Abstract
Objectives:
To determine characteristics of laboratory parameters of hematopoietic potential in umbilical cord blood and their association with maternal and neonatal factors.
Methods:
This prospective analysis was performed on 206 umbilical cord blood donations (50-200 ml) from King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia between January and September 2014. Samples were processed and analyzed for total nucleated cells (TNC’s), cluster designation (CD)45+, CD34+ counts, nucleated red blood cells (NRBCs) count, and viability testing.
Results:
Most of the study participants (63.6%) were on their first 3 deliveries and from women with age between 17 and 30 years (80.6%). The donated volume was 50.4-192.4 ml, TNCs ranged from 500.2×106 to 9430.3 ×106 cells, and CD34+ cells ranged from 1.25×106 to 12.82×106/unit. The volume was positively affected by bigger birth weight of the baby (p<0.0001), larger placenta (p=0.001), TNCs (p<0.0001), CD34+ (p<0.0001), NRBCs (p<0.0001), and viability (p=0.002). There were no statistically significant differences between baby boys and girls for laboratory variables.
Conclusion:
In the selection and identification of a possible donor of umbilical cord blood, several maternal and neonatal factors should be considered, as younger maternal age, neonatal birth weight >3300 grams, larger placental size, and first or second-born babies, were shown to be associated with higher TNCs, CD34+, CD45+, NRBCs, and viability.
Umbilical cord blood is an attractive alternative source of hematopoietic stem cells to bone marrow (BM) in stem cell transplantation.1 To date, umbilical cord blood stems cells are used in the treatment of malignant disorders, which includes acute and chronic myeloid and lymphoid leukemia’s, myelodysplastic syndromes,2 and a variety of solid tumors, and non-malignant disorders such as osteopetrosis,3,4 primary immune-deficiencies,5 leukodystrophies,6 and hemoglobinopathies.7 There are more advantageous on umbilical cord blood transplantation over BM transplantation, with lower risks to either the mother, or to the infant; lower frequency of pathogen transmission, and lower possibility of graft-versus-host disease (GVHD) in recipients.8,9 In addition, umbilical cord blood has a huge population of stem cells that are of hematopoietic origin.10 Apart from these hematopoietic stem cells, umbilical cord blood also contains non-hematopoietic progenitor cells known as mesenchymal stem cells, which have the capacity to differentiate into various types of mature cells, including neuronal cells, osteoblasts, adipocytes, chondroblasts, and myocytes.11 For this reason, the propensity of using umbilical cord blood as a source of stem cells has gained huge popularity in the area of transplantation.12 The use of umbilical cord blood for stem cell transplantation; however, is limited due to the few number of cluster designation (CD34+) present in umbilical cord blood, and the higher costs of cryopreservation that is needed to store the blood units.13 Therefore, it is important to determine the maternal and neonatal factors that can influence the higher yield of hematopoietic stem cells obtained from umbilical cord blood. Furthermore, there is a need to determine ways on how to optimize selection and identification of possible candidates for umbilical cord blood donors. The parameters commonly used to evaluate the suitability of umbilical cord blood and predict transplant outcomes were total volume of umbilical cord blood, total nucleated cells (TNC’s), and CD34+ cells concentration. The volume of umbilical cord blood collection was important for the high yield of TNCs and CD34+ cells concentration.14 Previous studies reported that there are some variables that affect the quality of the umbilical cord blood, especially those related to maternal and fetal factors such as maternal age, birth weight, placental weight, gender of the newborn, and birth order.15 Previous studies13,16,17 suggested that optimal results of umbilical cord blood units would be obtained by selecting babies with heavier weight larger placental size, and the first or second babies from young mothers irrespective of their ethnicity. In the present study, we aimed to determine the primarily donor-related variables (maternal age, weight of the new born, placental weight, gender of the newborn, and birth order) before cell processing in a Saudi population and investigate the association between the concentrations of these cellular components (umbilical cord blood volume, TNCs, CD34+ count, CD45+ count, nucleated red blood cells [NRBC] count and viability), and maternal and neonatal factors among Saudi pregnant women. Aside from determining the effect of the variables of umbilical cord blood, we also identify suitable characteristics for transplantation.
Methods
This observational study was conducted in the Cord Blood Bank, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia, between January and September 2014. The local ethics committee approved the study, which was carried out according to the Principles of the Helsinki Declaration.
