Abstract
An immune-suppressive role of myeloid-derived suppressor cells (MDSCs) in melanoma has long been speculated, whereas molecular mechanisms underlying this role are not well understood. Here, Chung and colleagues show that dendritic cell-associated, heparan sulfate proteoglycans-dependent integrin ligand (DC-HIL), a cell surface immune-modulatory molecule, is highly expressed on tumor-associated MDSCs. Genetic ablation or antibody blockade of DC-HIL delays the growth of transplantable B16 melanoma in syngeneic mice, which is accompanied by enhanced antitumor T-cell activities. These findings support a role for DC-HIL in immune evasion within the melanoma microenvironment.
Recent breakthroughs in the treatment of a broad spectrum of human metastatic solid tumors, including melanoma, through blockade of the PD-1/PD-L1 (B7-H1) immune-suppressive pathway represents a major conceptual advancement in cancer immunotherapy. Different from systemic enhancement of immune responses with immune-checkpoint blockade by anti-CTLA-4, selective modulation of the tumor microenvironment (TME) by blocking the interaction between PD-1 on effector T cells and PD-L1 on tumor cells and other TME cells can eliminate large clinically terminal-stage tumors and metastases with significantly greater clinical benefit and minimal auto-immune toxicity (Brahmer et al., 2012; Topalian et al., 2012). Therefore, understanding the molecular mechanisms that suppress immune responses selectively within the TME could be important for the optimal design of therapeutic interventions.
In addition to tumor cells and host stromal cells, the TME is also populated by various blood-derived immune cells, including MDSCs, that exhibit potent immune-suppressive activities. Tumor-induced granulocytic hyperplasia accompanying immune suppression was described more than half a century ago. However, the concept that MDSCs may reflect the abnormal nature of myelopoiesis in cancer has only been well received recently. MDSCs are highly heterogenic cells with immature and progenitor-like features of myeloid origin (Talmadge and Gabrilovich, 2013). These cell types are present in many patients with cancer and in animal tumor models, where they suppress immune responses on T cells and other tumor-reactive immune cells profoundly. Furthermore, other studies have revealed that the number of circulating MDSCs correlates poorly with cancer prognosis and that it correlates inversely with T-cell frequency in melanoma. Increases in the MDSC population depend on both tumor burden and on tumor-secreted factors that regulate myeloid progenitor cell survival and expansion. Several factors that may regulate MSDC accumulation and activities include the chemokines CCL2 and CXCL12 and proinflammatory mediators, such as GM-CSF, G-CSF, IL-1β, IL-6, and prostaglandin E2, as has been reported recently. Nevertheless, the mechanisms responsible for the suppressive function MDSCs remain uncertain, although l-arginine metabolism and reactive oxygen species production have been attributed to this effect. Moreover, several studies indicate that the suppressive activities of MDSCs for T cells, or natural killer cells, are contact-dependent, possibly requiring molecular crosstalk between MDSCs and immune effector cells (Talmadge and Gabrilovich, 2013).
Dendritic cell-associated, heparan sulfate proteoglycans (HSPG)-dependent integrin ligand (DC-HIL), also known as GPNMB, was identified originally using subtractive cDNA cloning by comparing human melanoma lines with high and low metastatic potential (Weterman et al., 1995). The DC-HIL gene encodes a transmembrane protein of 560 amino acids that displays high homology with the melanocyte antigen PMEL, and it may be associated with melanoma metastasis. Subsequently, another group cloned the same gene independently, as a mouse DC-specific protein with the capacity to bind endothelial cells in a HSPG-dependent manner. As this protein also contains an integrin ligand–like RGD domain, it was named DC-HIL (Shikano et al., 2001). DC-HIL could suppress T-cell responses in vitro, and infusion of soluble DC-HIL protein exacerbated T-cell responses to antigens in a mouse model of contact hypersensitivity (Chung et al., 2007b). This effect was found to be mediated via binding to syndecan-4 (SDC-4), one of the HSPGs, on T cells. Cross-linking of SDC-4 by antibodies also attenuated anti-CD3-induced T-cell responses in vitro. In contrast, ablation of SDC-4 by specific antibodies, knockdown of SDC-4, or treatment with soluble SDC-4 led to an enhanced T-cell response to antigen, in a DC-HIL-dependent manner, and in both human and mouse systems (Chung et al., 2007a; 2009). Importantly, by knocking down DC-HIL in a B16-F10 melanoma cell line, Tomihari et al. (2010) showed that melanoma-associated DC-HIL could inhibit melanoma-reactive T cells and promote tumor growth in vivo, and in an SDC-4-dependent manner. However, the role of DC-HIL in non-melanoma cells remains uncertain. In this issue of JID (Chung et al., 2014), further evidence demonstrates another mechanism of action: DC-HIL on MDSCs in the melanoma microenvironment may suppress T-cell responses via SDC-4 (Figure 1). Therefore, DC-HIL may have a much broader role than previously thought in suppressing melanoma immunity in the microenvironment.
