Abstract
Immune evasion is an important hallmark of cancer, and a better understanding of this mechanism is essential for the development of effective strategies against cancer. The B7 homolog 1 (B7-H1)/programmed cell death 1 (PD-1) pathway has been demonstrated as a major mechanism of immune evasion in tumor site, and its blockade therapy shows very encouraging results in clinical trials. Inducible B7-H1 expression in tumor microenvironment is complex, with multidimensional interactions and expression by different subsets of hematopoietic and nonhematopoietic cells. Understanding these interactions and how tumors take advantage of this pathway can help us design future strategies for better therapeutic efficacy and to overcome resistances.
Keywords: B7-H1 (PD-L1); B7-DC (PD-L2); PD-1, B7-1; immune evasion; tumor microenvironment
During the last decades, immunotherapy of cancer has achieved promising results in terms of specific T cell activation against tumor antigens.1 However, this immune response has not been correlated most of the time with tumor response in clinical trials.2 For instance, anticancer vaccines, including cell-based, DNA-based, and purified component-based preparations, could induce detectable tumor-specific T cell responses in peripheral blood but fails to regress growth of tumors in the majority of patients.3 This discrepancy reflects the failure of T cell activity in tumor sites, most likely because of the immunosuppressive nature of tumor microenvironment, which damper already successful tumor immune response.
It has been clearly shown that tumor microenvironment is composed of dysfunctional immune cells and other stromal host cells that are reprogrammed by active tumor-mediated process to evade immunity. Tumor and stromal cells in tumor site exploit multiple immunoregulatory pathways including production of immunosuppressive cytokines (ie, transforming growth factor β, interleukin 10) and enzymes (indoleamine-2,3-dyoxigenase), down-regulation of tumor cell surface major histocompability complex (MHC), or favoring conversion of immune effector cells to immunosuppressive cells population (ie, regulatory T cells, myeloid-derived suppressor cells).4 Nevertheless, although therapies targeting these mechanisms have demonstrated promising results in animal models, the efficacy in patients has been limitedly demonstrated, indicating that they may not play a central role in immune suppression on tumor sites.
Manipulation of coinhibitory molecules has been shown to enhance T cell responses to various antigens including tumor antigens. 5,6 Antibodies to cytotoxic T lymphocyte (CTL)–associated antigen 4 (CTLA-4), a T cell coinhibitory molecule showing to mainly suppress endogenous autoreactive T cell responses, have been tested clinically.7,8 As expected, in the context of CTLA-4–deficient mice data,9 a broad enhancement of T cell activity has been observed. Nevertheless, strong trend of this agent to induce autoreactivity and autoimmune toxicity has been observed when used alone10 and in combination.11,12 Recent studies indicate that one of the major mechanisms of antitumor effect related with anti–CTLA-4 monoclonal antibodies is based in depletion of T regulatory (Treg) cells.13,14 It has been shown that CTLA-4 expression on Treg cells is critical for its expansion and activity.15 Anti–CTLA-4 treatment increases T effector/Treg cells ratio selectively in tumor site, but in mice lacking FcγRIV anti–CTLA-4 treatment fails to elicit tumor protection.14
The B7-H1/programmed cell death 1 (PD-1) pathway represents a different class of immune-modulatory target. Current data indicate that this pathway works selectively in tumor site with minimal expression in other organs. This pathway mediates inhibitory signals in T cells and antiapoptotic signals in the tumor cells, being characterized as 1 of the major mechanisms of tumor immune escape. The importance of this pathway has been confirmed in clinic with an unprecedented tumor response ratio when this pathway is blocked16–18 (also see articles of Sznol et al, Gettinger et al, and Harshman et al in this series).
The expression and interrelation of B7-H1 and PD-1 in tumor microenvironment are complex and multidimensional. Here we summarize the main aspects in the regulation of this pathway at the tumor microenvironment and its consequence as a mechanism of immune evasion.
