Abstract
Multiple factors may contribute to the development of emergence delirium in a child. We present the case of a healthy 12-year-old girl who received preoperative midazolam with the desired anxiolytic effect, underwent a brief general anesthetic, and then exhibited postoperative delirium, consisting of a transient associative agnosia and expressive aphasia. Administration of flumazenil led to immediate and lasting resolution of her symptoms. We hypothesize that γ-aminobutyric acid type A receptor-mediated effects, most likely related to an atypical offset of midazolam, are an important subset of emergence delirium that is amenable to pharmacologic therapy with flumazenil.
Introduction
Emergence delirium (ED) is a common cause of pediatric postoperative distress and presents a challenging problem for children, families, and postanesthesia care unit (PACU) staff. With an incidence ranging from 10 – 63%,1–6 ED is characterized by nonpurposeful restlessness, agitation, thrashing, crying/moaning, disorientation, incoherence, and paranoid ideation.5–6 The Pediatric Anesthesia Emergence Delirium scale, which scores eye contact, purposeful actions, awareness, restlessness, and consolability, was developed and validated to better define and quantify ED.7 Many signs and symptoms of ED overlap with those of paradoxical midazolam reactions.8–10 Flumazenil, a competitive antagonist at the benzodiazepine binding site of the γ-aminobutyric acid type A (GABAA) receptor, has been used to successfully treat postoperative ED associated with midazolam exposure.11–12 Though some recommend midazolam for treatment of ED,13 additional midazolam may worsen symptoms for this subset of children with ED.12,14–15
The ED literature lacks high quality, patient-verified narrative and largely consists of medical staff observations in part because midazolam’s anterograde amnestic effects typically prevent subjects from recounting their own experiences. We present the unusual case of an articulate older child with ED who was fully responsive to flumazenil, able to recall her immediate postoperative condition, and thus capable of relating experiential details.
She assented and her parents consented to the publication of this case report.
Case Description
A healthy 12-year-old 39 kg right-handed female was scheduled for removal of a small abdominal wall nevus. Oral midazolam 10 mg was administered 40 minutes before inhaled induction with nitrous oxide and sevoflurane. Anesthesia was maintained with 2–4% sevoflurane via mask-assisted ventilation for a cumulative sevoflurane dose of 0.52 minimal alveolar concentration-hours.16 A 6 ml mixture of lidocaine 0.5% and bupivacaine 0.125% with epinephrine 2.5 mcg/ml was infiltrated at the surgical site. Morphine 2 mg IV and ondansetron 4 mg IV were administered intraoperatively. During the uneventful 13-minute procedure 250 ml IV lactated Ringer’s solution was infused.
The patient was transported to the PACU, initially sleeping peacefully with normal vital signs. However, upon awakening 10 minutes later, the patient began crying and hyperventilating. She was very anxious, unable to speak, and unresponsive to her parents’ attempts to console her. Aside from an increased respiratory rate of 28 breaths/minute, vital signs remained normal. Her Pediatric Anesthesia Emergence Delirium score was 13 (eye contact=0, purposeful action=3, aware of surroundings=2, restless=4, inconsolable=4), corresponding to a diagnosis of ED.7 Flumazenil 0.2 mg IV was administered with an instant calming effect. Within a few minutes she regained the ability to speak and interact normally. She reported that she remembered the entire episode, describing being unable to speak, but being aware of her surroundings. She had not fully recognized her parents and stated that she was crying and anxious due to her inability to speak and relate to those around her. She denied pain or nausea. She was discharged home after 2 hours of otherwise uneventful recovery.
Several months later we conducted a telephone interview with the child and her parents. The 5th grade honor student exceled in writing and had no history of migraines or other headaches. She confirmed taking no medications and had not consumed grapefruit. She recalled taking oral midazolam; however, she remembered neither entering the operating room nor facemask application. In the PACU she first remembered having difficulty opening her eyes, being unable to stop shaking, and then being unable to make purposeful movements. When she opened her eyes, she recognized her parents as familiar, that is, known to her, but she did not recall their names. She reported being “unable to match them up” with what she knew about them. She also had difficulty speaking and processing what was said to her, causing her to panic. She was so startled that, in retrospect, she claimed no words would have comforted her. With the administration of flumazenil, however, everything changed in an instant. She reported an immediate “cooling, relaxing, calming effect” and subsequent full recognition of parent identity. In that moment she regained the confidence that “I’ll be fine.” “I started to think again,” she said.
