Abstract
Radiation therapy is the primary treatment in nasopharyngeal carcinoma (NPC), and the effect of radiation therapy is strongly related to the oxygen content of cancer cells. That means, it is imperative to balance the interactions between radiotherapy and anti-angiogenesis therapy when giving combination therapy to improve clinical outcomes. The complicated mechanisms between antiangiogenic agents and radiation involve many interactions between the cancer cells, vasculature, and cancer stroma. The proliferation and metastasis of cancer depends on angiogenesis, while rapid growth of cancers will cause hypoxia, which contributes to radioresistance. Antiangiogenic agents can modulate the cancer blood flow and oxygenation through target cancer vasculature, leading to increased radiosensitivity. This study discusses the mechanisms of the synergistic effect of the antiangiogenic therapy with radiation therapy in metastatic NPC, and reviews the data supporting this strategy as a promising treatment for metastatic NPC.
Nasopharyngeal carcinoma (NPC), as a malignant head and neck cancer, is known for its atypical early symptoms and high-metastatic potential. Unlike other malignant cancers, due to the complexity structure of the nose-pharynx ministry, the characteristics of invasive growth and radiosensitivity, radiotherapy is the first choice for NPC. With the incessant development and update of radiotherapy-associated equipment and technology, the effect of treatment in NPC has been improved greatly, but there still exists some patients who are not sensitive to radiation, and may lead to failures of treatment. Increasing the sensitivity of radiation and improving the local control rate are important approaches to enhance the curative effect of NPC. Radiotherapy combined with chemotherapy has been proven to increase the effect to some extent.1,2 But more novel targeting strategies are needed in order to improve outcome. In the past years, anti-angiogenesis therapies have showed a rapid ascent into clinical practice. Since angiogenesis is associated with advanced and metastatic cancers, it has its unique characters in cancer. Combining antiangiogenic agents and radiotherapy seems to be feasible. Here, we briefly summarize the effects of antiangiogenic agents added to radiotherapy in NPC, and explain the mechanisms under the current knowledge.
Hypoxia-inducing factor 1-alpha and radiosensitivity of nasopharyngeal carcinoma
Like the repair of DNA damage, regulation of cell cycle, apoptosis, or others, the oxygenation state of cancer cells is one of the main factors that regulate cancer radiosensitivity. With further research of radiation biology, the influence of hypoxia of tissues or cells to radiosensitivity cannot be simply summarized as enhanced, or reduced. The mechanisms between them are very complicated, even paradoxical to some extent. On the one hand, hypoxia can result in reducing radiosensitivity. Radiation-induced DNA double strand breaks, which causes cell cycle arrest, and cell death is the main mechanism of radiotherapy. Meanwhile, as a potent radiosensitizer, oxygen can facilitate the production of free radicals, which is essential for the induction of radiation-associated DNA damage.3,4 That means, the growth of cancers, anti-angiogenesis or other factors, which can result in a lack of adequate blood supply or oxygen for regions will lead to radiation resistance as the cancer microenvironment in hypoxia cannot promote radiation-induced DNA damage.5 On the other hand, hypoxic cancer cells are characterized by up-regulating HIF-1α, an important regulatory factor that enables cancer cells to endure a hypoxic microenvironment.6,7 The hypoxia tolerance includes regulating the induction of various transcription factors involved in tumor metabolism, invasion, cell death and angiogenesis, including the key angiogenic molecule vascular endothelial growth factor (VEGF).8,9
It has been reported that the overexpression of HIF-1α in NPC correlates with carcinogenesis,10,11 proliferation,7 and surviving,12 as well as with poorer prognosis,13 and advanced cancer stage,14 while HIF-1α and VEGF play roles in these modulation. The HIF-1α has also been found to have connections with radio resistance.3,15,16 Hosokawa et al16 showed that oral squamous cell carcinoma (OSCC) cells of high-level HIF-1α were resistant to radiation and HIF-1α involved in controlling short-term radiosensitivity of cells. Xu et al17 found that down-regulating the expression of HIF-1α and osteopontin mRNA could radiosensitize the HNE-1 cell. As stated above, HIF-1α caused by radiation exposure can result in the up-regulation of VEGF, estimated glomerular filtration rate (EGFR) and others, followed