B7-H1/PD-1 Pathway Members
B7-H1 is a 290-amino-acid type I transmembrane glycoprotein belonging to B7-CD28 family of the immunoglobulin superfamily. 19 B7-H1 was initially named as the first gene homolog to B7 molecules.19 It was also renamed in murine system as PD-1 ligand 1 (PD-L1) after it has been identified as the first ligand of the receptor PD-1 (CD279).20 A subsequent study, however, showed that B7-H1 interacts with B7-1 (CD80) in addition to the ligand of PD-1.21 Furthermore, not only is B7-H1 a ligand, but it also has receptor function. It has been shown that PD-1 could act as a ligand to transmit antiapoptotic signal to tumor cells via binding to B7-H1.22 Therefore, one should not be misled by the nomenclature to consider B7-H1/PD-L1 is only a ligand for PD-1. The second known counter-receptor of PD-1, called B7-DC or PD-L2, is also a member of the B7 family. More recently, B7- DC/PD-L2 was found to interact with RGMb (repulsive guidance molecule b), and this interaction may involve in the induction of pulmonary tolerance.23
Programmed cell death 1 is a member of the CD28 family and is expressed on activated T cells, B cells, dendritic cells (DCs), and macrophages.51 Ligation of its ligands results in the formation of PD-1/T cell receptor inhibitory microclusters. SHP-2 is recruited to these sites and dephosphorylates multiple members of the T cell receptor signaling pathway, ultimately leading to the suppression of T cell activation.24 Through its ability to limit T cell signaling, the interaction between B7-H1 and PD-1 has been demonstrated of importance in limiting the activity of T cells in peripheral tissues at the time of an inflammatory response.25 The physiology of PD-1/B7-H1 pathway and biochemical signaling has been further reviewed in other numbers of this issue (also see articles of Yao et al and Boussiotis VA et al in this series).
Expression and Induction Mechanism of B7-H1
The expression of mRNA encoding B7-H1 has been found in all normal tissues in humans and mice examined so far. However, constitutive expression and location of B7-H1 protein on cell surface are rare and have been found in only a fraction of tissue macrophages-like cells in the liver, in the lung or tonsil,26 and in immune-privileged sites including the eyes and placenta.27 The discrepancy between the expression of mRNA and cell surface protein of B7-H1 suggests an important role of posttranscriptional mechanism in the control of B7-H1 protein expression. Overall, the B7-H1 expression is regulated by both extrinsic and intrinsic mechanisms.
Extrinsic induction of B7-H1 is largely mediated by proinflammatory cytokines. B7-H1 can be induced in vitro in numerous hematopoietic and nonhematopoietic cells by several inflammatory mediators, of which interferon γ (IFN-γ) is the most potent.26,28 Interferon γ can induce high levels of cell surface B7-H1 expression in normal epithelial cells, vascular endothelial cells, myeloid cells, and naive T cells.29,30 In addition to the regulation of B7-H1 in posttranscriptional level, IFN-γ could also increase transcription of B7-H1 via binding on interferon regulatory factor 1 two sites (200- and 320-base-pair upstream of transcriptional start site) in the promoter of B7-H1.31 Type I IFNs are also shown to stimulate cell surface B7-H1 expression by hepatocytes, monocytes, and DCs.32 Other inflammatory mediators related with B7-H1 expression are vascular endothelial growth factor, granulocyte-macrophage colony-stimulating factor, interleukin 4 (IL-4), and IL-10.25 However, except IFN-γ, physiological function of other inducing agents in the regulation of B7-H1 expression and their role in immune evasion by tumor remain to be elucidated.
Several oncogenic and transcriptional pathways are shown to mediate intrinsic induction of B7-H1 in transcriptional, posttranscriptional control. Loss of PTEN was found to posttranscriptionally correlate with B7-H1 up-regulation in glioblastoma.33 In addition, activity of PI3K and mTOR pathway or epidermal growth factor receptor–mitogen-activated protein kinase pathway is shown to correlate with overexpression of B7-H1 in non–small cell lung cancer and breast cancer.34,35,52 In light of high frequency of mutations in cancer tissues, biochemical signaling pathways may play an important role in aberrant B7-H1 expression on cancer cells.
