Abstract
Objectives:
To compare the mean total antioxidant status (TAS) among 3 glaucoma types, namely: pseudoexfoliation glaucoma (PEG), primary open angle glaucoma (POAG), and primary angle closure glaucoma (PACG), and study its potential association with various clinical glaucoma-parameters.
Methods:
In this case-control study, plasma samples were obtained between September 2013 and October 2014 from 340 glaucoma patients (PEG [n=54]; POAG [n=147]; PACG [n=139]), and 351 controls of matching age, gender, ethnicity, and 5 different systemic co-morbidities from King Abdulaziz University Hospital, Riyadh, Saudi Arabia. The TAS in all samples was determined by a colorimetric-based assay.
Results:
The mean±standard deviation of TAS was significantly lower among cases: 0.77±0.32 than controls: 1.1±0.22, p<0.0001. Moreover, the TAS levels were significantly different across the 3 types of glaucoma: 0.86±0.24 in PEG, 0.47±0.32 in POAG, and 0.98±0.41 in PACG (all p<0.0001). In addition, there was a significant correlation between TAS and age at onset (Pearson correlation coefficient [R] 0.17, p<0.0001), cup/disc ratio (R: -0.13, p=0.004), and number of anti-glaucoma medications (R: -0.16, p=0.001).
Conclusion:
Our findings provide evidence that plasma TAS levels are decreased in patients with glaucoma, more so in POAG and PEG than PACG, supporting the hypothesis that decreased antioxidative defense and/or increased oxidative stress may have a critical role in the pathogenesis of glaucoma.
Glaucoma is a progressive optic neuropathy associated with optic nerve damage, and is one of the most leading cause of blindness worldwide.1 Elevated intraocular pressure (IOP) as a result of reduction in normal aqueous outflow is a major causal risk factor that is well supported by animal studies.2-4 Although IOP is considered a major risk factor for glaucoma,2,3 other concomitant factors affecting the pathophysiology of glaucomatous retinal ganglion cell (RGC) death include retinal ischemia,5 nutritional status,6 and oxidative stress.7 There is evidence of oxidative damage in ocular diseases, such as cataract and age-related macular degeneration.8 In addition, significant oxidative damage has been demonstrated in human trabecular meshwork (TM) cells of patients with glaucoma,7 causing elevated IOP and visual field damage.9 Furthermore, our previous studies have documented mitochondrial abnormalities10-12 (oxidative stress marker), and glutathione-S-transferase (antioxidant) gene (GST) polymorphisms to be associated with various types of glaucoma.13 It is clearly evident from the literature, and our own studies, that oxidative stress mechanisms play a critical role in the pathogenesis of glaucoma. Previous studies had demonstrated reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma.14-17 To evaluate the role of oxidative stress in different types of glaucoma we had previously investigated total antioxidants status (TAS) in the plasma of pseudoexfoliation glaucoma (PEG) patients,18 primary angle closure glaucoma (PACG) patients,19 and in the plasma of primary open angle glaucoma (POAG) patients.20 As an extension to these studies, here, we compare the mean TAS level among these 3 glaucoma types, and study the potential association between the TAS level and various clinical parameters important to each type of glaucoma.18-20
Methods
Search method
PubMed and Google search engines were used to locate research work published in this area. Keywords such as ‘total antioxidants’, ‘oxidative stress’, ‘glaucoma’ and their combinations were used for searching the literature.
Study population
This case-control cross-sectional study was conducted between September 2013 and October 2014 at King Abdulaziz University Hospital, King Saud University, Riyadh, Saudi Arabia. The study adhered to the tenets of the Declaration of Helsinki, and was approved by our institutional review board committee. Informed consents were obtained from all the participants. We recruited 340 Saudi glaucoma patients (54 with PEG, 147 with POAG, and 139 with PACG). Inclusion and exclusion criteria for each type of glaucoma have been detailed previously.18-20 In our study, the team included 2 glaucoma consultants who carried out the measurements and reviewed the collected data. For this purpose, a standardized technique was meticulously followed. In our facility, in general, we adopt the European glaucoma society guidelines,21 where all assessment procedures are standardized. Information on all risk factors (smoking, diabetes mellitus [DM], hypertension, coronary artery disease [CAD] and hypercholesterolemia) was procured through their medical records. An ethnically-matched healthy control group (n=351) included individuals of age >40 years with normal IOP, open angles on gonioscopy, normal optic nerves, and free from glaucoma as described elsewhere.18 All our patients and controls declared that they were not taking any antioxidant supplements at the time when the blood was collected.
