Abstract
Since President Nixon officially declared a war on cancer with the National Cancer Act, billions of dollars have been spent on research in hopes of finding a cure for cancer. Recent reviews have pointed out that over the ensuing 42 years, cancer death rates have barely changed for the major cancers. Recently, several researchers have questioned the prevailing cancer paradigm based on recent discoveries concerning the mechanism of carcinogenesis and the origins of cancer. Over the past decade we have learned a great deal concerning both of these central issues. Cell signaling has taken center stage, particularly as regards the links between chronic inflammation and cancer development. It is now evident that the common factor among a great number of carcinogenic agents is activation of genes controlling inflammation cell-signaling pathways and that these signals control all aspects of the cancer process. Of these pathways, the most important and common to all cancers is the NFκB and STAT3 pathways. The second discovery of critical importance is that mutated stem cells appear to be in charge of the cancer process. Most chemotherapy agents and radiotherapy kill daughter cells of the cancer stem cell, many of which are not tumorigenic themselves. Most cancer stem cells are completely resistant to conventional treatments, which explain dormancy and the poor cure rate with metastatic tumors. A growing number of studies are finding that several polyphenol extracts can kill cancer stem cells as well as daughter cells and can enhance the effectiveness and safety of conventional treatments. These new discoveries provide the clinician with a whole new set of targets for cancer control and cure.
Keywords: Cancer stem cell, cell signaling, inflammatory oncogenes, stemness, tumor microenvironment
INTRODUCTION
Oncogene activation leading to the overstimulation of cell growth as a cause of Cancer.
We often hear it said that all the billions spent on the “war on cancer” was essentially wasted, as death rates from metastatic cancer have changed little since the war was declared 42 years ago under President Nixon's National Cancer Act. It is accepted that long-term survival, once a cancer metastasizes, is no more than 5–10% despite intensive chemotherapy and radiotherapy – a pretty dismal conclusion to a 40-year war.[32]
Much of the research was directed at cancer cell biology, in particular genetics and cell-signaling mechanisms. Based on early research, it was assumed that most cells in the body, under particular conditions, could transform into immortalized cancer cells through a specific gene-directed process. Oncogenes, as the paradigm concluded, were either mutated or overexpressed leading to excessive stimulation of cell cycling and growth signals and/or suppression of cancer suppressor signals – the bottom-line being that somatic cells had lost growth restraint signals and were transformed into cancer cells.
Further, it was assumed that carcinogenic agents affected cell signaling and their carcinogenicity was based on their effects on oncogenes, which could occur by a number of mechanisms. Based on this theory of carcinogenesis, chemotherapeutic treatments were mostly directed at controlling cell cycling, induction of apoptosis and reducing cell growth signaling.
WILL CANCER TREATMENT UNDERGO A PARADIGM SHIFT?
Consideration of the role of inflammation in cancer
Sarah Crawford in a series of important papers asks this critical question based on a considerable amount of research that indicates conventional treatments have failed to live up to early promises and that new discoveries suggest that we may have been following an incorrect paradigm.[31,32] That is, most conventional treatments do not prolong the life of patients with metastatic cancers making up the major aggressive types, but rather produce short-term improvements of survival and that perhaps directing treatment at the main cause of malignancy – inflammation and stem cells – treatments may be more fruitful.
