the progression of myoblasts through differentiation by adding nutlin-3 after the cells had already been subjected to differentiation conditions. To this end, we induced differentiation in C2C12 cells, and added nutlin-3 24 hours after addition of DM. As shown in Figure 3A, addition of nutlin-3 at 24 hours post-differentiation resulted in markedly reduced differentiation, as evidenced by decreased MyHC staining, compared to control cells. Likewise, MyHC expression was markedly down-regulated in nutlin-3-treated cells, as assessed by western blotting (Figure 3B). In addition, nutlin-3 strongly up-regulated MDM2 and down-regulated Rb. Moreover, DM induced the early differentiation marker Myogenin within 24 hours. Notably, in control cells Myogenin levels continued to increase throughout the course of the experiment. On the other hand, addition of nutlin-3 after 24 hours halted Myogenin up-regulation (Figure 3B).
Figure 3. Nutlin-3 treatment 24 hours post-differentiation dramatically inhibits C2C12 differentiation, correlated with down-regulation of Rb.
C2C12 cells were subjected to differentiation conditions in DMEM containing 2% horse serum for 24 hours prior to the addition of 10 µM nutlin-3. DMSO was used as a vehicle control. (A) At the indicated times, cells were immunostained for myosin heavy chain (MyHC) and counterstained with propidium iodide (PI). Images were captured by fluorescence or phase contrast microscopy. (B) Whole-cell lysates were subjected to western blot analyses, as indicated.
Next, we examined whether nutlin-3 affects myoblast differentiation when added 48 post-induction of differentiation, when most cells are already committed to differentiation. As show in Figure 4A, MyHC up-regulation and differentiation, which were underway at 48 hours, was halted by addition of nutlin-3, as evidenced by no changes in the levels of MyHC staining. Again, nutlin-3 led to increased MDM2 levels and Rb down-regulation. Importantly, Myogenin levels, which were reaching strong up-regulation by DM, were down-regulated after addition of nutlin-3 (Figure 4B). Together, these data indicate that nutlin-3 strongly inhibits progression through the differentiation process, even after initial commitment.
Figure 4. Nutlin-3 treatment 48 post-differentiation partially inhibits C2C12 differentiation at later stage of differentiation.
C2C12 cells were subjected to differentiation conditions in DMEM containing 2% horse serum for 48 hours and then the media was changed to differentiation media in the presence or absence of 10 µM nutlin-3 for an additional 48 hours. DMSO was used as a vehicle control. (A) The cells were analyzed by immunostaining for MyHC and counterstained with PI. Images were captured by fluorescence or phase contrast microscopy. (B) Whole-cell lysates were subjected to western blot analyses, as indicated.
Discussion
Targeted cancer therapies are designed to be highly effective at reducing tumor size, while minimizing side effects on normal tissues. As a potential chemotherapy drug, it is important to examine the effects of nutlin-3 on different cell types. In this study, we showed that both long-term and short-term nutlin-3 treatment block myoblast differentiation, which is concomitant with up-regulated MDM2 and markedly lower Rb protein levels. Importantly, we had previously shown that MDM2 induces Rb degradation in an ubiquitin-independent, but proteasome-dependent mechanism [11,27]. Since nutlin-3-bound MDM2 retains its ability to bind Rb via its central acidic domain and to promote Rb degradation, these results suggest that nutlin-3-mediated p53 up-regulation induces MDM2 expression, which leads to increased Rb degradation and impaired myoblast differentiation.
Nutlin-3 has been suggested as a treatment for rhabdomyosarcomas (RMS) with MDM2 amplification. Of note, it has been shown in xenograft studies using human RMS cell lines that standard chemotherapy treatment leads to a reduction of tumor size, but not to a complete elimination of the tumor, thus highlighting the need for developing more efficacious therapies against RMS [28]. For example, treatment of RMS cell lines with nutlin-3 was shown to induce cell cycle arrest and p53-dependent apoptosis [29]. Similarly, MI-63, a different small molecule inhibitor of the p53-MDM2 interaction that also fits in the p53-binding pocket in MDM2, was also shown to effectively impair cell proliferation and viability of RMS cells [30]. More recently, a newer generation nutlin family member termed RG7112 was shown to be a potent inhibitor of tumor growth in pre-clinical mouse xenograft models, especially in osteosarcoma cells with amplified MDM2 expression [31]. In addition, RG7112 was independently shown to inhibit RMS tumor growth and enhance survival of xenograft-bearing mice [32]. Nevertheless, our results indicate that nutlin-3 treatment disrupts myogenesis, therefore suggesting that there may be potential complications for clinical application.
