Abstract
Autophagy is an evolutionarily conserved catabolic process important in regulating the turnover of essential proteins and in elimination of damaged organelles and protein aggregates. Autophagy is observed in the lung in response to oxidative stress generated as a consequence of exposure to environmental toxicants. Whether autophagy plays role in promoting cell survival or cytotoxicity is unclear. In this article recent findings on oxidative stress-induced autophagy in the lung are reviewed; potential mechanisms initiating autophagy are also discussed. A better understanding of autophagy and its role in pulmonary toxicity may lead to the development of new strategies to treat lung injury associated with oxidative stress.
Keywords: Autophagy, Oxidative stress, Pulmonary toxicants, Cytotoxicity, Lung injury, p62
Introduction
Autophagy is a homeostatic process important in eliminating toxic protein aggregates, defective organelles and pathogens from cells. Diverse stimuli can induce autophagy including nutritional depletion, endoplasmic reticulum (ER) stress, hypoxia, hyperoxia, mitochondrial damage, oxidative stress, danger-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs) (Crighton et al., 2006; Levine et al., 2011). Deregulated autophagy has been implicated in cancer, neurodegeneration, altered innate immune defense and aging (Levine et al., 2011). However, its potential contribution to tissue injury is just beginning to be investigated.
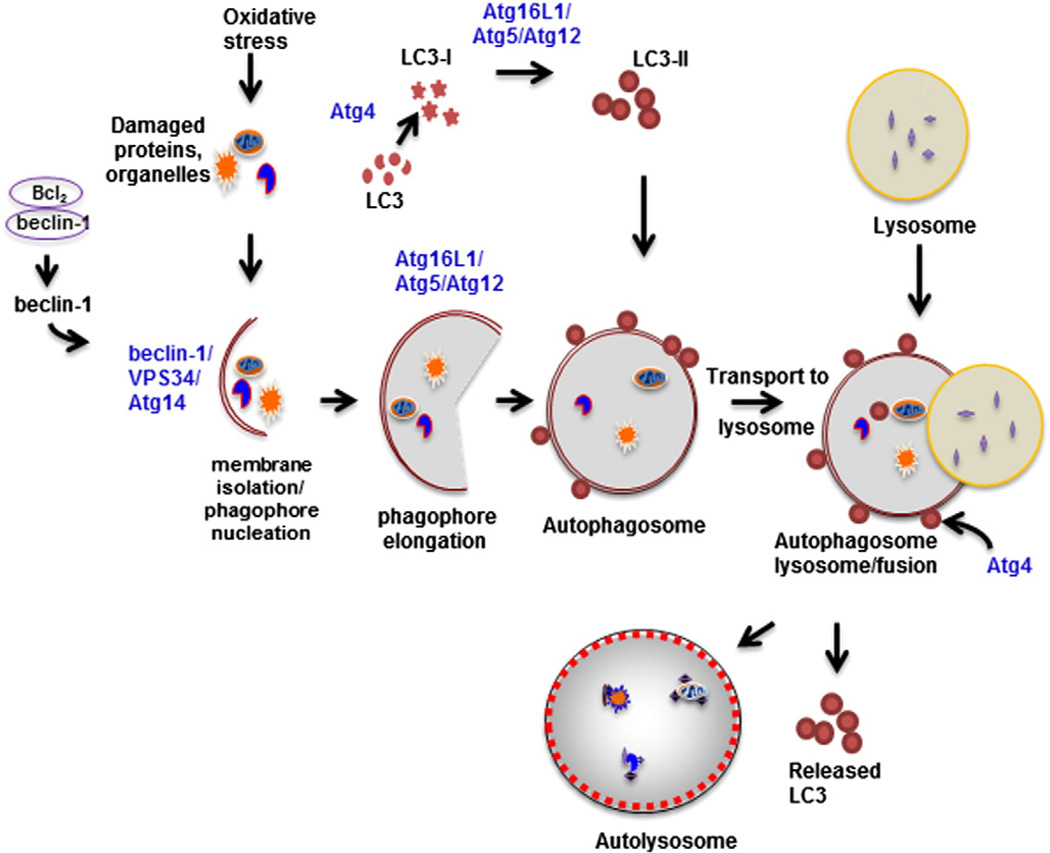
Autophagy is an evolutionarily conserved process first characterized in budding yeast. More than 30 autophagy-related (Atg) genes have been identified. Proteins encoded for by Atg genes function in an organized manner to orchestrate the process. The initial step in autophagy is isolation and enclosure of materials marked for degradation, including damaged organelles, ubiquitinated substrates or unfolded protein aggregates, into a vesicular membrane called a phagophore (Fig. 1). Elongation and maturation of the phagophore leads to the formation of a double membrane bound vesicle called an autophagosome which functions to transport cargos to be degraded to lysosomes. The outer membrane of the autophagosome then fuses with the lysosomal membrane forming an autolysosome. The contents of the autolysosome are subsequently degraded by lysosomal proteases. Several multiprotein complexes have been identified that mediate autophagy (Haspel and Choi, 2011). The first proteins activated are mammalian homolog of Atg1, Unc-51-like kinase 1 (ULK1), Atg101, a focal adhesion kinase (FAK) interacting protein (FIP200), and Atg13. These proteins are predominantly localized in the cytosol and exist together as a complex under nutrient rich conditions (Mizushima, 2010). Under homeostatic conditions mammalian target of rapamycin complex 1 (mTORC1) interacts with the ULK1 complex (ULK1–Atg101–FIP200–Atg13) to inhibit its function. Following cellular stress, mTORC1 dissociates from the ULK1 complex, allowing it to associate with the endoplasmic reticular membrane to initiate autophagosome formation. The ULK1 complex, in turn, controls the activity and localization of another multiprotein complex consisting of autophagy protein beclin-1 (Atg6), vacuolar protein sorting (VPS)-34, and barkor (Atg14), which is responsible for initiating membrane isolation and nucleation of phagophores. The activity of beclin-1 is negatively regulated by the antiapoptotic protein, Bcl-2, which, under homeostatic conditions, exists in an inhibitory complex (Maiuri et al., 2010). Induction of autophagy results in dissociation of beclin-1 from Bcl-2; beclin-1 then forms a complex with VPS-34. VPS possesses phosphoinositide (PI) 3 kinase activity which induces physical changes in the membrane facilitating phagophore formation and recruits binding proteins required for autophagosome maturation (Haspel and Choi, 2011). This macromolecular complex (VPS-34–Atg14–beclin-1) has the ability to integrate multiple proteins that can positively (Ambra, UVRAG, BIF-1) or negatively (Rubicon, Bcl-2, Bcl-xL) regulate autophagy (Chen and Klionsky, 2011). The next steps in autophagy are mediated by two ubiquitin-like reactions. The first reaction involves covalent conjugation of Atg5 to Atg12 via Atg7. The Atg5/Atg12 conjugate subsequently complexes with Atg16L1; this complex is essential for the elongation of the phagophore. The Atg16L1/Atg5/Atg12 complex also mediates the conversion of microtubule-associated protein light chain 3 (LC3)-I or Atg8 into LC3-II, a phosphatidylethanolamine (PE)-lipidated form. Atg4 participates in this conversion by facilitating the exposure of the C-terminal glycine residue in LC3, the site of PE conjugation. LC3-II associates with the outer and inner membranes of the autophagosome and mediates its expansion (Xie et al., 2008). Following fusion of the autophagosome to the autolysosome, inner membrane associated LC3-II is degraded by hydrolases whereas outer membrane LC-3II is recycled via Atg4-mediated delipidation and deconjugation.
