Abstract
Background
Neoadjuvant erlotinib and customized adjuvant therapy are appealing but controversial. The purpose of this study was to evaluate the role of biomarker-guided neoadjuvant treatment strategy in patients with IIIA-N2 non-small cell lung cancer (NSCLC) stratified by epidermal growth factor receptor (EGFR) mutation status.
Findings
Patients with resectable histologically documented stage IIIA-N2 NSCLC were assigned to a neoadjuvant erlotinib arm or a gemcitabine/carboplatin (GC) arm based on EGFR mutation status. The primary endpoint was response rate (RR). Secondary endpoints were progression-free survival (PFS) and overall survival (OS).
Twenty-four patients with IIIA-N2 NSCLC were enrolled in the trial from January 2008 until May 2011. The overall response rate was 41.7 % and the PFS and OS were 7.9 and 23.2 months, respectively, in overall population. The RR was 58.3 % (7/12) for the erlotinib arm with mutant EGFR and 25.0 % (3/12) for the GC arm with wild type EGFR (P = 0.18). Median PFS was 6.9 months versus 9.0 months, respectively (P = 0.071). Median OS was 14.5 months for the erlotinib arm and 28.1 months for the GC arm (P = 0.201). No unexpected toxicities were observed.
Conclusions
The primary endpoint was met and biomarker-guided neoadjuvant treatment strategy in patients with IIIA-N2 NSCLC is feasible. Erlotinib alone in neoadjuvant setting of EGFR mutant population showed an improved response but without survival benefits.
Trial registration
ClinicalTrials.gov NCT00600587 https://www.clinicaltrials.gov/ct2/show/NCT00600587?term=NCT00600587&rank=1
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-015-0151-3) contains supplementary material, which is available to authorized users.
Keywords: IIIA-N2, Biomarker guided, EGFR mutation, Lung cancer, Neoadjuvant therapy
Findings
Background
Patients with stage IIIA non-small cell lung cancer (NSCLC) represent a relatively heterogeneity with ipsilateral mediastinal lymph nodes (N2) involved, and relative roles of treatment modalities are not clearly defined. Chemoradiotherapy is an important treatment for stage IIIA disease but limited by treatment-related life-threatening toxicities [1]. And previous work showed that status of gene expression was related to different degrees of how much gemcitabine improved survival of patients with advanced NSCLC [2]. Recently, an individual participant data meta-analysis [3] found that neoadjuvant chemotherapy improves an absolute 5-year survival of 5 % and may be preferable for patients with poorer prognosis or larger tumors. However, chemotherapy has reached a therapeutic plateau in NSCLC. A literature-based meta-analysis reported [4] that tyrosine kinase inhibitors (TKIs) could provide more survival benefits for patients with advanced epidermal growth factor receptor (EGFR) mutant NSCLC, indicating the importance of selected patients with specific mutations when exploring efficacy of targeted therapy. In patients with EGFR mutation positive NSCLC, an EGFR-TKI may provide a dramatic response in a metastatic setting [5–7]. The primary analysis in the OPTIMAL study, comparing first-line erlotinib with gemcitabine/carboplatin (GC) in patients with advanced NSCLC with EGFR mutations, showed relatively higher response rate of 82.9 % (68/82) and significantly longer progression-free survival (PFS) with erlotinib than with GC therapy [8]. Since 2007, several case reports and retrospective studies with small sample sizes have shown that neoadjuvant EGFR-TKI therapy results in N2 downstaging in patients with stage IIIA-N2 NSCLC harboring EGFR mutation [9–12]. In two phase II studies, neoadjuvant EGFR-TKI showed low toxicity and sufficient activity in an enriched population [13, 14]. However, no survival data in neoadjuvant TKI therapy were obtained.
In the near future, lung cancer treatment will likely become more patient-tailored by a molecular-based strategy. Neoadjuvant EGFR-TKI therapy and customized adjuvant therapy (IFCT-0801, TASTE trial) are appealing but controversial strategies in patients with IIIA-N2 NSCLC [15]. The aim of this study was to investigate the efficacy of biomarker-guided neoadjuvant treatment strategy with erlotinib versus GC regimen in patients with stage IIIA-N2 NSCLC stratified by EGFR activating mutations and explore a new treatment strategy for this subset of patients.
Results and discussion
Results
Patient characteristics
Twenty-four patients with IIIA-N2 NSCLC diagnosed by mediastinoscopy or endobronchial ultrasound (EBUS) were enrolled from January 2008 to May 2011. The cutoff date for PFS and overall survival (OS) data was March 22nd, 2015. The median follow-up was 24.4 months (range, 1.7–68.7 months). Twelve cases with a mutant-type EGFR were assigned to the erlotinib arm and 12 cases with wild type EGFR to the GC arm. In the erlotinib arm, two patients with EGFR L858R mutation also had a KRAS mutation or EML4-ALK translocation. No T790M mutation was found in surgical specimens after neoadjuvant erlotinib therapy. Age, gender, smoking status, histology, postoperative radiotherapy, and the median follow-up were balanced between arms. The clinical and histological data for all patients are listed in Table 1.
Table 1.