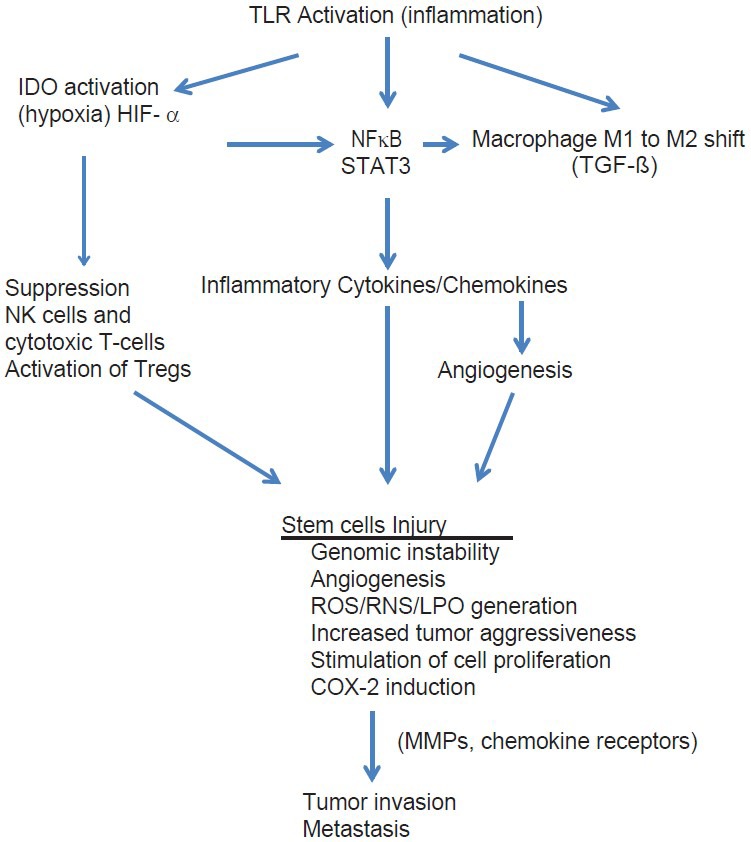
In this paper, I have reviewed some of these studies. What we have learned is that central to all cancers is inflammation and that the cell processes involved in inflammation not only are responsible for initiation of the cancer, but also persist during its growth and play a central role throughout every phase of the cancer's existence, including progression, invasion, angiogenesis, and metastasis [Figure 1].
Figure 1.
Tumor Microenvironment: The role of inflammation
The role of short term and chronic inflammation in cancer and disease
While short-term inflammation is rarely associated with cancer induction, chronic smoldering inflammation, as seen with a large number of disorders, is almost always linked to carcinogenesis.[35,98] All carcinogenic agents and conditions, such as chemicals, obesity, hyperglycemia, persistent infections, autoimmune diseases, and carcinogenic heavy metals, promote inflammation. Carcinogenic viruses, such as human papillomavirus (HPV-cervical carcinoma), herpes virus (lymphoma), hepatitis B and C (hepatocarcinoma), cytomegalovirus (glioblastoma), and Helicobacter pylori (gastric cancer), produce cancer by inducing chronic inflammation.[109]
Common molecular signaling pathways in cancer
What all of these carcinogenic events have in common is that they activate two main cell-signaling molecules – nuclear factor kappa (NFκB) and signal transducer and activator of transcription-3 (STAT3).[104] Both of these transcription molecules are linked to inflammatory gene activation and to genes controlling cell growth factors, angiogenesis, and cytokine/chemokine regulation.[70,72,104] They are also linked to a great number of other cell signaling pathways playing a critical role in cancer behavior.
The NFκB and STAT3 pathways are central pathways in both inflammation and tumorigenesis. Both are activated by a wide assortment of tumor-associated events, such as growth factors (epidermal growth factor [EGF]), hypoxia, acidic microenvironment, hyperglycemia (diabetes and insulin resistance), and proinflammatory cytokines (TNF-α). In fact, TNF-α is one of the most powerful activators of NFκB, which explain the strong association found between high levels of TNF-α and the aggressive behavior of several cancers, such as glioblastomas, head and neck squamous cell cancer, mantle cell lymphoma and acute myeloid leukemia, and others.[30,62,111,113,163]
Growth factors, such as EGF and growth receptors, such as HER2 and EGFR, are universally activated in a variety of cancers and they also activate NFκB.[2,46] Growth factors, in addition, activate STAT3.[2]
The proinflammatory cytokine IL-6, a major growth factor in prostate and other cancers, activates both NFκB and STAT3. NFκB is also a major controller of IL-6 production, a major cancer growth factor.[85,104] Interestingly, NFκB is linked to most tumorigenic genes, including cFLIP, Bcl-sl, Bcl-2, and survivin. It is also linked to genes controlling other carcinogenic pathways, such as cyclinD1, c-myc, cyclooxygenase-2, metalloproteinase, vascular endothelial growth factor, CXCR4, and TWIST.[64] These cell signaling mechanism control tumor proliferation, invasion, and metastasis.