A total of 206 umbilical cord blood samples were collected from the Al-Yamama Maternity Hospital in Riyadh, Saudi Arabia. The umbilical cord blood was collected after the umbilical cord is detached from the baby, and the placenta was delivered. An informed consent was obtained from the mothers for collection of cord blood as most of mothers were unscheduled and were walk-in patients for delivery. Prospective donors should be at least 17 years old and not more than 45 years old, at least 37 weeks age of gestation, the membranes not ruptured for more than 24 hours, with uterine dilatation of less than 7 centimeters, not on any medication/s and should have delivered via normal spontaneous vaginal delivery. Infants should have a birth weight of more than 2,500 grams; have a 5-minute Apgar score more than 8 and without signs of infections, and resuscitation efforts. Women with ex-utero delivery, cesarian delivery, and known history of viral, and congenital or genetic diseases congenital diseases were excluded. Infants with birth defects, preterm babies, twins, and low birth weight were excluded.
A preformed case report form was completed for each patient. This form contained information on maternal age, obstetric history, labor, and delivery, and family, and medical histories. The analysis included previous live births, and it considers other pregnancies that did not result in live births. The medical history form also included an evaluation of the risk factors or clinical evidence of infectious diseases, genetic diseases, and the presence of any hematological disorders. After delivery, the weight of the baby and the placental weight were also recorded. Two maternal age groups were studied, 17-30 years and 31-45 years.
Umbilical cord blood sample preparation
Umbilical cord blood was collected in a cord blood bag containing 35 ml of citrate phosphate dextrose anticoagulant (Suru International Pvt. Ltd., Mumbai, India). Approximately 5 ml of each blood sample was placed in ethylene-diamine-tetraacetic acid tube (Becton Dickinson Company, Franklin Lakes, New Jersey, USA) for detection of hemoglobin abnormalities by hemoglobin electrophoresis (Bio-Rad, Hercules, California, USA)
Pre-processing of samples
Three and a half ml of umbilical cord blood was taken from the AK 100 sample chamber using a Biosafe blue sample calibrator (Biosafe SA, Geneva, Switzerland). Aliquots were placed into separate tubes: 1 ml for TNC and complete blood count with the differential count determination, 0.5 ml for CD45, CD34, and viability testing, 1 ml for human leukocyte typing and 1 ml for sterility testing.
Processing of umbilical cord blood
Hespan containing solution (6% Hes in 0.9 sodium chloride; [B. Braun Medical, Melsung, Germany]) was added and mixed with the anticoagulated umbilical cord blood in a flow rate of 0.5 ml/second through the filter. The amount of hespan added to the umbilical cord blood was calculated based on the following formula: amount of hespan = volume of umbilical cord blood (umbilical cord blood weight/1.05) x 0.2 ml. The filter upstream was sealed-off by the clamp and the filter was removed for disposal. All blue clamps were closed to avoid kinks. A barcode label of the umbilical cord blood unit was attached on all the bags on the processing single-use kit. Then, the processing single-use kit was loaded on the Sepax-100 system (Biosafe SA, Geneva, Switzerland) in the umbilical cord blood bank. On-screen instructions were meticulously followed. At the end of the separation, the weight of buffy coat line, red cell pallet, and leukocyte poor plasma were recorded. After processing, 0.5 ml of the buffy coat sample was dispensed in a red tube (Becton Dickinson Company, Franklin Lakes, New Jersey, USA) for CBC with differential count, and 0.5 ml was reserved for CD45, CD34, and viability determination. Subsequently, a 6 ml sample of the red cell pellet were tested for ABO grouping (0.5 ml) using Dia Med-ID Microplates gel system (Diamed, Cressier, Switzerland), storage (2 × 2.0 ml), and CBC (1 ml). Apart from these, 1 ml of the leukocyte poor plasma were placed in a red top tube for CBC count, (2 × 2 ml) for storage and 1 ml for bacterial sterility test (Bactec, BD, New Jersey, USA). Afterwards, the cell bag (buffy coat) was placed in the cool mix at 4°C (Thermo Fisher Scientific Inc, New Baltimore, MI, USA) for stem cells cryopreservation preparation. Cryopreservation of the umbilical cord blood was carried out by adding 5 ml of 10% dimethyl sulfoxide (OriGen Biomedical, Helsingborg, Sweden) using a syringe pump at the rate of 0.650 ml/minute for 15 minutes. Then, the bag and the segment were sealed and placed in an appropriate canister in the Cryomed controlled rated freezer (Thermo Fisher Scientific Inc., New Baltimore, MI, USA). Cell bags were stored in liquid nitrogen in a BioArchive System (Thermogenesis, Rancho, Cordova, CA, USA) in the umbilical cord blood bank.