Fig. 1.
Oxidative stress causes damage to proteins and organelles. This results in dissociation of beclin-1 from Bcl2, and subsequent formation of a multiprotein complex, consisting of beclin-1/VPS34/Atg14. This complex facilitates membrane isolation and phagophore nucleation. The double membrane phagophore surrounds the ubiquitinated and aggregated proteins and damaged organelles to be degraded. The phagophore is then elongated, a process mediated by the Atg16L1/Atg5/Atg12 complex, resulting in the formation of an autophagosome. Conversion of LC3 to PE-lipidated LC3-II is mediated by the sequential actions of Atg4 and the Atg16L1/Atg5/Atg12 complex. LC3-II associates with autophagosomal membranes. Following fusion of the autophagosome with lysosomes, the intracellular proteins and organelles are degraded by lysosomal enzymes and LC3 is delipidated and recycled by Atg4.
Pulmonary toxicants and oxidative stress
Reactive oxygen species (ROS) including superoxide anion, hydrogen peroxide (H2O2), hydroxyl radicals and lipid peroxides are cytotoxic mediators derived from the metabolism of oxygen in aerobic organisms. Most ROS are formed as by-products of normal metabolic reactions, including energy generation from mitochondria or cytochrome P-450 mediated metabolism (Lenaz, 2012; Mishin et al., 2010). About 1–2% of molecular oxygen is converted into superoxide anion radicals during normal respiration and is regarded as the source of most ROS (Orrenius et al., 2011). ROS are also generated by macrophages and neutrophils during inflammatory responses. In addition, these cells produce reactive nitrogen species (RNS) including nitric oxide and peroxynitrite via inducible nitric oxide synthase (iNOS) (Laskin et al., 2010). Oxidative stress results from an imbalance between the production and elimination of ROS and RNS. These highly reactive species can modify lipids, proteins and DNA leading to cytotoxicity. Environmental oxidants including cigarette smoke, diesel exhaust, industrial pollutants, ozone, and ionizing radiation can also contribute to oxidative stress (Biswas and Rahman, 2009). Pulmonary exposure to these toxicants causes tissue injury and initiates an inflammatory response characterized by an accumulation of phagocytic leukocytes in the lung which generate additional oxidants. A number of pulmonary diseases and pathologies are associated with oxidative stress, characterized by increased production of ROS and RNS in the respiratory tract, and/or decreased levels of antioxidants.
For example, in patients with asthma, high levels of macrophage ROS production is observed, along with reduced levels of pulmonary glutathione (GSH) and superoxide dismutase (SOD) (Comhair and Erzurum, 2010). This is associated with increased sensitivity to air pollutants (Comhair and Erzurum, 2010). GSH is also decreased in bronchoalveolar lavage (BAL) from patients with idiopathic pulmonary fibrosis (IPF), acute respiratory syndrome (ARDS) and cystic fibrosis (Biswas and Rahman, 2009). Pulmonary toxicants containing oxidants including cigarette smoke, smoke derived from burning of biomass fuel, nitrogen dioxide and sulfur dioxide, have been shown to play a pathogenic role in the development of diseases such as chronic obstructive pulmonary disease (COPD) (Rahman, 2012). Recent evidence indicates that cigarette smoke irreversibly modifies reduced GSH levels in airway epithelial cells, leading to oxidative damage in the lung (van der Toorn et al., 2009). Similarly, exposure to oxidative air pollutants like particulate matter, ozone, and nitrogen dioxide has been linked to increases in asthma attacks and changes in lung function (Jackson et al., 2011). In fact, the ability of ozone, as well as particulate air pollutants, to oxidize cellular components through free-radical mediated reactions is key to their toxicity. Particulate air pollutants also induce the generation of free radicals via metals including iron, cobalt and chromium which undergo redox cycling, and cadmium and nickel, which deplete GSH (Stohs and Bagchi, 1995). Cadmium, which is also a component of cigarette smoke, has been shown to inhibit the activity of SOD and increase lipid peroxides in the lung (Stohs and Bagchi, 1995).
