Abstract
Context
Maternal pregestational diabetes (PGDM) is a risk factor for development of congenital heart defects (CHDs). Glycemic control before pregnancy reduces the risk of CHDs. A meta-analysis was used to estimate summary ORs and mathematical modeling was used to estimate population attributable fractions (PAFs) and the annual number of CHDs in the U.S. potentially preventable by establishing glycemic control before pregnancy.
Evidence acquisition
A systematic search of the literature through December 2012 was conducted in 2012 and 2013. Case–control or cohort studies were included. Data were abstracted from 12 studies for a meta-analysis of all CHDs.
Evidence synthesis
Summary estimates of the association between PGDM and CHDs and 95% credible intervals (95% CrIs) were developed using Bayesian random-effects meta-analyses for all CHDs and specific CHD subtypes. Posterior estimates of this association were combined with estimates of CHD prevalence to produce estimates of PAFs and annual prevented cases. Ninety-five percent uncertainty intervals (95% UIs) for estimates of the annual number of preventable cases were developed using Monte Carlo simulation. Analyses were conducted in 2013. The summary OR estimate for the association between PGDM and CHDs was 3.8 (95% CrI=3.0, 4.9). Approximately 2670 (95% UI=1795, 3795) cases of CHDs could potentially be prevented annually if all women in the U.S. with PGDM achieved glycemic control before pregnancy.
Conclusions
Estimates from this analysis suggest that preconception care of women with PGDM could have a measureable impact by reducing the number of infants born with CHDs.
Introduction
Congenital heart defects (CHDs) collectively are the most common birth defect, affecting approximately 80 per 10,000 births annually in the U.S.1 CHDs account for significant infant morbidity and mortality, causing approximately 2.4 deaths per 10,000 live births in U.S. each year.2,3 Women with diabetes before pregnancy, that is, pregestational diabetes mellitus (PGDM), have 2–5 times the risk of having a CHD-affected pregnancy compared to women without diabetes4–7; however, the magnitude of the association varies among studies and by CHD phenotype.5,6,8–10 The major teratogen in diabetic pregnancies is presumed to be hyperglycemia caused by poor glycemic control during organogenesis.11 Potential mechanisms for hyperglycemia-induced birth defects include decreased levels of inositol, arachidonic acid metabolic disturbances, increased oxidative stress, and alterations in gene expression.12–14 Some studies suggest that the excess risk can be eliminated with optimal diabetes management prior to and during early pregnancy.8,13,15–17
Results from a previous meta-analysis indicated an increased risk of specific CHDs in pregnancies affected by type 1 diabetes mellitus, but studies included were limited to those published before 2002.4 Other meta-analyses have suggested that hyperglycemia increases adverse effects in pregnancies affected by type 1 and type 2 diabetes,18 and that preconception care can be effective in establishing glycemic control prior to pregnancy and improving maternal and fetal outcomes, including reducing the risk of congenital anomalies.19–21 Establishing glycemic control prior to and early in pregnancy may decrease the risk of having a CHD-affected pregnancy, possibly to the same level as pregnancies not affected by diabetes.8,13,15–17 However, only 40%–60% of women with PGDM are estimated to achieve glycemic control prior to pregnancy.22,23 Given the effectiveness of diabetes management before and early in pregnancy, it may be possible to reduce the annual number of cases of CHD occurring as a consequence of PGDM.
The prevalence of type 1 and type 2 diabetes has increased in the U.S. among men and women of all ages,24,25 including reproductive-aged women and women who become pregnant. The majority of this increase is likely due to increased prevalence of type 2 diabetes.26,27 Among women at risk of becoming pregnant, diabetes prevalence estimates vary from 1.9% to 4.0%,24,26 with prevalence varying by race, age, and SES. An additional 0.5%–1% of reproductive-aged women with diabetes have not been diagnosed and are not being clinically managed.24,28,29
The objectives of this study were to conduct a systematic review and meta-analysis to estimate the magnitude of the association between PGDM and CHDs (all CHDs and specific subtypes), and to use these results to estimate population attributable fractions (PAFs) and the annual number of CHDs that could potentially be prevented in the U.S. with established glycemic control in women with PGDM prior to and early in pregnancy.
Evidence Acquisition
Systematic Review
CDC librarians conducted literature searches of Medline, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Population Information Online (POPLINE) in April 2012 to obtain published estimates of the associations of PGDM with all CHDs and specific CHDs. This search was repeated in March 2013 to include all publications through December 2012. Each database was searched for studies published from database inception through December 2012. The search strategy used keywords that combined diabetes, CHDs, and pregnancy (Appendix, available online).
Papers were considered for inclusion if they were in English, included women with PGDM, included a comparison group of women without a diagnosis of PGDM, contained one or multiple CHDs as an outcome, were case–control or cohort studies, and were retrospective or prospective. Papers were excluded if they did not include CHDs (n=151), were not original research studies (e.g., editorials, conference abstracts; n=325), had estimates of diabetes exposure limited to or including gestational diabetes without the capacity to separate PGDM (n=29), did not include PGDM as an exposure (women with PGDM were excluded or papers did not include a measure of PGDM; n=27), did not have an unexposed comparison group (n=222), did not exclude chromosomal and genetic defects from estimates of CHDs (n=7), or did not include a study sample that was a representative population (e.g., only women aged older than 40 years, only women at increased risk of having an infant with a CHD [e.g., because of family history, previous infant with CHD], only women with parity greater than six, only women experiencing pregnancy complications and in which the CHD was a secondary outcome; n=11).