In this study, we found that nutlin-3 treatment inhibits both initiation of differentiation, as well as progression through the differentiation program. Notably, these effects were accompanied by up-regulated MDM2 expression and a dramatic down-regulation of Rb protein levels. These observations are consistent with our previous study showing that nutlin-3 treatment of cancer cells leads to marked reductions in Rb protein levels [33]. Since it has been shown that Rb is required for myoblasts to exit the cell cycle and initiate differentiation [19], it is likely that nutlin-3 inhibits myoblast differentiation via down-regulation of Rb protein by MDM2. Surprisingly, nutlin-3 still blocked differentiation when added 48 hours after differentiation, thus indicating that Rb is required not only for exit from the cell cycle, but also for induction of the muscle differentiation program, as has been previously shown [23,24]. Analogously, low MDM2 levels have been shown to be important for maintaining myoblast differentiation potential, as MDM2 over-expression inhibits myoblast differentiation [34]. On the other hand, the role of p53 in muscle differentiation is not yet clear. While p53−/− mice have normal skeletal muscle development [35], it has been shown that activation of p53 promotes the differentiation of myogenic lineages [36]. In addition, it was shown that p53 directly regulates Rb gene transcription and thereby MyoD activity during initiation of differentiation in C2C12 cells, and that p53 deficiency prevents differentiation, which can be rescued by reintroducing Rb expression [37].
Nutlin-3 has been found to affect differentiation of other cell types. For example, nutlin-3 was shown to inhibit pre-osteoclast proliferation and differentiation in a p53-dependent manner [38]. On the other hand, nutlin-3 induced apoptosis in primary acute myeloid leukemia and promoted maturation of the surviving cells [39]. In addition, nutlin-3 has been shown to induce rapid differentiation of embryonic stem cells [40]. Interestingly, it was shown that short-term nutlin-3 treatment of p53 wild-type osteosarcoma U2-OS and colon adenocarcinoma HCT116 cancer cells can lead to polyploidy and drug resistance [41], thus suggesting that polyploidy in myoblast satellite cells could potentially lead to sporadic RMS.
Supplementary Material
Highlights.
Nutlin-3 inhibits myoblast proliferation and prevents differentiation into myotubes.
Nutlin-3 increases MDM2 expression and down-regulates Rb protein levels.
This study has implication in nutlin-3 treatment of rhabdomyosarcomas
Acknowledgements
We thank Dr. Jason Chen (University of Massachusetts Medical School, Worcester, MA) for kindly providing antibodies against mouse MDM2. This work was supported by the National Key Basic Research Program (973 Program) of China (2012CB910700) and National Natural Science Foundation of China grants (31171362) to Z-X.X., and (31350110216) to J.B., and NIH grant (CA79804) to Z-X.X.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 3.Vassilev LT. Small-molecule antagonists of p53-MDM2 binding: research tools and potential therapeutics. Cell Cycle. 2004;3:419–421. [PubMed] [Google Scholar]
- 4.Vassilev LT. p53 Activation by small molecules: application in oncology. J Med Chem. 2005;48:4491–4499. doi: 10.1021/jm058174k. [DOI] [PubMed] [Google Scholar]
- 5.Stock N, Chibon F, Binh MBN, Terrier P, Michels JJ, Valo I, et al. Adult-type rhabdomyosarcoma: analysis of 57 cases with clinicopathologic description, identification of 3 morphologic patterns and prognosis. Am J Surg Pathol. 2009;33:1850–1859. doi: 10.1097/PAS.0b013e3181be6209. [DOI] [PubMed] [Google Scholar]