Plasma preparation and storage
Ethylene-diamine-tetraacetic acid (EDTA) blood samples were used to collect the plasma layer after centrifugation at 5,500 xg for 5 min. The plasma samples were stored at -80°C until use for TAS assay.
Plasma total antioxidant status
A colorimetric-based assay from Randox (Randox Laboratories Ltd, Antrim, UK) was used to estimate the plasma TAS levels. The method is based on detection of a reduction in the generation of the ABTS®+(2, 2’-Azinobis-di [3-ethylbenzthiazoline sulphonate]) radical cations by plasma antioxidants, which is proportional to the total antioxidant concentration. The assay was performed in duplicate on an automated biochemical analyzer, ChemWell-T (Awareness Technology Inc., Palm City, FL, USA), as per the manufacturer’s instructions (Randox Laboratories, Antrim, UK) and has been detailed elsewhere.18 The plasma TAS levels were expressed in mmol/L.
Statistical methods
Data were retrieved and recorded in a specific hard copy of the study form. Data were then reviewed and stored into a specifically designed database using Microsoft Access 2010® software (Microsoft Corporation, Redmond, WA, USA). Data management was then performed after a process of data cleaning. Data coding and analysis was carried out using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, USA), and StatsDirect® statistical software, version 2.7.2 (StatsDirect Ltd., Cheshire, UK). Descriptive analysis was primarily carried out, where categorical variables were presented in the form of frequencies and percentages and continuous variables in the form of mean ± standard deviation (SD) and range (minimum - maximum). Alternatively, Chi-squared test was conducted to detect the association between types of glaucoma and different systemic comorbidities (Fisher’s exact test, when indicated). Inferential analysis was carried out in forms of comparison of means using Student’s t-test, and analysis of variance (ANOVA) while comparing different glaucoma groups. A consecutive post-hoc analysis was conducted to variables that showed significant differences in the ANOVA test to identify the source of a cross-groups variation. Test of correlation was carried out using where Pearson correlation coefficient (R) and the corresponding p-value were calculated, while the Spearman’s test was alternatively used whenever indicated. To detect an adjusted effect size of different study variables on the TAS concentration, linear regression analysis was finally carried out including all potentially associated factors. A 95% confidence interval (CI) was calculated and p<0.05 was considered statistically significant.
Results
In the current study, we recruited a total of 691 subjects. These included 340 (49.2%) cases with a confirmed diagnosis of various types of glaucoma, and 351 (50.8%) healthy individuals free from glaucoma that served as controls. Overall, the mean ± SD TAS level was found to be significantly lower among cases as compared with controls (0.77 ± 0.32 versus 1.1 ± 0.22; p<0.0001, 95% CI: -0.291 - -0.175). Moreover, the levels were also found to be significantly different across the 3 glaucoma types as shown in Figure 1. The demographic details of the subjects included in the study is shown in Table 1. Table 2 shows the distribution of different systemic co-morbidities, glaucoma related indices, and glaucoma specific indices including TAS levels among the 3 groups of glaucoma cases. Glaucoma awareness and age at onset were found to be significantly distributed (p<0.0001) among the cases. Similarly, except for cup/disc ratio other glaucoma specific indices such as IOP (p=0.005), number of anti-glaucoma medications (p<0.0001), Logarithm of the minimum angle of resolution (LogMAR) visual acuity (p=0.034), and TAS (p<0.0001) showed significant distribution. In addition, significant distribution was noted for diabetes mellitus (p=0.003) and hypertension (p=0.022) among cases. However, no significant difference was seen for any of the 5 different systemic co-morbidities when total cases were compared with controls (Table 3). To identify the source of variations between groups showing significant distributions, post hoc analysis was performed, and the results are indicated in Table 4. Importantly, post hoc analysis for TAS revealed that the source of variation was reciprocal among all different glaucoma categories (PEG versus POAG, p<0.0001; PEG versus PACG, p=0.003 and POAG versus PACG, p<0.0001). Overall, correlation testing showed that there was significant correlation between TAS and age at onset (R: 0.17, p<0.0001), cup/disc ratio (R: -0.13, p=0.004), and number of anti-glaucoma medications (R: -0.16, p=0.001) (Table 5). As indicated in Table 6, linear regression analysis showed that TAS levels were significantly associated with age (p=0.020), diabetes (p=0.001), hypertension (p=0.024), awareness of having glaucoma (p=0.001), type of glaucoma (p=0.032), and cup/disc ratio (p=0.001).