During the first few postoperative days, the patient slept in her parents’ bedroom, but this did not continue. At the time of the phone interview, the patient had had no nightmares about the event. Because of the extraordinary detail recalled and because of the patient’s exceptional language and writing skills, we invited her to compose a longer narrative and, if she wished, to become an author. She declined, expressing fear that she would be identified.
An in-depth family medication/anesthetic history was significant. Her father had prolonged sedation after general anesthesia for 3 different elective day surgery procedures. It is unknown whether benzodiazepines were administered. With codeine, her mother reported having pruritus and hallucinations described as “bugs under my skin.” There was no family history of migraines, neurologic, or anxiety disorders.
Discussion
After flumazenil-specific reversal of ED, our patient had complete recall of postoperative events and was able to describe her perceptions and feelings. This allowed us to diagnose both a transient expressive aphasia and associative agnosia, providing new insight into the enigma of ED. Though suggested in some observational reports,9,11 these neurologic conditions have neither been well-defined nor named. Expressive aphasia, or Broca’s aphasia, results from abnormalities in the frontotemporal cortex as found in cognitively intact adults who have suffered strokes or injuries affecting speech production, and as demonstrated by functional magnetic resonance imaging.17 Our patient further displayed an associative prosopagnosia, or associative visual agnosia, in which her parents’ faces were familiar but for which semantic knowledge was impaired. Older studies have mapped this condition to the occipitotemporal region,18 but more recent work using functional magnetic resonance imaging suggests association with the right anterior temporal lobe.19–20 Because voice recognition might also have been compromised, our patient may have suffered a form of multimodal person recognition disorder.21 Regardless, this constellation of neurologic entities contributed to her intense fear and anxiety and may explain the outward fear that younger, nonverbal children exhibiting ED project.
Other authors have theorized that paradoxical benzodiazepine reactions could underlie a subset of ED.12 Short-duration surgeries, facilitated by anesthesia with insoluble volatile anesthetics leading to rapid emergence, clearly contribute. Rapidly changing volatile drug brain concentrations along with short-acting benzodiazepine offset likely restores primitive arousal networks in the absence of proper reconnection of higher order neural networks required for full cognitive processing. Based on our patient’s end-tidal sevoflurane concentration, minute ventilation, weight, estimated cardiac output, and anesthetic duration, the predicted sevoflurane vessel-rich concentration would have decreased by > 90% even before PACU arrival.22 Although it is possible that low sevoflurane blood levels contributed to ED, we believe that midazolam is the more likely causal drug because significant effect-site concentrations are certain and because flumazenil is specific for the GABAA receptor benzodiazepine binding site. Although absolute serum midazolam concentrations after oral administration are difficult to predict, the reported maximum serum concentration after a 0.25 mg/kg dose is 55.6 ± 30.2 ng/mL, while the tmax = 2.0 ± 1.5 hours and the t1/2 = 4.8 ± 3.3 hours for a 0.5 mg/kg dose.23 Importantly, midazolam pharmacokinetics were not altered by concomitant use of anticonvulsants, imidazole antifungals, macrolide antibiotics, herbal medications or grapefruit juice.24,25,26 Our patient exhibited symptoms approximately 80 minutes after receiving oral midazolam (Figure 1), and given the wide variability of the reported pharmacologic data, it is impossible to estimate her serum midazolam level at that time point. Flumazenil has not been reported to treat sevoflurane-associated ED in the absence of benzodiazepine exposure, but it has been reported to reverse paradoxical midazolam reactions.9–12 Typically only 1 dose is required despite large prior doses of midazolam. Most reports note that flumazenil administration stops disruptive behavior but does not completely reverse sedation or amnesia.
Figure 1.
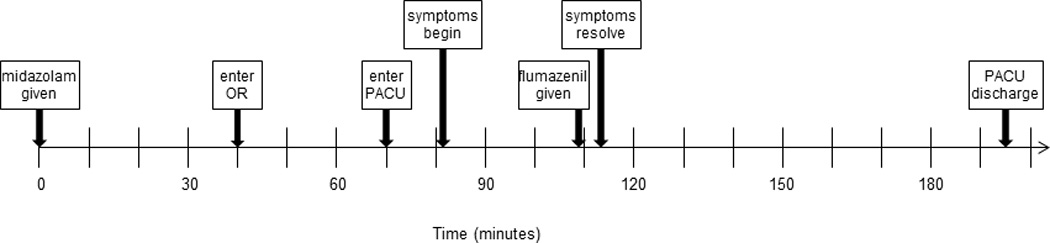
Timeline of events.