Cell count assays
Post processing of red blood cell samples and leukocyte-poor plasma umbilical cord blood samples were reserved for CBC testing included TNC’s while the crude product of umbilical cord blood and buffy coat were reserved for CBC with differential included TNC and NRBC, which were all determined with the Sysmex XE-2100 automated hematology analyzer (Sysmex America Inc., Illinois, USA) in the hematology laboratory.
Analysis of CD34+, CD45+ cells, and viability
Umbilical cord blood samples were submitted to the flowcytometry section in a 2 ml cryogenic vial. A 50 µl sample of umbilical cord blood was incubated with 10µl of anti-CD45 fluorescein isothiocyanate, 10µl of anti-CD34 phycoerythrin monoclonal antibody (anti HPCA-2PE), and 10 µl of viability dye 7-amino actinomycin in trucount tube (Becton Dickinson Company, Franklin Lakes, New Jersey, USA). The tube was vortexed for a few seconds and then incubated in the dark for 20 minutes. Then, 1 ml of ammonium chloride lysing solution was added to the tube and incubated in the dark for 5 minutes. Samples were run immediately using fluorescence-activated cell sorting (BD FACS Calibur; Becton Dickinson Company, Franklin Lakes, New Jersey, USA). A CD45 Trucount single platform template was used for sequential (Boolean) gating technique to define the CD34+ stem cells. The threshold on the FL1 (CD45 FITC) was set and moved up to 270 to ensure that the dim CD45/low side scattered/CD34+ cells were not excluded from the analysis. The CD34+ cells were selected based upon forward and 90° scatter properties, and dim CD45 expression. Subsequently, an exclusion gate R6 was used to eliminate signals arising from debris, while allowing collection of all the bead data. The numbers of absolute CD34+ cells, viability, and CD45+ cells were calculated by using the fluorescence-activated cell sorting method.
Statistical analysis
Statistical analysis was performed by the IBM SPSS Statistics for Windows version 20.0 (IBMCorp, Armonk, NY, USA). Non-parametric Kruskal-Wallis test was used for univariate analyses to compare the birth order in different laboratory variables. The significance of differences between baby’s gender groups was determined by non-parametric Mann-Whitney U test. Spearman rank association coefficient was calculated for univariate analyses to ascertain the relationship between mother’s age and other variables, relationship between birth weight and other variables, relationship between birth order and other variables, and the relationship between placental weight and other variables. A p-value less than 0.05 was considered statistically significant for all analysis.
Results
The characteristics of the donating mothers and babies, including maternal age, birth weight, placental weight, baby’s gender, and birth order are shown in Table 1. Most of the study participants (63.6%) were on their first 3 deliveries, while the rest of the study participants were at least in their fourth pregnancy. Summary of laboratory results are shown in Table 2.
Table 1.
Characteristics of the 206 umbilical cord blood donations (donor and baby) from the cord blood bank.
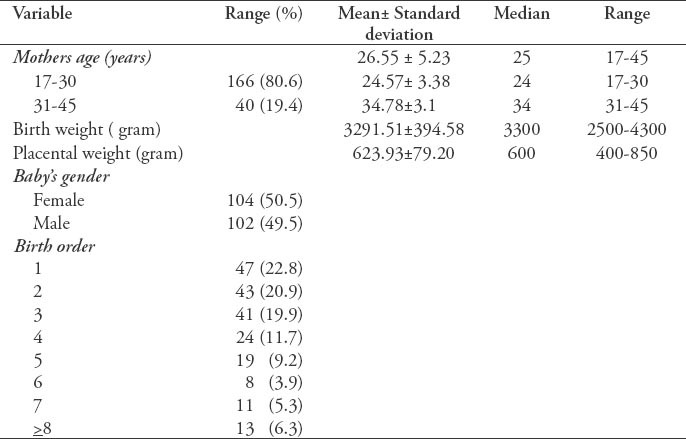
Table 2.
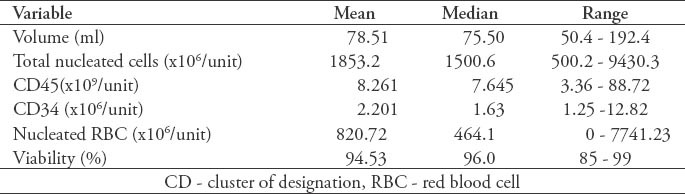
Summary of 206 laboratory results umbilical cord blood units (donor and baby) from the cord blood bank.