A primary consequence of oxidative stress is lipid peroxidation. Membrane-bound polyunsaturated fatty acids including arachidonic acid, oleic acid and linoleic acid are major targets of ROS and RNS. Lipid peroxidation products derived from these unsaturated fatty acids can act as mitogens, activate membrane receptors, and induce airway hyperresponsiveness (Rahman, 2005). Lipid hydroperoxides generated during fatty acid metabolism react with metals to produce highly reactive epoxides and aldehydes. Major aldehyde products of lipid peroxidation are malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE). MDA is highly mutagenic whereas 4-HNE activates various signal transduction pathways (Rahman, 2005). High levels of MDA are formed in the lung following exposure to cigarette smoke and ambient air pollutants (Pereira et al., 2007); lipid hydroperoxides and MDA have also been observed in the lungs of patients with cystic fibrosis indicating a potential role of lipid peroxides in chronic lung inflammation (Brown et al., 1996). The arachidonic acid metabolite and oxidative stress marker, 8-isoprostane is a potent bronchial smooth muscle constrictor, and it plays an important role in asthma and COPD. Increased levels of 8-isoprostane in exhaled breath condensate, plasma and BAL of smokers and patients with COPD indicate persistent oxidative stress in the lung (Louhelainen et al., 2008). Various redox sensitive signaling molecules including the mitogen-activated protein kinases (MAPKs), c-Jun N terminal kinase (JNK), and extracellular signal regulated kinase (ERK), are activated in response to oxidative stress culminating in increased activity of transcription factors like NF-κB, AP-1, c-Jun, c-Fos and ATF-2. This leads to increased expression of genes important in inflammation and antioxidant defense (Xanthoudakis et al., 1992). For example, following ozone inhalation, a rapid induction of PI3-kinase and its downstream target protein kinase B/Akt has been observed (Laskin et al., 2002). This is thought to be mediated by the lipid peroxidation product 4-HNE formed after ozone exposure (Fakhrzadeh et al., 2004a). Subsequent activation of NF-κB leads to upregulation of iNOS and increased RNS production, key mediators of ozone toxicity (Fakhrzadeh et al., 2004b). Another transcription factor involved in the oxidative stress response is nuclear factor erythroid 2-related factor 2 (Nrf2) which regulates the expression of antioxidants and cytoprotective genes (Baird and Dinkova-Kostova, 2011). Nrf2 plays a protective role in the lung against various environmental oxidants including ozone, cigarette smoke, particulate matter and hyperoxia (Cho and Kleeberger, 2010). In fact, loss of Nrf2 has been shown to exacerbate ozone toxicity (Cho and Kleeberger, 2010). Similarly, Nrf2-deficient mice exhibit excessive oxidative stress, increased apoptosis, inflammation, and exacerbated emphysema in response to cigarette smoke (Rangasamy et al., 2004). A role for Nrf2 has also been established in the pathogenesis of oxidative pulmonary diseases including COPD, asthma and interstitial pulmonary fibrosis (Cho and Kleeberger, 2010).
Another mechanism whereby oxidants can exert their pathological effects is by damaging DNA. ROS-mediated single- and double-strand DNA breaks, DNA adduct formation, and/or transformation of bronchial epithelial cells into mesenchymal cells have been described (Lenaz, 2012). DNA damage and neoplastic transformation of the airways have been reported in the lungs of animals exposed to crystalline silica or sulfurmustard (Malaviya et al., 2010; Saffiotti et al., 1994). Lung macrophages and epithelial cells also exhibit DNA damage following exposure to ozone (Sunil et al., 2012), an effect inhibited by pretreatment with vitamin E (Cheng et al., 2003), indicating an association between oxidative stress and DNA damage. ROS-mediated DNA damage has also been described in cells treated with metal nanoparticles, sodium selenite, or retinol (Murata and Kawanishi, 2000; Park et al., 2012; Wan et al., 2012).
Oxidative stress and autophagy
Oxidative stress is a major inducer of autophagy, which is important in the removal of oxidized proteins and damaged mitochondria (Ryter et al., 2012; Scherz-Shouval and Elazar, 2011). Moreover, defects in autophagy lead to excessive oxidative stress (Lee et al., 2012). Chen et al. (2009) reported that amino acid starvation-induced autophagy in HeLa cells is mediated by increased levels of ROS. Findings that loss of MnSOD augments autophagy in the kidney and in mouse embryonic fibroblasts are consistent with this observation (Parajuli and Macmillan-Crow, 2013; Zhang et al., 2010). Antioxidants, including N-acetyl cysteine (NAC) and catalase, have been reported to inhibit autophagosome formation and consequent protein degradation (Duan et al., 2010). Mitochondria are a major source of ROS and it has been suggested that they are also important in the formation of autophagosomes (Chen and Gibson, 2008). This is supported by findings that mitochondrial electron transport chain inhibitors such as H2O2, rotenone, thenoyltrifluoroacetone (TTFA), and 2-methoxyestradiol, which is also an inhibitor of SOD, induce autophagy (Chen and Gibson, 2008). Further evidence for a role of mitochondria generated ROS in induction of autophagy comes from studies in which autophagy genes have been disrupted. For example, deletion of Atg3 or Atg5 genes in T cells leads to deformed or dysfunctional mitochondria and increases in ROS accumulation (Lee et al., 2012). Similarly, Atg7−/− mouse embryonic fibroblasts exhibit dysfunctional mitochondrial respiration due to increased accumulation of ROS and p62 protein (Wu et al., 2009). Damaged, nonfunctional mitochondria undergo a specialized type of autophagy called mitophagy (Gomes and Scorrano, 2013). The antioxidant, heme oxygenase-1 (HO-1) has been reported to induce mitophagy and regulate the elimination of damaged mitochondria (Unuma et al., 2013).