Figure 1.
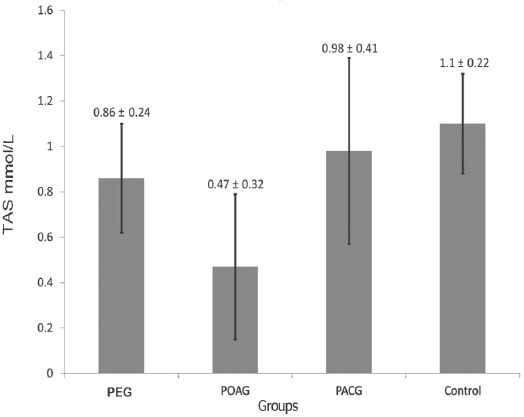
Mean ± standard deviation of total antioxidant concentration for different types of glaucoma. PEG - pseudoexfoliation glaucoma, POAG - primary open angle glaucoma, PACG - primary angle closure glaucoma, TAS - total antioxidant status
Table 1.
Demographic characteristics of subjects included in an ophthalmology study in Saudi Arabia.
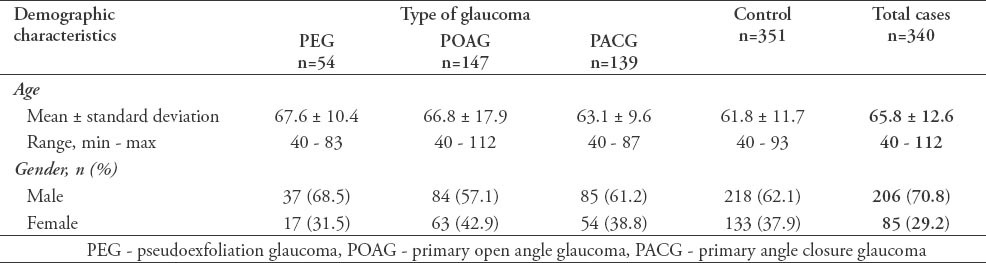
Table 2.
Clinical and systemic diseases at presentation of subjects included in an ophthalmology study in Saudi Arabia.
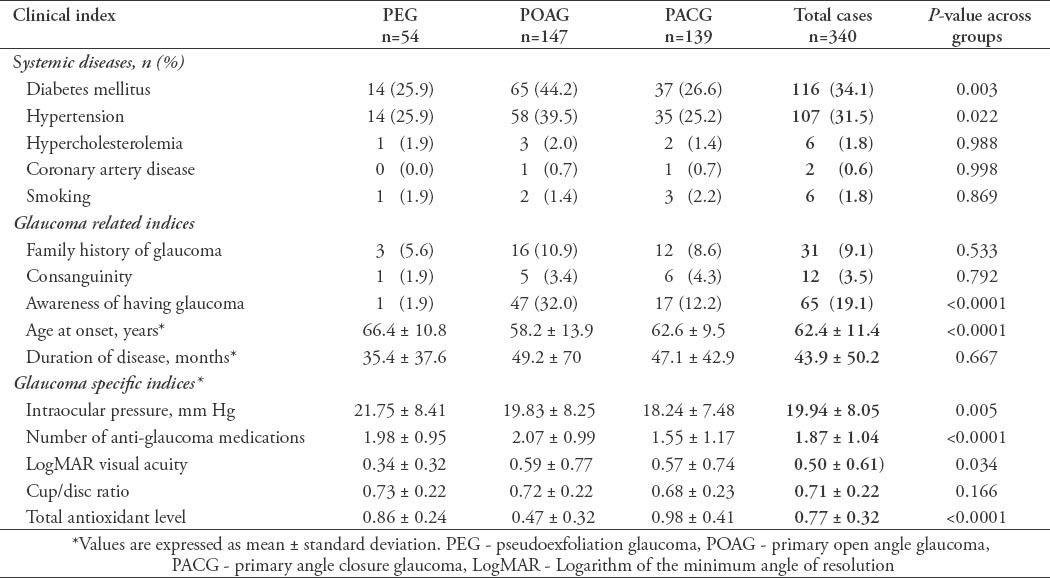
Table 3.