Maternal and neonatal factors
Among the maternal factor examined, mothers age had a weakly negative effect with CD45+ cell count only (rs = -0.150, p=0.016). No relationship was found between mothers age and the other laboratory measurement as volume, TNC counts, CD34+ cell counts, NRBC counts, and viability (p>0.05). Among the neonatal factors examined, baby’s birth weight had a weakly positive effect with the volume of umbilical cord blood (rs=0.269, p<0.0001), TNC counts (rs=0.279, p<0.0001), CD34+ cell counts (rs=0.209, p=0.001), CD45+ cell counts (rs=0.137, p=0.025), NRBC counts (rs=0.158, p=0.012), and viability (rs=0.128, p=0.033). There was also a weakly positive effect between placental weight and the volume of umbilical cord blood (rs=0.212, p=0.001), TNC counts (rs=0.196, p=0.002), CD34+ cell counts (rs=0.134, p=0.028), and CD45+ cell counts (rs=0.143, p=0.02). There was no correlation between placental weight and other laboratory measurements as NRBC and viability (p>0.05). Baby’s gender had no effect on any of the outcome measures (p>0.05).
Birth order had a weakly negative effect with TNC counts (rs=-0.211, p=0.001), CD34+ cell (rs=-0.196, p=0.002), CD45+ cell (rs=-0.233, p<0.0001), and NRBC counts (rs=-0.162, p=0.01). There was a weakly positive effect between birth order and viability (rs=0.119, p=0.044). No relationship was found between birth order and the volume of umbilical cord blood (p=0.057). Among the maternal and neonatal factors examined, bigger birth weight of a baby (p<0.0001) and larger placenta (p=0.001) were associated with a large volume of umbilical cord blood. A higher TNC count was associated with a bigger baby (p<0.0001), larger placenta (p=0.002), and with a first born baby (p=0.001). A higher CD34+ cell concentration was associated with larger placenta (p=0.028), bigger baby (p=0.001), and first born baby (p=0.002). A higher CD45+ cell count was associated with younger maternal age (p=0.016), bigger baby (p=0.025), larger placenta (p=0.02), and first born baby (p<0.0001). A higher NRBC count was associated with a bigger baby (p=0.012), and first born baby (p=0.01). A higher viability was associated with a bigger baby (p=0.033) and high number of birth order (p=0.044). There was no significant difference between baby boys and girls regarding laboratory variables. Among the neonatal factors examined, birth order had a significant effect with the first baby with higher TNCs count, CD34+ cells count, CD45+ cell count, and NRBC count. In this study, the first baby had a median TNC count of 1909.34×106, second baby had 1479.92×106, third baby had 1322.48×106, fourth baby 1220.14×106, fifth baby 1114.09×106, sixth baby 1768.02×106, seventh baby 1366.36×106, and subsequent babies 1737.64 ×106 (p=0.001). There was a similar decrease in CD34+ cell count, CD45+ cell count, and NRBC count with subsequent deliveries while there was a decrease in viability with the first baby. The first baby had a median viability of 95%, second baby 96%, third baby 96%, fourth baby 97%, fifth baby 96%, sixth baby 96.5%, seventh baby 95%, and subsequent babies 96%. There is no significant effect between birth order and the volume of umbilical cord blood (p=0.057).
Association of umbilical cord blood volume
A large umbilical cord blood volume is significantly affected by bigger birth weight of a baby (p<0.0001) and larger placenta (p=0.001) (Figures 1A-1D).
Figure 1.
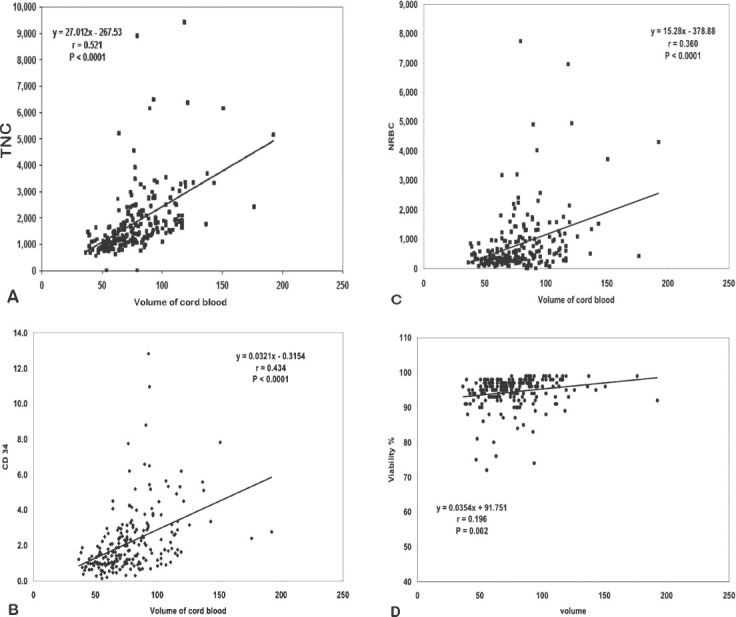
Association between umbilical cord blood volume with A) total nucleated cells (TNCs) (r =0.521, p<0.0001), B) CD34 (r=0.434, p<0.0001) C) nucleated red blood cells (NRBDs) (r=0.360, p<0.0001), and D) viability (r= 0.196, p=0.002).