Selective Expression of B7-H1 in Tumor Site
Our early study shows that the majority of cultured tumor cell lines derived from human or mouse do not constitutively express cell surface B7-H1. In sharp contrast, cell surface B7-H1 was detected by immunohistochemistry analysis in many surgical and biopsy specimens of cancer.30 This finding implicates a mechanism in tumor microenvironment, which is required for the maintenance of B7-H1 expression in cancer tissues. Minimal expression of B7-H1 in normal tissues but inducible expression in tumor site is the most unique feature of this pathway distinguished from other coinhibitory pathways. This selective expression of B7-H1 allows “tumor-targeted” immune modulation and may be responsible for high antitumor efficacy and limited toxicity of blocking antibodies in clinical trials.16–18
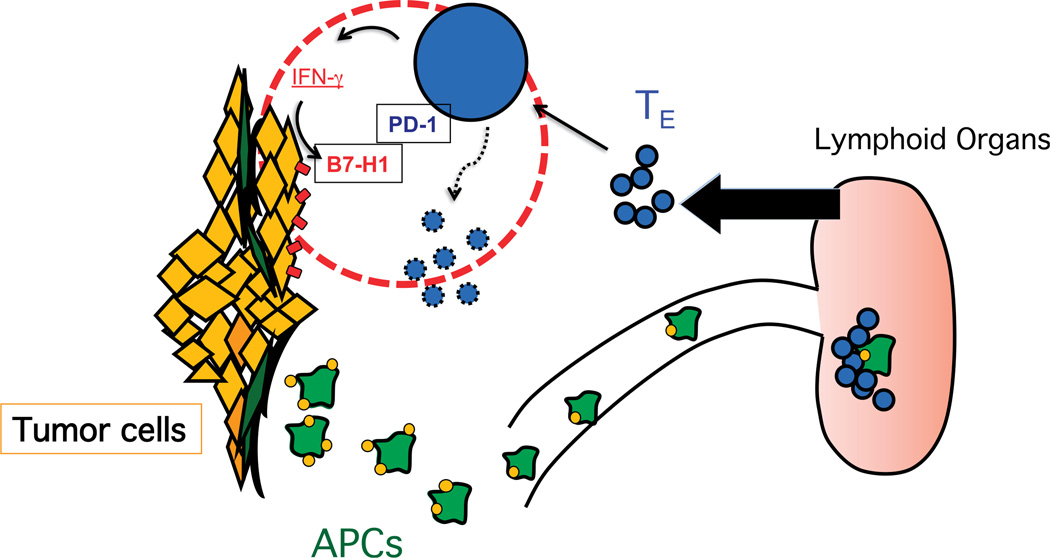
In addition to tumor cells, cell surface B7-H1 protein has been observed in human tumor-associated DCs,28 fibroblasts,36 and T cells.37 It is likely that this expression is also due to T cell-derived IFN-γ as IFN-γ appears to be a universal inducer of B7-H1. Interferon γ is a major cytokine released by T cells after antigen recognition and activation. Primary function of T cell–derived IFN-γ is to further amplify and maintain T cell functions such as up-regulation of MHC molecules, enhancement of antigen processing and presentation by target cells, or promotion of T cell differentiation. Thus, induction of B7-H1 in inflammatory tissues by IFN-γ represents a self-limiting mechanism of immune system, called “adaptive resistance.”30,38 Tumors utilize the natural physiological function of B7-H1 that normally occurs to protect a tissue from an exaggerated immune activation in the context of infection and inflammation in order to protect itself from an anti-tumor immune response. Because this mechanism operates specifically in tumor site and even in the interface of tumor–T cell interaction, this “adaptive resistance” is now believed to play a major role in the resistance of tumor cells to T cell attack in tumor microenvironment38 (Fig. 1).
Figure 1.