by high levels of angiogenic growth factors, especially VEGF, endothelial cell survival can be increased, which may participate in radioresistance.18 Meanwhile, an increased proliferation of cancer cells may result from the promotion or maintenance of cancer vascular system via up-regulated radiation-induced VEGF.19,20 This may contribute to radiation resistance in many ways, including improved interstitial fluid pressure, or vascular permeability, increased oxygen consumption, and hypoxic microenvironment. There is also evidence to support that HIF-1α can enhance the sensitivity of radiation. The HIF-1α can promote cell cycle arrest and apoptosis to enhance cellular radiosensitivity.21,22 However, recently Sendoel et al23 reported HIF-1 could antagonize p53-mediated apoptosis through a secreted neuronal tyrosinase. The outcomes will vary from different conditions. Oike et al24 found that the expression of HIF-1α did not contribute primarily to the radiosensitivity of lung adenocarcinoma cells under acute hypoxia. At present, most scholars support that increasing HIF-1α can result in radiation resistance, while silencing HIF-1α contributes to an increased radiosensitivity.16,25
Vascular endothelial growth factor and its role in nasopharyngeal carcinoma
The VEGF, known as a potent promoter for angiogenesis, plays a primary role in the formation of new blood vessels. Its role in NPC has also been well established.26 There are 7 ligands of VEGF family, including VEGF A-E. The VEGFR-1/2, which are primarily involved in angiogenesis is known to bind VEGF A-D and PLGF.27 The VEGF-C and VEGF-D were also found to bind to VEGFR-3, which is involved in lymphatic metastasis. Previous reports indicate that VEGF, especially VEGF-A can bind to 2 receptor tyrosine kinases (VEGFR-1/2) to promote endothelial cell differentiation, proliferation, migration, and induction of matrix metalloproteinase (MMPs). Signaling pathways, such as phosphatidylinositol-3-kinase/Silk threonine protein kinase (PI3K/AKT) and Ras/Mitogen-activated protein kinase (Ras/MAPK) was also activated to help with endothelial cell survival.18
In NPC, VEGF-inducted MMPs not only participate in the formation of new blood vessels though degrading endothelial extracellular matrix, but also regulate the invasion and metastasis of cancer, leading to a progression of NPC.28,29 In addition, it was reported that VEGF has a strong connection with varied regulatory factors, which are involved in angiogenesis. Chen et al30 indicated that the effects of angiopoietin-2, which can maintain the mature blood vessels, highly rely on the level of VEGF expression. Chen et al30 found that Celecoxib, an inhibitor of cyclooxygenase-2 -2, has the ability to inhibit the capacity of invasion, suppress the level of VEGF-A expression, and enhance radiosensitivity in NPC.31 Thus, these could be effective targets to inhibit angiogenesis for the treatment of NPC.
Anti-angiogenesis combined with radiation in nasopharyngeal carcinoma
As the angiogenesis plays an important role in the progress of cancer, targeting angiogenesis agents will be a significant part of the treatment of NPC. Recently, the treatment of anti-angiogenesis combined with radiation has been used in clinical trials, and it has some effects. Bevacizumab had been used in the clinical trial of Head Neck Squamous Cell Carcinoma (HNSCC), and the results showed that combined therapy was feasible.32,33 Lee et al34 followed-up 46 NPC patients, and found that adding bevacizumab to standard chemoradiation treatment was feasible. The estimated 2 year locoregional progression-free interval was 83.7% (95% confidence interval [CI]: 72.6-94.9), 2 year distant metastasis-free interval was 90.8% (95% CI: 82.2-99.5), 2 year progression-free survival was 74.7% (95% CI: 61.8-87.6), and 2 year overall survival 90.9% (95% CI: 82.3-99.4). Bevacizumab may delay the progression of subclinical distant disease.34 Elser et al35 evaluated 27 patients and determined the efficacy and safety of sorafenib, which could inhibit the growth and angiogenesis of cancer in NPC. They found the median time of progression was 1.8 months (95% CI: 1.6-3.4 months), and overall survival was 4.2 months (95% CI: 3.6-8.7 months). While fatigue, mucositis, lymphopenia, anemia, and hand-foot skin reaction were the most common toxicities.35,36 Xue et al37 found that it was tolerable and feasible for a combination of sorafenib, cisplatin (80 mg/m2), and 5 fluorouracil (FU) (3000 mg/m2) in NPC recurrent or metastatic, but then requires further randomized trials. Huang et al38 reported that sorafenib and sunitinib could markedly increase the cytotoxic sensitivity of cancer cells to natural killer cells by up-regulating NKG2D ligands.