An explanation for resistance of cancer to radiation and chemotherapy
Of major interest is that activation of NFκB plays a major role in resistance to chemotherapy and radiation therapy.[148] It appears to promote this resistance by controlling the expression of P-glycoprotein, the multidrug resistance (MDR) factor that expels chemotherapeutic agents from cancer cells.[148] Ironically, most chemotherapy agents, such as paclitaxel, vinblastine, vincristine, doxorubicin, daunomycin, 5-fluorouricil, cisplatin, and tamoxifen activate NFκB, and it is thought that this plays a major role in resistance to these modalities of treatment. That is, the chemotherapy agent itself is initiating chemoresistance. Radiation is also a powerful activator of NFκB.[94] Essentially, conventional treatments, such as chemotherapy and radiation treatments, are fairly efficient in eliminating the daughter cells produced by the cancer stem cells, but rarely kill the cancer stem cells themselves. Studies have shown that implantation of the daughter cancer cells are rarely successful in producing cancers when transplanted to test animals, yet implantation of as few as 100 cancer stem cells can induce growth and invasion of malignant tumor cell implants.[110,114]
Reactive oxygen and nitrogen species as the initiator of the cancer cascade
Within the microenvironment of the stem cells, before conversion to cancer stem cells, one witnesses a transition of the stem cell niche into an area of high concentrations of reactive oxygen species (ROS) and reactive nitrogen species (RNS), lipid peroxidation products (LPPs) and inflammatory cytokines and chemokines.[83,98] This inflammatory change can occur because of chronic systemic inflammatory illnesses, such as autoimmune diseases (colitis, gastritis, hepatitis), diet or persistent viral infections. The inflammation can also develop locally within the niche itself without systemic inflammation. The cancer itself becomes a source of inflammation because of its antigenicity and destruction of cells within the region of the tumor. Because inflammation and associated free radical accumulation persist throughout the lifetime of the cancer it also affects tumor behaviors concerned with long-term survival of the cancer, such as invasion and metastasis.[83,98]
The cancer stem cell, its microenvironment and inflammation: effects on cancer biology
As basic research further expanded our understanding of the biology of the cancer process, a different story began to appear. Ironically, it was a story that had been suggested almost 150 years ago by pathologist Rudolph Virchow.[11] What has changed is the concept of the cell of origin of all cancers and the central importance of the microenvironment surrounding these cells. It now appears that inflammation is essential to not only cancer induction by its mutagenic effects on stem cell DNA, but also that the subsequent long-term behavior of these tumors is, to a large degree, determined by the tumor's microenvironment.[83]
The frequent association of various tumor types with known chronic inflammatory diseases suggested that inflammation was playing an essential role in cancer biology. For example, colon cancer risk was associated with inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease; pancreatitis with pancreatic cancer; obesity with breast cancer; gastric reflux with esophageal cancer and Schistosmoma infections with bladder cancer. Further support came from the observation that certain antiinflammatory drugs not only reduced the risk of cancer development but also reduced recurrence, metastasis and tumor size.[7,12,23,54]
CANCER STEM CELLS AND STEMNESS
Early hypotheses on the genesis of tumors from dormant cells, trophoblasts
One of the most important discoveries in cancer biology is one that actually surfaced over 100 years ago. And that is the idea that uncommitted cells lying dormant throughout the body are the source of most cancers.[154] Until fairly recently, this idea was lost among a sea of studies and accumulation of data concerning oncogenes and gene-related cell signaling. It briefly arose again in a series of papers by John Beard written from 1905 to 1911 in which he proposed that embryonic trophoblast were scattered throughout the tissues and organs or they represented dedifferentiated somatic cells and that certain events could reactivate these uncommitted cells to produce cancerous tumors.[19,49,92]
Unfortunately, his ideas soon fell into oblivion. I say unfortunately, because so much time was lost examining other theories that did not lead to treatments that could make a significant impact against the major killer cancers.