A number of toxicants including sodium selenite, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), acetaminophen and cigarette smoke extract have been reported to activate autophagy by increasing ROS formation. For example, autophagy is readily induced in hepatocytes by acetaminophen, a process inhibited by the antioxidant NAC (Ni et al., 2012). Bovine kidney cells undergo LC3 conversion and acidic vesicle formation when exposed to TCDD suggesting that autophagy is involved in TCDD-induced kidney cell death (Fiorito et al., 2011). Exogenous H2O2 can also induce autophagy in transformed cells by increasing intracellular superoxide anion levels (Y. Chen et al., 2008). Sodium selenite has been reported to induce ROS production, loss of mitochondrial membrane potential, entrapment of damaged mitochondria in autophagosomes and autolysosomes leading to mitophagy in malignant glioma cells (Kim et al., 2007). Selenium containing histone deacetylase inhibitors are effective in suppressing alveolar epithelial cell growth by inducing autophagy (Karelia et al., 2010). Arsenic is a human carcinogen known to induce oxidative stress in mammalian cells (Hughes et al., 2011; Zhang et al., 2012). Evidence suggests a role for ski-related novel protein N (SnoN) in arsenic trioxide (As2O3)-induced autophagy in ovarian cancer cells (Smith et al., 2010). This process is beclin-1-independent and associated with a dramatic upregulation of ROS-mediated SnoN levels which parallel levels of LC3-II in the cells. The addition of NAC to As2O3 treated cells reverses changes in SnoN protein, and consequent autophagy. Moreover, knockdown of SnoN using siRNA results in a decrease in LC3-II/I ratio indicating that SnoN can regulate LC3 levels (Smith et al., 2010).
Autophagy is also thought to be protective by mitigating toxicity and inhibiting cell death. Free heme, generated by hemolysis of red blood cells or muscle damage, induces endothelial cell toxicity. Lipid peroxidation and mitochondrial dysfunction induced by heme are also associated with activation of autophagy and mitophagy in endothelial cells (Higdon et al., 2012). A role of autophagy in reducing oxidative stress has been demonstrated in arterial endothelial dysfunction caused by reduced nitric oxide bioavailability (Brandes et al., 2005). Similarly, LaRocca et al. (2012) reported that treatment of endothelial cells with trehalose, an autophagy inducer, reduces oxidative stress. The antioxidant, curcumin, has also been shown to protect endothelial cells from oxidative stress by inducing autophagy (Han et al., 2012). Collectively, these studies indicate that autophagy can ameliorate oxidative stress-induced toxicity. Additional studies have identified Atg4 as a key cytoplasmic target of ROS (Scherz-Shouval et al., 2007). A highly conserved cysteine (Cys81) residue in Atg4 is redox sensitive and mediates its conjugating and deconjugating functions. Following the initial cleavage of LC3 to LC3-I and its lipidation, Atg4 is inactivated resulting in conjugation of LC3-II to the autophagosomal membrane. Subsequently, LC3-II is delipidated by Atg4 protease and recycled. ROS have been reported to inactivate Atg4 and consequently promote LC3 lipidation, leading to increases in autophagosome formation. Inactivation of Atg4 by ROS represents one of the few established mechanisms of oxidant control of autophagy. A role for poly(ADP-ribose) polymerase-1 (PARP-1) has also been established in autophagy. Thus, while overactivation of PARP-1 in response to DNA damage triggers autophagy, suppression of PARP-1 protects against autophagy (Munoz-Gamez et al., 2009).
Autophagy in the lung following exposure to toxicants
Increasing evidence suggests a role for autophagy in the pathogenic response to toxicants and oxidative stress in the lung (Table 1). Among pulmonary toxicants, cigarette smoke has been the most widely investigated (Ryter and Choi, 2010). Cigarette smoke extract (CSE), a potent source of oxidants, has been reported to increase the LC3B-II/LC3B-I ratio in human bronchial epithelial cells (HBECs) (Z.H. Chen et al., 2008; Shi et al., 2012). Morphological and molecular markers of autophagy including Atg4, Atg7, Atg5–Atg12, and LC3B-II are also upregulated in the lungs of patients with COPD (Z.H. Chen et al., 2008). A characteristic feature of COPD is emphysema or enlargement of air spaces, a process which increases during the progression of the disease. The fact that autophagy markers appear early in the disease process and remain elevated suggest that it may contribute to the enlargement of airspaces. This is supported by findings that mice lacking LC3B exhibit reduced air space enlargement when exposed chronically to cigarette smoke (Z.H. Chen et al., 2008). Moreover, treatment of pulmonary epithelial cells with NADPH oxidase inhibitors such as diphenylene iodonium or apocyanin, or the antioxidant NAC, attenuates CSE-induced autophagy suggesting that altered cellular redox homeostasis is involved in the process. Similarly, overexpression of transcription factor Nrf2 which is involved in antioxidant signaling, suppressed CSE-induced LC3B expression and autophagosome formation in airway epithelial cells (Zhu et al., 2013). Additionally, overexpression of HO-1 decreased CSE-induced increases in beclin-1 and LC3 lipidation in lung epithelial cells, suggesting cytoprotective effects of this stress-induced protein (Kim et al., 2008; Ryter et al., 2012). Exposure to particulate matter (PM) with an aerodynamic diameter <2.5 µM (PM2.5) has also been shown to induce oxidative stress and upregulate expression of autophagy-related proteins in lung epithelial cells supporting a link between ROS and autophagy (Deng et al., 2013).
Table 1.
Pulmonary toxicants associated with autophagy
| Toxicants | In vitro | In vivo |
|---|---|---|
| Cigarette smoke/CSE | Z.H. Chen et al. (2008, 2010) | Z.H. Chen et al. (2008, 2010) |
| Kim et al. (2008) | Hwang et al. (2010) | |
| Hwang et al. (2010) | Shi et al. (2012) | |
| Monick et al. (2010) | Bodas et al. (2011) | |
| Guzik et al. (2011) | ||
| Fujii et al. (2012) | ||
| Shi et al. (2012) | ||
| Zhu et al. (2013) | ||
| Ozone | Sunil et al. (2012) | |
| Radiation | Zois et al. (2011) | |
| Carbon monoxide | Lee et al. (2011) | Lee et al. (2011) |
| Sulfur mustard | Malaviya et al. (2010) | |
| Metal nanoparticles | Li et al. (2010) | |
| Khan et al. (2012) | ||
| Arsenic trioxide | Zhang et al. (2012) | |
| Sodium selenite | Park et al. (2012) | |
| Karelia et al. (2010) | ||
| Bleomycin | Patel et al. (2012) | Mi et al. (2011) |
| Particulate matter | Deng et al. (2013) |
Alveolar macrophages respond to CSE-mediated oxidative stress by upregulating autophagy proteins. Like epithelial cells, alveolar macrophages isolated from the lungs of smokers express increased levels of autophagy proteins and contain greater numbers of autophagy vesicles and autophagosomes; additionally, abnormal cytoplasmic accumulation of damaged mitochondria, ubiquitinated proteins and autophagosomes have also been detected (Monick et al., 2010). Similar defects in autophagy have been described in human alveolar macrophages treated with CSE (Monick et al., 2010). Defects in autophagy lead to reduced capacity of alveolar macrophages to clear damaged organelles and proteins which may impact innate immune responses in the lung. Impaired autophagy can also lead to attenuated clearance of dead cells in the lung contributing to the buildup of damaged materials and autophagosomes. CSE has been reported to induce autophagy in neutrophils, and alter their phagocytic capacity (Guzik et al., 2011). Accumulation of these compromised neutrophils, along with autophagy-defective macrophages, may render cigarette smoke-exposed lung more susceptible to pathogenic infections and degradative diseases.