Comparing cases to controls in terms of systemic co-morbidity of subjects included in an ophthalmology study in Saudi Arabia.
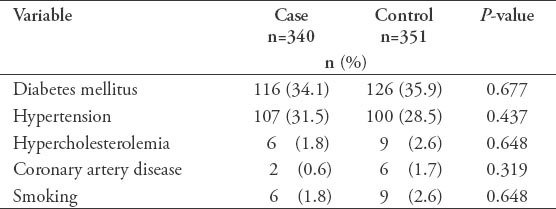
Table 4.
Post hock analysis for pairwise mean differences among different glaucoma groups.
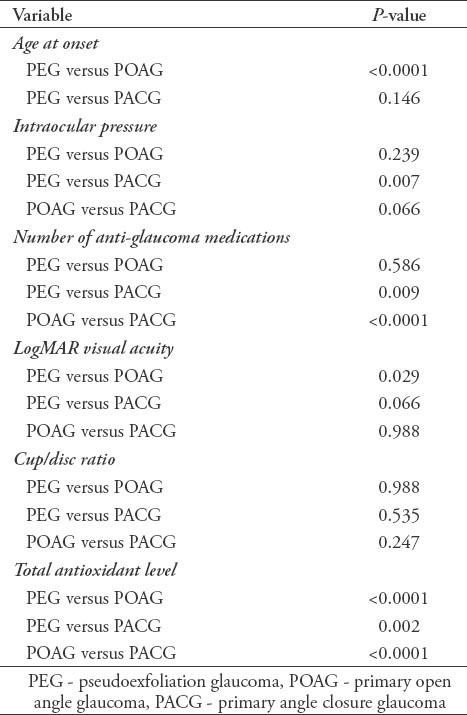
Table 5.
Correlation between total antioxidant concentration and different glaucoma indices among subjects included in an ophthalmology study in Saudi Arabia.
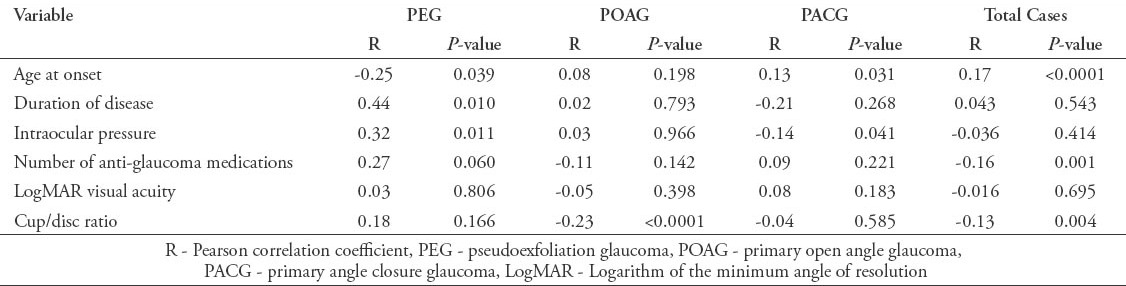
Table 6.
Adjusted estimated effect size using multiple regression analysis of subjects included in an ophthalmology study in Saudi Arabia.