Baseline patient demographics and clinical characteristics
| Characteristic | Erlotinib | GC | P value |
|---|---|---|---|
| Median age at diagnosis (years) | 60.17 ± 13.31 | 58.75 ± 12.12 | 0.71 |
| Gender, n (%) | 0.68 | ||
| Male | 6 (6/12, 50.00 %) | 8 (8/12, 66.67 %) | |
| Female | 6 (6/12, 50.00 %) | 4 (4/12, 33.33 %) | |
| Smoking duration | 6.25 ± 11.89 | 20.83 ± 21.09 | 0.10 |
| Daily cigarette consumption, n | 7.92 ± 14.99 | 17.92 ± 18.27 | 0.18 |
| Pathology, n (%) | 1.00 | ||
| Adeno | 11 (11/12, 91.67 %) | 11 (11/12, 91.67 %) | |
| LCNEC | 0 (0.00 %) | 1 (1/12, 8.33 %) | |
| Adenoid cystic carcinoma | 1 (1/12, 8.33 %) | 0 (0.00 %) | |
| Mutation status, n (%) | <0.001 | ||
| EGFR/KRAS wild type | 0 | 11 | |
| KRAS | 0 | 1 | |
| Exon 19 deletion | 6 | 0 | |
| L858R | 4 | 0 | |
| EGFR mutation with KRAS codon | 1 | 0 | |
| 12/13* | |||
| EGFR mutation with EML4-ALK* | 1 | 0 | |
| Deletion in BIM | 2/8 | 0/9 | |
| Postoperative radiotherapy, n | 3/6 | 2/7 | 0.59 |
| Median follow-up (months) | 19.3 (5.8–64.0) | 35.6 (1.7–68.7) | 0.41 |
EGFR epidermal growth factor receptor, LCNEC large-cell neuroendocrine carcinoma, GC gemcitabine/carboplatin, BIM Bcl-2-interacting mediator of cell death
*Two patients in the erlotinib arm with the EGFR L855R mutation also had the KRAS mutation or EML4-ALK translocation
Efficacy
The overall response rate was 41.7 %(10/24). The RR was 58.3 % (7/12) in the erlotinib arm and 25.0 % (3/12) in the GC arm (P = 0.18). Overall, 54.2 % (13/24) patients received surgical resection. Three of six cases in the erlotinib arm and five of seven cases in the GC arm underwent complete resection (R0). The clinical N2 downstaging rate was 25.0 % (3/12) in both arms. The pathological N2 complete response rates were 16.7 % (2/12) versus 25 % (3/12) (P = 0.64) (Table 2 and Fig. 1).
Table 2.
Evaluation of neoadjuvant therapy efficacy
| Index | Evaluation | Erlotinib arm n = 12, (%) | GC arm n = 12, (%) | P value |
|---|---|---|---|---|
| RECIST | PR | 7/12 (58.33) | 3/12 (25.00) | 0.18 |
| SD | 2/12 (16.67) | 6/12 (50.00) | ||
| PD* | 3/12 (25.00) | 2/12 (16.67) | ||
| NA | 0 (0.00) | 1/12 (8.33) | ||
| Clinical N2 downstaging | 3/12 (25.00) | 3/12 (25.00) | 1.00 | |
| Pathological N2 downstaging | 2/12 (16.67) | 3/12 (25.00) | 0.64 | |
| Resection | R0 | 3/6 (50.00) | 5/7 (71.43) | 0.59 |
| R1 | 3/6 (50.00) | 2/7 (28.57) |
Values are presented as n (percentage)
GC gemcitabine/carboplatin, RECIST Response Evaluation Criteria in Solid Tumors, PR partial response, SD stable disease, PD progressive disease, NA not available
*Two patients with the EGFR L858R mutation and the KRAS mutation or EML4-ALK translocation developed primary resistance to induction erlotinib
Fig. 1.
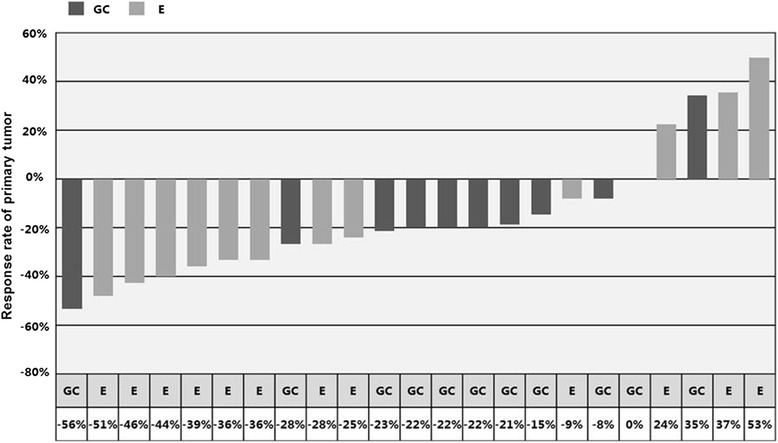
Waterfall plot of response to neoadjuvant treatment. Abbreviations: GC, gemcitabine/carboplatin; E, erlotinib. Note: The response rate of one case in the GC arm was not available
Survival and failure models [16]
Overall, the PFS and OS were 7.9 and 23.2 months, respectively (Fig. 2a). The median PFS was 6.9 months (95 % confidence interval (CI), 3.8–10.0) for erlotinib arm and 9.0 months (95 % CI, 3.1–15.0) for GC arm (P = 0.071). The median OS was 14.5 months (95 % CI, 1.0–28.1) for the erlotinib arm and 28.1 months (95 % CI, 0.0–66.2) for the GC arm (P = 0.201) (Fig. 2b, c). Among patients receiving surgery, the median PFS was 8.6 months (95 % CI, 5.8–11.3) for the erlotinib arm and 28.9 months (95 % CI, 0.0–64.0) for the GC arm (P = 0.018) and the median OS was 25.5 months and 57.3 months, respectively (P = 0.162) (Fig. 2d, e). The local recurrence rate (5/12) was similar to the distant metastasis rate (7/12) in the GC arm, whereas the common initial failure model in the erlotinib arm was distant metastasis (10/12), particularly brain metastases (3/10) and bilateral lung metastases (8/10) (Additional file 1).