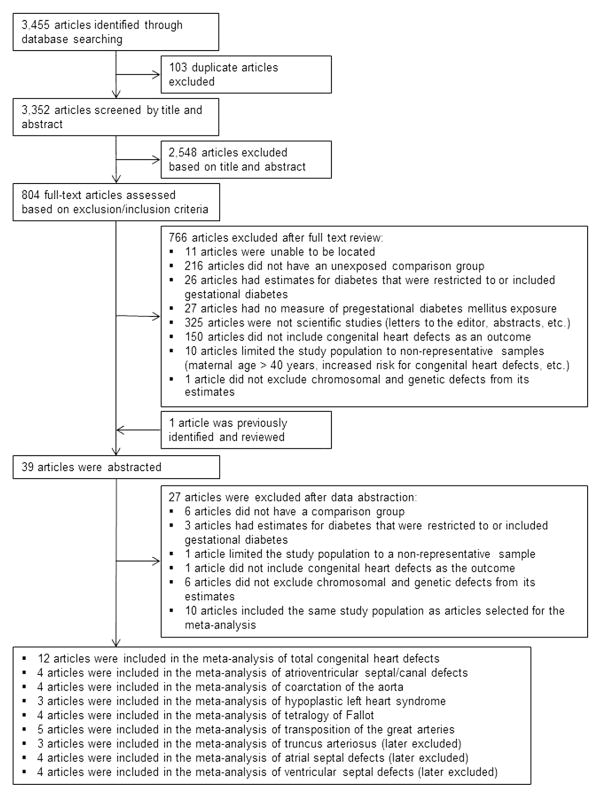
The search yielded 3,455 references, with 103 articles identified as duplicates and excluded. Two co-authors (J.A.M., R.M.S.) independently screened each title and abstract. Based on title and abstract, 2,548 references were excluded, leaving 804 references for full-text review. Full texts were reviewed independently by the same co-authors (J.A.M., R.M.S.). Reference lists of full-text articles were searched for additional references. A third co-author (S.M.G.) resolved any decisions regarding whether an article’s data should be abstracted (n=23). After review, 766 articles were excluded and 39 articles were abstracted (including one that had been previously identified) (Figure 1).
Figure 1.
Systematic review. Literature review and exclusions for the association between pregestational diabetes and congenital heart defects for systematic review and meta-analysis, publications through December 2012.
Using a standard abstraction form created in Microsoft Word (Redmond WA), two co-authors (J.A.M., R.M.S.) independently abstracted data from each article, including information on the type of study (case–control or cohort), method of data collection (retrospective or prospective), study population, population size, type of comparison group, type of diabetes (type 1, type 2, or not specified), type of CHD, type of results available (crude or adjusted), and counts of exposed/unexposed CHD cases. Crude data were abstracted from each article and unadjusted ORs or risk ratios (RRs) and 95% CIs were calculated as effect estimates for each study. Data were entered into a Microsoft Excel spreadsheet and discrepancies were identified and resolved by J.A.M. and R.M.S. Different subsets of articles were used for analyses of total CHDs and individual CHDs. Estimates from overlapping study populations, CHDs with fewer than three published effect estimates for the association with PGDM, and CHDs with at least three published effect estimates, but in which the effect estimates were too heterogeneous to obtain stable summary estimates in the meta-analysis were excluded. For studies with overlapping study populations, only the largest or most recent study was included in the analysis (Figure 1). Owing to the limited number of studies with effect estimates for CHDs, no formal analysis of study bias was conducted. However, exclusion criteria were intended to minimize bias in study design and ensure that well-designed, population-based studies were included in the meta-analysis. After exclusions, 12 studies contributed to the meta-analysis for all CHDs,5,6,8,10,30 –37 and three to five studies contributed data to the meta-analysis for the five individual CHDs assessed (Appendix Table 1, available online; Figure 1).5,6,8–10,38
Measures of the association with PGDM were available for eight individual CHDs from at least three studies; meta-analyses were conducted for five of these defects: atrioventricular septal defect (AVSD), coarctation of the aorta, hypoplastic left heart syndrome (HLHS), tetralogy of Fallot (ToF), and transposition of the great arteries (TGA). Unadjusted ORs or RRs were estimated for each study and used for the meta-analyses. Meta-analyses were not conducted for ventricular septal defects, atrial septal defects, and truncus arteriosus because of the small number of studies focused on these outcomes or the extensive heterogeneity of the effect estimates among the reported results, which may suggest systematic ascertainment bias among the different studies (Figure 1).
Prevalence of Selected Congenital Heart Defects and Annual Number of Expected Outcomes
National prevalence estimates for TGA, ToF, HLHS, and AVSD were obtained from a publication of 2004–2006 data from pooled state birth defect surveillance programs (Table 1).39 Because prevalence estimates of total CHDs and coarctation of the aorta were not available from this publication, estimates for total CHDs and coarctation of the aorta were estimated from an evaluation from the Metropolitan Atlanta Congenital Defects Program (MACDP) and were assumed to be nationally representative (Table 1).1 Race-specific prevalence estimates were also obtained from a recent evaluation from the MACDP.40 SEs for prevalence estimates of each CHD were estimated by assuming that the reported prevalence values were samples from a normal distribution. The annual number of U.S. cases were estimated by multiplying estimated prevalence by the number of U.S. live births in 2011 (n=3,953,590), the most recent year for which final birth data were available (Table 1).41 Race-specific number of cases were estimated by multiplying race-specific estimated prevalence by number of U.S. live births in 2011 (non-Hispanic white, n=2,146,566; non-Hispanic black, n=582,345; and Hispanic, n=918,129).41
Table 1.