Cigarette smoke-induced epithelial cell senescence is characterized by an accumulation of damaged organelles and ubiquitinated proteins, and has been implicated in the pathogenesis of COPD. Fujii et al. (2012) investigated the role of autophagy in the response of HBECs to CSE. Transient activation of autophagy in HBECs by CSE was followed by cellular senescence and an accumulation of the LC3A binding protein, p62 in the cells, along with ubiquitinated proteins. Inhibition of autophagy in HBECs enhanced p62 accumulation and senescence. This was reversed by Torin1, an mTOR inhibitor known to activate autophagy. Interestingly, bronchial epithelial cells from COPD patients exhibit an attenuated autophagy response to CSE, despite significantly higher baseline levels of autophagy in cells from non-smokers. These data indicate that insufficient clearance of damaged cellular components due to reduced autophagy in COPD patients contributes to cell senescence, supporting a protective role for autophagy in this disease.
Exposure to radiation is associated with inflammation and oxidative stress culminating in the development of pulmonary fibrosis. Using a mouse model of whole body exposure, Zois et al. (2011) showed that radiation results in an accumulation of LC3A and p62 in the lung, and deregulated autophagy. p62 accumulation has been linked to suppression of autophagy (Lee et al., 2008). Whether this contributes to lung fibrosis has not been established. Fibroblasts and endothelial cells in bronchial biopsies from patients with asthma have been reported to contain double membrane autophagosomes (Poon et al., 2012). Asthma is also associated with increased release of IL-13 which promotes transforming growth factor (TGF)-β1 mediated lung remodeling, a response enhanced by ROS (Poon et al., 2012). These findings suggest that autophagy plays role in airway remodeling in asthma, and it may similarly contribute to the development of fibrosis. Direct evidence for a role of autophagy in lung fibrosis has recently been established in the pathogenesis of cystic fibrosis (Luciani et al., 2011). Using a mouse model itwas observed that restoring autophagy ameliorated cystic fibrosis associated ROS generation, and favored the clearance of protein aggregates, leading to the resolution of lung inflammation. Recent findings suggest that proinflammatory cytokines TGF-β1 and IL-17A promote fibrosis by inhibiting autophagy and autophagy-associated cytotoxicity, as evidenced by co-localization of markers of autophagy and cell death (Mi et al., 2011; Nakahira and Choi, 2013; Patel et al., 2012).
Metal nanoparticles are widely used for tumor therapy owing to their potential to induce oxidative stress and increase cellular toxicity. Evidence suggests that in addition to inducing oxidative stress, nanoparticles activate autophagy (Khan et al., 2012; Li et al., 2010). Gold nanoparticles have been shown to induce autophagy in lung fibroblasts concomitant with increases in lipid hydroperoxide and MDA levels, whereas iron oxide nanoparticles are selective, only inducing autophagy in cancer cells (Khan et al., 2012; Li et al., 2010). Induction of autophagy in iron oxide treated cells also correlated with increases in ROS and loss of mitochondrial membrane potential.
Autophagy has also been observed in lung endothelial cells of animals exposed to vesicants which are known to cause oxidative stress (Malaviya et al., 2010; Weinberger et al., 2010). Thus, following exposure of rodents to sulfur mustard or nitrogen mustard, pulmonary expression of iNOS increases (Malaviya et al., 2010; Weinberger et al., 2010). This is correlated with expression of LC3-II protein in the lung, and the appearance of autophagic vacuoles in pulmonary endothelial cells within 6 h of exposure. It remains to be determined if autophagy is protective or pathologic in this model.
Autophagy proteins have also been identified in the lungs of animals exposed to ozone and bleomycin (Mi et al., 2011; Patel et al., 2012; Sunil et al., 2012). The toxicity of each of these pulmonary irritants involves oxidative stress suggesting a potential link to autophagy. Arsenic exposure has also been shown to induce oxidative stress and activate autophagy in HBECs (Zhang et al., 2012). Inhibition of autophagy in these cells promoted arsenic-induced cell transformation suggesting autophagy as a self-protective mechanism in arsenic-induced tumorigenesis. Cellular accumulation of LC3-II and autophagic flux has also been described in human lung cells following exposure to sodium selenite (Park et al., 2012). Findings that bafilomycin A1, an inhibitor of autophagic vacuole maturation, enhances sodium selenite-induced apoptosis suggest that autophagy serves a protective role in this model. A protective role of autophagy has similarly been described following exposure of lung epithelial cells to hyperoxia (Lee et al., 2011).