Fig. 2.
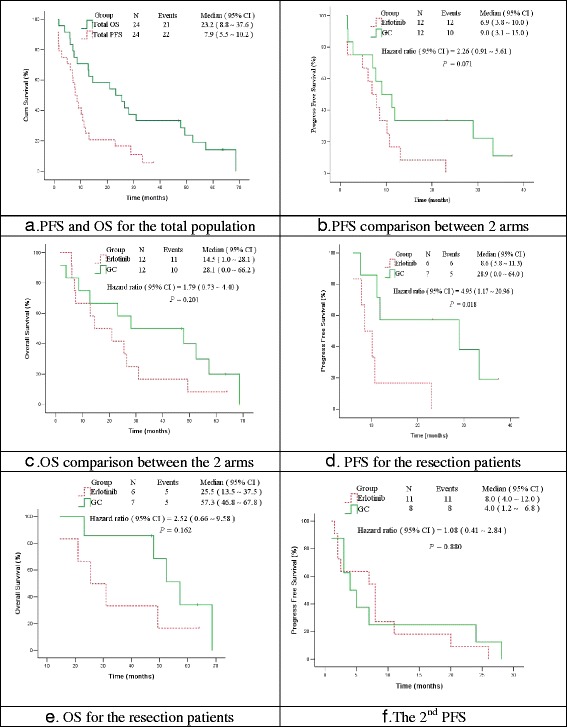
PFS and OS. a PFS and OS for the total population; b PFS comparison between two arms; c OS comparison between two arms; d PFS for the resection patients; e OS for the resection patients; f The 2nd PFS. Abbreviations: GC, gemcitabine/carboplatin; PFS, progression-free survival; OS, overall survival
Treatment toxicity and feasibility
Overall, neoadjuvant therapies were well tolerated. The most common side effects in the erlotinib arm were rash (100 %; 16.7 % as grade 3–4) and diarrhea (41.6 %). Only one case had postoperative bleeding. Another case in the erlotinib arm suffered from acute radiotherapy-induced pneumonitis related to death. Three cases in the GC arm exhibited grade 4 thrombocytopenia, two of which received blood transfusion.
EGFR-TKI retreatment
The second median PFS, after the first progression, was 8.0 months (95 % CI, 4.0–12.0) for the erlotinib arm and 4.0 months (95 % CI, 1.2–6.8) for the GC arm (P = 0.880) (Fig. 2f). In addition, all six cases undergoing R0/R1 resection in the erlotinib arm achieved PR to TKI retreatment at progression, with a median PFS of 9.4 months.
Biomarker
Genetic profiles in two arms are summarized in Additional file 1, indicating rare genetic heterogeneity between initial specimens and surgical samples after neoadjuvant therapy. There was only one patient in each arm whose gene mutation status transferred from mutant type to wild type or contrariwise. The BIM deletion polymorphism had no correlation with TKI efficacy (Additional file 1). Immunohistochemistry (IHC) was conducted to detect protein expressions on resected samples after induced elotinib therapy. In all six cases, pEGFR (Tyr1068) has been deregulated. Two cases with L858R mutation enjoyed the longest PFS, among which the p44/42MAPK (Erk1/2) (137 F5) was deregulated and the pAkt (Thr308) (244 F9) was most activated compared with other five cases. (Additional file 2).
Discussion
Several randomized controlled trials (RCTs) have established the foundation of EGFR-TKI as first-line therapy in advanced NSCLC with EGFR mutation [5–8]. Indications for EGFR-TKIs have been transferred from second-line to first-line in targeted populations. However, NSCLC is a heterogeneous disease between early and advanced stages and between wild and mutant EGFR lung cancer. Therefore, principles for TKI therapy might be different between first-line, neoadjuvant, and adjuvant treatment [17]. The use of EGFR-TKI in neoadjuvant treatment of NSCLC has been evaluated in limited numbers of phase II studies without survival data. Furthermore, customized NSCLC adjuvant therapy (IFCT-0801, TASTE trial) [15] and systematic therapy (BATTLE) [18] had validated its feasibility. Thus, biomarker-guided neoadjuvant treatment should be further evaluated in neoadjuvant settings for locally advanced but operable diseases.
