Abstract
Objectives:
To examine the trends of kidney cancer over the last 2 decades in a subset of a Saudi Arabian population.
Methods:
We conducted a retrospective study in a tertiary care center including all adult patients with primary kidney cancer who presented and were managed between 1990 and 2010. The time period was split into 4 quartiles, and variables tested and compared using chi-square, T-test, and Kaplan-Meier curves for survival.
Results:
The total was 215 patients with a mean age of 57.8 years. There was an increase in the number of kidney cancer cases over the last 2 decades. There was no significant difference in the mode of presentation or stage distribution between quartiles. A significant change was observed in the management towards minimally invasive and nephron-sparing surgeries (p<0.001). There was no change in recurrence-free and disease-specific survival over the last 20 years.
Conclusions:
There have been an increasing number of kidney cancer patients over the last 2 decades with no observed migration towards more incidental and low stage tumors as compared with developed countries.
Kidney cancer is considered to be one of the most common neoplasms in the genitourinary tract. It is one of the most basic and one of the most recurrent types of cancers. Its management and outcome is solely dependent upon the stage of the disease. There are 4 stages of the disease that are usually known as primary tumor, regional lymph node, metastasis, and distant metastasis.1 These are usually defined or identified on the basis of the condition of the patient and the growth or the occurrence of the tumor. In recent years, the disease has been increasing at a very high rate and the incidence of the disease has seen new peaks. In the year 2007, 87 people out of 100,000 were diagnosed with the deadly disease. The estimated data of the incidence in United States have shown an increase by 2%.2 However, in Europe, studies have shown variability in the trend of kidney cancer.3 Over the last 2 decades with the development of imaging techniques and extensive use of ultrasound and CT, the presentation of kidney cancer has shifted towards more incidental diagnoses at earlier stages, which has resulted in a dramatic improvement in the management; however, various studies have provided evidence that the improved management of the disease has not induced a decrease in the mortality rate of the disease.2,4,5 Due to the scarcity of literature available on this topic in Saudi Arabia, our study aims to examine the trends of kidney cancer in terms of mode of presentation, stage distribution, management, and outcomes over the last 2 decades. This study will contribute to understanding the nature of kidney cancer in the sample population. It will be helpful in the formulation and planning of health care policies and strategies that improve cancer management and outcome of the disease in the future. A large population-based cohort study in Japan6 revealed that risk factors for renal cell carcinoma (RCC) are male gender, old age, medical history of hypertension, high blood pressure level, diabetes mellitus, high body mass index, medical history of kidney disease, smoking, low physical activity, and westernized dietary habits.6 According to studies conducted in Asian countries,1,6,7 some of the common risk factors responsible for RCC include gender, old age, hypertension, high blood pressure level, diabetes mellitus, high body mass index, medical history of kidney disease, smoking, low physical activity, and westernized dietary habits (repetition of previous sentence, please rephrase). Sivaramakrishna et al7 discussed patterns of RCC in an Indian population, while a study conducted by Washio et al,6 discussed risk factors of RCC in a Japanese population. Singam et al9 discussed clinical characteristics of renal cancer in Malaysia. Chow et al,1 discussed increased risks of kidney cancer in many Asian countries such as Korea, China, Hong Kong, Singapore, and Japan.
Methods
Selection and description of participants
We conducted a retrospective study analysis of kidney cancer patients who were presented or managed in a tertiary care center in Riyadh, Saudi Arabia from 1990 to 2010. Cases were identified through medical records and were defined as: all adult patients (>18 years old) that were diagnosed with a primary kidney cancer.
Variables that were used to generate results
Demographics (age, year of presentation, gender), risk factors (hypertension), mode of presentation (incidental versus symptomatic), pathological stage and grade, type of surgical management (open radical nephrectomy, open partial nephrectomy, laparoscopic radical nephrectomy, laparoscopic partial nephrectomy), and prognosis (mortality rate, recurrence) were utilized in order to collect data. Information on diabetes mellitus, chronic kidney disease, obesity, smoking and drinking habits, and physical activity were not sufficient in the medical records. Symptomatic cases were defined as cases presenting with some or any of the following complaints: flank pain, hematuria, or abdominal lump. Histopathological type and grading were identified according to patient’s pathological conditions that were derived from the patient’s pathology report. If the stage was not mentioned in the pathology report, staging was recorded based on tumor size, and lymph nodes involvement. However, staging was not utilized as in the long run it would contaminate the data and distant metastasis as described in recent TNM (Tumor, Lymph Node, and Metastasis) classification. Recurrence was defined as any recurring tumor that was detected after complete treatment of the primary tumor. Cause of death was obtained from the patient’s death report.