In mouse models, it had been reported that the function of radiation in antitumor and antiangiogenesis could significantly increase in NPC by Endostar™ (rh-endostatin, YH-16) (a new recombinant human endostatin developed by Medgenn Bioengineering Co. Ltd., Yantai, Shandong, China), while promoting apoptosis of endothelial cells and cancer cells, increasing hypoxia of cancer cells, and changing proangiogenic factors that contributed to it.39 Zhou et al40 found that by Endostar significantly inhibited the growth of NPC cells, the cancer inhibition rates of Endostar + radiation was 86.1%, Endostar was 27.1%, and radiation was 60.5%. Additional, Endostar could enhance the radiosensitivity of NPC cells by lowering VEGF expression. Zhou et al41 had a similar conclusion. Peng et al42 also found that Endostar is involved in normalizing tumor vasculature, which could lead to alleviating hypoxia, and sensitizing the antitumor effect of radiation. The increase of pericyte coverage in NPC tumor vessels by the up-regulated PEDF and down-regulated VEGF might play a role in this.42 In addition to the phase II trial, the efficacy and safety of Endostar combined with gemcitabine and cisplatin chemotherapy in metastatic NPC was determined. Twenty-eight patients were included for evaluation. The median progression-free survival (PFS) was 19.4 months (95% CI: 13.6-25.1 months). The confirmed objective response rate was 85.7% (95% CI: 66.4-95.3%) including complete response in 14 patients (50%). The one-year PFS rate was 69.8%, and the one-year overall survival rate was 90.2%. The most common grade 3/4 adverse events were neutropenia (46.4%), and thrombocytopenia (14.3%). This indicated that Endostar combining with gemcitabine and cisplatin chemotherapy would be a potential treatment for NPC.43
The mechanism by which the combined treatment has an effect is complicated. On one hand, the formation of cancer blood vessels would be inhibited by targeting VEGF and other targets, as a result, cancer blood supply is insufficient to meet the needs of growth and metastasis, and resulted in an inhibition of cancer progress. Meanwhile, VEGF/VGFR can also activate signaling pathways, such as Ras/MAPK, and PI3K/AKT pathways to promote endothelial cell proliferation and survival.18,44 Thus, endothelial cells are easily damaged, and the radiosensitivity will be increased by targeting VEGF/VGFR. Then in terms of the paradoxical effect that hypoxia caused by anti-angiogenesis will reduce the sensitivity of radiation, the theory of vascular normalization window can explain it.42,45,46 Antiangiogenic therapy can induce a specific ‘‘vascular normalization window’’. During this time, the function, structure of cancer blood vessels, and microenvironment temporarily become normalized, meaning the interstitial fluid pressure is decreased, and blood perfusion is increased. As a consequence, the anticancer drugs can easily penetrate into the cancers; in addition, hypoxia will be temporarily overcome and leads to more DNA damage, cell death, and high sensitivity of radiotherapy by producing more free radicals. Thus, administering radiotherapy during the window period is the key to improve the antitumor efficacy. The effect of radiation for NPC is summarized in Table 1, and the effect of antiangiogenic + radiation for NPC is summarized in Table 2.