Figure 1. Critical role of DC-HIL expression on myelomonocytic cells in melanoma growth.
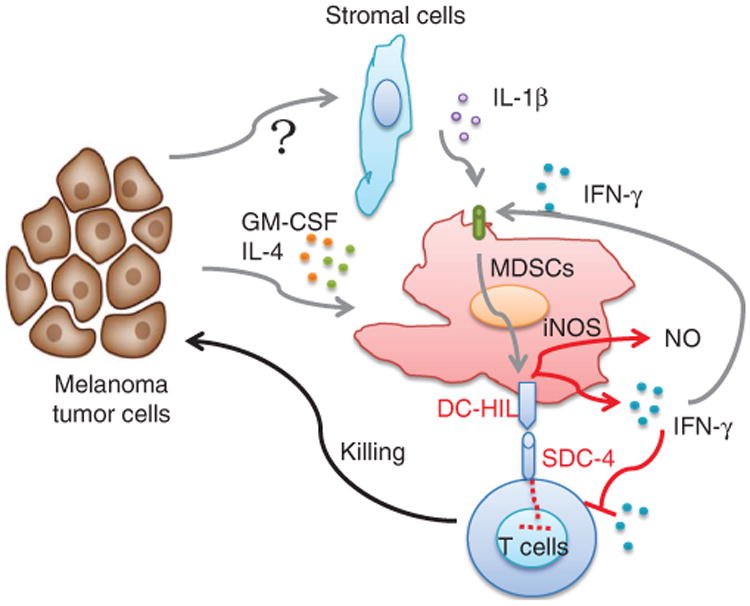
B16-F10 melanoma could induce significant expansion of CD11b+ Gr-1+ myeloid-derived suppressor cells (MDSCs) in vivo, possible through GM-CSF or IL-4 from tumor or tumor-associated microenvironment. Concurrently, large amounts of IL-1β and IFN-γ are secreted by tumor stromal cells or other immune cells, resulting in the upregulation of DC-HIL on MDSCs. Upon ligation of DC-HIL counter-receptor SDC-4 on T cells, DC-HIL itself delivers an important signal to MDSCs, inducing IFN-γ, as well as iNOS, thus inhibiting T-cell responses and promoting tumor growth. DC-HIL, dendritic cell-associated, heparan sulfate proteoglycans-dependent integrin ligand; iNOS, inducible nitric oxide synthase; NO, nitric oxide; SDC-4, syndecan-4.
In their article, Chung et al. (2014) demonstrate that DC-HIL has an indispensable role in MDSCs' T cell–suppressive function in the melanoma microenvironment. Using a DC-HIL-deficient B16 tumor cell, they found that these tumors regressed more rapidly in DC-HIL knockout versus wild-type mice. These data suggest that DC-HIL expression on host cells, but not on melanoma cells, has an important role in immune invasion. The authors also found that DC-HIL was overexpressed on CD11b+ Gr-1+ myeloid cells, either in the bone marrow, blood, and spleen or at the tumor site. Interestingly, CD11b+ Gr-1+ cells from tumor-free mice expressed little DC-HIL, suggesting a tumor-induced phenotype for DC-HIL expression. DC-HIL-rich CD11b+ Gr-1+ cells, but not F4/80+ macrophages or CD11c+ DCs, showed potent immune-suppressive activities, resembling the phenotype of MDSCs. In addition, 40% of splenic CD11b+Gr-1+ cells expressed DC-HIL, whereas ∼20 and ∼70% of these cells were positive for PD-L1 or CD80/CD86, respectively. Although several reports suggest possible roles for PD-L1 or CD80/86 in MDSC function, Chung et al. (2014) found that infusion of DC-HIL antibodies to block the DC-HIL/SDC-4 interaction restored T-cell activities in an MSDC and T-cell coculture system; this was not the case when anti-PD-L1 or anti-CD80/86 antibodies were used. Consistent with these findings, DC-HIL-deficient myeloid cells failed to suppress antitumor T-cell responses both in vitro and in vivo. Therefore, DC-HIL may be a critical molecule that mediates the suppressive activities of MSDCs.