Data was split into 4 quartiles of 5-year intervals according to the date of presentation. Cohort 1 (January 1, 1990 to December 31, 1994). Cohort 2 (January 1, 1995 to December 31, 1999). Cohort 3 (January 1, 2000 to December 31, 2004). Cohort 4 (January 1, 2005 to December 31, 2010).
Inclusion and exclusion criteria
The cases of renal cancer in the past 20 years have been included for the reason of examination. All patients aged >18 years were included in the research study. Patients suffering from other chronic medical conditions were not included in the research study.
Statistical analysis
The collected data was analyzed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA) via Chi-square for all of the identified variables in each time period. The T-test was also used for a comparison between the 4 time periods. Kaplan-Meier curves were used for survival.
Ethical approval was obtained from our institute retrospective research committee. All of the ethical considerations were followed during the research study. The privacy and confidentiality of the data was an extreme priority. Other than the study investigators, no one was allowed access to the medical records. The Helsinki Declaration that directly addresses the ethical issues were considered; however, as we used secondary data with no direct human contact, the declaration was not applicable for this study.
Results
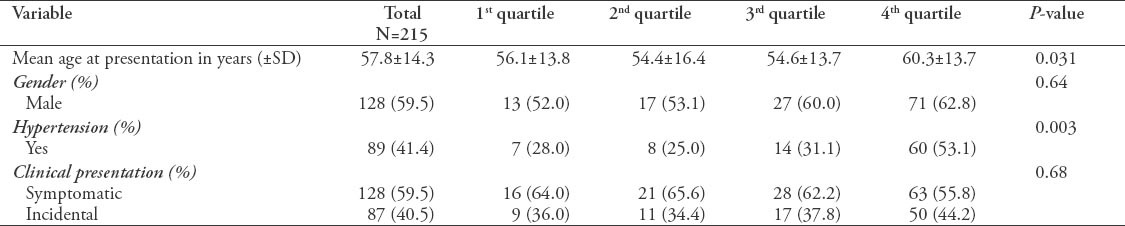
A total of 215 patients were included in the study. As shown in Figure 1 Trends of kidney cancer over 20 years. There was a significant change in the age at presentation with mean age of 57.8 years (p=0.031). Most of the disease population was male. The number of patients diagnosed incidentally with kidney tumors did not show a significant difference between the various quartiles. Table 1 shows the demographics and clinical presentation in total and comparing quartiles. Large numbers of population tested had hypertension at the time of diagnosis with kidney cancer, most of them were from the last decade (3rd quartile (31.1%) and 4th quartile (53.1%) with statistically significant difference compared to previous years.
Figure 1.
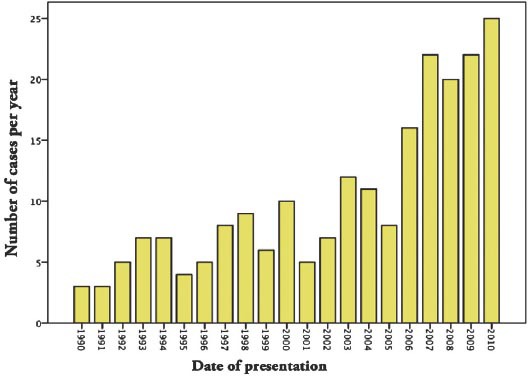
Trends of kidney cancer over 20 years (1990-2010).
Table 1.
Demographics and clinical presentation in total and comparing quartiles.
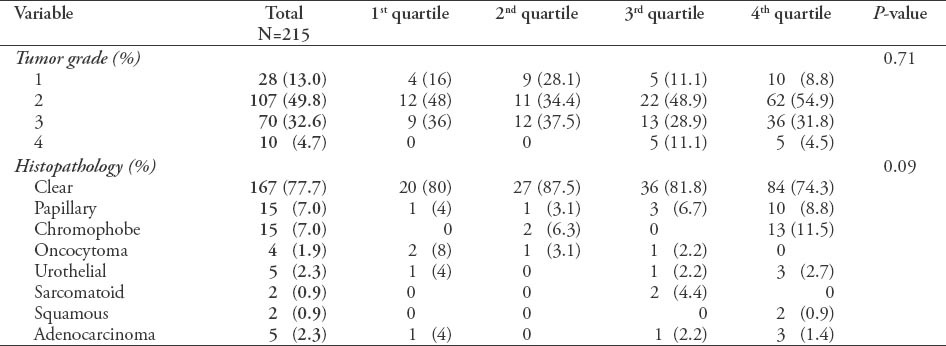
Regarding the tumor grade and histopathology, there was no significant difference between the 4th quartiles. However, there was an increase in the number of tumors diagnosed in all stages over the different quartiles (Table 2).