STEM CELL HYPOTHEIS OF CANCER
Cancerous tumors are said to represent aberrant attempts to produce organs and contain heterogenous populations of cells that differ in their accumulated mutations and degree of differentiation.[20,162] One would think that based on this observation alone, one would consider uncommitted, embryonic-like cells as the source of these aberrant organs.
Considerable evidence suggests that cancer stem cells closely resemble stem cells themselves.[147,162] The main characteristic of both stem cells and cancer stem cells is their ability for self-renewal, which gives them a lifetime existence.[147] Key to their survival is their ability to block apoptosis and this is mainly accomplished by increased expression of the antiapoptotic factor bcl-2.
Because stem cells can exist for a lifetime they are vulnerable to varying episodes of attack by ROS/RNS as well as LPPs, such as 4-hydroxynonenal and acrolein.[117,166] Generation of these harmful molecules can be frequently intense during one's lifespan. For example, during periods of infections, trauma, stress, chronic illness, exposure to chemical toxic substances and even by eating a poor diet one may experience intense generation of these harmful particles. Aging itself is associated with a progressive increase in the generation of free radicals and LPPs.[16]
Exposure of these stem cells’ DNA to intense or prolonged, unrepaired assaults by ROS/RNS and LPPs can produce varying degrees of genetic mutations that over time can convert a somatic stem cell into a cancer stem cell.[97,98] Once converted, the cancer stem cell would produce increasing numbers of uncommitted progenitor cells that would rapidly produce more mature daughter cells giving the aberrant organ architecture of the tumor. In other words, the cancer stem cells undergo self-renewal (duplicating more cancer stem cells) as well as generating great numbers of daughter cells. One can see that the microenvironment of the stem cell niche would be a powerful determining factor on the makeup of the bulk of the tumor. A principally inflammatory microenvironment, even locally, would expose the stem cells to intense oxidant DNA damage.
The literature on cancer stem cells speaks of stemness, indicating that certain influences can alter progenitor cells to revert back to stem cells or cancer stem cells, depending on the conditions. Normally, progenitor cells are less likely to produce tumor formation, as they proliferate for a shorter time before terminally differentiating.[110] When alteration of progenitor cells toward cancer stem cell morphology does occur, it is referred to as induction of stemness.
One of the important findings is that cancer stem cells generally make up only a very small proportion of the cellular structure of the tumor; most cells being daughter cells derived from the cancer stem cells.[162] A large number of these daughter cells are nontumorigenic and can even metastasize to distant locations without producing true cancers.[114] It may be that only the spreading of actual cancer stem cells can produce tumorigenic metastasis.[110]
Cancer stem cells are isolated using flow cytometry according to the expression pattern of surface markers such as CD24, CD44, and CD133.[4] In vitro, they will grow indefinitely as spheres and are tumorigenic in vivo. In serum-free cultures, cancer stem cells characteristically form sphere bodies.[162]
The first isolation of cancer stem cells from a solid tumor was from breast cancer.[4] Less than 5% of the cells within the tumor expressed CD44, characteristic of these particular cancer stem cells.[4] Interestingly, less than 100 cells with this phenotype were able to form tumors in implanted immunocompromised mice.[101] In mouse models of leukemia, only 1 in 10,000 to 1 in 100 of the cancer cells can form colonies characteristic of tumorigenic cells.[101] For solid tumors, only 1 in 1000 to 1 in 5000 lung, ovarian cancer or neuroblastoma cells were found to have characteristics of tumorigenic cells.[50] Melanoma stem cells, unlike the other cells taken from the same tumor (not having the stem cell marker CD271), are able to maintain tumor growth in vivo.[15] Yet, the daughter cells not having this stem cell marker cannot support implanted tumor growth. What these observations suggest is that only the cancer stem cells themselves participate in tumor recurrence and metastasis, the main factors that make cancers deadly.