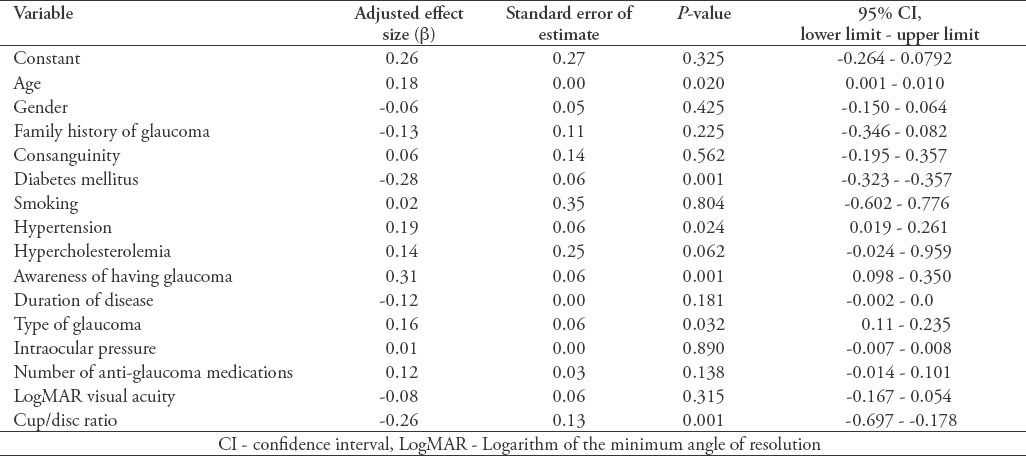
Discussion
We obtained plasma samples from 340 cases of extensively diagnosed glaucoma patients and 351 healthy controls. Our control group was carefully selected in terms of age, gender, and ethnicity. We established that they were free of glaucoma by extensive clinical examination. Age and gender are known to affect TAS levels independent of glaucoma status; therefore, age-and-gender matched control groups were selected for this study.22 In addition, as indicated in Table 3 there was no difference in the distribution of 5 different systemic co-morbidity (DM, hypertension, hypercholesterolemia, CAD, and smoking) between patients and controls thus eliminating any other factors which may influence the TAS level and allowing us to compare TAS level as independent risk factor for glaucoma. There is strong evidence of oxidative mechanisms in POAG,10 and PEG.23 However, optic nerve injury in PACG is mainly considered to be caused by increased IOP as a result of anatomic changes in the anterior,24 and posterior globe.25,26 The role of oxidative stress in PACG has perhaps therefore received limited attention, despite the fact that PACG affects 3.9 million people worldwide.1 We previously investigated the TAS level in a PACG group of patients.19 Among the 340 glaucoma cases we had 54 PEG cases, 147 POAG, and 139 PACG cases. When every group of glaucoma was compared to controls independently, we detected the following: i) TAS levels among PEG were decreased in relation to controls;18 ii) TAS level was also decreased in POAG patients versus controls, and TAS level was associated with increased cup-to-disc ratio;20,27 and iii) TAS level was almost similar in PACG patients versus controls; however, there was an inverse correlation of TAS level with IOP.19,28 From these results, it is clear that decreased TAS level plays a bigger role in PEG and POAG compared with PACG. This may indicate that oxidative stress represented indirectly by the TAS levels; play a bigger role in cases of POAG and PEG than for PACG.
Our current observation that oxidative stress may play a minor role in PACG development compared to other types of glaucoma is well supported by our previous findings in PACG.29 It is also possible that decreased levels of TAS are more linked to particular clinical indices important for PACG rather than the disease per se. Collectively, the mean total antioxidant concentration was significantly lower among cases compared with controls. This fact confirms our previous findings in the Saudi population,18-20 and agrees with the published literature. Gherghel et al30 reported decreased levels of plasma glutathione in patients with glaucoma suggesting compromised antioxidative defense. Erdurmus et al15 reported decreased serum antioxidant capacity and increased blood oxidative stress in patients with POAG and PEG. Zanon-Moreno et al31 reported a significant increase in oxidative status measured as malondialdehyde-thiobarbituric acid reactive substances (MDA-TBARS), and decreased total antioxidant activity in the aqueous humor of glaucomatous eyes than the cataract eyes. In addition; however, our current study highlights the association between type and severity of glaucoma and the mean TAS level. Our findings suggest that not only the mean TAS level may be decreased due to glaucoma, but it is also more decreased in open angle than in closed angle or pseudoexfoliation types, enforcing our earlier investigations that oxidative stress may play a minor role in PACG development compared with other types of glaucoma.29 Oxidative stress in the anterior chamber of glaucomatous eyes can be an ocular manifestation as a result of systemic insult. A study by Sorkhabi et al16 provided evidence for increased oxidative damage and decreased TAS in serum and aqueous humor of glaucoma patients suggesting that in glaucoma the increased oxidative burden in the anterior chamber of glaucomatous eyes may be due more to a faulty antioxidative defense and increased oxidative stress than to a systemic insult, compromising the trabecular meshwork and its functioning. Hence, we speculate that decreased levels of TAS, as a result of increased oxidative stress in the glaucomatous eyes, may at least in part play a role in occurrence and progression of POAG and PEG.