Mechanisms regulating expression of autophagy genes in the lung after exposure to toxicants and oxidative stress are largely unknown. Z.H. Chen et al. (2008) identified transcription factors including early growth response-1 (Egr-1) and E2F, which bind to consensus regions in the LC3B promoter in the lung after cigarette smoke exposure, indicating transcriptional control of the process. An involvement of epigenetic factors has also been suggested. Levels of histone deacetylase (HDAC) protein and mRNA decline with disease progression in COPD patients, as well as in the lungs of smokers (Ito et al., 2005). Cigarette smoke-mediated inhibition of HDAC activity is associated with upregulation of LC3B expression in lung epithelial cells (Z.H. Chen et al., 2008). Cystic fibrosis transmembrane conductance regulator (CFTR), is an ATP- and cAMP-dependent protein kinase A-regulated chloride channel present at the apical surface of epithelial cells, important in the progression of the disease (Dudez et al., 2008). CFTR-deficient mice show increased cigarette smoke-induced accumulation of p62 and LC3B in the lung suggesting defective autophagy in the absence of CFTR, which may be important in pathogenesis of emphysema (Bodas et al., 2011).
Sirtuin1 (SIRT1), an evolutionarily conserved protein kinase, has been reported to deacetylate the pro-autophagic proteins, Atg5, Atg7 and Atg8, and the transcription factor forkhead box O3a (FoxO3a) during starvation (Kume et al., 2010; Lee et al., 2008). This leads to increased expression of Bnip3 (Kume et al., 2010). SIRT1−/− mouse embryonic fibroblasts are unable to activate autophagy in response to starvation, supporting a role of SIRT1 in autophagy (Lee et al., 2008). Interestingly, SIRT1 activity decreases in the lungs of smokers and patients with COPD, as well as in lung macrophages and epithelial cells exposed to CSE (Rajendrasozhan et al., 2008; Yang et al., 2007). Moreover, CSE can induce autophagy in the lungs of SIRT1−/− mice (Hwang et al., 2010); these data suggest that SIRT1 is not required for oxidant-induced autophagy. The activity of SIRT1 is regulated by oxidative stress mediated activation of the nicotinamide adenine dinucleotide (NAD)+ dependent enzyme PARP-1 (Hwang et al., 2010). CSE-induced activation of PARP-1 results in depletion of NAD+ and reduced SIRT1 activity. Consistent with a role of PARP-1 in autophagy are findings that PARP-1 inhibitors attenuate CSE-induced autophagy and increase SIRT1 activity (Hwang et al., 2010). It appears that SIRT1 and PARP-1 coordinately regulate autophagy in response to CSE.
Relationship between autophagy and apoptosis
The extent to which autophagy and apoptosis contribute to cell death is related to the specific pathology. In mammalian cells evidence suggests that depending on triggering mechanisms, apoptosis and autophagy can operate independently, or may regulate one another (Chen and Klionsky, 2011). In the lungs of patients with COPD, both autophagy and apoptosis have been described (Z.H. Chen et al., 2008). Concurrent induction of both processes has also been observed in rat lung following exposure to sulfur mustard or to ozone (Malaviya et al., 2010; Sunil et al., 2012). Induction of autophagy and apoptosis in the lung is likely influenced by the extent of cellular damage. It is possible that autophagy is activated in response to mild oxidative stress at early stages of the injury while apoptosis is induced later to remove damaged cells (Z.H. Chen et al., 2008).
Z.H. Chen et al. (2008, 2010) observed significantly reduced levels of apoptosis in the lungs of LC3B−/− mice relative to wild type mice, and increased resistance to emphysema following CSE exposure. Additionally, decreasing levels of autophagy proteins, beclin-1 or LC3B in lung epithelial cells results in increased activation of caspases-8, -9, and -3 in response to cigarette smoke. LC3B regulates cigarette smoke-induced apoptosis through its interaction with Fas protein (Chen et al., 2010; Ryter et al., 2011). A similar interaction between LC3B and components of the Fas apoptotic pathway has been described following hyperoxic lung injury (Tanaka et al., 2012). Bcl-2 family members and caspases have also been shown to control the process of autophagy. Bcl-2 and Bcl-xL inhibit autophagy by binding to beclin-1 (Zhou et al., 2010). Activated caspases have also been reported to cleave beclin-1, Atg5 and Atg4D proteins facilitating their interaction with Bcl-2 in mitochondria and amplifying apoptosis (Djavaheri-Mergny et al., 2010). These data support the idea that caspases can regulate cell fate by producing truncated forms of autophagy proteins that can either promote apoptosis or inhibit autophagy.
Conclusion
Although autophagy was initially thought to be a relatively nonselective process, recent evidence suggests an active role in normal physiology and in pathologic responses to oxidative stress. Whether autophagy represents an adaptive response of the lung or merely a process required to clear damaged organelles or proteins during oxidative stress remains to be determined. Conceptually, removing damaged mitochondria and protein aggregates through autophagy supports survival; however, the exact role of autophagy in lung injury has not been established. The use of knockout mice deficient in different components of the autophagy pathway may shed light on the role of these molecules in the progression of lung injury and inflammation and diseases such as COPD and emphysema. It will also be important to consider various processes of cell death activated in response to pulmonary toxicants in different cell types in the lung and assess their contribution to injury. Studies correlating the magnitude of injury to the activation of autophagy or apoptosis or both may be beneficial for developing strategies to mitigate pulmonary toxicity.