One of the unsettled questions is whether the daughter cells can at some time dedifferentiate into cancer stems cells, which would create a moving target for cancer treatment and make cures much more difficult. Important in any context is the importance of killing both cancer stem cells and daughter cells of the tumor.
Three studies examined this issue in some detail.[24,38,119] The Chen et al. study, an in vivo study using glioblastoma tumors, demonstrated that only killing the daughter cells, which make up the bulk of the tumor and are targeted by conventional treatments of cancer, always led to recurrence. Killing both the glioma stem cells and the daughter cells dramatically impeded growth. Importantly, killing both the glioma stem cells and the daughter cells appears to be essential to preventing recurrence.
The strongest evidence of cancer stem cells as the origin of cancers comes from two studies, the Dressens et al. study and the Schepers et al. study mentioned above.[38,119] In both studies, the researchers used a permanent in vivo fluorescent marker of stem cells. By using this method, both groups demonstrated that the tumors progressed from stem cell populations and as the tumor progressed, the number of cancer stem cells increased proportionally. They also demonstrated that cancers have a much higher number of stem cells than do benign tumors, and as benign tumors undergo a transition to a malignant form, they attain a greater number of cancer stem cells. Of real interest was the observation that nonstem cell tumors may revert to a stem cell-like state, even in the absence of mutations.[89,160] In essence, it is the microenvironment that determines stemness.
THE CENTRAL IMPORTANCE OF THE MICROENVIRONMENT OF THE TUMOR
Early history of inflammation in the development of cancer
Rudolph Virchow, over 150 years ago, noted that at its earliest stages, all cancerous tumors were infiltrated with leukocytes of various kinds and that advanced tumors had characteristics of infectious boils.[11] In fact, he noted that physicians at the time described pus extruding from cancerous tumors – more likely a mixture of leukocytes and necrotic tumor matter.
More recent studies have confirmed his observations and that leukocyte infiltration occurs even in the precancerous phase of cancer development.[53,87] The story that is now unfolding is that this inflammatory microenvironment is essential to malignant transformation and the subsequent biological behavior of cancers, and that mere stimulation of cell proliferation, as was previously thought, is insufficient for cancer development.[35] While it is still accepted that multiple mutations, often numbering in the hundreds, are responsible for the malignant transformation of cells, there is significant evidence to convince us that inflammatory generation of high concentrations of ROS and RNS, and LPPs are the damaging elements.
The link between inflammation and viral and chemical transformation of cells
An example of the central role played by inflammation is seen with malignancies induced by the Rous sarcoma virus. Without inflammation, the virus cannot induce malignant transformation.[88] This also appears to be true with other carcinogenic viruses, such as the HPV virus, Epstein–Barr virus, and the hepatitis C virus.[14,133] Stomach cancers induced by H. pylori are also dependent on the proinflammatory cytokine IL-6 for their development.[142] The most important question is not only what is the link between inflammation and stem cell transformation into cancer stem cells, but also, how is inflammation affecting ongoing tumor behavior.
Solid tumors and Inflammation – How it works; other observations
It is becoming evident that inflammation is playing a central role in tumor initiation, progression, invasion and metastasis – that is, in every phase of the carcinogenic process.[87,98] Common to all tumors thus far examined is a combination of high levels of ROS and RNS and inflammation, which are closely linked.[120] This is especially so with solid tumors such as pancreatic cancer, breast cancer, prostate cancer, lung cancer, cervical cancer, liver cancer, stomach cancer, and many others.