Birth Prevalence and Estimated Annual Number of U.S. Cases for Selected Congenital Heart Defect Outcomes
| Defect | Prevalence per 10,000 live births | 95% CI for prevalence | Estimated annual number of U.S. cases | 95% CI for annual number of U.S. cases |
|---|---|---|---|---|
| Total congenital heart defectsa | 81.4 | 78.6, 84.2 | 32,182 | 31,075, 33,301 |
| Non-Hispanic whiteb | 97.3 | 91.5, 103.0 | 20,878 | 19,642, 22,114 |
| Non-Hispanic blackb | 79.8 | 74.6, 84.9 | 4,645 | 4,347, 4,943 |
| Hispanicb | 91.5 | 83.8, 99.3 | 8,402 | 7,682, 9,113 |
| Atrioventricular septal defectsc | 4.7 | 4.6, 5.0 | 1,858 | 1,759, 1,961 |
| Non-Hispanic whiteb | 4.1 | 2.9, 5.3 | 884 | 629, 1,140 |
| Non-Hispanic blackb | 5.1 | 3.8, 6.4 | 296 | 221, 372 |
| Hispanicb | 2.1 | 0.9, 3.2 | 190 | 82, 297 |
| Coarctation of the aortaa | 4.5 | 2.9, 4.9 | 1,767 | 1,147, 1,937 |
| Non-Hispanic whiteb | 5.5 | 4.1, 6.8 | 1,173 | 878, 1,467 |
| Non-Hispanic blackb | 4.0 | 2.8, 5.1 | 231 | 164, 298 |
| Hispanicb | 3.6 | 2.1, 5.2 | 332 | 190, 474 |
| Hypoplastic left heart syndromec | 2.3 | 2.2, 2.5 | 909 | 850, 969 |
| Non-Hispanic whiteb | 2.0 | 1.1, 2.8 | 423 | 246, 600 |
| Non-Hispanic blackb | 2.5 | 1.6, 3.4 | 146 | 93, 199 |
| Hispanicb | 1.9 | 0.8, 3.0 | 174 | 71, 276 |
| Tetralogy of Fallotc | 4.0 | 3.8, 4.2 | 1,570 | 1,491, 1,649 |
| Non-Hispanic whiteb | 4.6 | 3.3, 5.8 | 980 | 711, 1,249 |
| Non-Hispanic blackb | 5.5 | 4.2, 6.9 | 321 | 243, 400 |
| Hispanicb | 3.4 | 1.9, 4.9 | 316 | 177, 454 |
| Transposition of the great arteriesc | 3.0 | 2.8, 3.2 | 1,186 | 1,119, 1,253 |
| Non-Hispanic whiteb | 2.2 | 1.4, 3.1 | 481 | 292, 669 |
| Non-Hispanic blackb | 2.3 | 1.5, 3.2 | 136 | 84, 187 |
| Hispanicb | 2.6 | 1.3, 3.9 | 237 | 117, 357 |
Evidence Synthesis
Meta-Analysis
A Bayesian approach, with study-level random effects to account for inter-study heterogeneity, was used to summarize data on the association between PGDM and CHDs. Posterior estimates were developed using Markov Chain Monte Carlo methods and summarized using posterior medians and equal-tailed 95% credible intervals (95% CrIs).42,43 Models were not adjusted for maternal characteristics such as age, parity, or race. Details on the models and the estimation process used in the meta-analysis are given in the Appendix (available online). The meta-analysis was conducted using WinBUGS, version 1.4.3.
Sensitivity Analyses
A series of alternative meta-analysis models were evaluated to assess the sensitivity of results to underlying assumptions. For example, posterior estimates of the OR relating PGDM to all CHDs were estimated using alternative assumptions for the prior distributions of both the true OR and the study-level random effects. In addition, separate analyses were conducted to examine the potential impact of study design, cohort versus case–control, on the posterior OR estimates. Separate analyses were also conducted to examine the impact of restricting the meta-analysis only to studies that included both type 1 and type 2 diabetes. Because the studies selected for the meta-analysis spanned a wide range of years, a supplemental meta-analysis, limited to those studies published in 2000–2012, was also conducted. Details on the sensitivity assessment for the meta-analysis portion of the analyses are provided in the Appendix (available online); all sensitivity analyses were conducted in 2013.
Prevalence of Pregestational Diabetes
Prevalence estimates of measured or self-reported PGDM are not available for large, population-based studies of women who had a pregnancy with or without a CHD. Therefore, overall and race-specific estimates of diabetes among women aged 20–44 years were obtained from Health Data Interactive software provided by the National Center for Health Statistics and used as a proxy for PGDM.28 Health Data Interactive utilizes data from the National Health and Nutrition Examination Survey and includes women, regardless of pregnancy status, surveyed in 2007–2010. Estimates include diagnosed and undiagnosed diabetes; diagnosed diabetes was defined as diabetes diagnosed by a doctor/health professional outside of pregnancy, and undiagnosed diabetes was defined as having fasting blood glucose ≥126 mg/dL or hemoglobin A1c ≥6.5%, but no diabetes diagnosis.44 Among all women, 3.2% (95% CI=2.5%, 4.0%) were estimated to have diabetes; by race, 2.7% (95% CI=1.8%, 4.1%) of non-Hispanic white, 4.6% (95% CI=3.3%, 6.4%) of non-Hispanic black, and 3.7% (95% CI=2.2%, 6.2%) of Hispanic women were estimated to have diabetes.28
Population Attributable Fraction and Estimated Preventable Number of Congenital Heart Defects with Elimination of Risk Associated with Pregestational Diabetes
The PAF for the number of CHDs due to PGDM was estimated using the model45:
where P[D] is the prevalence of diabetes among women at risk for pregnancy and OR is the estimate of the true OR relating PGDM and the CHD of interest developed in the meta-analysis.45 This model is adapted from the traditional estimate of the PAF using RRs and is used when the OR approximates the RR (when the disease is rare, as is the case with birth defects, and controls have been sampled from the nondiseased population). Race-specific diabetes prevalence was used to obtain race-specific PAFs. This model assumes no confounding of the diabetes–CHD association and that estimated effect estimates were not adjusted for potential confounders. The model did not control for maternal age, parity, or other maternal factors. The estimate for PAF was multiplied by the estimated race-specific annual number of births with CHD of various types to obtain the annual number of births attributable to PGDM. A key assumption in the approach to estimating PAFs and the attributable number of cases is that an intervention results in optimal glycemic control prior to pregnancy among all women with PGDM and reduces their CHD risk to that of nondiabetic women. Because complete reduction of the risk of PGDM-attributable CHD might be unrealistic, the impact assuming a 50% reduction in risk was also estimated. The same approach was used to produce race-specific and overall estimates of PAFs and the number of PGDM-attributable CHD cases. Although race-specific estimates of diabetes prevalence and the number of live births were used to develop the race-specific effects, a common estimate of the OR across race categories was used.