Table 1.
The effect of radiation for nasopharyngeal carcinoma cells.
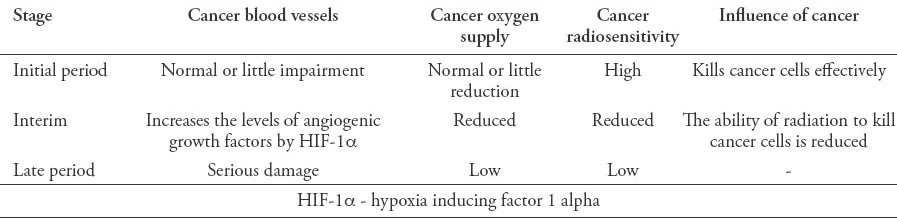
Table 2.
The effect of antiangiogenic therapy for nasopharyngeal carcinoma cells in radiation.
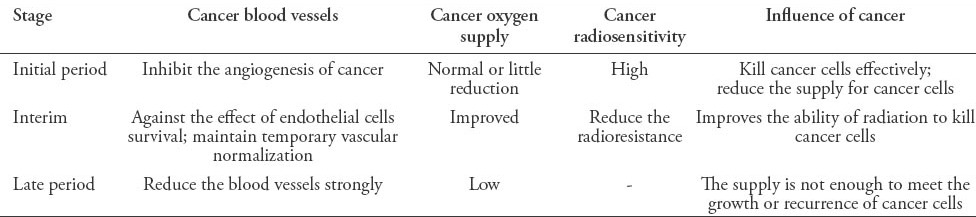
In conclusion, due to the characteristics of NPC, radiotherapy is the main means of treatment. However, the single treatment often cannot meet the need of the expected goal, and combination therapy is a trend for NPC. Anti-angiogenesis, as the main mechanism for blocking the supply of tumor cell growth is a promising treatment for NPC. A high expression of HIF-1α is often induced by radiation, and it regulates the radiosensitivity by modulating the expression of VEGF, or other signaling pathways. Moreover, the vascular normalization window in anti-angiogenesis is considered to be an important factor for the promotion of cancer radiosensitivity. Therefore, the combined therapy does not equate a simple addition of the 2 therapies. More research is needed to obtain a better understanding of the interactive effect. It was found that combining anti-angiogenic therapy with radiotherapy has a clinical value in improving the effect of NPC, but of note, the number of patients in trials is still low, and more specimens are needed to confirm the outcomes. Considering the importance of the vascular normalization window in such treatment, some issues, such as the formative time and duration of the vascular normalization, whether the normalization relies on the dose, or type of drugs is worth further study. In addition, to inhibit the formation of new blood vessels, targeting the existing blood vessels and reducing its function is also involved in antiangiogenic therapy.47 Radiotherapy combined with antiangiogenic is a promising model for NPC treatment. Considering various factors, such as the type of drugs, delivery time, dose, and the type of ray,48 and a reasonable therapy scheme are critical to improve the effect of NPC.
Footnotes
Related Articles.
Xu XH, Liu Y. Study of the correlationship link between microRNAs and nasopharyngeal carcinoma. Saudi Med J 2014; 35: 329-335.
Golshiri A, Shabani Z, Mokhtaree MR, Sayadi AR, Faezi H. Effect of opium smoking cessation on the nasopharyngeal microbial flora. Saudi Med J 2010; 31: 25-28.
Abuidris DO, Elgaili EM, Elhaj AH, Elmustafa OM. Histopathological patterns of nasopharyngeal carcinoma in Sudan. Saudi Med J 2008; 29: 962-965.