Adaptive resistance in tumor microenvironment. Antigen-presenting cells (APCs) capture antigens in tumor site and migrate into the lymphoid organs where antigens are presented to naive T cells. Selectively, antigen-specific T cells are activated, differentiated to T effector (Teff) cells, and expanded. Teff cells migrate to the tumor microenvironment where tumor cells will present their tumor antigens in MHC-I, stimulating IFN-γ production by Teff cells in tumor microenvironment. The presence of IFN-γ will induce in tumor cells and tumor-associated stroma cells the expression of B7-H1 (PD-L1) as an “adaptive resistance” mechanism of immune evasion, which will lead in last term to the immune suppression of T cells. TE, T effector cells.
The B7-H1/PD-1 Pathway in Immune Evasion of Tumor
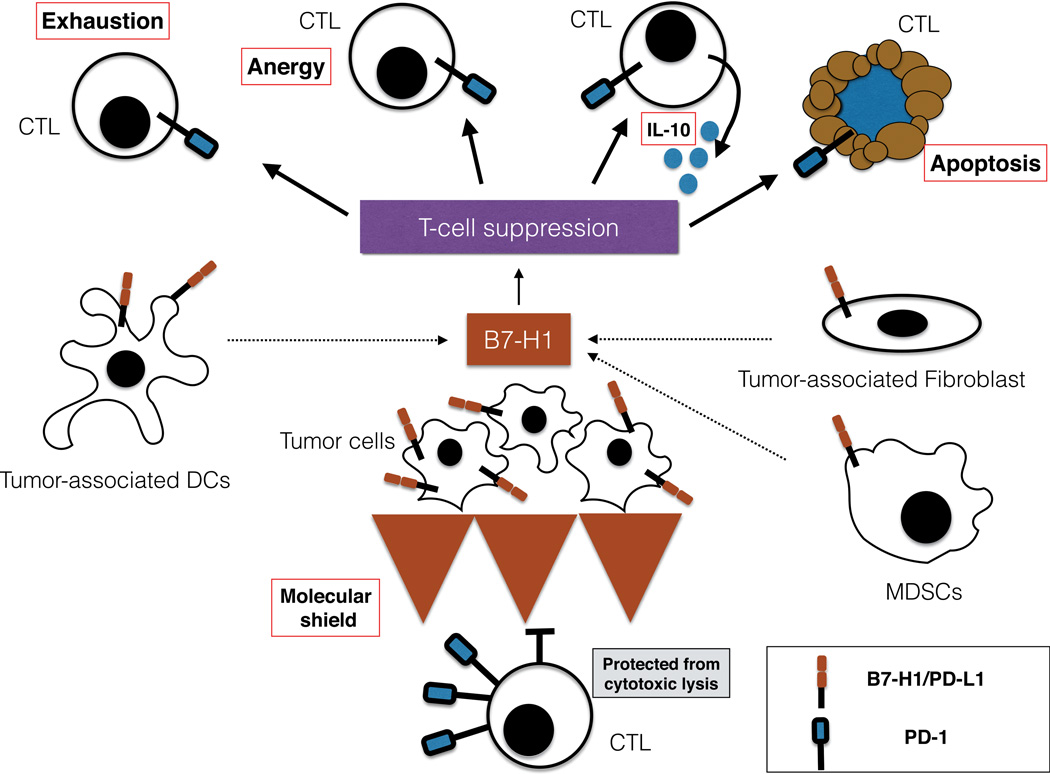
Several distinct mechanisms are identified and characterized that may be responsible for T cell dysfunction upon engagement of the B7-H1 in tumor site and PD-1 on T cells (Fig. 2). Although these mechanisms represent multiple potential possibilities, it is yet to be elucidated whether these mechanisms play roles in the evasion of tumor immunity in vivo, especially in human cancer patients.
Figure 2.
Mechanism of B7-H1/PD-1 pathway immune evasion. B7-H1/PD-1 pathway has been established as a major mechanism in the resistance of tumor to T cells’ attack. It is probable that a combination of mechanism, rather than a single model of action, can explain this resistance. B7-H1–positive tumor and tumor-associated cells might induce T cell apoptosis, anergy, functional exhaustion, or IL-10 production. Inducible B7-H1 expression by tumor cells in contact with CTLs acts as a “molecular shield” protecting tumor cells from cytotoxic lysis. Even though these mechanisms have been demonstrated in animal models, clinical significance in cancer patients remains poorly studied.