Inflammation is known to induce genomic instability, angiogenesis, alterations in the epigenomic state, stimulation of cell proliferation, increase in cytokine growth factors, generation of reactive oxygen and nitrogen species, induction of chemokine receptors on malignant cells, induction of COX-2 and activation of NFκB and STAT3.[28,31] This inflammatory milieu in the microenvironment remains in the vicinity of cancer cells throughout the malignant process.
The degree of inflammation appears to determine the proliferative potential of the tumor as well as its invasive and metastatic aggressiveness.[138] Conditions that increase inflammation also promote aggressiveness and include tumor promoters, radiation, chemotherapeutic agents, dietary components, and persistent viral and bacterial infections.[5,66]
Recruitment of macrophages, neutrophils and mast cells increase nitric oxide (NO) levels within the tumor microenvironment and this promotes tumor proliferation.[6] NO in the presence of superoxide, both increased in the tumor microenvironment, produces the powerful, DNA damaging radical peroxynitrite. In addition, these cells release high levels of proinflammatory cytokines, chemokines, and other immune mediators. It is becoming obvious that a successful immune attack, principally by the cellular immune arm, depends on the phenotype of macrophages and T-cells.[33] Macrophages are thought to exists either in a cytotoxic M1 phenotype or a M2 anticytotoxic phenotype. Switching of the macrophage to an M1 phenotype provides powerful antitumor effects and explains the reports that have found high levels of immune cells in some tumors as conveying a better prognosis.[130,164] More often, invasion of inhibitory macrophages (tumor associated macrophages; TAMs) and mast cells indicates protumor immune-related growth stimulation. In addition, lymphocyte infiltration was assumed in the past to indicate a successful cytotoxic T-cell attack. Newer studies suggest that far too often these lymphocytes are regulatory T-cell (Tregs) that inhibit cytotoxic attacks on cancer cells.[33] As a result, the Tregs and M1 macrophages protect the cancer from a successful immune attack.
Unfortunately, for most malignancies, macrophages are switched to an M2 immune-suppressing phenotype that allows the tumor to escape immune detection and destruction.[40] The M2 phenotype also switches T-lymphocytes to Tregs that suppress tumor immunity. In this way, inflammatory mediators within the tumor microenvironment provide the tumor “immune invisibility.”
One of the central control elements for immune tolerance under a variety of conditions is the tryptophan metabolizing enzyme indoleamine 2,3-dioxygenase (IDO), an enzyme found in all tissues, including tumor and immune cells.[93] Activation of IDO induces immune tolerance to the tumor and promotes metastasis.[136] IDO is overexpressed in malignant tumors and this leads to inflammatory suppression of antitumor immunity by suppressing cytotoxic T-lymphocytes. M1 macrophages and natural killer cells (NK cells), by stimulating the generation of high levels of immune suppressing Tregs, M2 macrophages and myeloid-derived suppressor cells (MDSCs), allows the tumor to grow and infiltrate unimpeded by the immune system.[61,145]
Studies have shown that small molecule inhibitors of IDO can cause rapid regression of aggressive tumors that are otherwise known to be treatment resistant.[65] Chronic inflammation is a major factor causing prolonged upregulation of IDO in all tissues, including the brain.[123] Under nonmalignant conditions, upregulation of IDO in the face of chronic inflammatory states is designed to reduce damage by Th1-type immune activation (increased immune reactivity). In the case of malignant disease, tumors hijack this enzyme to protect itself from immune detection and destruction.[136]
Inflammation and oncogene activation within stem-like cells – how cancers develop
It is generally accepted that the trigger for conversion of normal somatic cells into malignant cells involved alteration in their genes controlling cell proliferations and/or tumor suppression/apoptosis. Newer evidence suggests that it is the stem cells in which activation of oncogenes is occurring.[83,162] The main driving force for this mutagenic transformation is exposure of these stem cells to high concentrations of ROS/RNS and LPPs, that is, within the presence of chronic inflammation either systemically or locally. Several oncogenes were identified as being commonly activated in a number of tumor types, such as RAS and MYC.[100,144] The RAS family are among the most frequently mutated dominant oncogenes in human cancers and are known to induce the production of tumor promoting chemokines and cytokines, that is, inflammatory mediators.[48] In essence, what we are seeing is a transformation of stem-like cells into immortal cancer stem cells and their subsequent long-term production of additional inflammation by generating cytokines and chemokines. Because the tumor itself is antigenic and is releasing chemokines (immune cell attractants), it attracts an additional array of immune cells into the tumor's microenvironment as well. This creates a tumor microenvironment that is inflammatory throughout the life of the tumor.