We also observed that the more reduced mean TAS level, the more acute or severe the glaucoma type. Additionally, the inheritance factors are playing a major role in this causative pathway, as our findings show that the prevalence of consanguinity and a family history of glaucoma are much more among both POAG and PACG groups than the PEG group. Although most of our recruited patients are usually set on anti-glaucoma medications, there was a statistically significant difference across the 3 subgroups of cases in terms of both mean IOP and number of glaucoma mediations levels. It is well established that chronic glaucoma patients suffer an increasing pattern of IOP until reaching the maximum tolerated level of medication and are indicated for surgical intervention. This fact was also true for the visual acuity level. Furthermore, our findings suggest that the mean TAS level may be used as an indicator of the severity of glaucoma, and possibly on the duration and compliance to treatment among chronic cases. Nevertheless, this area requires more investigation and recruitment of a large cohort of patients in a longitudinal multi-centric study to provide concrete evidence.
This study has its own limitations. There is a great debate in the literature regarding the role of cataract in glaucoma surgery. Visual acuity assessment as a glaucoma severity index used in our study could therefore be affected by other diseases common in these patients including cataract. However, among our subjects we did not have enough cases to compare vision with and without combined cataract. Reviewing our data, we found only 2 cases with combined cataract, where visual acuity was better than 20/40; so, it was not worth controlling for them. In addition, our current study used age at first diagnosis and self reported age at onset as a surrogate to the actual age at onset. The reason for that was to roughly estimate the duration of having glaucoma. We understand that this measurement would face a lot of potential bias. However, we think that in terms of age, it would be much more appropriate to use “age at onset” than using the “current patient age” where this approach is expected to minimize the estimation error. Although significant correlation has been reported between serum and aqueous levels of TAS,16 it should be noted that the results reflect the antioxidant status in the plasma samples and not aqueous fluid and hence require cautious interpretations. The study is purely descriptive in nature and provides no evidence of mechanistic conclusions. In a perfect scenario an ideal study group would include glaucoma patients without anti-glaucoma medications; however, these cannot be included for ethical reasons. Our findings do not necessarily indicate that low levels of TAS is a primary cause of glaucoma, but suggest future avenues for research.
In conclusion, our findings provide evidence that plasma TAS levels are decreased in patients with glaucoma, more so in open-angle and pseudoexfoliation than angle-closure glaucoma. The study adds to the growing body of evidence supporting the hypothesis that decreased antioxidative defense and/or increased oxidative stress may have a critical role in the pathogenesis of glaucoma. As far as we know, this study is unique in its approach, and may open the gate for more investigations in the area of antioxidant research in glaucoma that would yield a more in-depth understanding of the association between pathogenic mechanisms of the disease and antioxidants level, and provide a new therapeutic approach to control the increasing prevalence of blinding glaucoma.
Footnotes
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krakau CE. Intraocular pressure elevation-cause or effect in chronic glaucoma? Ophthalmologica. 1981;182:141–147. doi: 10.1159/000309104. [DOI] [PubMed] [Google Scholar]
- 3.Bonomi L, Marchini G, Marraffa M, Morbio R. The relationship between intraocular pressure and glaucoma in a defined population. Data from the Egna-Neumarkt Glaucoma Study. Ophthalmologica. 2001;215:34–38. doi: 10.1159/000050823. [DOI] [PubMed] [Google Scholar]
- 4.Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010;81:349–358. doi: 10.1016/j.brainresbull.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt Z, O’Brien C, McKillop G, Aspinall P, Allan P. Color Doppler imaging in untreated high- and normal-pressure open-angle glaucoma. Invest Ophthalmol Vis Sci. 1997;38:690–696. [PubMed] [Google Scholar]
- 6.Veach J. Functional dichotomy: glutathione and vitamin E in homeostasis relevant to primary open-angle glaucoma. Br J Nutr. 2004;91:809–829. doi: 10.1079/BJN20041113. [DOI] [PubMed] [Google Scholar]