Total nucleated cells cell count, CD34+, CD45+, NRBC count, and viability
Figure 2 shows the association between CD34+ and TNCs (r=0.653, p<0.0001). Figure 3 shows the association between CD45+ and TNCs (r=0.139, p=0.023). Figure 4 shows the association between NRBC and TNCs (r=0.947, p<0.0001). The NRBC is moderately positively affected by CD34+ cell count (r=0.545, p<0.0001) (Figure 5) while there is no relationship between NRBC and viability (r=0.027, p=0.352).
Figure 2.
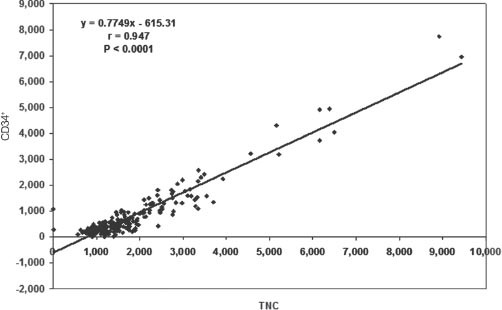
Association between CD34+ and total nucleated cells (TNC) (r=0.653, p<0.0001).
Figure 3.
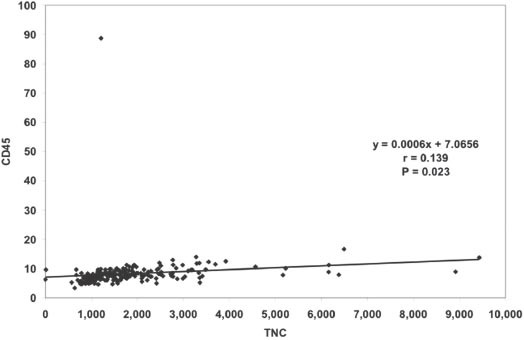
Association between CD45+ and total nucleated cells (TNCs) (r=0.139, p=0.023).
Figure 4.
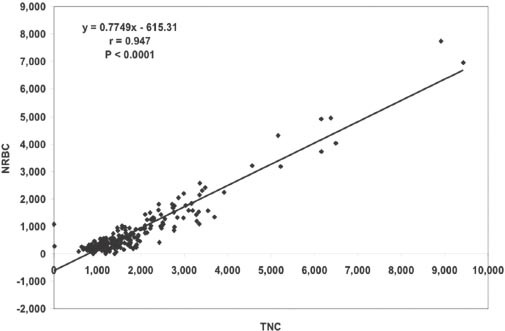
Association between nucleated red blood cells (NRBDs) and total nucleated cells (TNC) (r=0.947, p<0.0001).
Figure 5.
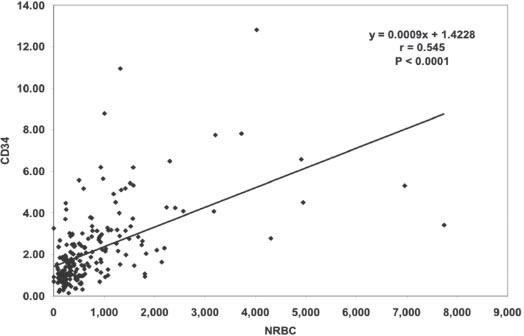
Association between CD34+ and nucleated red blood cells (NRBDs) (r=0.545, p<0.0001).
Discussion
The efficiency of an umbilical cord blood unit correlates with its capability for engraftment after transplantation. Umbilical cord blood banks aim to store cold blood units with high potency in their inventories. Efficiency is a measure of the overall health of an umbilical cord blood unit and reflects its ability to resist the stresses produced during processing, cryopreservation, and thaw. In a large American retrospective study (n=5267),18 high-quality umbilical cord blood samples were defined as those with larger collection volume, higher TNCs, high CD34+ cell count, and higher birth weight.