Inducing T cell apoptosis: B7-H1 mediates apoptosis in T cells in a physiological manner to maintain homeostasis in peripheral tissues. Control of the number of CD8+ cells in liver39 and CD4+ cells in eye microenvironment40 is a good example of this. Tumor cell–associated B7-H1 increases apoptosis of human tumor-specific T cells in vitro, and this process can be partially inhibited by using B7-H1 blocking monoclonal antibodies. However, at least in 1 human T cell clone, B7-H1 can induce apoptosis of PD-1–negative T cells, suggesting B7-H1 may induce apoptosis via a different receptor. 30 As previously mentioned, B7-H1 can regulate T cell function through binding to B7-1 as well as PD-1, and this pathway could also be involved with induction of T cell apoptosis. 41 Although apoptosis is identified as a potential mechanism of immune evasion, the detailed molecular mechanism of B7-H1–mediated T cell apoptosis remains to be elucidated.
Induction of IL-10: Increased T cell production of IL-10 following activation of purified human T cells with CD3-specific antibody and B7-H1–immunoglobulin fusion protein was initially observed.19 Tumor-associated B7-H1+ DC culture with allogeneic human T cells also produced high level of IL-10. Subsequent studies have demonstrated a correlation between the up-regulation of B7-H1 expression and increased levels of IL-10 in patients with HIV.42 Recently, it has been established that monocytes up-regulate PD-1 during HIV infection, and PD-1/B7-H1 interaction induces IL-10 production in these cells. These results may explain in part the increased levels of IL-10 in HIV patients and suggest a possible mechanism of immunosuppression in these patients.43 Although increased IL-10 production could be induced via B7-H1/PD-1 interaction in different immune subsets, it remains to be determined if IL-10 increase has a major role in B7-H1/PD-1–mediated suppression in tumor microenvironment.
Anergy of T cells: B7-H1 and PD-1 have been demonstrated to be crucial for T cell tolerance and the induction of anergy. 27 The role of B7-H1 in T cell anergy was determined with the use of a cell culture system with alloreactive T cell and monocyte-derived myeloid DCs. Pretreatment of DCs with IL-10 induced unresponsiveness in T cells, although T cells could be rescued by restimulation with DCs treated with a blocking antibody specific for human B7-H1.44 Tumor-associated DCs highly express B7-H1 conferring an environment of immunosuppression to the T cells. However, while the role of B7-H1/PD-1 interaction has been well studied in vitro, its ability to induce anergy of human tumor-specific naive and effector T cells in vivo is still unclear.
Exhaustion of T cells: In chronic infection, a mechanism of T cell exhaustion has been demonstrated to be dependent on the B7-H1/PD-1 pathway.45 Exhausted T cell results from maintaining antigen exposure, which is characteristic of chronic infection or in the tumor microenvironment and is a reversible state. Exhausted T cell expresses high levels of inhibitory molecules including PD-1, LAG-3, or TIM-3. Programmed cell death 1 is up-regulated on a significant fraction of tumor-infiltrating lymphocytes (TILs) in cancer patients, and blockade PD-1 46 or PD-L1 28 restores tumor-specific function, indicating that B7-H1/PD-1 could be mediating human tumor-specific T cell exhaustion.
B7-H1 as an antiapoptotic receptor (“molecular shield”): B7-H1 acts not only as a ligand of PD-1, but can also serve as a receptor transmitting reverse signals that protect cancers cells to apoptosis mediated by FAS-FASL pathway21 and potentially against other types of anticancer agents. In tumor cell lines, in vitro it has been demonstrated that B7-H1 expression confers a resistance to CTL cytolysis in a PD-1–dependent and –independent manner.30,47 B7-H1+ and B7-H1− tumor cells mixed together with antigen-specific CTL in short-term in vitro assays demonstrate preferential lysis of B7-H1− cells, suggesting that resistance to killing is not an impairment in PD-1+ T cells in the first hours.47 Tumors expressing B7-H1 with a truncated intracellular domain also become more susceptible to T cell–mediated immunity in vivo. In contrast, the intracellular truncated domain of PD-1 on T cells does not eliminate the “molecular shield” effect,21 demonstrating that the “molecular shield” is critically dependent of B7-H1 signaling on tumor cells rather than T cell suppression due to PD-1 signaling.