To our knowledge, this is the first trial to evaluate the feasibility of biomarker-guided neoadjuvant therapy among patients with N2 NSCLC receiving TKI or chemotherapy as neoadjuvant regimen based on EGFR mutation status. The RR was 41.7 %, higher than 35.4 % reported in Crystalloid Versus Hydroxyethyl Starch Trial (CHEST) [19] with chemotherapy alone [20, 21], 5–11 % with TKI alone in phase II trial in the total population [13, 14], and close to 46 % of the chemo-TKI sequential treatment strategy in enriched population [22] (Table 3). There were two cased achieving downstaging of lymph nodes (2/6), while three of seven in GC arm; and all of these five cases receiving complete resection. Besides, cases whose primary tumor responded to neoadjuvant therapy had more chances of R0 surgeries, although tumors might invade visceral pleura affecting downstaging of T.
Table 3.
Comparison of neoadjuvant trials in lung cancer
| Trial | Phase | TNM | Population | Regimen | Sample | RR |
|---|---|---|---|---|---|---|
| Chemotherapy | ||||||
| Roth 1994 [21] | II | IIIA | Total | CEP | 28 | 35 % |
| Rosell 1994 [20] | II | IIIA | Total | MIC | 30 | – |
| Scagliotti 2012 [19] (CHEST) | III | I-IIIA | Total | GC | 129 | 35.4 % |
| TKI | ||||||
| Lara-Guerra 2009 [13] | II | I | Total | G | 36 | 11 % |
| II | I | Mut EGFR | G | 6 | 50 % | |
| Schaake 2012 [14] | II | I-IIIA | Total | E | 60 | 5 % |
| II | I-IIIA | Enriched | E | 29 | 34 % | |
| Chemo-TKI | ||||||
| Lu 2013 [22] (CTONG 1101) | II | IIIA-N2 | Enriched | E + GC | 39 | 46 % |
| Bio-maker guided | ||||||
| Zhong 2014 (CSLC 0702) | II | IIIA-N2 | Total | E or GC | 24 | 42 % |
| II | IIIA-N2 | Mut EGFR | E | 12 | 58.3 % | |
| II | IIIA-N2 | WT-EGFR | GC | 12 | 25 % |
CEP cyclophosphamide/etoposide/cisplatin, MIC mitomycin/ifosfamide/cisplatin, GC gemcitabine/carboplatin, E erlotinib, G gefitinib, TKI tyrosine kinase inhibitor, RR response rate, EGFR epidermal growth factor receptor, Mut mutant, WT wild type
Overall, the PFS and OS were 7.9 and 23.2 months, respectively, similar to the INT 0139 trial in radiotherapy plus chemotherapy with/without surgical resection for stage IIIA NSCLC [23]. Therefore, biomarker-guided neoadjuvant treatment strategy in patients with IIIA-N2 NSCLC based on EGFR mutation status is feasible.
However, benefits for improved response to neoadjuvant erlotinib therapy in this trial did not transfer to survival benefits. According to previous work [16], the most common failure model in the erlotinib arm was distant metastasis (10/12). The PFS and OS did not differ significantly between two arms, although the PFS and OS tended to be longer for all patients in the GC arm. There appears to be no appropriate explanations for why TKIs are not potentially contributable to better PFS in the neoadjuvant setting but so dramatically better than chemotherapy in patients with EGFR mutation in the metastatic settings. In our study, an analysis of EGFR mutation abundance in sequential plasma samples showed that the abundance of plasma L858R dropped significantly 1 week after R0 resection, but it rebounded soon after progression (Fig. 3). Furthermore, longer PFS contributed partly to the expression of pEGFR and the downstream molecules (Additional file 2). Riely and colleagues also reported a rebound effect or disease flare phenomenon after discontinuing TKI in patients with advanced EGFR mutant NSCLC developing acquired resistance to TKI and that the optimal treatment strategy should be to add another agent to TKI or switch to systemic chemotherapeutic drugs [24]. Similarly, after the termination of neoadjuvant TKI before receiving surgery in patients with IIIA-N2 disease, the sudden removal of oncogene inhibition may promote potential residual circulating tumor cells to accelerate and rebound, resulting in more aggressive diseases. So it’s imperative to add standard care of neoadjuvant chemotherapy to reduce disease flare and improve complete resection rate of patients with IIIA-N2 NSCLC. Besides, this might partially explain why patients in erlobinib relapsed and developed distant metastasis with higher incidence. RADIANT study, a randomized double-blind phase III trial of adjuvant erlotinib versus placebo following complete tumor resection with/without adjuvant chemotherapy in patients with stage IB-IIIA EGFR positive (IHC/FISH) non-small cell lung, reported in ASCO (American Society of Clinical Oncology, 2014) by Karen Kelly and colleagues, figured out that more patients developed brain relapse in erlotinib group than in placebo group (40.0 % vs. 12.9 %, respectively) and that no statistically significant difference of survival benefits were observed, indicating that TKIs just delay tumor recurrence rather than eliminate chances of recurrence and that poor ability of TKI permeating through blood–brain barrier results in relative lower concentration of erlotinib in central nervous system. Another recently activated trial, the ALCHEMIST study (registered on ClinicalTrials.gov as NCT02194738), aimed at comparing erlotinib and placebo as adjuvant therapy based on genetic testing. Although this study detects EGFR mutation status by direct sequencing which is with less sensitivity, its results indeed deserve expectation to improve tailored treatment in real world. Thus, it is warranted to combine neoadjuvant and adjuvant TKI treatment and enhance longer duration of TKI and strength of systemic chemotherapy as standard care when diseases relapse based on genetic analysis [25]. In our study, there was one patient receiving surgical resection in each arm whose genetic mutation status transferred from mutant type to wild type or contrariwise (Additional file 1). Chin et al. [26] figured out that first-line chemotherapy to patients with EGFR mutant NSCLC would decrease sensitivity to TKIs as second-line therapy. Similar work conducted by Wang J [27] indicated that intra-tumoral heterogeneity may be contributable to reduced EGFR mutation frequency caused by chemotherapy. According to Zhou Q [28], EGFR mutation abundance is significantly related to identity of mutation status and sensitivity of detecting methods. Gene mutation switching to EGFR wild type in erlotinib arm after neoadjuvant TKI therapy is possibly due to relative low abundance of EGFR mutation. Therefore, detecting methods with more sensitivity are extremely essential to identify pseudo-heterogeneity. Direct sequencing (DS) could detect samples with more than 10 % EGFR mutation frequency, whereas ARMS is with higher sensitivity (0.1–1 %) [29]. However, DS was most widely used when our study was designed and activated; lowing EGFR mutation abundance might not be detected and potential bias could cloudy the interpretation of current data. That is why there is an ongoing study in our team to compare efficacy and accuracy of DS vs. ARMS.