References
- 1.Ji X, Xie C, Hu D, Fan X, Zhou Y, Zheng Y. Survival benefit of adding chemotherapy to intensity modulated radiation in patients with locoregionally advanced nasopharyngeal carcinoma. PLoS One. 2013;8:e56208. doi: 10.1371/journal.pone.0056208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komatsu M, Tsukuda M, Matsuda H, Horiuchi C, Taguch T, Takahashi M, et al. Comparison of concurrent chemoradiotherapy versus induction chemotherapy followed by radiation in patients with nasopharyngeal carcinoma. Anticancer Res. 2012;32:681–686. [PubMed] [Google Scholar]
- 3.Wu Y, Zheng Y, Shen Z, Ge W, Xie Y, Li C. Endostar combined with radiotherapy increases radiation sensitivity by decreasing the expression of TGF-β1, HIF-1α and bFGF. Exp Ther Med. 2014;79:911–916. doi: 10.3892/etm.2014.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karar J, Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther. 2009;8:1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeom CJ, Zeng L, Zhu Y, Hiraoka M, Harada H. Strategies to assess hypoxic /HIF-1-active cancer cells for the development of innovative radiation therapy. Cancers (Basel) 2011;3:3610–3631. doi: 10.3390/cancers3033610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi D, Xie F, Zhang Y, Tian Y, Chen W, Fu L, et al. TFAP2A Regulates Nasopharyngeal Carcinoma Growth and Survival by Targeting HIF-1a Signaling Pathway. Cancer Prev Res. 2013;7:266–277. doi: 10.1158/1940-6207.CAPR-13-0271. [DOI] [PubMed] [Google Scholar]
- 7.Shi D, Guo W, Chen W, Fu L, Wang J, Tian Y, et al. Nicotine promotes proliferation of human nasopharyngeal carcinoma cells by regulating a7AChR, ERK, HIF-1a and VEGF/PEDF signaling. PLOS One. 2012;7:e43898. doi: 10.1371/journal.pone.0043898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khong TL, Thairu N, Larsen H, Dawson PM, Kiriakidis S, Paleolog EM. Identification of the angiogenic gene signature induced by EGF and hypoxia in colorectal cancer. BMC Cancer. 2013;13:518. doi: 10.1186/1471-2407-13-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi SH, Kwon OJ, Park JY, Kim do Y, Ahn SH, Kim SU, et al. Inhibition of tumour angiogenesis and growth by small hairpin HIF-1a and IL-8 in hepatocellular carcinoma. Liver Int. 2014;34:632–642. doi: 10.1111/liv.12375. [DOI] [PubMed] [Google Scholar]
- 10.Shi D, Xie F, Zhang Y, Tian Y, Chen W, Fu L, et al. TFAP2A regulates nasopharyngeal carcinoma growth and survival by targeting HIF-1a signaling pathway. Cancer Prev Res (Phila) 2014;7:266–277. doi: 10.1158/1940-6207.CAPR-13-0271. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Li X, Wu S, Xu G, Zhou Y, Gong L, et al. Expression of HIF-1a and CAIX in nasopharyngeal carcinoma and their correlation with patients’ prognosis. Med Oncol. 2014;31:304. doi: 10.1007/s12032-014-0304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, Guo L, et al. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy. 2010;6:395–404. doi: 10.4161/auto.6.3.11303. [DOI] [PubMed] [Google Scholar]
- 13.Shou Z, Lin L, Liang J, Li JL, Chen HY. Expression and prognosis of FOXO3a and HIF-1a in nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138:585–593. doi: 10.1007/s00432-011-1125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Q, Wang S, Li C, Yang C, Lin X, Lin X, et al. [Expressions and correlation of HPA, CK2beta and HIF-1alpha in nasopharyngeal carcinoma] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28:157–161. Chinese. [PubMed] [Google Scholar]
- 15.Khan Z, Khan N, Tiwari RP, Patro IK, Prasad GB, Bisen PS. Down-regulation of survivin by oxaliplatin diminishes radioresistance of head and neck squamous carcinoma cells. Radiother Oncol. 2010;96:267–273. doi: 10.1016/j.radonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Hosokawa Y, Okumura K, Terashima S, Sakakura Y. Radiation protective effect of hypoxia-inducible factor-1a (HIF-1a) on human oral squamous cell carcinoma cell lines. Radiat Prot Dosimetry. 2012;152:159–163. doi: 10.1093/rpd/ncs215. [DOI] [PubMed] [Google Scholar]