A Monte Carlo simulation was used to combine lack of knowledge concerning the true PGDM and CHD OR, as estimated by the posterior distribution for the parameter produced in the meta-analysis, with additional sources of uncertainty associated with the other inputs used to model PAF and the number of live births affected by CHD. The Monte Carlo process was used to propagate these various uncertainties through to the modeled estimates of PAF and number of prevented cases (Appendix, available online).46,47 The Monte Carlo simulations were implemented using SAS, version 9.3. All estimates of preventable numbers were rounded to the nearest multiple of five to avoid overstating the precision of these estimates.
For all CHDs, ORs and RRs from individual studies that were used in the meta-analysis of the association with PGDM ranged from estimates of 1.3 to 8.4. More variation was observed for individual CHDs, with ORs ranging from 1.3 for coarctation of the aorta to 13.3 for AVSD (Appendix Table 1, available online). The estimated number of affected U.S. births per year for all CHDs was 32,182 (95% CI=31,075, 33,301). Of the individual CHDs included in the meta-analyses, the estimated annual number of U.S. births ranged from 909 for HLHS to 1862 for AVSD (Table 1).
The summary OR for PGDM and all CHDs estimated in the meta-analysis was 3.8 (95% CrI=3.0, 4.9), based on 12 studies (Table 2). PGDM and AVSD had the largest summary OR (10.6), and PGDM and coarctation of the aorta had the smallest summary OR (3.7). The PAF for PGDM and total CHDs in the population was 8.3% (95% uncertainty interval [UI]=5.6%, 11.8%). Accounting for differences in the prevalence of PGDM by race, the PAF for PGDM and total CHDs ranged from 7.1% for non-Hispanic white to 11.5% for non-Hispanic black women (Table 2). Among women of all races, the PAF for PGDM with individual CHDs ranged from 7.9% for coarctation of the aorta to 23.4% for AVSD. In the U.S., for all CHDs considered in the analysis, reducing the risk for women with PGDM to that of women without PGDM could potentially reduce the annual number of CHD cases by 2670 (95% UI=1795, 3795) (Table 2); by CHD subtype, it could potentially prevent 435 cases of AVSD, 140 cases of coarctation of the aorta, 75 cases of HLHS, 230 cases of ToF, and 130 cases of TGA. Assuming a 50% reduction in the risk of PGDM-associated CHDs, approximately 1,335 (95% UI=295, 485) PGDM-associated CHD cases could be prevented annually.
Table 2.
Summary OR, Population Attributable Fraction, and Preventable Cases of Congenital Heart Defects
| Congenital heart defect | Summary OR (95% CrI)b | Attributable fraction (%) (95% UI)c | Annual preventable number of CHD (95% UI)a | |
|---|---|---|---|---|
| 100% Elimination of risk associated with PGDM | 50% Reduction in risk associated with PGDMd | |||
| All congenital heart defects | 3.8 (3.0, 4.9) | 8.3 (5.6, 11.8) | 2,670 (1,795, 3,795) | 1,335 (900, 1,900) |
| Non-Hispanic white | 7.1 (4.2, 11.4) | 1,480 (880, 2,385) | 740 (440, 1,195) | |
| Non-Hispanic black | 11.5 (7.4, 17.1) | 535 (340, 800) | 270 (170, 400) | |
| Hispanic | 9.5 (5.3, 16.2) | 795 (440, 1,370) | 400 (220, 685) | |
| Atrioventricular septal defects | 10.6 (4.7, 20.9) | 23.4 (10.6, 40.0) | 435 (195, 745) | 220 (100, 375) |
| Non-Hispanic white | 20.4 (8.5, 38.1) | 180 (70, 355) | 90 (35, 180) | |
| Non-Hispanic black | 30.6 (14.1, 49.8) | 90 (40, 155) | 45 (20, 80) | |
| Hispanic | 26.1 (11.1, 46.5) | 50 (15, 105) | 25 (20, 80) | |
| Coarctation of the aorta | 3.7 (1.7, 7.4) | 7.9 (2.1, 17.6) | 140 (35, 315) | 70 (20, 160) |
| Non-Hispanic white | 6.7 (1.8, 15.8) | 80 (20, 195) | 40 (10, 100) | |
| Non-Hispanic black | 11.0 (2.9, 24.1) | 25 (5, 60) | 15 (5, 30) | |
| Hispanic | 8.9 (2.4, 21.8) | 30 (5, 75) | 15 (5, 40) | |
| Hypoplastic left heart syndrome | 3.7 (1.5, 8.9) | 8.0 (1.6, 20.4) | 75 (15, 185) | 40 (10, 95) |
| Non-Hispanic white | 6.9 (1.3, 18.8) | 30 (55, 85) | 15 (5, 45) | |
| Non-Hispanic black | 11.2 (2.2, 27.5) | 15 (5, 45) | 10 (5, 25) | |
| Hispanic | 9.1 (1.7, 24.6) | 15 (0, 50) | 10 (0, 25) | |
| Tetralogy of Fallot | 6.5 (3.3, 11.8) | 14.8 (6.6, 26.3) | 230 (105, 415) | 115 (55, 210) |
| Non-Hispanic white | 12.8 (5.4, 24.4) | 125 (50, 250) | 60 (25, 125) | |
| Non-Hispanic black | 20.0 (8.9, 34.7) | 65 (30, 115) | 35 (15, 60) | |
| Hispanic | 16.7 (7.0, 32.0) | 50 (20, 115) | 25 (10, 60) | |
| Transposition of the great arteries | 4.8 (2.7, 8.3) | 10.9 (5.1, 19.8) | 130 (60, 235) | 65 (30, 120) |
| Non-Hispanic white | 9.4 (4.0, 18.6) | 45 (15, 100) | 25 (10, 50) | |
| Non-Hispanic black | 15.0 (6.8, 27.0) | 20 (10, 40) | 10 (5, 20) | |
| Hispanic | 12.4 (5.2, 24.9) | 30 (10, 65) | 15 (5, 35) | |
All estimates and uncertainty intervals rounded to nearest multiple of five.