Fig. 3.
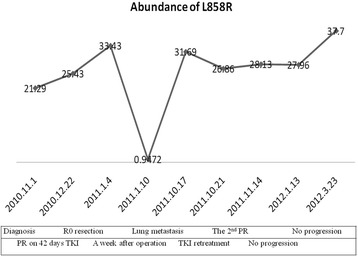
Plasma L858R abundance in the neoadjuvant TKI setting. Abbreviations: PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor. One week after the R0 resection, the abundance of plasma L858R decreased significantly, but it rebounded soon after progression. However, the PR in the first- and second-line TKI therapy did not decrease the level of plasma L858R for one case
Several limitations existed in our study. Firstly, according to clinical characteristics in Table 1, smoking duration in erlotinib arm was apparently shorter than that in GC arm despite of P > 0.05. However, EGFR mutant NSCLC is indeed different from EGFR wild type NSCLC involving more smokers in clinical characteristics. Besides, more genetic heterogeneity of EGFR mutation was observed in erlotinib, which might result in relative lower response rate of TKI and cloudy interpretation of data in our study. Our study were based on populations with different biological features, with EGFR mutant and wild type, which is the basic principle of biomarker-guided study design. Although biases related to non-randomized clinical trials existed, our trial contributed to neoadjuvant TKI and biomarker-guided therapy in patients with IIIA-N2 NSCLC. Furthermore, based on this trial, CTONG has launched a multicenter RCT to elucidate the role of perioperative TKIs [CTONG1103, a national, multicenter, randomized, controlled, open-label, phase II trial of erlotinib versus GC as (neo)adjuvant therapy in stage IIIA-N2 NSCLC with EGFR mutation in exon 19 or 21 (EMERGING); registered in ClinicalTrials.gov (NCT01407822)] in 2011. Ninety patients with resectable stage IIIA-N2 NSCLC harboring EGFR mutations will be randomized to neoadjuvant erlotinib arm for 42 days or GC arm for two cycles. After complete resections, patients will continue erlotinib for 1 year or the GC regimen for two cycles (Fig. 4).
Fig. 4.
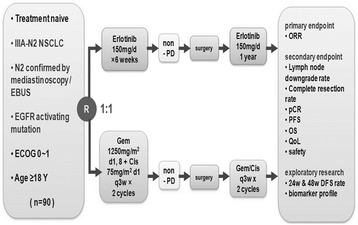
Study design of the CTONG 1103 (EMERGING). Abbreviations: NSCLC, non-small cell lung cancer; EBUS, endobronchial ultrasound; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group; PD, progressive disease; ORR, object response rate, CR, complete response; PFS, progression-free survival; OS, overall survival; QOL, quality of life
Conclusions
To our knowledge, CSLC 0702 is the first phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 NSCLC based on EGFR mutation status with PFS and OS data. The trial met its primary outcome and validated the feasibility of this strategy. Nevertheless, erlotinib alone in neoadjuvant setting tended to show an improved response but without better PFS or OS. Brain and lung metastases were most common failure models. The role of TKIs in first-line setting of advanced NSCLC should not be simply extrapolated to neoadjuvant therapy. More RCTs combining neoadjuvant with adjuvant EGFR-TKI therapy in a larger population are warranted to validate the role of perioperative TKI therapy. We look forward to results of these trials to provide convincing evidences for customized therapy for patients with resectable NSCLC [30].
Methods
Study design
This study, conducted in Guangdong General Hospital, China, was designed as an open-label, single-center, non-randomized, phase II clinical trial. It was approved by a local independent ethics committee and designed in accordance with Good Clinical Practice Guidelines. Written informed consents were obtained from patients before the start of treatment. Patients with resectable stage IIIA-N2 NSCLC diagnosed by mediastinoscopy or EBUS were assigned at a ratio of 1:1 to the neoadjuvant erlotinib arm or the GC arm based on EGFR mutation status. This study was sponsored by Chinese Society of Lung Cancer (CSLC 0702), the predecessor of Chinese Thoracic Oncology Group (CTONG), and was registered at ClinicalTrials.gov as NCT00600587.