With various blocking agents, Chung et al. (2014) discovered a critical role for inducible nitric oxide synthase in the T-cell suppression activities of DC-HIL+ MDSCs. Interestingly, they also found that blocking IFN-γ, but not IL-10 or transforming growth factor-β, reversed almost completely the inhibitory effect of MDSCs in a T-cell proliferation assay. These data are contradictory to a recent report, which suggested a minimal role for IFN-γ and IL-4R in MDSC differentiation and function in several mouse tumor models, including B16 melanoma (Sinha et al., 2012). This contradiction may be attributed to the different roles for IFN-γ in the periphery versus the TME, wherein IFN-γ may induce specific gene products selectively. Although IFN-γ is an essential cytokine in augmenting immune responses, including the upregulation of major histocompatibility complexes, and stimulation of antigen processing and presentation, IFN-γ is also shown to be a major cytokine responsible for upregulating the co-inhibitory molecule PD-L1 in the TME (Sznol and Chen, 2013). Therefore, DC-HIL may be another suppressor molecule, induced by IFN-γ in the melanoma microenvironment.
One of the most interesting aspects of this study is the therapeutic potential of this pathway as a target in melanoma immunotherapy. Currently, it is not known how broad DC-HIL expression is in human cancer. Administration of anti-DC-HIL antibodies markedly suppresses melanoma growth and prevents the expansion of CD11b+ myeloid cells in mice. As DC-HIL has minimal expression on CD11b+ Gr-1+ cells from tumor-free mice, factors from melanoma and/or the melanoma microenvironment could be responsible for the selective expression of DC-HIL in melanoma and in MDSCs. The authors determined that IL-1β and IFN-γ, which were elevated in the B16-bearing mouse sera, could trigger DC-HIL expression synergistically. However, DC-HIL knockout mice showed similar kinetics of tumor growth compared with wild-type mice in EL-4 lymphoma and LL-2 lung carcinoma. Furthermore, MDSCs from these tumors did not show significant upregulation of DC-HIL and indeed demonstrated less suppressive activities. These observations, although implicating melanoma-specific mechanisms to promote DC-HIL expression and to foster MDSC functions, does not necessarily rule out a broader mechanism of action in other types of cancer.
Clinical Implications.
The expression of dendritic cell-associated, heparan sulfate proteoglycans-dependent integrin ligand (DC-HIL) on myeloid-derived suppressor cells (MDSCs) may contribute to immune suppression in the melanoma microenvironment.
Therapeutic blockade of the interaction between DC-HIL and its counter-receptor syndecan-4 (SDC-4) is a promising, novel approach to enhancing melanoma immunity.
Acknowledgments
We thank Edward Quinlan and Beth Cadugan for editing the manuscript. This work is partially supported by the National Institutes of Health grants CA121979 and CA142779, and endowment from the United Technologies Corporation.
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Tamura K, Cruz PD, Jr, Ariizumi K. DC-IL-expressing myelomonocytic cells are critical promoters of melanoma growth. J Invest Dermatol. 2014;134:2784–94. doi: 10.1038/jid.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Bonkobara M, Tomihari M, et al. The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses. Eur J Immunol. 2009;39:965–74. doi: 10.1002/eji.200838990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Dougherty I, Cruz PD, Jr, et al. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J Immunol. 2007a;179:5778–84. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- Chung JS, Sato K, Dougherty II, et al. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007b;109:4320–7. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikano S, Bonkobara M, Zukas PK, et al. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J Biol Chem. 2001;276:8125–34. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- Sinha P, Parker KH, Horn L, et al. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-gamma and IL-4Ralpha. Eur J Immunol. 2012;42:2052–9. doi: 10.1002/eji.201142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer—response. Clin Cancer Res. 2013;19:5542. doi: 10.1158/1078-0432.CCR-13-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–52. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomihari M, Chung JS, Akiyoshi H, et al. DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res. 2010;70:5778–87. doi: 10.1158/0008-5472.CAN-09-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weterman MA, Ajubi N, van Dinter IM, et al. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]