- 6.Bouron-Dal Soglio D, Rougemont A-L, Absi R, Barrette S, Montpetit A, Fetni R, Fournet J-C. SNP genotyping of a sclerosing rhabdomyosarcoma: reveals highly aneuploid profile and a specific MDM2/HMGA2 amplification. Hum Pathol. 2009;40:1347–1352. doi: 10.1016/j.humpath.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Keleti J, Quezado MM, Abaza MM, Raffeld M, Tsokos M. The MDM2 oncoprotein is overexpressed in rhabdomyosarcoma cell lines and stabilizes wild-type p53 protein. Am J Pathol. 1996;149:143–151. [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi K, Wettach GR, Ryan CW, Hung A, Hooper JE, Beadling C, Warrick A, Corless CL, Olson SB, Keller C, Mansoor A. MDM2 Amplification and PI3KCA Mutation in a Case of Sclerosing Rhabdomyosarcoma. Sarcoma. 2013;2013:520858. doi: 10.1155/2013/520858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ragazzini P, Gamberi G, Pazzaglia L, Serra M, Magagnoli G, Ponticelli F, Ferrari C, Ghinelli C, Alberghini M, Bertoni F, Picci P, Benassi MS. Amplification of CDK4, MDM2, SAS and GLI genes in leiomyosarcoma, alveolar and embryonal rhabdomyosarcoma. Histol Histopathol. 2004;19:401–411. doi: 10.14670/HH-19.401. [DOI] [PubMed] [Google Scholar]
- 10.Xia SJ, Pressey JG, Barr FG. Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther. 2002;1:97–104. doi: 10.4161/cbt.51. [DOI] [PubMed] [Google Scholar]
- 11.Sdek P, Ying H, Zheng H, Margulis A, Tang X, Tian K, Xiao Z-XJ. The central acidic domain of MDM2 is critical in inhibition of retinoblastoma-mediated suppression of E2F and cell growth. J Biol Chem. 2004;279:53317–53322. doi: 10.1074/jbc.M406062200. [DOI] [PubMed] [Google Scholar]
- 12.Xiao ZX, Chen J, Levine AJ, Modjtahedi N, Xing J, Sellers WR, Livingston DM. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995;375:694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- 13.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevaux O, Dyson NJ. A revised picture of the E2F transcriptional network and RB function. Curr Opin Cell Biol. 2002;14:684–691. doi: 10.1016/s0955-0674(02)00388-5. [DOI] [PubMed] [Google Scholar]
- 15.de Bruin A, Wu L, Saavedra HI, Wilson P, Yang Y, Rosol TJ, Weinstein M, Robinson ML, Leone G. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc Natl Acad Sci USA. 2003;100:6546–6551. doi: 10.1073/pnas.1031853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003;421:942–947. doi: 10.1038/nature01417. [DOI] [PubMed] [Google Scholar]
- 17.Zacksenhaus E, Jiang Z, Chung D, Marth JD, Phillips RA, Gallie BL. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev. 1996;10:3051–3064. doi: 10.1101/gad.10.23.3051. [DOI] [PubMed] [Google Scholar]
- 18.De Falco G, Comes F, Simone C. pRb: master of differentiation. Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene. 2006;25:5244–5249. doi: 10.1038/sj.onc.1209623. [DOI] [PubMed] [Google Scholar]
- 19.Huh MS, Parker MH, Scimè A, Parks R, Rudnicki MA. Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J Cell Biol. 2004;166:865–876. doi: 10.1083/jcb.200403004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mal A, Chattopadhyay D, Ghosh MK, Poon RY, Hunter T, Harter ML. p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J Cell Biol. 2000;149:281–292. doi: 10.1083/jcb.149.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 22.Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, Olson EN, Harper JW, Elledge SJ. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 23.Novitch BG, Spicer DB, Kim PS, Cheung WL, Lassar AB. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr Biol. 1999;9:449–459. doi: 10.1016/s0960-9822(99)80210-3. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 25.Anderson J, Gordon A, Pritchard-Jones K, Shipley J. Genes, chromosomes, and rhabdomyosarcoma. Genes Chromosomes Cancer. 1999;26:275–285. [PubMed] [Google Scholar]
- 26.Burattini S, Ferri P, Battistelli M, Curci R, Luchetti F, Falcieri E. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem. 2004;48:223–233. [PubMed] [Google Scholar]