Based on detection of mutation status, patients with mutant type EGFR in tumor tissue received 42-day administration of neoadjuvant erlotinib, and patients with wild type EGFR received an intended three cycles of GC regimen to serve as concurrent assignment. A CT scan or a positron emission tomography/computed tomography (PET/CT) was performed at 1–7 days after neoadjuvant treatment discontinuation. Patients with stable disease (SD) or a partial response (PR) underwent a thoracotomy which was scheduled to be done within 1 week after discontinuation. A radical resection of the tumor preferably by lobectomy and regional lymph node dissection with sampling of at least three N1 and three mediastinal lymph node stations was involved. Patients with progressive disease received second-line therapy or combined chemoradiotherapy. The primary outcome was response to neoadjuvant therapy. Secondary outcome measures were safety, PFS, and OS (Fig. 5).
Fig. 5.
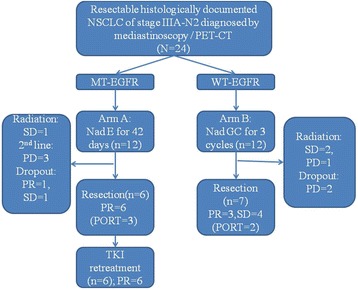
Flow chart. Abbreviations: NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; E, erlotinib; GC, gemcitabine/carboplatin; PR, partial response; SD, stable disease; PD, progressive disease; NA, not available; PORT: postoperative radiotherapy; Nad,neoadjuvant; MT, mutant type; WT, wild type
Patient eligibility
Patients with newly diagnosed resectable stage IIIA-N2 NSCLC and confirmed by mediastinoscopy or EBUS (i.e., clinical T1-3 N2) were enrolled. All patients were evaluated in a multidisciplinary tumor board discussion. The diagnosis had to be histologically or cytologically confirmed with sufficient tissue samples to perform gene analysis. Candidates, having ECOG performance status of 0–1, adequate hematological and hepatic-renal functions, and qualified lung function, were required to tolerate neoadjuvant therapy and a lobectomy and radical lymph node dissection. No pregnant or breast feeding patients were included. In addition, patients with a small cell lung cancer component, any unstable systemic disease, or exposure to investigational drug therapy or other concurrent anticancer therapies outside of this trial were excluded.
Efficacy assessment
Tumor specimens and imaging data were reviewed and analyzed by the Guangdong Lung Cancer Institute. The CT or FDG-PET/CT scans were performed after study treatments were compared with baseline scans. Radiological tumor response after neoadjuvant therapy was assessed according to the Response Evaluation Criteria in Solid Tumors measurement criteria, version 1.1.
Molecular analyses
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) samples or frozen resection samples with macroscopically viable tumor tissue. Mutation testing was performed at the certified laboratory of Guangdong Lung Cancer Institute. EGFR and KRAS mutations in the initial biopsy, postoperative material, and recurrent tumor tissue were determined by Sanger sequencing, and EGFR mutations in plasma were tested using ARMS according to the protocol of the DxS EGFR mutation test kit (DxS). EML4-ALK translocation was analyzed by FISH using Vysis ALK Break Apart FISH Probe Kit according to the manufacturer’s instruction. In addition, the deletion polymorphism of the Bcl-2-interacting mediator of cell death (BIM) gene in intron 2 was retrospectively examined by Sanger sequencing to validate its predictive role for TKI efficacy. IHC was conducted to detect the protein expressions of mutant EGFR and downstream molecules using rabbit mAbs from Cell Signaling Technology according to the protocols recommended by the manufacturer [31, 32].
Statistical analysis
Power analysis of one proportion non-inferiority was applied to provide 95 % power to declare the treatment sufficiently active for a response rate ≥42.5 % (the average of 50 % of TKI in EGFR mutant lung cancer and 35 % of GC regimen in neoadjuvant setting) in the biomarker-guided neoadjuvant treatment strategy and 11 % for the history reference of neoadjuvant TKI therapy [13, 19]. A sample size of 22 achieves 96 % power to detect a difference of −0.01 using a one-sided binomial test. The target significance level is 0.05. The actual significance level achieved is 0.0344. These results assume a baseline proportion of 0.12 and that the actual proportion is 0.417 [33].
Response rates were analyzed by use of the Fisher’s Exact Test. Survival was estimated with Kaplan-Meier methodology and was summarized as a median value with range and a two-sided 95 % CI. A Cox proportional hazards model was utilized to estimate hazard ratios (HR) and 95 % CI. SPSS version 21 was used for statistical analyses. All analyses were exploratory only.
Acknowledgements
We would like to thank all patients who took part in and contributed to this research. We thank the investigators (Binchao Wang, She-Juan An, Congrui Xu, Huajun Chen, Benjiang Yuan, Yisheng Huang, Zhiyong Chen, Ying Huang, Hong-Yan Tang, Zhi Xie, and Shi-Lang Chen) and study nurses (Sufen Luo, Bin Gan) who participated in this study.
This work was supported by grants from the National Natural Science Foundation of China [81001031 to W.Z. Zhong, 81372285 to W.Z. Zhong] and the grant S2013010016354 from the Natural Science Foundation of Guangdong, Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (Grant No. 2012A061400006), Special Fund for Research in the Public Interest from National Health and Family Planning Commission of PRC (Grant No. 201402031), and Research Fund from Guangzhou Science and Technology Bureau (Grant No. 2011Y2-00014).