Posterior estimates for the summary OR estimated using Bayesian meta-analyses.
Attributable fraction estimated based on race-specific estimates of the prevalence of diabetes. Uncertainty intervals estimated via Monte Carlo sampling
Estimated by multiplying the attributable fraction by half of the estimated prevalence.
CHD, congenital heart defect; CrI, credible interval; PGDM, pregestational diabetes mellitus; UI, uncertainty interval.
In some cases, posterior estimates have been shown to be sensitive to the form of the assumed prior distribution for the OR even when that prior appears to be non-informative. To assess this possibility, the estimated posterior distributions derived under a vague normal prior for the log OR, the primary result, were compared to those derived under a noninformative uniform prior for the log of the OR and a similarly noninformative uniform prior on the untransformed OR. In both cases, the selection of alternative forms for the prior on the OR had negligible effects on the posterior estimates. In addition, no difference was observed in meta-analyses results when studies were stratified by design type, that is, cohort versus case–control. Nor were meaningful differences in the meta-analyses results observed when the analyses were restricted to include only studies based on populations with a mixture of both type 1 and type 2 diabetes. OR estimates based on a random-effects meta-analysis in which estimates were developed using approximate maximum likelihood methods were virtually identical to those produced using the Bayesian approach (Appendix Figure 1, available online). Limiting studies to those published between 2000 and 2012 led to an estimated median posterior OR of 3.6 (95% CrI=2.6, 4.8), which is consistent with the estimate based on all studies of 3.8 (95% CrI=3.0, 4.9).
Conclusions
Interventions identifying and increasing the number of women with PGDM who achieve glycemic control prior to pregnancy could substantially reduce the number of CHD-affected infants each year. Assuming complete elimination of CHD risk due to PGDM, about 2670 cases of CHDs may be prevented annually in the U.S. Among some specific CHDs, 435 cases of AVSD, 140 cases of coarctation of the aorta, 75 cases of HLHS, and 230 cases of ToF could potentially be prevented in the U.S. annually.
The prevalence of diabetes increased significantly among young adults (aged 20–34 years) from 1988–1994 to 2005–2010.48 Projections of diabetes incidence among those aged younger than 20 years indicates that the number with type 1 and type 2 diabetes will triple and quadruple by 2050, respectively.49 A greater burden of disease at younger ages will place more women at risk prior to pregnancy. Presence of PGDM and hyperglycemia has important maternal and fetal health consequences beyond the development of CHDs. Compared to women without PGDM, women with PGDM are at increased risk of having infants affected by noncardiac birth defects,7,50 macrosomia,51 preterm birth,51,52 spontaneous abortion,21 and perinatal mortality.51,53
If all reproductive-aged women with PGDM planned their pregnancies, sought preconception care, and achieved glycemic control prior to and throughout pregnancy, they could reduce the risk of CHDs as well as other adverse maternal and fetal outcomes. However, there are challenges to achieving this reduction. First, about 0.5%–1% of all women of reproductive age in the U.S. (310,000–625,000 women) have undiagnosed diabetes.24,29,54 These women are not receiving diabetes management prior to a potential pregnancy and are likely not under glycemic control. Lack of glycemic control will cause these women to remain at increased risk for congenital anomalies and other adverse maternal and fetal outcomes if they become pregnant. Second, despite recommendations that women with diabetes plan their pregnancies and receive preconception care,55,56 fewer than half of women with diabetes who become pregnant plan their pregnancies, and women with diabetes may have poorer contraceptive practices than women without diabetes.23,57–59 Furthermore, although achieving glycemic control is possible for some women, not all women will achieve glycemic control prior to pregnancy, even with preconception care.21,60 Generally, only 40%–60% of women with diagnosed PGDM achieve glycemic control prior to and early in pregnancy.22,23 Improved screening and diagnosis of diabetes among women who are overweight with additional risk factors for diabetes (e.g., physical inactivity or first-degree relative with diabetes),61 coupled with improved diabetes management and access to care both at preconception and throughout pregnancy may prevent numerous adverse outcomes associated with PGDM.13,19,21,60
The analysis was subject to several limitations. First, the Bayesian meta-analyses for individual CHDs were based on limited data and risk of bias was not formally assessed owing to the limited number of studies. However, sensitivity analyses indicated that results were robust to prior assumptions on the true values of the PGDM–CHD association and on the level of heterogeneity among studies (Appendix, available online). Second, hyperglycemia due to PGDM was assumed to be the only cause of PGDM-related CHDs and achievement of glycemic control prior to pregnancy was assumed to eliminate the PGDM–CHD risk. The true biological mechanism of the PGDM–CHD association is unknown and could arise from multiple factors.13 Third, because of limited data, risk of having a CHD-affected pregnancy was assumed to be the same regardless of the presence of type 1 or type 2 diabetes.62 However, type 1 and type 2 diabetes may differentially impact the risk of CHDs. Currently, women with type 2 PGDM tend to be older during pregnancy, more socially disadvantaged, overweight or obese, and report later entry into prenatal care compared to women with type 1 PGDM.20 In addition, as the studies contributing data to the meta-analysis spanned a wide range of years, differences in the presentation of diabetes could have existed; however, a supplemental analysis indicated that the summary OR was not impacted by the changing prevalence of type 1 and type 2 diabetes in the population. Fourth, the present models assumed no confounding by maternal factors, such as age and parity; however, confounding by maternal age and parity was not expected.63,64 These limitations could have biased the estimate of the true PAFs and thus overstated or understated the impact of achieving glycemic control prior to pregnancy.