- 7.Izzotti A, Saccà SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 8.Ohia SE, Opere CA, Leday AM. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res. 2005;579:22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–2541. doi: 10.1167/iovs.05-1639. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Amero KK, Cabrera VM, Larruga JM, Osman EA, González AM, Al-Obeidan SA. Eurasian and Sub-Saharan African mitochondrial DNA haplogroup influences pseudoexfoliation glaucoma development in Saudi patients. Mol Vis. 2011;17:543–547. [PMC free article] [PubMed] [Google Scholar]
- 12.Bosley TM, Hellani A, Spaeth GL, Myers J, Katz LJ, Moster MR, et al. Down-regulation of OPA1 in patients with primary open angle glaucoma. Mol Vis. 2011;17:1074–1079. [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Amero KK, Morales J, Mohamed GH, Osman MN, Bosley TM. Glutathione S-transferase M1 and T1 polymorphisms in Arab glaucoma patients. Mol Vis. 2008;14:425–430. [PMC free article] [PubMed] [Google Scholar]
- 14.Nucci C, Di Pierro D, Varesi C, Ciuffoletti E, Russo R, Gentile R, et al. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol Vis. 2013;19:1841–1846. [PMC free article] [PubMed] [Google Scholar]
- 15.Erdurmuş M, Yağcı R, Atış Ö, Karadağ R, Akbaş A, Hepşen IF. Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr Eye Res. 2011;36:713–718. doi: 10.3109/02713683.2011.584370. [DOI] [PubMed] [Google Scholar]
- 16.Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Rashtchizadeh N, Moharrery M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis. 2011;17:41–46. [PMC free article] [PubMed] [Google Scholar]
- 17.Engin KN, Yemişci B, Yiğit U, Ağaçhan A, Coşkun C. Variability of serum oxidative stress biomarkers relative to biochemical data and clinical parameters of glaucoma patients. Mol Vis. 2010;16:1260–1271. [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Amero KK, Kondkar AA, Mousa A, Osman EA, Al-Obeidan SA. Decreased total antioxidants status in the plasma of patients with pseudoexfoliation glaucoma. Mol Vis. 2011;17:2769–2775. [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Amero KK, Azad TA, Mousa A, Osman EA, Sultan T, Al-Obeidan SA. Total antioxidant level is correlated with intra-ocular pressure in patients with primary angle closure glaucoma. BMC Res Notes. 2014;7:163. doi: 10.1186/1756-0500-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Amero KK, Kondkar AA, Mousa A, Osman EA, Al-Obeidan SA. Decreased total antioxidants in patients with primary open angle glaucoma. Curr Eye Res. 2013;38:959–964. doi: 10.3109/02713683.2013.794246. [DOI] [PubMed] [Google Scholar]
- 21.European Glaucoma Society. Terminology and guidelines for glaucoma. 3rd ed. [Updated: 2015; Accessed 2014 October 2014] Available from: http://www.eugs.org/eng/EGS_guidelines.asp)
- 22.Tulunoglu O, Demirtas S, Tulunoglu I. Total antioxidant levels of saliva in children related to caries, age, and gender. Int J Paediatr Dent. 2006;16:186–191. doi: 10.1111/j.1365-263X.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Amero KK, Bosley TM, Morales J. Analysis of nuclear and mitochondrial genes in patients with pseudoexfoliation glaucoma. Mol Vis. 2008;14:29–36. [PMC free article] [PubMed] [Google Scholar]
- 24.Ritch R, Shields M, Krupin T, editors. Angle-closure glaucoma: mechanisms and epidemiology. In: The Glaucomas. 2nd ed. St Louis (MO): Mosby; 1996. p. 801-819. [Google Scholar]
- 25.Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12:167–180. doi: 10.1097/00061198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 26.de Kater AW, Melamed S, Epstein DL. Patterns of aqueous humor outflow in glaucomatous and nonglaucomatous human eyes. A tracer study using cationized ferritin. Arch Ophthalmol. 1989;107:572–576. doi: 10.1001/archopht.1989.01070010586035. [DOI] [PubMed] [Google Scholar]
- 27.Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 1997;6:123–132. doi: 10.1097/00061198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Amero KK, Morales J, Osman MN, Bosley TM. Nuclear and mitochondrial analysis of patients with primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2007;48:5591–5596. doi: 10.1167/iovs.07-0780. [DOI] [PubMed] [Google Scholar]
- 30.Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–883. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- 31.Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, Pons-Vazquez S, Garcia-Medina JJ, Vinuesa-Silva I, et al. Oxidative stress in primary open-angle glaucoma. J Glaucoma. 2008;17:263–268. doi: 10.1097/IJG.0b013e31815c3a7f. [DOI] [PubMed] [Google Scholar]