The characteristics of umbilical cord blood collected from participants was highly relevant for the research investigation. In this study, the average isolation procedure generated approximately 78.5 ml of umbilical cord blood, of which would contain 1853.2×106 TNC per unit, 2.2×106 CD34+ count per unit, 8.3 ×109 CD45+ count per unit, 820×106 NRBC per unit, and a viability of 94.5%. Our analysis showed that TNC increases with the extracted volume of cord blood. This data is in agreement with previous studies.19,20 This observation further strengthens the suggestion that the collection of more umbilical cord blood during delivery provides a greater chance of success in transplantation efforts, as the volume can support a larger population of TNC.20
In our study, the number of CD34+ cells in umbilical cord blood was positively associated with the volume of extracted umbilical cord blood, similar to those reported by previous investigators.16 It should be understood that the survival of a patient is largely dependent on the number of CD34+ cells that are present in the transplanted cord blood, and it is one of the main reasons that the population of this cell type should be increased during umbilical cord blood banking.21,22 Many research groups have developed an ex vivo culture procedure that results in the further expansion of CD34+ cells resulting in more rapid engraftment of neutrophils following infusion to transplant recipients.23
We found a strong positive relationship between NRBC and CD34+ cell count, in agreement with the findings of Cairo et al in 2005.24 Under normal conditions, NRBCs were found only in the blood of the fetus and the newborn. The concentration of NRBCs in umbilical cord blood unit has been reported to range between 0.03-4.8×109, the presence of a high number of NRBCs can be an indicator of a pathologic process.25
Our results showed a weak positive association between the viability of the umbilical cord blood cells and the extracted volume of cord blood. This positive relationship indicates that there is a need to further determine conditions and other factors that would increase the viability of these cells during umbilical cord blood banking. It is essential to maintain the viability of cells during storage, as it is highly likely that subjecting these tissues to extremely low temperatures may decrease the cell count and viability.26 Furthermore, our study showed that CD34+ cells are positively affected by the TNCs present in the umbilical cord blood, as reported by Chandra et al in 2010.14 This significant association appeared to be a promising characteristic of the tissue resource, as there are main efforts for cell expansion that could be focused on the proliferation of CD34+ cells, thereby, it can increase the usefulness of the umbilical cord blood during transplantation. This relationship can also assist in the understanding of the cellular content of the cord blood.
Due to the scanty published information on the CD45+ leukocyte antigen count in cord blood, we studied the association between CD45+ with maternal and neonatal factors, TNCs, umbilical cord blood volume, and CD34+ count in Saudi women. The CD45+ count was observed to have a weak positive relationship with the TNCs in the cord blood. It is possible that the CD45+ cell dose, which reflects the viable infused cells expressing the common CD45 leukocyte antigen, may be more potent criteria to predict engraftment than the TNC count, which includes nonviable cells and mature erythroblasts. Barlogis et al27 suggested that a minimum CD45 cell dose of 3.35 × 107/kg at infusion must be targeted to achieve a faster lymphocyte reconstitution. Furthermore, CD45+ count may be used as a predictive factor for the quality of T cell recovery and consequently for the risk assessment of life-threatening infections. In addition, the antigen CD45 is one of the most important factors in the detection of CD34+ by different qualitative and quantitative protocols.28 This is why it was included in our study panels.
In our analysis, we found that the birth weight were associated positively with umbilical cord blood volume, placental weight, CD34+, and TNC counts. These data are in agreement with findings from previous reports.13,16,17,20,29 This significant observation may be largely due to the birth weight, which could be directly affected by placental volume.30 Thame et al30 suggested that placental volume may be a more reliable predictor of size at birth than fetal measurements, and may be useful in the early identification of a fetus at risk in the perinatal period. In addition, birth order had a significant effect, with the first-born baby having higher CD45+ cell count, CD34+ cell count, TNCs count, and NRBCs count. These findings are also consistent with those previously reported.30 Another significant finding in our study is the inversely proportional relationship between maternal age and CD45+ cell count. Previous reports14 suggested that maternal age is not significantly related to the umbilical cord blood and CD34+ cell count, as well as the number of NRBC’s in the umbilical cord blood. However, a previous report28 showed that women >25 years old carry more TNCs, and younger maternal age was associated with a higher CD34+ cell concentration.16 This observation may serve as a parameter during the selection and evaluation of the usefulness of a specific umbilical cord blood specimen.