Despite these limitations, the analysis benefited from strict inclusion criteria and abstraction of raw data from the published literature. Uncertainty and heterogeneity between studies was accounted for using a Bayesian procedure. Additionally, sensitivity analyses examined the assumptions that type 1 and type 2 PGDM impacted risk of CHDs in the same way, and that studies spanning all years could be used to examine the PGDM–CHD association despite changes in presentation, treatment, and management of PGDM. These sensitivity analyses suggested that the estimates are robust and stable.
Findings from this meta-analysis and modeling project suggest that effective preconception care for women with PGDM could reduce the number of cases of CHD in the U.S. each year. In addition, considerable reductions in the number of individual, serious CHDs such as ToF and HLHS could be achieved. These results underscore the need for improved screening for PGDM, preconception care, and pregnancy planning to ensure that women with PGDM are under proper care for optimal pregnancy outcomes.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge librarians, Christy Cechman, MLIS, of the American Public University System, and Gail Bang, MLIS, of the CDC Public Health Library and Information Center, who conducted the literature searches; Jaynia Anderson, MPH, a graduate student from Emory University’s Rollins School of Public Health, who assisted with the collection and maintenance of the manuscripts; and Amy Cordero, MPA, who developed the original search strategy for this project. This research (J.A.M., H.R., R.M.S.) was supported in part by an appointment to the Research Participation Program at CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Appendix. Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.amepre.2014.09.002.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
No financial disclosures or conflicts of interest were reported by the authors of this paper.
References
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153(6):807–13. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–63. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Chen H, Correa A, Devine O, Mathews TJ, Honein MA. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A Clin Mol Teratol. 2006;76(10):706–13. doi: 10.1002/bdra.20308. [DOI] [PubMed] [Google Scholar]
- 4.Lisowski LA, Verheijen PM, Copel JA, et al. Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis Herz. 2010;35(1):19–26. doi: 10.1007/s00059-010-3244-3. [DOI] [PubMed] [Google Scholar]
- 5.Erickson JD. Risk factors for birth defects: data from the Atlanta Birth Defects Case-Control Study. Teratology. 1991;43(1):41–51. doi: 10.1002/tera.1420430106. [DOI] [PubMed] [Google Scholar]
- 6.Correa A, Gilboa SM, Botto LD, et al. Lack of periconceptional vitamins or supplements that contain folic acid and diabetes mellitus associated birth defects. Am J Obstet Gynecol. 2012;206(3):218 e1–e13. doi: 10.1016/j.ajog.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237 e1–e9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell R, Glinianaia SV, Tennant PW, Bilous RW, Rankin J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia. 2012;55(4):936–47. doi: 10.1007/s00125-012-2455-y. [DOI] [PubMed] [Google Scholar]
- 9.Ferencz C, Rubin JD, McCarter RJ, Clark EB. Maternal diabetes and cardiovascular malformations: predominance of double outlet right ventricle and truncus arteriosus. Teratology. 1990;41(3):319–26. doi: 10.1002/tera.1420410309. [DOI] [PubMed] [Google Scholar]
- 10.Sharpe PB, Chan A, Haan EA, Hiller JE. Maternal diabetes and congenital anomalies in South Australia 1986–2000: a population-based cohort study. Birth Def Res A Clin Mol Teratol. 2005;73(9):605–11. doi: 10.1002/bdra.20172. [DOI] [PubMed] [Google Scholar]
- 11.Reece EA, Homko CJ, Wu YK. Multifactorial basis of the syndrome of diabetic embryopathy. Teratology. 1996;54(4):171–82. doi: 10.1002/(SICI)1096-9926(199610)54:4<171::AID-TERA1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198(1):130 e1–e7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson UJ, Cederberg J, Wentzel P. Congenital malformations in offspring of diabetic mothers—animal and human studies. Rev Endocr Metab Disord. 2003;4(1):79–93. doi: 10.1023/a:1021879504372. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z, Reece EA. Experimental mechanisms of diabetic embryopathy and strategies for developing therapeutic interventions. J Soc Gynecol Investig. 2005;12(8):549–57. doi: 10.1016/j.jsgi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Kitzmiller JL, Wallerstein R, Correa A, Kwan S. Preconception care for women with diabetes and prevention of major congenital malformations. Birth Defects Res A Clin Mol Teratol. 2010;88(10):791–803. doi: 10.1002/bdra.20734. [DOI] [PubMed] [Google Scholar]
- 16.Kitzmiller JL, Buchanan TA, Kjos S, Combs CA, Ratner RE. Preconception care of diabetes, congenital malformations, and spontaneous abortions. Diabetes Care. 1996;19(5):514–41. doi: 10.2337/diacare.19.5.514. [DOI] [PubMed] [Google Scholar]