The interindividual variability associated with these reactions could have pharmacokinetic and/or pharmacodynamic components. Within the affected brain regions there could be different receptor densities, receptor subtype populations, or unusual genetic variants. These symptoms could be mediated by GABAA receptors in Broca’s area and along other neural pathways in the temporal lobe. Specifically, α-2 and α-3 GABAA receptor subtypes and their allosteric modulation by midazolam and flumazenil may have altered the balance of anxiety/anxiolysis,27 whereas the α-1 GABAA receptor subtype may have mediated the visual recognition impairment.28 Whether genetic differences, as suggested decades ago,29 might cause a change in GABAA receptor structure/distribution, benzodiazepine metabolism, or many other potential factors, is unknown.
In summary, postanesthetic ED with transient associative agnosia and expressive aphasia may occur with the atypical offset of midazolam. This phenomenon may be obscured by the drug’s amnestic properties and may be difficult to detect in younger, less articulate children. Rapid emergence and, though not a factor in the present case, untreated pain triggering sudden changes in arousal state, are likely contributory. Patients experiencing these reactions should be treated in a calm manner because forceful voices can be frightening to both patients and parents. Expeditious diagnosis and proper treatment can minimize the duration of this terrifying state. Further work is needed to determine which patients are predisposed to this phenomenon so that they can be identified and managed appropriately.
Acknowledgments
Funding: None
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Julie K. Drobish, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (Current Affiliation: Department of Anesthesiology, Penn State College of Medicine, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania.
Max B. Kelz, Department of Anesthesiology and Critical Care, Perelman School of Medicine, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania.
Patricia M. DiPuppo, Department of Nursing, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Scott D. Cook-Sather, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Department of Anesthesiology and Critical Care, Perelman School of Medicine, Philadelphia, Pennsylvania.
References
- 1.Bajwa SA, Costi D, Cyna AM. A comparison of emergence delirium scales following general anesthesia in children. Paediatr Anaesth. 2010;20:704–711. doi: 10.1111/j.1460-9592.2010.03328.x. [DOI] [PubMed] [Google Scholar]
- 2.Bong CL, Ng AS. Evaluation of emergence delirium in Asian children using the Pediatric Anesthesia Emergence Delirium Scale. Paediatr Anaesth. 2009;19:593–600. doi: 10.1111/j.1460-9592.2009.03024.x. [DOI] [PubMed] [Google Scholar]
- 3.Breschan C, Platzer M, Jost R, Stettner H, Likar R. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth. 2007;17:347–352. doi: 10.1111/j.1460-9592.2006.02101.x. [DOI] [PubMed] [Google Scholar]
- 4.Pieters BJ, Penn E, Nicklaus P, Bruegger D, Mehta B, Weatherly R. Emergence delirium and postoperative pain in children undergoing adenotonsillectomy: a comparison of propofol vs sevoflurane anesthesia. Paediatr Anaesth. 2010;20:944–950. doi: 10.1111/j.1460-9592.2010.03394.x. [DOI] [PubMed] [Google Scholar]
- 5.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 6.Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96:1625–1630. doi: 10.1213/01.ANE.0000062522.21048.61. [DOI] [PubMed] [Google Scholar]
- 7.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100:1138–1145. doi: 10.1097/00000542-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Golparvar M, Saghaei M, Sajedi P, Razavi SS. Paradoxical reaction following intravenous midazolam premedication in pediatric patients – a randomized placebo controlled trial of ketamine for rapid tranquilization. Paediatr Anaesth. 2004;14:924–930. doi: 10.1111/j.1460-9592.2004.01349.x. [DOI] [PubMed] [Google Scholar]
- 9.Massanari M, Novitsky J, Reinstein LJ. Paradoxical reactions in children associated with midazolam use during endoscopy. Clin Pediatr. 1997;36:681–684. doi: 10.1177/000992289703601202. [DOI] [PubMed] [Google Scholar]