- 27.Sdek P, Ying H, Chang DLF, Qiu W, Zheng H, Touitou R, Allday MJ, Xiao Z-XJ. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20:699–708. doi: 10.1016/j.molcel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Seitz G, Warmann SW, Vokuhl CO, Heitmann H, Treuner C, Leuschner I, Fuchs J. Effects of standard chemotherapy on tumor growth and regulation of multidrug resistance genes and proteins in childhood rhabdomyosarcoma. Pediatr Surg Int. 2007;23:431–439. doi: 10.1007/s00383-006-1852-z. [DOI] [PubMed] [Google Scholar]
- 29.Miyachi M, Kakazu N, Yagyu S, Katsumi Y, Tsubai-Shimizu S, Kikuchi K, Tsuchiya K, Iehara T, Hosoi H. Restoration of p53 pathway by nutlin-3 induces cell cycle arrest and apoptosis in human rhabdomyosarcoma cells. Clin Cancer Res. 2009;15:4077–4084. doi: 10.1158/1078-0432.CCR-08-2955. [DOI] [PubMed] [Google Scholar]
- 30.Canner JA, Sobo M, Ball S, Hutzen B, DeAngelis S, Willis W, Studebaker AW, Ding K, Wang S, Yang D, Lin J. MI-63: a novel small-molecule inhibitor targets MDM2 and induces apoptosis in embryonal and alveolar rhabdomyosarcoma cells with wild-type p53. Br J Cancer. 2009;101:774–781. doi: 10.1038/sj.bjc.6605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tovar C, Graves B, Packman K, Filipovic Z, Higgins B, Xia M, Tardell C, Garrido R, Lee E, Kolinsky K, To K-H, Linn M, Podlaski F, Wovkulich P, Vu B, Vassilev LT. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73:2587–2597. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 32.Carol H, Reynolds CP, Kang MH, Keir ST, Maris JM, Gorlick R, Kolb EA, Billups CA, Geier B, Kurmasheva RT, Houghton PJ, Smith MA, Lock RB. Initial testing of the MDM2 inhibitor RG7112 by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2013;60:633–641. doi: 10.1002/pbc.24235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du W, Wu J, Walsh EM, Zhang Y, Chen CY, Xiao Z-XJ. Nutlin-3 affects expression and function of retinoblastoma protein: role of retinoblastoma protein in cellular response to nutlin-3. J Biol Chem. 2009;284:26315–26321. doi: 10.1074/jbc.M109.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiddler TA, Smith L, Tapscott SJ, Thayer MJ. Amplification of MDM2 inhibits MyoD-mediated myogenesis. Mol Cell Biol. 1996;16:5048–5057. doi: 10.1128/mcb.16.9.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 36.Molchadsky A, Shats I, Goldfinger N, Pevsner-Fischer M, Olson M, Rinon A, Tzahor E, Lozano G, Zipori D, Sarig R, Rotter V. p53 plays a role in mesenchymal differentiation programs, in a cell fate dependent manner. PLoS ONE. 2008;3:e3707. doi: 10.1371/journal.pone.0003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porrello A, Cerone MA, Coen S, Gurtner A, Fontemaggi G, Cimino L, Piaggio G, Sacchi A, Soddu S. p53 regulates myogenesis by triggering the differentiation activity of pRb. J Cell Biol. 2000;151:1295–1304. doi: 10.1083/jcb.151.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zauli G, Rimondi E, Corallini F, Fadda R, Capitani S, Secchiero P. MDM2 antagonist Nutlin-3 suppresses the proliferation and differentiation of human pre-osteoclasts through a p53-dependent pathway. J Bone Miner Res. 2007;22:1621–1630. doi: 10.1359/jbmr.070618. [DOI] [PubMed] [Google Scholar]
- 39.Secchiero P, Zerbinati C, Melloni E, Milani D, Campioni D, Fadda R, Tiribelli M, Zauli G. The MDM-2 antagonist nutlin-3 promotes the maturation of acute myeloid leukemic blasts. Neoplasia. 2007;9:853–861. doi: 10.1593/neo.07523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maimets T, Neganova I, Armstrong L, Lako M. Activation of p53 by nutlin leads to rapid differentiation of human embryonic stem cells. Oncogene. 2008;27:5277–5287. doi: 10.1038/onc.2008.166. [DOI] [PubMed] [Google Scholar]
- 41.Shen H, Moran DM, Maki CG. Transient nutlin-3a treatment promotes endoreduplication and the generation of therapy-resistant tetraploid cells. Cancer Res. 2008;68:8260–8268. doi: 10.1158/0008-5472.CAN-08-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.