The MYC oncogene is overexpressed in many human cancers and promotes the first wave of angiogenesis by stimulating the production of the inflammatory cytokine IL-1ß.[127] In addition, this oncogene is responsible for the production of mast cell recruitment of chemokines.[137] Mast cells drive angiogenesis.[1]
The essential nature of inflammation in the initial stem cell transformation is emphasized by the findings that in the case of pancreatic adenocarcinoma both mutation of the oncogene K-RAS and pancreatitis are necessary for tumor cell development.[48] Several lines of evidence show that a variety of types of oncogenes all coordinate inflammatory transcription programs necessary for angiogenesis and recruitment of myeloid immune suppressor cells, essential for tumor growth, and invasion.[137]
According to the present hypothesis, activation of oncogenes results from damage to DNA by high levels of ROS/RNS and these are generated by smoldering inflammation, either systemically or locally at the site of tumor development. The tumor-initiating inflammation can results from a number of insults, such as trauma, chronic, smoldering infections, latent viruses, parasitic infections, chemical carcinogens, or autoimmune disorders. Most types of cancer are found to have high levels of ROS/RNS.[82] These reactive species are associated with DNA-strand breaks, point mutations, and aberrant DNA cross-linking – that is, genetic instability. As a result of this DNA damage, oncogenes are mutated or over expressed or in the case of tumor suppressor genes, are mutated and inactivated. In addition, mitochondrial DNA is also damaged and mitochondrial DNA is significantly more sensitive to free radical damage than is nuclear DNA.
Cell signaling and control of cancer stem cell behavior
In general, adult stem cells are normally quiescent and this state is dependent on the microenvironment of the stem cell niche. This quiescence requires interaction with various cell types within and surrounding the niche or tumor bed.[139] This can include endothelial cells and other stromal cells within the tumor microenvironment, as mentioned.[89]
Quiescence is controlled by a number of cell signaling pathways, including p53, FoxO, HIF-1α, nuclear factor of activated T cells c1 (NFATc1), Phosphatase and tensin homolog (PTEN), mammalian Target of Rapamycin (mTOR), bone morphagenic proteins (BMPs), transforming growth factor beta (TGF-β), thrombopoietin, angiopoietin-1 (ang-1), and Wnt/B-caterin signaling.[77] Cancer stem cells can remain dormant for decades by utilizing these mechanisms and are totally resistant to traditional treatments during quiescence.
