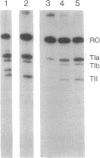
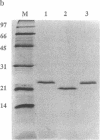
Abstract
RNA polymerases encounter a variety of types of blocks to elongation during transcription in eukaryotic cells. At least one protein, TFIIS, can promote read-through of many types of blocks to elongation by RNA polymerase II, and this protein stimulates cleavage of the nascent transcript in stalled elongation complexes as a prelude to read-through. The C-terminal half of the TFIIS protein is sufficient for stimulating the cleavage and read-through reactions in vitro. To study how TFIIS changes the response of RNA polymerase II elongation complexes to such blocks, targeted amino acids in the C terminus of HeLa TFIIS were mutated to alanines. Two mutant TFIIS proteins as well as the unmutated C-terminal half of the TFIIS protein were purified following overexpression in Escherichia coli. Each protein was examined for read-through activity and ability to stimulate transcript cleavage in ternary elongation complexes. Mutant TFIIS5 (E174A, E175A) was reduced in read-through and cleavage activities relative to the unmutated, truncated TFIIS (delta TFIIS). Mutant TFIIS7 (K187A, K189A) was able to stimulate cleavage nearly at the rate and to the extent of the TFIIS5 mutant. In contrast to what was observed with TFIIS5, no detectable read-through was observed in the presence of the TFIIS7 mutant during the course of the reaction. Thus, there is no simple, direct correlation between the ability of TFIIS to promote cleavage and its ability to promote read-through by RNA polymerase II. These results suggest that although TFIIS is necessary to mediate the cleavage reaction that precedes the read-through event, the cleavage event itself is not sufficient to allow read-through by RNA polymerase II.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K., Baek K. H., Jeon C. J., Miyamoto K., Ueno A., Yoon H. S. Stimulation of transcript elongation requires both the zinc finger and RNA polymerase II binding domains of human TFIIS. Biochemistry. 1991 Aug 6;30(31):7842–7851. doi: 10.1021/bi00245a026. [DOI] [PubMed] [Google Scholar]
- Bengal E., Flores O., Krauskopf A., Reinberg D., Aloni Y. Role of the mammalian transcription factors IIF, IIS, and IIX during elongation by RNA polymerase II. Mol Cell Biol. 1991 Mar;11(3):1195–1206. doi: 10.1128/mcb.11.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S., Polyakov A., Nikiforov V., Goldfarb A. GreA protein: a transcription elongation factor from Escherichia coli. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8899–8902. doi: 10.1073/pnas.89.19.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S., Sagitov V., Goldfarb A. Transcript cleavage factors from E. coli. Cell. 1993 Feb 12;72(3):459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai H., Luse D. S. Variations in template protection by the RNA polymerase II transcription complex during the initiation process. Mol Cell Biol. 1987 Oct;7(10):3371–3379. doi: 10.1128/mcb.7.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. J. New models for the mechanism of transcription elongation and its regulation. Harvey Lect. 1992 1993;88:1–21. [PubMed] [Google Scholar]
- Chen H. C., England L., Kane C. M. Characterization of a HeLa cDNA clone encoding the human SII protein, an elongation factor for RNA polymerase II. Gene. 1992 Jul 15;116(2):253–258. doi: 10.1016/0378-1119(92)90522-q. [DOI] [PubMed] [Google Scholar]
- Christie K. R., Awrey D. E., Edwards A. M., Kane C. M. Purified yeast RNA polymerase II reads through intrinsic blocks to elongation in response to the yeast TFIIS analogue, P37. J Biol Chem. 1994 Jan 14;269(2):936–943. [PubMed] [Google Scholar]
- Gu W., Powell W., Mote J., Jr, Reines D. Nascent RNA cleavage by arrested RNA polymerase II does not require upstream translocation of the elongation complex on DNA. J Biol Chem. 1993 Dec 5;268(34):25604–25616. [PMC free article] [PubMed] [Google Scholar]
- Guo H., Price D. H. Mechanism of DmS-II-mediated pause suppression by Drosophila RNA polymerase II. J Biol Chem. 1993 Sep 5;268(25):18762–18770. [PubMed] [Google Scholar]
- Hagler J., Shuman S. Nascent RNA cleavage by purified ternary complexes of vaccinia RNA polymerase. J Biol Chem. 1993 Jan 25;268(3):2166–2173. [PubMed] [Google Scholar]
- Horikoshi M., Sekimizu K., Hirashima S., Mitsuhashi Y., Natori S. Structural relationships of the three stimulatory factors of RNA polymerase II from Ehrlich ascites tumor cells. J Biol Chem. 1985 May 10;260(9):5739–5744. [PubMed] [Google Scholar]