The current study has several limitations that need to be addressed. Most of our cases were of the younger age group (17-30 years), due to the inherent nature of the reproductive capacity of the human body for pregnancy and delivery of babies at a younger age range. The older age group comprised of women within the range of 31-45 years, which represented a 20% of our study population; therefore, they do not represent our study population. Further studies should have an approximately equal numbers of mothers in both age groups. Another limitation is the small number of umbilical cord blood samples. However, the collection of cord blood in Saudi Arabia is only at limited hospitals. Therefore, it is difficult to recruit a larger number of donors.
Finally, our data suggests that umbilical cord blood units should be selected aside from the gender of the baby, or maternal age. Optimal results can be obtained by selecting the first or second babies, babies with weight more than 3,300 grams, and with a larger placental size.
This research could help in future regarding the selecting of the optimal umbilical cord blood donors in our region, as at the present time there are no such records. Moreover, there are only a few studies on the association in the parameters of hematopoietic potential in umbilical cord blood. This study may help reduce the costs of history taking, determine the best selection processes for donors of umbilical cord blood (to improve quality) and storage of umbilical cord blood units (to prevent storage of ineffective blood units), processing of umbilical cord blood units, optimize the collection procedure, and minimize the number of rejected umbilical cord blood units.
In conclusion, maternal and neonatal factors could be useful in the identification and selection of potential best umbilical cord blood units. This study demonstrates that the neonatal factors examined weakly influence the umbilical cord blood volume, CD34+ cell, CD45+ cell, NRBC, viability, and TNC. The maternal age has no effect on the laboratory variables except for CD45 counts; which was negatively correlated regarding the age of the mother. There were no significant of differences between baby boys and girls with laboratory variables.
Acknowledgment
I would like to thank Dr. Hind A. Al-Humaidan, Consultant Hematopathologist, Department of Pathology & Laboratory Medicine, King Faisal Specialist Hospitals and Research Centre, Riyadh, Saudi Arabia, for her constructive review and comments. We also thank Dr. Morad Al-Kaff, Supervisor, Stem Cell Processing Laboratory & Cord Blood Bank, King Faisal Specialist Hospitals and Research Centre, Riyadh, Saudi Arabia for his guidance.
Footnotes
Related Articles.
Han ZC, Zhang HN, Wang YZ, Lv CY, Xu ZY. Effect of the human insulin-like growth factor 1 gene transfection to human umbilical cord blood mesenchymal stem cells. Saudi Med J 2014; 35: 435-441.
Huang JL, Yang SX. Safety of umbilical cord blood-derived mesenchymal stem cells (MSCs) following 5-azaserine induction and inhibition of human cardiac myocyte apoptosis by MSCs. Saudi Med J 2009; 30: 1144-1149.
References
- 1.Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: place of umbilical cord blood. Br J Haematol. 2009;147:246–261. doi: 10.1111/j.1365-2141.2009.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad VK, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses. Semin Hematol. 2010;47:59–69. doi: 10.1053/j.seminhematol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions. Br J Haematol. 2010;148:356–372. doi: 10.1111/j.1365-2141.2009.07974.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith AR, Gross TG, Baker KS. Transplant outcomes for primary immunodeficiency disease. Semin Hematol. 2010;47:79–85. doi: 10.1053/j.seminhematol.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Orchard PJ, Tolar J. Transplant outcomes in leukodystrophies. Semin Hematol. 2010;47:70–78. doi: 10.1053/j.seminhematol.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 7.MacMillan ML, Walters MC, Gluckman E. Transplant outcomes in bone marrow failure syndromes and hemoglobinopathies. Semin Hematol. 2010;47:37–45. doi: 10.1053/j.seminhematol.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Reboredo NM, Díaz A, Castro A, Villaescusa RG. Collection, processing and cryopreservation of umbilical cord blood for unrelated transplantation. Bone Marrow Transplant. 2000;26:1263–1270. doi: 10.1038/sj.bmt.1702728. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JE, Gluckman E. Umbilical cord blood transplantation: the first 20 years. Semin Hematol. 2010;47:3–12. doi: 10.1053/j.seminhematol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Tolosa JN, Park DH, Eve DJ, Klasko SK, Borlongan CV, Sanberg PR. Mankind’s first natural stem cell transplant. J Cell Mol Med. 2010;14:488–495. doi: 10.1111/j.1582-4934.2010.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Rev Hematol. 2010;3:273–283. doi: 10.1586/ehm.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenna D, Sheth J. Umbilical cord blood: current status & promise for the future. Indian J Med Res. 2011;134:261–269. [PMC free article] [PubMed] [Google Scholar]