B7-H1 Expression as a Predictive Marker
Expression of B7-H1 on tumor site implicates not only an important mechanistic insight but also clinical perspectives in cancer patients. The first important question is the prognostic value of B7-H1 expression on tumor site. Whereas several large retrospective studies show that B7-H1 expression correlates with poor prognosis, other studies indicate lack of association between B7-H1 expression and prognosis48 or even that B7-H1 expression is associated with improved survival.38,49 These data, however, should be interpreted with caution largely because of technical issues: (i) difficulty to correctly assess cell surface B7-H1 expression in formalin-fixed paraffin-embedded samples due to conformational change38 and lack of specificity of several commercially available antibodies; (ii) requirement to assess B7-H1 protein rather than intracellular protein or mRNA; (iii) heterogeneity of B7-H1 expression in tissue section due to its association with infiltrating lymphocytes.38
On the other hand, B7-H1 expression in the tumor site has been hypothesized that may be associated with response to B7-H1/PD-1 blockade therapy. Preliminary data suggest that B7-H1 expression in tumor biopsy correlates with response to anti–PD-116 and anti–B7-H150 therapies. However, immune response is dynamic, and B7-H1 expression at a single time point may not reflect the whole value of this marker, especially if the tumor biopsy is not close in time to the beginning of the B7-H1/ PD-1 blockade treatment. Because of all these technical details, preliminary data should be interpreted carefully, although further evaluation of B7-H1 as a potential predictive marker is warranted.
B7-H1 Expression and Therapeutic Implications
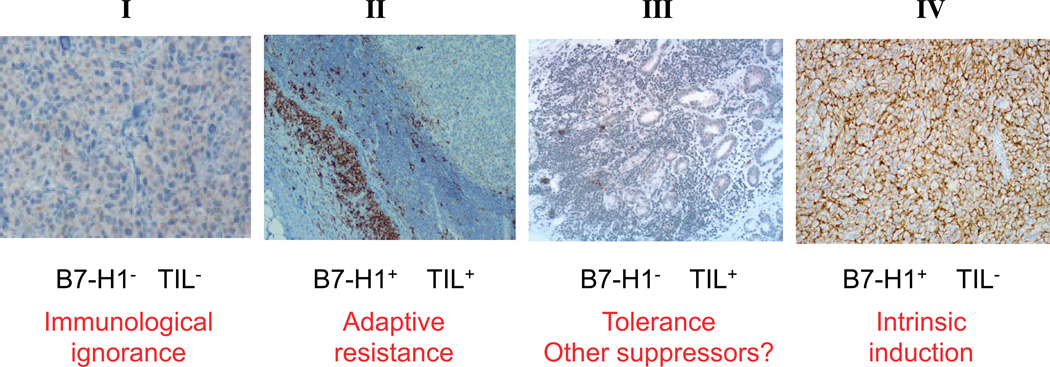
Based on B7-H1 expression and the presence of TILs in the tumor biopsies of melanoma patients, 4 different groups have been identified: (I) TIL(−)B7-H1(−); (II) TIL(+)B7-H1(+); (III) TIL(+)B7-H1(−); (IV) TIL(−)B7-H1(+)38 (Fig. 3). Group I (TIL −B7-H1−) could explain lack of response in most of patients treated with anti–B7-H1 or anti–PD-1 monoclonal antibodies, because the TILs are not in the tumor microenvironment. In accordance with the cause of the lack of TILs, one or other strategy should be developed to overcome this potential mechanism of primary resistance to anti–B7-H1/PD-1 pathway. Group II (TIL+B7- H1+) seems to be the group that is ready to receive anti–B7-H1/ PD-1 treatment and where it would expect the best responses. Following the previous ideas, group III (TIL+B7-H1) should represent a scenario where TILs are present but show lack of IFN-γ production. If B7-H1 is not expressed, anti–B7-H1 will not be effective, and other strategies according to the mechanism of T cell suppression should be developed. The last group (TIL–B7-H1+) has been related with intrinsic induction of B7-H1 by oncogenic pathways in absence of IFN-γ. These findings should have direct implications for future design of combination therapy.