- 10.Saltik IN, Ozan H. Role of flumazenil for paradoxical reaction to midazolam during endoscopic procedures in children. Am J Gastroenterol. 2000;10:3011–3012. doi: 10.1111/j.1572-0241.2000.03235.x. [DOI] [PubMed] [Google Scholar]
- 11.Sanders JC. Flumazenil reverses a paradoxical reaction to intravenous midazolam in a child with uneventful prior exposure to midazolam. Paediatr Anaesth. 2003;13:369–370. doi: 10.1046/j.1460-9592.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 12.Voepel-Lewis T, Mitchell A, Malviya S. Delayed postoperative agitation in a child after preoperative midazolam. J Perianesth Nurs. 2007;5:303–308. doi: 10.1016/j.jopan.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Dahmani S, Mantz J, Veyckemans F. Case scenario: severe emergence agitation after myringotomy in a 3-yr-old child. Anesthesiology. 2012;117:399–406. doi: 10.1097/ALN.0b013e31825fb069. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigo CR. Flumazenil reverses paradoxical reaction to midazolam. Anesth Prog. 1991;38:65–68. [PMC free article] [PubMed] [Google Scholar]
- 15.Roelofse JA, Stegmann DH, Hartshorne J, Joubert JJ. Paradoxical reactions to rectal midazolam as premedication in children. Int J Oral Maxillofac Surg. 1990;19:2–6. doi: 10.1016/s0901-5027(05)80558-2. [DOI] [PubMed] [Google Scholar]
- 16.Nickalls RWD, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane, and desflurane in man. Br J Anaesth. 2003;91:170–174. doi: 10.1093/bja/aeg132. [DOI] [PubMed] [Google Scholar]
- 17.Burns MS, Fahy J. Broca’s area: rethinking classical concepts from a neuroscience prospective. Top Stroke Rehabil. 2010;17:401–410. doi: 10.1310/tsr1706-401. [DOI] [PubMed] [Google Scholar]
- 18.Feinberg TE, Schindler RJ, Ochoa E, Kwan PC, Farah MJ. Associative visual agnosia and alexia without prosopagnosia. Cortex. 1994;30:395–411. doi: 10.1016/s0010-9452(13)80337-1. [DOI] [PubMed] [Google Scholar]
- 19.Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 20.Pancaroglu R, Busigny T, Johnston S, Sekunova A, Duchaine B, Barton JS. The right anterior temporal lobe variant of prosopagnosia. J Vision. 2011;11:573. [Google Scholar]
- 21.Gainotti G. Is the right anterior temporal variant of prosopagnosia a form of ‘associative prosopagnosia’ or a form of ‘multimodal person recognition disorder’? Neuropsychol Rev. 2013;23:99–110. doi: 10.1007/s11065-013-9232-7. [DOI] [PubMed] [Google Scholar]
- 22.Bailey JM. Context-sensitive half-times and other decrement times of inhaled anesthetics. Anesth Analg. 1997;85:681–686. doi: 10.1097/00000539-199709000-00036. [DOI] [PubMed] [Google Scholar]
- 23.Reed MD, Rodarte A, Blumer JL, Khoo K, Akmari B, Pou S, Kearns GL. The single-dose pharmacokinetics of midazolam and its primary metabolite in pediatric patients after oral and intravenous administration. J Clin Pharmacol. 2001;41:1359–1369. doi: 10.1177/00912700122012832. [DOI] [PubMed] [Google Scholar]
- 24.Liu YT, Hao HP, Liu CX, Wang GJ, Xie HG. Drugs as CYP3A probes, inducers and inhibitors. Drug Met Rev. 2007;39:699–721. doi: 10.1080/03602530701690374. [DOI] [PubMed] [Google Scholar]
- 25.Kupferschmidt HH, Ha HR, Ziegler WH, Meier PJ, Krahenbuhl S. Interaction between grapefruit juice and midazolam in humans. Clin Pharm Ther. 1995;58:20–28. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 26.Kiani J, Imam SZ. Medicinal importance of grapefruit juice and its interaction with various drugs. Nutrition J. 2007;6:33. doi: 10.1186/1475-2891-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- 28.Soto PL, Ator NA, Rallapalli SK, Biawat P, Clayton T, Cook JM, Weed MR. Allosteric modulation of GABAA receptor subtypes: effects on visual recognition and visuospatial working memory in rhesus monkeys. Neuropsychopharmacology. 2013;38:2315–2325. doi: 10.1038/npp.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Short TG, Forrest P, Galletly DC. Paradoxical reactions of benzodiazepines – a genetically determined phenomenon? Anaesth Intens Care. 1987;15:330–345. doi: 10.1177/0310057X8701500314. [DOI] [PubMed] [Google Scholar]