This study was presented in part at the World Conference on Lung Cancer (WCLC) (e-poster on July 31st–August 4th, 2009; San Francisco, California, USA), the American Society of Clinical Oncology (ASCO) (poster on June 4th–8th, 2010; Chicago, Illinois, USA) (poster on June 1st–5th, 2012; Chicago, Illinois, USA) (poster on May 30th–June 3rd, 2014; Chicago, Illinois, USA), and the Japanese Society of Medical Oncology (JSMO) (oral presentation on July 26th–28th, 2012; Osakasayama City, Osaka, Japan). What is more, our team has won CAHON’s 2014 ASCO YIA awards for this trial.
Additional files
Comparison of gene mutation status and tumor staging before and after neoadjuvant therapy and corresponding disease failure model. Abbreviations: EGFR, epidermal growth factor receptor; LN, lymph node; GC, gemcitabine/carboplatin; PFS, progression-free survival; RT, radiotherapy; PORT, postoperative radiotherapy; RR, response rate; PR, partial response; SD, stable disease; PD, progressive disease; NA, not available; WT, wild type; BIM, BCL2L11; cT staging: primary staging of tumor before neoadjuvant therapy; cN staging: primary staging of lymph nodes before neoadjuvant therapy; pT staging: restaging of tumor based on pathological results of surgical resection; pN staging: re-staging of lymph nodes based on pathological results of surgical resection. (LOG 0 bytes).
Immunohistochemistry for mutant EGFR and downstream molecules on the resected samples after induction erlotinib. Abbreviations: EGFR, epidermal growth factor receptor; PFS, progression-free survival. Note: All six cases in the erlotinib arm that underwent resection gained partial response to tyrosine kinase inhibitor. Immunohistochemistry was conducted to detect the protein expressions on the resected samples after induction elotinib. In all the 6 cases, pEGFR (Tyr1068) has been deregulated. Cases 2 with L858R mutation enjoyed the longest PFS of 23 months, among which the p44/42MAPK (Erk1/2) (137 F5) was deregulated and the pAkt (Thr308) (244 F9) was most activated compared with other 5 cases.
Footnotes
Wenzhao Zhong and Xuening Yang contributed equally to this work.
Competing interests
Yi-Long Wu has received honorarium from Roche, Eli Lilly, AstraZeneca, Pfizer, and Sanofi. Wenzhao Zhong, Xuening Yang, Xuchao Zhang, Qing Zhou, and Jinji Yang have received payment for lectures from Roche, Eli Lilly, and AstraZeneca. All other authors declare that they have no competing interests.
Authors’ contributions
WZ designed the study, carried out most of surgeries to obtain eligible tumor tissues, supervised the whole procedure of the study, and drafted this manuscript. XY participated in the design of the study and performed parts of surgeries. SD, RL, and QN took responsibility of surgeries to ensure enough issues for further tests. RL performed EBUS for pathological confirmation of N2. XZ, JS, and ZC carried out the molecular genetic analyses, including gene sequencing, ARMS, and so on. HY participated in designing the study and performed the statistical analysis. QZ, JY, and HT participated in the design and coordination of the study, helped to collect samples, and follow-up. YW designed and supervised the study and participated in drafting and revising the manuscript. All authors read and approved the final manuscript.
Contributor Information
Wenzhao Zhong, Email: 13609777314@163.com.
Xuening Yang, Email: yangxncn@qq.com.
Honghong Yan, Email: yanhonghong385@126.com.
Xuchao Zhang, Email: zhxuchao3000@hotmail.com.
Jian Su, Email: 13725338633@139.com.
Zhihong Chen, Email: wczh2002@163.com.
Riqiang Liao, Email: gzfekl@hotmail.com.
Qiang Nie, Email: bulaier6480@163.com.
Song Dong, Email: dsong@aliyun.com.
Qing Zhou, Email: gzzhouqing@126.com.
Jinji Yang, Email: yangjinji2003@163.com.
Haiyan Tu, Email: thoraciconcology88@163.com.
Yi-Long Wu, Email: syylwu@live.cn.