- 17.Xu P, Huang JM, Ren Y, Zha X, Deng BF, Wu JH, et al. Regulation of hypoxia-induced mRNA expressions of HIF-1alpha and osteopontin and in vitro radiosensitization by tirapazamine in human nasopharyngeal carcinoma HNE-1 and CNE-1 cells. Chin J Cancer. 2010;29:126–130. doi: 10.5732/cjc.009.10500. [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Pan SL, Wang JC, Kuo SH, Cheng JC, Teng CM. Radiation-induced VEGF-C expression and endothelial cell proliferation in lung cancer. Strahlenther Onkol. 2014;190:1154–1162. doi: 10.1007/s00066-014-0708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Mohan S, Natarajan M. Radiation-triggered NF-kB activation is responsible for the angiogenic signaling pathway and neovascularization for breast cancer cell proliferation and growth. Breast Cancer (Auckl) 2012;6:125–135. doi: 10.4137/BCBCR.S9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erkal EY, Bora H, Tepeoğlu M, Akmansu M. Role of vascular endothelial growth factor in clinically localized prostate cancer treated with radiation therapy. Balkan Med J. 2014;31:43–49. doi: 10.5152/balkanmedj.2014.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer. 2006;95:1–5. doi: 10.1038/sj.bjc.6603201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Yu Z. siRNA targeting HIF-1a induces apoptosis of pancreatic cancer Cells through NF-κB-independent and dependent pathways under hypoxic conditions. Anticancer Res. 2009;29:1367–1372. [PubMed] [Google Scholar]
- 23.Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010;465:577–583. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oike T, Suzuki Y, Al-Jahdari W, Mobaraki A, Saitoh JI, Torikai K, et al. Suppression of HIF-1a expression and radiation resistance in acute hypoxic conditions. Exp Ther Med. 2012;3:141–145. doi: 10.3892/etm.2011.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YH, Yoo KC, Cui YH, Uddin N, Lim EJ, Kim MJ, et al. Radiation promotes malignant progression of glioma cells through HIF-1alpha stabilization. Cancer Lett. 2014;354:132–141. doi: 10.1016/j.canlet.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Zhao Y, Zhang S, Lv J. [The expressions of EphrinB2 and VEGF in nasopharyngeal carcinoma and their clinical significance] Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:178–180. Chinese. [PubMed] [Google Scholar]
- 27.Dorsey K, Agulnik M. Promising new molecular targeted therapies in head and neck cancer. Drugs. 2013;73:315–325. doi: 10.1007/s40265-013-0025-3. [DOI] [PubMed] [Google Scholar]
- 28.Li WW, Long GX, Liu DB, Mei Q, Wang JF, Hu GY, et al. Cyclooxygenase-2 inhibitor celecoxib suppresses invasion and migration of nasopharyngeal carcinoma cell lines through a decrease in matrix metalloproteinase-2 and -9 activity. Pharmazie. 2014;69:132–137. [PubMed] [Google Scholar]
- 29.Li XY, Lin YC, Huang WL, Hong CQ, Chen JY, You YJ, et al. Zoledronic acid inhibits proliferation and impairs migration and invasion through downregulating VEGF and MMPs expression in human nasopharyngeal carcinoma cells. Med Oncol. 2012;29:714–720. doi: 10.1007/s12032-011-9904-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen HH, Weng BQ, Cheng KJ, Liu HY, Wang SQ, Lu YY. Effect of the vascular endothelial growth factor expression level on angiopoietin-2 mediated nasopharyngeal carcinoma growth. Vasc Cell. 2014;6:4. doi: 10.1186/2045-824X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Ran Y, Hong C, Chen Z, You Y. Anti-cancer effects of celecoxib on nasopharyngeal carcinoma HNE-1 cells expressing COX-2 oncoprotein. Cytotechnology. 