Figure 3.
B7-H1 expression and TILs. Four different melanoma groups with potential implication for mechanism and therapy have been identified according to B7-H1 expression and the presence of TILs inmelanoma biopsies: I, B7-H1–negative tumors without TILs, considered as a immunological ignorance because immune cells do not go to the tumor site; II, B7-H1–positive tumors with TILs, considered as a paradigm of adaptive resistance of tumor mediated by B7-H1/PD-1 pathway; III, B7-H1–negative tumors with TILs, considered as a situation of tolerance because TILs are present, but they are not inducing B7-H1 expression in tumor microenvironment through IFN-γ production; and IV, B7-H1–positive tumors without TILs, considered as a scenario of intrinsic induction of B7-H1 expression in tumor cells. TILs, tumor infiltrating lymphocytes. Adapted from Szol and Chen,25 Copyright ©2013, with permission from the American Association for Cancer Research.
CONCLUSIONS
Understanding of underlying immune evasion mechanism by the B7-H1/PD-1 pathway gives us the clues to develop effective antitumor treatments and a rationale to overcome primary and eventually secondary resistance to anti–B7-H1 or anti–PD-1 treatment. Many important questions remain to be clarified about the mechanism of action of this molecular pathway such as the effect of PD-1 blockade versus B7-H1 blockade or the role of PD-1 and B7-H1 on a different subset of immune cells present in the tumor bed. All in all, discovery of the B7-H1/PD-1 pathway, its role in the evasion of tumor immunity and development of therapeutic antibody has become a great story of “from the bench to bedside” with a high impact in clinical oncology.
Acknowledgments
Source of Funding: This work was partially supported by Sociedad Española de Oncología Médica to M.F.S.; National Institutes of Health grants CA121974, CA142779, and CA16359; and the United Technologies Corporation professorship to L.C.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
- 1.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Slingluff CL. The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011;17:343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaddanapudi K, Mitchell RA, Eaton JW. Cancer vaccines: looking to the future. Oncoimmunology. 2013;2:e23403. doi: 10.4161/onci.23403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 5.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu J, Malm I-J, Kadayakkara DK, et al. Preclinical evidence that PD-1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. [published online ahead of print May 8, 2014] Cancer Res. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 10.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 11.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 14.Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) inmelanoma. N Engl JMed. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 20.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Y, Yu S, Zhu B, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211:943–959. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer—response. Clin Cancer Res. 2013;19:5542. doi: 10.1158/1078-0432.CCR-13-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 27.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 29.Mazanet MM, Hughes CCW. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 30.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 31.Lee S-J, Jang B-C, Lee S-W, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 32.Kryczek I, Wei S, Gong W, et al. Cutting edge: IFN-gamma enables APC to promote memory TH17 and abate TH1 cell development. J Immunol. 2008;181:5842–5846. doi: 10.4049/jimmunol.181.9.5842. [DOI] [PubMed] [Google Scholar]
- 33.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 34.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang C-Y, Lin M-W, Chang Y-L, Wu C-T, Yang P-C. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361–1369. doi: 10.1016/j.ejca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Nazareth MR, Broderick L, Simpson-Abelson MR, et al. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007;178:5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 37.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127–137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H, Zhu G, Tamada K, et al. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 40.Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 41.Park J-J, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trabattoni D, Saresella M, Biasin M, et al. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101:2514–2520. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 43.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selenko-Gebauer N, Majdic O, Szekeres A, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 45.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 46.Wong RM, Scotland RR, Lau RL, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 47.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 48.Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 49.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbst RS, Gordon MS, Fine GD, et al. A study of {MPDL3280A}, an engineered {PD-L1} antibody in patients with locally advanced or metastatic tumors. J Clin Oncol. 2013;31 (suppl; abstract 3000) [Google Scholar]
- 51.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]