References
- 1.Robinson LA, Ruckdeschel JC, Wagner HJ, Stevens CW. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:243S–65S. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 2.Dong S, Guo AL, Chen ZH, Wang Z, Zhang XC, Huang Y, et al. RRM1 single nucleotide polymorphism -37C → A correlates with progression-free survival in NSCLC patients after gemcitabine-based chemotherapy. J Hematol Oncol. 2010;3:10. doi: 10.1186/1756-8722-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NSCLC Meta-analysis Collaborative Group Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–1571. doi: 10.1016/S0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu C, Zhou Q, Wu YL. Can EGFR-TKIs be used in first line treatment for advanced non-small cell lung cancer based on selection according to clinical factors? - A literature-based meta-analysis. J Hematol Oncol. 2012;5:62. doi: 10.1186/1756-8722-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 9.Hishida T, Nagai K, Mitsudomi T, Yokoi K, Kondo H, Horinouchi H, et al. Salvage surgery for advanced non-small cell lung cancer after response to gefitinib. J Thorac Cardiovasc Surg. 2010;140:e69–71. doi: 10.1016/j.jtcvs.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Kappers I, Klomp HM, Burgers JA, Van Zandwijk N, Haas RL, van Pel R. Neoadjuvant (induction) erlotinib response in stage IIIA non-small-cell lung cancer. J Clin Oncol. 2008;26:4205–7. doi: 10.1200/JCO.2008.16.3709. [DOI] [PubMed] [Google Scholar]
- 11.Takamochi K, Suzuki K, Sugimura H, Funai K, Mori H, Bashar AH, et al. Surgical resection after gefitinib treatment in patients with lung adenocarcinoma harboring epidermal growth factor receptor gene mutation. Lung Cancer. 2007;58:149–55. doi: 10.1016/j.lungcan.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Wang H, Li P, Zhu H, He C, Wei B, et al. Erlotinib-based perioperative adjuvant therapy for a case of unresectable stage IIIA (N2) nonsmall cell lung cancer. Am J Med Sci. 2010;340:321–5. doi: 10.1097/MAJ.0b013e3181e59ac2. [DOI] [PubMed] [Google Scholar]
- 13.Lara-Guerra H, Waddell TK, Salvarrey MA, Joshua AM, Chung CT, Paul N, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol. 2009;27:6229–36. doi: 10.1200/JCO.2009.22.3370. [DOI] [PubMed] [Google Scholar]
- 14.Schaake EE, Kappers I, Codrington HE, Valdes OR, Teertstra HJ, van Pel R, et al. Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2012;30:2731–8. doi: 10.1200/JCO.2011.39.4882. [DOI] [PubMed] [Google Scholar]
- 15.Wislez M, Barlesi F, Besse B, Mazieres J, Merle P, Cadranel J, et al. Customized adjuvant phase II trial in patients with non-small-cell lung cancer: IFCT-0801 TASTE. J Clin Oncol. 2014;32:1256–61. doi: 10.1200/JCO.2013.53.1525. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Chen H, Yan H, Zhang X, Zhou Q, Su J, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. 2013. p. 33–9. [DOI] [PubMed]
- 17.Goss GD, O’Callaghan C, Lorimer I, Tsao MS, Masters GA, Jett J, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31:3320–6. doi: 10.1200/JCO.2013.51.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GJ, Tsao A, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scagliotti GV, Pastorino U, Vansteenkiste JF, Spaggiari L, Facciolo F, Orlowski TM, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol. 2012;30:172–8. doi: 10.1200/JCO.2010.33.7089. [DOI] [PubMed] [Google Scholar]
- 20.Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, Canto A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–8. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 21.Roth JA, Fossella F, Komaki R, Ryan MB, Putnam JJ, Lee JS, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86:673–80. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 22.Lu S, Jiang G, Chen Z. A single arm, multi-center, Phase II study of intercalated erlotinib with gemcitabine/cisplatin as neoadjuvant treatment in Stage IIIA non-small cell lung cancer (CTONG 1101, NCT01297101): preliminary result. WCLC Abstract #P1.09-016. In: the 15th World Conference on Lung Cancer; October 27–30, 2013. Sydney, Australia; 2013.
- 23.Albain KS, Swann RS, Rusch VW, Turrisi AR, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011;17:6298–303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Q, Cheng Y, Yang JJ, Zhao MF, Zhang L, Zhang XC, et al. Pemetrexed versus gefitinib as a second-line treatment in advanced nonsquamous nonsmall-cell lung cancer patients harboring wild-type EGFR (CTONG0806): a multicenter randomized trial. Ann Oncol. 2014;25:2385–91. doi: 10.1093/annonc/mdu463. [DOI] [PubMed] [Google Scholar]
- 26.Chin TM, Quinlan MP, Singh A, Sequist LV, Lynch TJ, Haber DA, et al. Reduced erlotinib sensitivity of epidermal growth factor receptor-mutant non-small cell lung cancer following cisplatin exposure: a cell culture model of second-line erlotinib treatment. Clin Cancer Res. 2008;14:6867–76. doi: 10.1158/1078-0432.CCR-08-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai H, Wang Z, Chen K, Zhao J, Lee JJ, Wang S, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:3077–83. doi: 10.1200/JCO.2011.39.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Zhang XC, Chen ZH, Yin XL, Yang JJ, Xu CR, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:3316–21. doi: 10.1200/JCO.2010.33.3757. [DOI] [PubMed] [Google Scholar]
- 29.Yi S, Zhuang Y, Zhou J, Ma H, Huang J, Wang L, et al. A comparison of epidermal growth factor receptor mutation testing methods in different tissue types in non-small cell lung cancer. Int J Mol Med. 2014;34:464–74. doi: 10.3892/ijmm.2014.1789. [DOI] [PubMed] [Google Scholar]
- 30.Mitsudomi T, Suda K, Yatabe Y. Surgery for NSCLC in the era of personalized medicine. Nat Rev Clin Oncol. 2013;10:235–44. doi: 10.1038/nrclinonc.2013.22. [DOI] [PubMed] [Google Scholar]
- 31.An SJ, Chen ZH, Su J, Zhang XC, Zhong WZ, Yang JJ, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One. 2012;7:e40109. doi: 10.1371/journal.pone.0040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–79. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3. New York: John Wiley & Sons; 2003. [Google Scholar]