- 17.Starikov R, Bohrer J, Goh W, et al. Hemoglobin A1c in pregestational diabetic gravidas and the risk of congenital heart disease in the fetus. Pediatr Cardiol. 2013;34(7):1716–22. doi: 10.1007/s00246-013-0704-6. [DOI] [PubMed] [Google Scholar]
- 18.Inkster ME, Fahey TP, Donnan PT, Leese GP, Mires GJ, Murphy DJ. Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: systematic review of observational studies. BMC Pregnancy Childbirth. 2006;6:30. doi: 10.1186/1471-2393-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray JG, O’Brien TE, Chan WS. Preconception care and the risk of ‘ congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM. 2001;94(8):435–44. doi: 10.1093/qjmed/94.8.435. [DOI] [PubMed] [Google Scholar]
- 20.Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94(11):4284–91. doi: 10.1210/jc.2009-1231. [DOI] [PubMed] [Google Scholar]
- 21.Wahabi HA, Alzeidan RA, Bawazeer GA, Alansari LA, Esmaeil SA. Preconception care for diabetic women for improving maternal and fetal outcomes: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2010;10:63. doi: 10.1186/1471-2393-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casele HL, Laifer SA. Factors influencing preconception control of glycemia in diabetic women. Arch Intern Med. 1998;158(12):1321–4. doi: 10.1001/archinte.158.12.1321. [DOI] [PubMed] [Google Scholar]
- 23.Holing EV, Beyer CS, Brown ZA, Connell FA. Why don’t women with diabetes plan their pregnancies? Diabetes Care. 1998;21(6):889–95. doi: 10.2337/diacare.21.6.889. [DOI] [PubMed] [Google Scholar]
- 24.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–8. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 25.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33(3):562–8. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence JM, Contreras R, Chen WS, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5):899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 27.Albrecht SS, Kuklina EV, Bansil P, et al. Diabetes trends among delivery hospitalizations in the US, 1994–2004. Diabetes Care. 2010;33(4):768–73. doi: 10.2337/dc09-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC. National Center for Health Statistics. Health Data Interactive. www.cdc.gov/nchs/hdi.htm.
- 29.Razzaghi H, Marcinkevage J, Peterson C. Prevalence of undiagnosed diabetes among non-pregnant women of reproductive age in the United States, 1999–2010. Prim Care Diabetes. 2013 Nov 8; doi: 10.1016/j.pcd.2013.10.004. pii: S1751-9918(13)00120-4. http://dx.doi.org/10.1016/j.pcd.2013.10.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 30.Chung CS, Myrianthopoulos NC. Factors affecting risks of congenital malformations. II. Effect of maternal diabetes on congenital malformations. Birth Defects. 1975;11(10):23–38. [PubMed] [Google Scholar]
- 31.Loffredo CA, Wilson PD, Ferencz C. Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology. 2001;64(2):98–106. doi: 10.1002/tera.1051. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen GL, Norgard B, Puho E, Rothman KJ, Sorensen HT, Czeizel AE. Risk of specific congenital abnormalities in offspring of women with diabetes. Diabetic Med. 2005;22(6):693–6. doi: 10.1111/j.1464-5491.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 33.Eidem I, Stene LC, Henriksen T, et al. Congenital anomalies in newborns of women with type 1 diabetes: nationwide population-based study in Norway, 1999–2004. Acta Obstet Gyn Scand. 2010;89(11):1403–11. doi: 10.3109/00016349.2010.518594. [DOI] [PubMed] [Google Scholar]
- 34.Peticca P, Keely EJ, Walker MC, Yang Q, Bottomley J. Pregnancy outcomes in diabetes subtypes: how do they compare? A province-based study of Ontario, 2005–2006. J Obstet Gynaecol Can. 2009;31(6):487–96. doi: 10.1016/S1701-2163(16)34210-4. [DOI] [PubMed] [Google Scholar]
- 35.Sheffield JS, Butler-Koster EL, Casey BM, McIntire DD, Leveno KJ. Maternal diabetes mellitus and infant malformations. Obstet Gynecol. 2002;100(5 Pt 1):925–30. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 36.Knight KM, Pressman EK, Hackney DN, Thornburg LL. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. J Matern-Fetal Neonatal Med. 2012;25(6):611–5. doi: 10.3109/14767058.2011.587059. [DOI] [PubMed] [Google Scholar]
- 37.Janssen PA, Rothman I, Schwartz SM. Congenital malformations in newborns of women with established and gestational diabetes in Washington State, 1984–91. Paediatr Perinat EP. 1996;10(1):52–63. doi: 10.1111/j.1365-3016.1996.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 38.Agopian AJ, Moulik M, Gupta-Malhotra M, Marengo LK, Mitchell LE. Descriptive epidemiology of non-syndromic complete atrioventricular canal defects. Paediatr Perinat Epidemiol. 2012;26(6):515–24. doi: 10.1111/ppe.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A. 2010;88(12):1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 40.Bjornard K, Riehle-Colarusso T, Gilboa SM, Correa A. Patterns in the prevalence of congenital heart defects, metropolitan Atlanta, 1978 to 2005. Birth Defects Res A Clin Mol Teratol. 2013;97(2):87–94. doi: 10.1002/bdra.23111. [DOI] [PubMed] [Google Scholar]
- 41.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Mathews TJ. Births: final data for 2011. Natl Vital Stat Rep. 2013;62(1):1–69. 72. [PubMed] [Google Scholar]