Table 2.
Grade and pathological distribution along with their respective p-values among 215 patients.
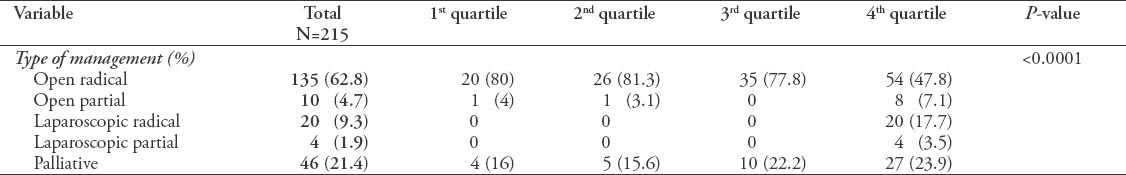
The highest percentage of minimally invasive and nephron sparing surgeries (laproscopic radical, laproscopic partial) performed was in the fourth quartile. This indicates a significant change in the management towards more minimally invasive and more nephron sparing surgeries, especially in the last 5 years (Table 3).
Table 3.
Type of management comparing quartiles among 215 patients.
In terms of outcome, neither recurrence free survival nor disease specific survival significantly changed over quartiles as shown by the 2 Kaplan-Meier survival curves (Figure 2).
Figure 2.
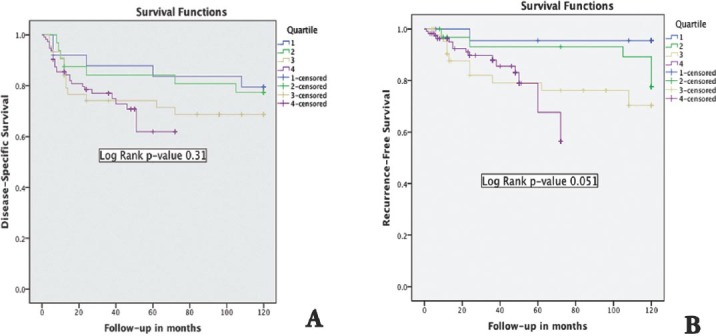
Kaplan-Meier survival curves showing the A) disease specific survival changes over quartiles and B) the recurrence free survival changes over quartiles
Discussion
This is a study of kidney cancer in the Saudi population representing patients seen at a tertiary care center. The increase in the number of patients observed, correlates well with the observed increasing incidence of kidney cancer both nationally and internationally. One of the possible reasons for the increase in the incidence of the disease is the relationship between obesity and hypertension. Two past studies addressing the topic suggested that these rates could be managed with an improved surveillance system and avoiding early surgical procedures.6,10 Other studies further suggested that the disease can be easily managed through early imaging techniques and by avoiding surgical treatments in the early stages.2,6,9 In the selected cohort, there was an evident decrease in the number of patients diagnosed with incidental kidney tumors and no stage migration, suggesting these are much more localized tumors in contrary to the recent evidence.11,12 One of the possible explanations for these localized tumors is that the patients in the selected group had a higher mean age as evident by the significant increase in the mean age at presentation in the selected cohort. This could be due to the difficult access to health care and radiological investigations, especially in rural areas, and smaller cities, where a large number of the patients reside, not only the patients of the selected group but also in the general population. There was a significant increase in the prevalence of hypertension in patients diagnosed with kidney tumors, and this could be due to established increased risk of kidney cancer with hypertension. McLaughlin et al13 and Fryzek et al14 have suggested that smoking, diabetes, and higher BMI have a close relationship with hypertension; however, there is no direct relationship of above identified risk factors with renal cancer. Simply, the studies have mentioned that smoking, diabetes, and obesity have the ability to exaggerate the state of hypertension, which in turn results in increased risk of kidney cancer. The results of this study also revealed that there is a significant increase in the prevalence of hypertension among patients having kidney tumor. Approximately 41% of the population tested was suffering from hypertension at the time of diagnosis with kidney cancer (84.2%) and a statistical significant difference (p=0.003). Out of 215 patients, 89 were diagnosed with hypertension as a major risk factor for RCC. Smoking is one of the major risk factors as it is connected to various diseases; however, smoking status could not to be obtained from medical records due to lack of documented status.