Acknowledgment
This work was supported by NIH Grants U54AR055073, K08HL096426, R01ES004738, R01CA132624 and P30ES005022.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol. Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodas M, Min T, Vij N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L811–L820. doi: 10.1152/ajplung.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc. Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Brown RK, Wyatt H, Price JF, Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur. Respir. J. 1996;9:334–339. doi: 10.1183/09031936.96.09020334. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gibson SB. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008;4:246–248. doi: 10.4161/auto.5432. [DOI] [PubMed] [Google Scholar]
- Chen Y, Klionsky DJ. The regulation of autophagy — unanswered questions. J. Cell Sci. 2011;124:161–170. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, Nakahira K, Pilewski JM, Lee JS, Zhang Y, Ryter SW, Choi AM. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18880–18885. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TJ, Kao HP, Chan CC, Chang WP. Effects of ozone on DNA single-strand breaks and 8-oxoguanine formation in A549 cells. Environ. Res. 2003;93:279–284. doi: 10.1016/s0013-9351(03)00041-0. [DOI] [PubMed] [Google Scholar]
- Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol. Appl. Pharmacol. 2010;244:43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, Chen D, Ding W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. In Vitro. 2013;27:1762–1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010;29:1717–1719. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- Duan W, Jin X, Li Q, Tashiro S, Onodera S, Ikejima T. Silibinin induced autophagic and apoptotic cell death in HT1080 cells through a reactive oxygen species pathway. J. Pharmacol. Sci. 2010;113:48–56. doi: 10.1254/jphs.09315fp. [DOI] [PubMed] [Google Scholar]
- Dudez T, Borot F, Huang S, Kwak BR, Bacchetta M, Ollero M, Stanton BA, Chanson M. CFTR in a lipid raft-TNFR1 complex modulates gap junctional intercellular communication and IL-8 secretion. Biochim. Biophys. Acta. 2008;1783:779–788. doi: 10.1016/j.bbamcr.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Gardner CR, Laskin DL. Superoxide dismutase-overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-alpha. Am. J. Respir. Cell Mol. Biol. 2004a;30:280–287. doi: 10.1165/rcmb.2003-0044OC. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNF-alpha and tissue injury are dependent on NF-kappaB p50. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004b;287:L279–L285. doi: 10.1152/ajplung.00348.2003. [DOI] [PubMed] [Google Scholar]
- Fiorito F, Ciarcia R, Granato GE, Marfe G, Iovane V, Florio S, De Martino L, Pagnini U. 2,3,7,8-tetrachlorodibenzo-p-dioxin induced autophagy in abovinekidney cell line. Toxicol. 2011;290:258–270. doi: 10.1016/j.tox.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Fujii S, Hara H, Araya J, Takasaka N, Kojima J, Ito S, Minagawa S, Yumino Y, Ishikawa T, Numata T, Kawaishi M, Hirano J, Odaka M, Morikawa T, Nishimura S, Nakayama K, Kuwano K. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology. 2012;1:630–641. doi: 10.4161/onci.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Scorrano L. Mitochondrial morphology in mitophagy and macroautophagy. Biochim. Biophys. Acta. 2013;1833:205–212. doi: 10.1016/j.bbamcr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Guzik K, Skret J, Smagur J, Bzowska M, Gajkowska B, Scott DA, Potempa JS. Cigarette smoke-exposed neutrophils die unconventionally but are rapidly phagocytosed by macrophages. Cell Death Dis. 2011;2:e131. doi: 10.1038/cddis.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, Pan Y, Li XJ. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8:812–825. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- Haspel JA, Choi AM. Autophagy: a core cellular process with emerging links to pulmonary disease. Am. J. Respir. Crit. Care Med. 2011;184:1237–1246. doi: 10.1164/rccm.201106-0966CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon AN, Benavides GA, Chacko BK, Ouyang X, Johnson MS, Landar A, Zhang J, Darley-Usmar VM. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1394–H1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol. Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, Rahman I. Cigarette smoke-induced autophagy is regulated by SIRT1–PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch. Biochem. Biophys. 2010;500:203–209. doi: 10.1016/j.abb.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J. Allergy Clin. Immunol. 2011;128:1165–1174. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelia N, Desai D, Hengst JA, Amin S, Rudrabhatla SV, Yun J. Selenium-containing analogs of SAHA induce cytotoxicity in lung cancer cells. Bioorg. Med. Chem. Lett. 2010;20:6816–6819. doi: 10.1016/j.bmcl.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–1488. doi: 10.1016/j.biomaterials.2011.10.080. [DOI] [PubMed] [Google Scholar]
- Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, Choi KS. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007;67:6314–6324. doi: 10.1158/0008-5472.CAN-06-4217. [DOI] [PubMed] [Google Scholar]
- Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, Ryter SW, Choi AM. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4:887–895. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J. Physiol. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Fakhrzadeh L, Heck DE, Gerecke D, Laskin JD. Upregulation of phosphoinositide 3-kinase and protein kinase B in alveolar macrophages following ozone inhalation. Role of NF-kappaB and STAT-1 in ozone-induced nitric oxide production and toxicity. Mol. Cell Biochem. 2002;234–235:91–98. [PubMed] [Google Scholar]
- Laskin DL, Sunil VR, Fakhrzadeh L, Groves A, Gow AJ, Laskin JD. Macrophages, reactive nitrogen species, and lung injury. Ann. N. Y. Acad. Sci. 2010;1203:60–65. doi: 10.1111/j.1749-6632.2010.05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am. J. Respir. Cell Mol. Biol. 2011;45:867–873. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: crosstalk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv. Exp. Med. Biol. 2012;942:93–136. doi: 10.1007/978-94-007-2869-1_5. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Hartono D, Ong CN, Bay BH, Yung LY. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials. 2010;31:5996–6003. doi: 10.1016/j.biomaterials.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Louhelainen N, Myllarniemi M, Rahman I, Kinnula VL. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. Int. J. Chron. Obstruct. Pulmon. Dis. 2008;3:585–603. doi: 10.2147/copd.s3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina DL, Settembre C, Gavina M, Raia V, Ballabio A, Maiuri L. Cystic fibrosis: a disorder with defective autophagy. Autophagy. 2011;7:104–106. doi: 10.4161/auto.7.1.13987. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010;29:515–516. doi: 10.1038/emboj.2009.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, Conti ML, Gordon RE, Laskin JD, Laskin DL. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol. Appl. Pharmacol. 2010;248:89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J. Immunol. 2011;187:3003–3014. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- Mishin V, Gray JP, Heck DE, Laskin DL, Laskin JD. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic. Biol. Med. 2010;48:1485–1491. doi: 10.1016/j.freeradbiomed.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW. Identification of an autophagy defect in smokers' alveolar macrophages. J. Immunol. 2010;185:5425–5435. doi: 10.4049/jimmunol.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Gamez JA, Rodriguez-Vargas JM, Quiles-Perez R, Aguilar-Quesada R, Martin-Oliva D, deMurcia G, Menissier deMurcia J, Almendros A, Ruiz de Almodovar M, Oliver FJ. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5:61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- Murata M, Kawanishi S. Oxidative DNA damage by vitamin A and its derivative via superoxide generation. J. Biol. Chem. 2000;275:2003–2008. doi: 10.1074/jbc.275.3.2003. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Choi AM. Autophagy: a potential therapeutic target in lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;305:L93–L107. doi: 10.1152/ajplung.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol. Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- Parajuli N, Macmillan-Crow LA. Role of reduced manganese superoxide dismutase in ischemia–reperfusion injury: a possible trigger for autophagy and mitochondrial biogenesis? Am. J. Physiol. Renal Physiol. 2013;304:F257–F267. doi: 10.1152/ajprenal.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Kim JH, Chi GY, Kim GY, Chang YC, Moon SK, Nam SW, Kim WJ, Yoo YH, Choi YH. Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol. Lett. 2012;212:252–261. doi: 10.1016/j.toxlet.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira CE, Heck TG, Saldiva PH, Rhoden CR. Ambient particulate air pollution from vehicles promotes lipid peroxidation and inflammatory responses in rat lung. Braz. J. Med. Biol. Res. 2007;40:1353–1359. doi: 10.1590/s0100-879x2006005000164. [DOI] [PubMed] [Google Scholar]
- Poon A, Eidelman D, Laprise C, Hamid Q. ATG5, autophagy and lung function in asthma. Autophagy. 2012;8:694–695. doi: 10.4161/auto.19315. [DOI] [PubMed] [Google Scholar]
- Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem. Biophys. 2005;43:167–188. doi: 10.1385/CBB:43:1:167. [DOI] [PubMed] [Google Scholar]
- Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim. Biophys. Acta. 2012;1822:714–728. doi: 10.1016/j.bbadis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Choi AM. Autophagy in the lung. Proc. Am. Thorac. Soc. 2010;7:13–21. doi: 10.1513/pats.200909-101JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Lam HC, Chen ZH, Choi AM. Deadly triplex: smoke, autophagy and apoptosis. Autophagy. 2011;7:436–437. doi: 10.4161/auto.7.4.14501. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Nakahira K, Haspel JA, Choi AM. Autophagy in pulmonary diseases. Annu. Rev. Physiol. 2012;74:377–401. doi: 10.1146/annurev-physiol-020911-153348. [DOI] [PubMed] [Google Scholar]
- Saffiotti U, Daniel LN, Mao Y, Shi X, Williams AO, Kaighn ME. Mechanisms of carcinogenesis by crystalline silica in relation to oxygen radicals. Environ. Health Perspect. 1994;102(Suppl. 10):159–163. doi: 10.1289/ehp.94102s10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Yin N, Xuan LL, Yao CS, Meng AM, Hou Q. Vam3, a derivative of resveratrol, attenuates cigarette smoke-induced autophagy. Acta Pharmacol. Sin. 2012;33:888–896. doi: 10.1038/aps.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 2010;17:1867–1881. . doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Patel-Vayas K, Shen J, Laskin JD, Laskin DL. Classical and alternative macrophage activation in the lung following ozone-induced oxidative stress. Toxicol. Appl. Pharmacol. 2012;263:195–202. doi: 10.1016/j.taap.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Jin Y, Lee SJ, Zhang M, Kim HP, Stolz DB, Ryter SW, Choi AM. Hyperoxia-induced LC3B interacts with the Fas apoptotic pathway in epithelial cell death. Am. J. Respir. Cell Mol. Biol. 2012;46:507–514. doi: 10.1165/rcmb.2009-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unuma K, Aki T, Matsuda S, Funakoshi T, Yoshida K, Uemura K. Inducer of heme oxygenase-1 cobalt protoporphyrin accelerates autophagy and suppresses oxidative damages during lipopolysaccharide treatment in rat liver. Hepatol. Res. 2013;43:91–96. doi: 10.1111/j.1872-034X.2012.01049.x. [DOI] [PubMed] [Google Scholar]
- van der Toorn M, Rezayat D, Kauffman HF, Bakker SJ, Gans RO, Koeter GH, Choi AM, van Oosterhout AJ, Slebos DJ. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L109–L114. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Mo Y, Feng L, Chien S, Tollerud DJ, Zhang Q. DNA damage caused by metal nanoparticles: involvement of oxidative stress and activation of ATM. Chem. Res. Toxicol. 2012;25:1402–1411. doi: 10.1021/tx200513t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger B, Laskin JD, Sunil VR, Sinko PJ, Heck DE, Laskin DL. Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity. Pulm. Pharmacol. Ther. 2010;24:92–99. doi: 10.1016/j.pupt.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging. 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos–Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol. Biol. Cell. 2008;19:3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang HM, Shi Y, Lustgarten M, Li Y, Qi W, Zhang BX, Van Remmen H. Loss of manganese superoxide dismutase leads to abnormal growth and signal transduction in mouse embryonic fibroblasts. Free Radic. Biol. Med. 2010;49:1255–1262. doi: 10.1016/j.freeradbiomed.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Qi Y, Liao M, Xu M, Bower KA, Frank JA, Shen HM, Luo J, Shi X, Chen G. Autophagy is a cell self-protective mechanism against arsenic-induced cell transformation. Toxicol. Sci. 2012;130:298–308. doi: 10.1093/toxsci/kfs240. [DOI] [PubMed] [Google Scholar]
- Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2010;278:403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- Zhu L, Barret EC, Xu Y, Liu Z, Manoharan A, Chen Y. Regulation of cigarette smoke (CS)-induced autophagy by Nrf2. PLoS One. 2013;8:e55695. doi: 10.1371/journal.pone.0055695. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zois CE, Giatromanolaki A, Kainulainen H, Botaitis S, Torvinen S, Simopoulos C, Kortsaris A, Sivridis E, Koukourakis MI. Lung autophagic response following exposure of mice to whole body irradiation, with and without amifostine. Biochem. Biophys. Res. Commun. 2011;404:552–558. doi: 10.1016/j.bbrc.2010.12.024. [DOI] [PubMed] [Google Scholar]