2010;62:431–438. doi: 10.1007/s10616-010-9296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argiris A, Kotsakis AP, Hoang T, Worden FP, Savvides P, Gibson MK, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2013;24:220–225. doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozec A, Sudaka A, Fischel JL, Brunstein MC, Etienne-Grimaldi MC, Milano G. Combined effects of bevacizumab with erlotinib and irradiation: a preclinical study on a head and neck cancer orthotopic model. Br J Cancer. 2008;99:93–99. doi: 10.1038/sj.bjc.6604429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee NY, Zhang Q, Pfister DG, Kim J, Garden AS, Mechalakos J, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma(RTOG 0615):a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, Chin SF, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25:3766–3773. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 36.Yadav A, Kumar B, Teknos TN, Kumar P. Sorafenib enhances the antitumor effects of chemoradiation treatment by downregulating ERCC-1 and XRCC-1DNA repair proteins. Mol Cancer Ther. 2011;10:1241–1251. doi: 10.1158/1535-7163.MCT-11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue C, Huang Y, Huang PY, Yu QT, Pan JJ, Liu LZ, et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 2013;24:1055–1061. doi: 10.1093/annonc/mds581. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Wang Y, Li Y, Guo K, He Y. Role of sorafenib and sunitinib in the induction of expressions of NKG2D ligands in nasopharyngeal carcinoma with high expression of ABCG2. J Cancer Res Clin Oncol. 2011;137:829–837. doi: 10.1007/s00432-010-0944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen QL, Meng MB, Yang B, Tu LL, Jia L, Zhou L, et al. Endostar, a recombined humanized endostatin, enhances the radio response for human nasopharyngeal carcinoma and human lung adenocarcinoma xenografts in mice. Cancer Sci. 2009;100:1510–1519. doi: 10.1111/j.1349-7006.2009.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Wang L, Xu X, Tu Y, Qin S, Yin Y. Antitumor activity of Endostar combined with radiation against human nasopharyngeal carcinoma in mouse xenograft models. Oncol Lett. 2012;4:976–980. doi: 10.3892/ol.2012.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou N, Hu G, Mei Q, Qiu H, Long G, Chen C, et al. Inhibitory effect of Endostar in combination with radiotherapy in a mouse model of human CNE2 nasopharyngeal carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2011;31:62–66. doi: 10.1007/s11596-011-0151-7. [DOI] [PubMed] [Google Scholar]
- 42.Peng F, Xu Z, Wang J, Chen Y, Li Q, Zuo Y, et al. Recombinant human endostatin normalizes tumor vasculature and enhances radiation response in xenografted human nasopharyngeal carcinoma models. PLoS One. 2012;7:e34646. doi: 10.1371/journal.pone.0034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin T, Li B, Chen XZ. A phase II trial of Endostar combined with gemcitabine and cisplatin chemotherapy in patients with metastatic nasopharyngeal carcinoma ( NCT01612286) Oncol Res. 2013;21:317–323. doi: 10.3727/096504014X13983417587401. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 45.Wicki A, Wild D, Prêtre V, Mansi R, Orleth A, Reubi JC, et al. Synergism of peptide receptor-targeted Auger electron radiation therapy with anti-angiogenic compounds in a mouse model of neuroendocrine tumors. EJNMMI Res. 2014;4:9. doi: 10.1186/2191-219X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu HW, Wall NR, Hsueh CT, Kim S, Ferris RL, Chen CS, et al. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 2014;50:19–26. doi: 10.1016/j.oraloncology.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Iversen AB, Busk M, Horsman MR. Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol. 2013;52:1320–1326. doi: 10.3109/0284186X.2013.825050. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Liu Y, Sun C, Gan L, Zhang L, Mao A, et al. Carbon ion radiation inhibits glioma and endothelial cell migration induced by secreted VEGF. PLoS One. 2014;6:e98488. doi: 10.1371/journal.pone.0098448. [DOI] [PMC free article] [PubMed] [Google Scholar]