- 42.Velez Edwards DR, Likis FE, Andrews JC, et al. Progestogens for preterm birth prevention: a systematic review and meta-analysis by drug route. Arch Gynecol Obstet. 2013;287(6):1059–66. doi: 10.1007/s00404-013-2789-9. [DOI] [PubMed] [Google Scholar]
- 43.Gajic-Veljanoski O, Tomlinson G, Srighanthan J, et al. Effect of Odanacatib on BMD and fractures: estimates from Bayesian univariate and bivariate meta-analyses. J Clin Endocrinol Metab. 2014;99(9):3070–9. doi: 10.1210/jc.2014-1162. [DOI] [PubMed] [Google Scholar]
- 44.National Center for Health Statistics. Health, United States, 2010: with special feature on death and dying. Hyattsville, MD: DHHS, CDC; 2011. DHHS publ. no. 2011–1232. [PubMed] [Google Scholar]
- 45.Coughlin SS, Benichou J, Weed DL. Attributable risk estimation in case-control studies. Epidemiol Rev. 1994;16(1):51–64. doi: 10.1093/oxfordjournals.epirev.a036144. [DOI] [PubMed] [Google Scholar]
- 46.Vos T, Astbury J, Piers LS, et al. Measuring the impact of intimate partner violence on the health of women in Victoria, Australia. Bull World Health Organ. 2006;84(9):739–44. doi: 10.2471/blt.06.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Land CE, Bouville A, Apostoaei I, Simon SL. Projected lifetime cancer risks from exposure to regional radioactive fallout in the Marshall Islands. Health Phys. 2010;99(2):201–15. doi: 10.1097/HP.0b013e3181dc4e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng YJ, Imperatore G, Geiss LS, et al. Secular changes in the age-specific prevalence of diabetes among U.S. adults: 1988–2010. Diabetes Care. 2013;36(9):2690–6. doi: 10.2337/dc12-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–20. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garne E, Loane M, Dolk H, et al. Spectrum of congenital anomalies in pregnancies with pregestational diabetes. Birth Defects Res A Clin Mol Teratol. 2012;94(3):134–40. doi: 10.1002/bdra.22886. [DOI] [PubMed] [Google Scholar]
- 51.Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328(7445):915. doi: 10.1136/bmj.38043.583160.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sibai BM, Caritis SN, Hauth JC, et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. The National institute of Child health and Human Development Maternal- Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;183(6):1520–4. doi: 10.1067/mob.2000.107621. [DOI] [PubMed] [Google Scholar]
- 53.Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004;27(12):2819–23. doi: 10.2337/diacare.27.12.2819. [DOI] [PubMed] [Google Scholar]
- 54.Howden LM, Meyer JA. Age and sex composition: 2010. U.S. Census Bureau, Census Briefs; 2011. www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. [Google Scholar]
- 55.American Diabetes Association. Preconception care of women with diabetes. Diabetes Care. 2004;27(Suppl 1):S76–S78. doi: 10.2337/diacare.27.2007.s76. [DOI] [PubMed] [Google Scholar]
- 56.Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an Endocrine Society clinical practice guideline. J Clin Endocr Metab. 2013;98(11):4227–49. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xaverius PK, Salas J, Kiel D. Differences in pregnancy planning between women aged 18–44, with and without diabetes: behavioral risk factor surveillance system analysis. Diabetes Res Clin Pr. 2013;99(1):63–8. doi: 10.1016/j.diabres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 58.Chuang CH, Chase GA, Bensyl DM, Weisman CS. Contraceptive use by diabetic and obese women. Womens Health Issues. 2005;15(4):167–73. doi: 10.1016/j.whi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Vahratian A, Barber JS, Lawrence JM, Kim C. Family-planning practices among women with diabetes and overweight and obese women in the 2002 National Survey For Family Growth. Diabetes Care. 2009;32(6):1026–31. doi: 10.2337/dc08-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen R, Ben-Haroush A, Weismann-Brenner A, Melamed N, Hod M, Yogev Y. Level of glycemic control and pregnancy outcome in type 1 diabetes: a comparison between multiple daily insulin injections and continuous subcutaneous insulin infusions. Am J Obstet Gynecol. 2007;197(4):404 e1–e5. doi: 10.1016/j.ajog.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Executive summary: Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S4–10. doi: 10.2337/dc13-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell R, Bailey K, Cresswell T, et al. Trends in prevalence and outcomes of pregnancy in women with pre-existing type I and type II diabetes. BJOG. 2008;115(4):445–52. doi: 10.1111/j.1471-0528.2007.01644.x. [DOI] [PubMed] [Google Scholar]
- 63.Fung A, Manlhiot C, Naik S, et al. Impact of prenatal risk factors on congenital heart disease in the current era. J Am Heart Assoc. 2013;2(3):e000064. doi: 10.1161/JAHA.113.000064. http://dx.doi.org/10.1161/JAHA.113.000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duong HT, Hoyt AT, Carmichael SL, et al. Is maternal parity an independent risk factor for birth defects? Birth Defects Res A Clin Mol Teratol. 2012;94(4):230–6. doi: 10.1002/bdra.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.