Surgical management of kidney cancer has evolved over the last 2 decades where the utilization of minimally invasive and nephron-sparing surgery has become the standard of care in localized disease.4,5 In our cohort, there was a significant increase in the number of those surgeries over the last 5 years meeting the standard of care despite the fact that we are still seeing the same number of advanced disease as compared to the first 3 quartiles.
In this study, the outcome of kidney cancer in terms of recurrence and disease-specific mortality did not change over the last 20 years. On the contrary, there is a trend towards poorer recurrence-free and disease-specific survival, although this was not statistically significant. This phenomenon was observed by others,12,15,16 who showed the same trend of poorer outcome despite their findings of stage migration towards lower stage disease, and this may reflect a change in the biology of kidney cancer towards more aggressive disease. One of the reasons for the low survival rates in the fourth quartile could be the medical condition of the patients. However, the lower survival rates in the first quartile could be due to the surgical therapeutic procedures. Stage has been shown to be the most significant predictor of outcome in kidney cancer.17,18 We observed the same findings with a significant correlation between stage of disease and recurrence free survival and disease specific survival. There are various associated factors with the disease that can certainly increase the incidence rate or the chances of acquiring the disease. It has been further evaluated that alcoholic drink consumption,19 lack of physical activity,20,21 and hypertension13 pose a great threat because these medical conditions can cause various deadly diseases such as renal cancer.
Another research20 provides evidence that physical activity, smoking, obesity, blood pressure, insulin resistance, and lipid peroxidation are related to kidney cancer, and if these issues are controlled and decreased then the kidney cancer incidence can also be reduced.20,21 There are several limitations to our study. As the study was based on data collected retrospectively, in which, important variables were lacking in patients records. That is particularly problematic because it can be very difficult to make accurate comparisons in terms of risk factors and incidence. Second, there might be a bias in this cohort towards more advanced cases due to the referral pattern as we are a tertiary care center. However, the second reason also provided an advantage to this study as it addresses the recent patients and the recent developments in the incidence and mortality rates of the disease.
Implications
According to the research conducted by Weber et al,22 many effective policies are designed to lead to improvements in therapeutic interventions, increasing clinical benefits for the patients. For example, phase I trials are very effective for improving the clinical outcomes in patients with cancer. According to study conducted by Al-Rubeaan et al,23 which discussed the effectiveness of policies for Saudi National Diabetes Registry (SNDR) as an electronic medical file to register geographic information system (GIS) for disease management and health care planning. Albejaidi24 discussed effective policies for implementing total quality management (TQM), and emphasized that the Ministry of Health should focus on encouraging quality management and reducing turnover rate of professionals.22 The implementation of such measures will be significant for controlling the increased rates of kidney cancers. Albejaidi24 also suggested establishment of an NHIS in Saudi Arabia to develop Regional Quality Health Information System (RQHIS). The WHO also provided complete plan for healthcare policies in Saudi Arabia25 to improve cancer management and outcome of the disease in the society. In order to implement these strategies, the main intention should be focused on the early detection of the disease with an inclination towards more therapeutic solutions rather than surgical methods. Moreover, an extensive investigation must also be carried out into the relationship between hypertension and kidney cancer. For improvement in early detection, patients with obesity, and hypertension must be recommended to have kidney tests so that any hidden markers of cancer, or any other related disease can be detected.
In conclusion, there has been an increase in the number of cases diagnosed with kidney cancer in recent years. However, we are still seeing fewer patients with incidental kidney tumors and localized disease as compared to developed countries. These results need to be confirmed through various population based studies and if this trend is identified and confirmed, measures should be taken to improve early diagnosis of kidney cancer at a nationwide scale. In order to understand the increase in the incidence rate of renal cancer and its relation with age, further research needs to be carried out to address this connection. Through the trends observed in the kidney cancer incidence over the previous 2 decades it can be suggested that the incidence of the disease increases with age; however, a clear cut explanation of the interconnection still needs to be researched. One of the possible reasons could be obesity and hypertension, which are a cause of renal cancer as these medical conditions are also found in the older population.
Footnotes
Copyright.
Whenever a manuscript contains material (tables, figures, etc.) which is protected by copyright (previously published), it is the obligation of the author to obtain written permission from the holder of the copyright (usually the publisher) to reproduce the material in Saudi Medical Journal. This also applies if the material is the authors own work. Please submit copies of the material from the source in which it was first published.