- 13.Urciuoli P, Passeri S, Ceccarelli F, Luchetti B, Paolicchi A, Lapi S, et al. Pre-birth selection of umbilical cord blood donors. Blood Transfus. 2010;8:36–43. doi: 10.2450/2009.0081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra T, Afree S, Kumar A, ingh U. Correlation of umbilical cord blood volume with CD34+cells concentration. International Journal of Blood Transfusion and Immunohematology. 2010;1:11–15. [Google Scholar]
- 15.Nunes RD, Zandavalli FM. Association between maternal and fetal factors and quality of cord blood as a source of stem cells. Rev Bras Hematol Hemoter. 2015;37:38–42. doi: 10.1016/j.bjhh.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa R, Watanabe T, Kawano Y, Kanai S, Suzuya H, Kaneko M, et al. Analysis of maternal and neonatal factors that influence the nucleated and CD34+cell yield for cord blood banking. Transfusion. 2004;44:262–267. doi: 10.1111/j.1537-2995.2004.00645.x. [DOI] [PubMed] [Google Scholar]
- 17.Urciuoli P, Passeri S, Ceccarelli F, Luchetti B, Paolicchi A, Lapi S, et al. Pre-birth selection of umbilical cord blood donors. Blood Transfus. 2010;8:36–43. doi: 10.2450/2009.0081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page KM, Mendizabal A, Betz-Stablein B, Wease S, Shoulars K, Gentry T, et al. Optimizing donor selection for public cord blood banking: influence of maternal, infant, and collection characteristics on cord blood unit quality. Transfusion. 2014;54:340–352. doi: 10.1111/trf.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaime-Pérez JC, Monreal-Robles R, Rodríguez-Romo LN, Mancías-Guerra C, Herrera-Garza JL, Gómez-Almaguer D. Evaluation of volume and total nucleated cell count as cord blood selection parameters: a receiver operating characteristic curve modeling approach. Am J Clin Pathol. 2011;136:721–726. doi: 10.1309/AJCPFB6EXO7BJVLR. [DOI] [PubMed] [Google Scholar]
- 20.Wen SH, Zhao WL, Lin PY, Yang KL. Associations among birth weight, placental weight, gestational period and product quality indicators of umbilical cord blood units. Transfus Apher Sci. 2012;46:39–45. doi: 10.1016/j.transci.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Canabarro R, Sporleder H, Gomes T, Zanatta G, Scribel L, Freitas F, et al. Imunophenotypic evaluation, and physiological and laboratory correlations of hematopoietic stem cells from umbilical cord blood. Bibliographic Details. 2007;31:397–404. [Google Scholar]
- 22.Yang H, Loutfy MR, Mayerhofer S, Shuen P. Factors affecting banking quality of umbilical cord blood for transplantation. Transfusion. 2011;51:284–292. doi: 10.1111/j.1537-2995.2010.02826.x. [DOI] [PubMed] [Google Scholar]
- 23.Chotinantakul K, Prasajak P, Leeanansaksiri W. Wnt1 accelerates an ex vivo expansion of human cord blood CD34+CD38-cells. Stem Cells Int 2013. 2013:909812. doi: 10.1155/2013/909812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cairo MS, Wagner EL, Fraser J, Cohen G, Van De Ven C, Carter SL, et al. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion. 2005;45:856–866. doi: 10.1111/j.1537-2995.2005.04429.x. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy JM, Capullari T, Thompson Z, Zhu Y, Spellacy WN. Umbilical cord nucleated red blood cell counts: normal values and the effect of labor. J Perinatol. 2006;26:89–92. doi: 10.1038/sj.jp.7211437. [DOI] [PubMed] [Google Scholar]
- 26.Sachdeva N. Analysis of the viability of umbilical cord blood stem cells. J Stem Cells Regen Med. 2009;5:44–48. doi: 10.46582/jsrm.0502009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlogis V, Glasman L, Brunet C, Loundou AD, Lemarie C, Galambrun C, et al. Impact of viable CD45 cells infused on lymphocyte subset recovery after unrelated cord blood transplantation in children. Biol Blood Marrow Transplant. 2011;17:109–116. doi: 10.1016/j.bbmt.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Ben Azouna N, Berraeis L, Regaya Z, Jenhani F. Immunophenotyping of hematopoietic progenitor cells: Comparison between cord blood and adult mobilized blood grafts. World J Stem Cells. 2011;3:104–112. doi: 10.4252/wjsc.v3.i11.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;58:894–900. doi: 10.1038/sj.ejcn.1601909. [DOI] [PubMed] [Google Scholar]