- Izban M. G., Luse D. S. Factor-stimulated RNA polymerase II transcribes at physiological elongation rates on naked DNA but very poorly on chromatin templates. J Biol Chem. 1992 Jul 5;267(19):13647–13655. [PubMed] [Google Scholar]
- Izban M. G., Luse D. S. SII-facilitated transcript cleavage in RNA polymerase II complexes stalled early after initiation occurs in primarily dinucleotide increments. J Biol Chem. 1993 Jun 15;268(17):12864–12873. [PubMed] [Google Scholar]
- Izban M. G., Luse D. S. The increment of SII-facilitated transcript cleavage varies dramatically between elongation competent and incompetent RNA polymerase II ternary complexes. J Biol Chem. 1993 Jun 15;268(17):12874–12885. [PubMed] [Google Scholar]
- Kerppola T. K., Kane C. M. Intrinsic sites of transcription termination and pausing in the c-myc gene. Mol Cell Biol. 1988 Oct;8(10):4389–4394. doi: 10.1128/mcb.8.10.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Kane C. M. RNA polymerase: regulation of transcript elongation and termination. FASEB J. 1991 Oct;5(13):2833–2842. doi: 10.1096/fasebj.5.13.1916107. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Linn S. C., Luse D. S. RNA polymerase II elongation complexes paused after the synthesis of 15- or 35-base transcripts have different structures. Mol Cell Biol. 1991 Mar;11(3):1508–1522. doi: 10.1128/mcb.11.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote J., Jr, Ghanouni P., Reines D. A DNA minor groove-binding ligand both potentiates and arrests transcription by RNA polymerase II. Elongation factor SII enables readthrough at arrest sites. J Mol Biol. 1994 Feb 25;236(3):725–737. doi: 10.1006/jmbi.1994.1185. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Natori S. Stimulatory proteins of RNA polymerase II from Ehrlich ascites tumor cells. Mol Cell Biochem. 1982 Aug 6;46(3):173–187. doi: 10.1007/BF00239666. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. How RNA polymerase II terminates transcription in higher eukaryotes. Trends Biochem Sci. 1989 Mar;14(3):105–110. doi: 10.1016/0968-0004(89)90132-1. [DOI] [PubMed] [Google Scholar]
- Reinberg D., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II. Transcription factor IIS stimulates elongation of RNA chains. J Biol Chem. 1987 Mar 5;262(7):3331–3337. [PubMed] [Google Scholar]
- Reines D., Chamberlin M. J., Kane C. M. Transcription elongation factor SII (TFIIS) enables RNA polymerase II to elongate through a block to transcription in a human gene in vitro. J Biol Chem. 1989 Jun 25;264(18):10799–10809. [PubMed] [Google Scholar]
- Reines D., Ghanouni P., Li Q. Q., Mote J., Jr The RNA polymerase II elongation complex. Factor-dependent transcription elongation involves nascent RNA cleavage. J Biol Chem. 1992 Aug 5;267(22):15516–15522. [PMC free article] [PubMed] [Google Scholar]
- Reines D., Mote J., Jr Elongation factor SII-dependent transcription by RNA polymerase II through a sequence-specific DNA-binding protein. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1917–1921. doi: 10.1073/pnas.90.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. A., Chamberlin M. J., Kane C. M. Contacts between mammalian RNA polymerase II and the template DNA in a ternary elongation complex. Nucleic Acids Res. 1993 Jan 11;21(1):113–118. doi: 10.1093/nar/21.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. A., Kane C. M., Chamberlin M. J. Footprinting analysis of mammalian RNA polymerase II along its transcript: an alternative view of transcription elongation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4245–4249. doi: 10.1073/pnas.88.10.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Reines D., Kane C. M. Purified elongation factor SII is sufficient to promote read-through by purified RNA polymerase II at specific termination sites in the human histone H3.3 gene. J Biol Chem. 1990 Aug 25;265(24):14554–14560. [PubMed] [Google Scholar]
- Sluder A. E., Greenleaf A. L., Price D. H. Properties of a Drosophila RNA polymerase II elongation factor. J Biol Chem. 1989 May 25;264(15):8963–8969. [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Transcription elongation and eukaryotic gene regulation. Oncogene. 1990 Jun;5(6):777–785. [PubMed] [Google Scholar]
- Wiest D. K., Wang D., Hawley D. K. Mechanistic studies of transcription arrest at the adenovirus major late attenuation site. Comparison of purified RNA polymerase II and washed elongation complexes. J Biol Chem. 1992 Apr 15;267(11):7733–7744. [PubMed] [Google Scholar]