References
- 1.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nature Reviews Urology. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Linehan JA, Nguyen MM. Kidney cancer: the new landscape. Curr Opin Urol. 2009;19:133–137. doi: 10.1097/MOU.0b013e328323f5ab. [DOI] [PubMed] [Google Scholar]
- 4.Gupta NP, Ishwar R, Kumar A, Dogra PN, Seth A. Renal tumors presentation: changing trends over two decades. Indian J Cancer. 2010;47:287–291. doi: 10.4103/0019-509X.64728. [DOI] [PubMed] [Google Scholar]
- 5.Nalavenkata S, Jarvis TR, Rashid P. Incidental small renal mass: current management. ANZ J Surg. 2011;81:797–803. doi: 10.1111/j.1445-2197.2011.05788.x. [DOI] [PubMed] [Google Scholar]
- 6.Washio M, Mori M, Mikami K, Miki T, Watanabe Y, Nakao M, et al. Risk factors for renal cell carcinoma in a Japanese population. Asian Pac J Cancer Prev. 2014;15:9065–9070. doi: 10.7314/apjcp.2014.15.21.9065. [DOI] [PubMed] [Google Scholar]
- 7.Sivaramakrishna B, Gupta NP, Wadhwa P, Hemal AK, Dogra PN, Seth A, et al. Pattern of metastases in renal cell carcinoma: a single institution study. Indian J Cancer. 2005;42:173–177. [PubMed] [Google Scholar]
- 8.Singam P, Ho C, Hong GE, Mohd A, Tamil AM, Cheok LB, et al. Clinical characteristics of renal cancer in Malaysia : a ten year review. Asian Pac J Cancer Prev. 2010;11:503–506. [PubMed] [Google Scholar]
- 9.Jewett MA, Mattar K, Basiuk J, Morash CG, Pautler SE, Siemens DR, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 12.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin JK, Chow WH, Mandel JS, Mellemgaard A, McCredie M, Lindblad P, et al. International renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertension. Int J Cancer. 1995;63:216–221. doi: 10.1002/ijc.2910630212. [DOI] [PubMed] [Google Scholar]
- 14.Fryzek JP, Poulsen AH, Johnsen SP, McLaughlin JK, Sørensen HT, Friis S. A cohort study of antihypertensive treatments and risk of renal cell cancer. Br J Cancer. 2005;92:1302–1306. doi: 10.1038/sj.bjc.6602490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank I, Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol. 2005;173:1889–1892. doi: 10.1097/01.ju.0000158043.94525.d6. [DOI] [PubMed] [Google Scholar]
- 16.Mancuso A, Sternberg CN. New treatment approaches in metastatic renal cell carcinoma. Curr Opin Urol. 2006;16:337–341. doi: 10.1097/01.mou.0000240305.78205.77. [DOI] [PubMed] [Google Scholar]
- 17.Kanao K, Mizuno R, Kikuchi E, Miyajima A, Nakagawa K, Ohigashi T, et al. Preoperative prognostic nomogram (probability table) for renal cell carcinoma based on TNM classification. J Urol. 2009;181:480–485. doi: 10.1016/j.juro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Lane BR, Kattan MW. Prognostic models and algorithms in renal cell carcinoma. Urol Clin North Am. 2008;35:613–625. doi: 10.1016/j.ucl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 20.Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer. 2013;108:798–811. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paz-Filho G, Lim EL, Wong ML, Licinio J. Associations between adipokines and obesity-related cancer. Front Biosci (Landmark Ed) 2011;16:1634–1650. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 22.Weber JS, Levit LA, Adamson PC, Bruinooge S, Burris HA, Carducci MA, et al. American Society of Clinical Oncology Policy Statement Update: The Critical Role of Phase I Trials in Cancer Research and Treatment. J Clin Oncol. 2015;33:278–284. doi: 10.1200/JCO.2014.58.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Rubeaan KA, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, Ibrahim HM. A Web-based interactive diabetes registry for health care management and planning in Saudi Arabia. J Med Internet Res. 2013;15:e202. doi: 10.2196/jmir.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albejaidi FM. Healthcare system in Saudi Arabia: An analysis of structure, total quality management and future challenges. Journal of Alternative Perspectives in the Social Sciences. 2010;2:794–818. [Google Scholar]
- 25.World Health Orgzanization. Country Cooperation Strategy for WHO and Saudi Arabia 2012-2016. Geneva (CH): WHO; 2013. Available from URL: http://www.who.int/countryfocus/cooperation_strategy/ccs_sau_en.pdf . [Google Scholar]