Granulocyte colony stimulating factor (G-CSF), interferon-α, and the chemokine CXCL12 can all mobilize dormant cancer stem cells into the circulation.[77] Wnt signaling plays a major role in maintaining quiescence, thus allowing cancer stem cells to escape destruction by conventional treatments.[77]
Nanog: A master controller of cancer behavior
Newer studies are finding that the transcription factor nanog plays a major and central role in regulating pluipotency and tumorigenesis of cancer stem cells.[17,58,151] The Nanog protein, as a transcription factor, is transported in and out of the cell nucleus where it activates a set of genes that can reprogram human somatic fibroblast into embryonic stem cell-like pluipotent cells.[58] Nanog is found only in pluripotent cells and is absent from differentiated cells. It appears to be a gate-keeper during embryogenesis.[17] The expression of Nanog is regulated by the cell-signaling factors Oct4 and Sox2. Leukemia inhibitory factor (LIF), a downstream effector of STAT3 (LIF/STAT3 pathway) is indispensible in maintain a pluripotent state as well.[58]
Nanog is expressed in a number of cancers including cancer of the breast, cervix, kidney, prostate, lung, brain, ovary, gastric carcinoma, and oral cancers.[58] Strong expression of nanog is an indicator of a poor prognosis in ovarian serous carcinoma, colorectal cancer, and breast cancer patients.[76,95] The expression of nanog is higher in cancer stem cells than nonstem cells. Importantly, overexpression of nanog increases cancer drug resistance and positively regulates cell motility and tumor metastasis. Knockdown of nanog impedes proliferation, migration, and invasion of cancer cells.[134]
Another way overexpression of nanog promotes tumor aggressiveness, invasion, and metastasis is by activating Wnt signaling, which allows the cancer cells to adapt to the immune system, that is, it leads to immune escape. Both the cancer cells and surrounding stromal cells can express high levels of nanog.[134] Interestingly, p53, the regulator of cell DNA damage, can also suppress nanog transcription, which decreases cancer stem cell self-renewal and promotes differentiation to nontumorigenic daughter cells. In essence, p53 can interfere with successful cancer stem cell survival. The p53 gene is mutated (suppressed) in over half of all cancers and may be operating at low activity in most others. This not only increases the risk that damaged stem cells would undergo malignant transformation, but would also releases p53 suppression by nanog, which would have the effect of increasing cancer cell proliferation, invasion, and metastasis. High levels of nanog are seen in malignant, high grade, and poorly differentiated cancers.
Sonic hedgehog, another cell signaling mechanism, also promotes cancer stem cell survival, tumor growth, and invasion in human glioma cells.[27] STAT3, which plays a central role in immune escape of tumors by switching M1 macrophages to immunosuppressive M2 macrophages, also interacts with nanog to regulate its expression. By this interaction STAT3 plays an essential role in maintaining pluipotency.[17] ß-catenin, a downstream activator of Wnt signaling, can stimulate self-renewal and proliferation of stem cells and enhance generation of cancers.[45] Activation of ß-catenin and dysregulation of Wnt is commonly found in human cancers.[105]
Micro RNA: New guys on the block
These cell signaling pathways, in conjunction with the previously described quiescence cell signaling, play a major role in controlling tumor behavior, especially as regards invasiveness and metastatic potential. A great deal of attention is now being paid to another regulator of stem cells and this includes microRNA, short, noncoding fragments of RNA. MicroRNAs appear to control a great number of processes in cells and are especially important in regulation of stem cells. This is well documented both in embryogenesis and in cancers.[86]
During brain development, CD133+ stem cells regulate cell differentiation and orientation.[132] In the adult, these stem cells can undergo malignant transformation and dysregulation of stem cell control appears to play a major role in brain tumor development.[132] Cancer stem cells have been identified in several primary brain tumors including glioblastomas, medulloblastomas, and ependymomas.[132,160] The CD133+ cancer stem cells are highly tumorigenic when implanted but even high concentrations of CD133-negative cells (daughter cells) do not form tumors when implanted in immune-suppressed animals.[160] This indicates that the cancer stem cells are acting as the tumor seed cells and not the daughter cells.
As controllers of a number of cell processes, microRNA dysfunction can result in tumor progression and aggressiveness by inhibiting the normal microRNA functions that control tumor suppressor genes and by overexpression of microRNAs that promote stemness and stem cell self-renewal.[141]
Studies have shown abundant levels of the microRNAs miR-9, miR-9± in cancer stem cells of glioblastomas.[122] One of the most highly expressed microRNAs in the adult brain, especially in neurons is miR128. Patients with high-grade gliomas show significant downregulation of miR128, whose main function is to inhibit stem cell self-renewal – a process essential for tumor formation and aggression.[135]
Downregulation of the microRNA miR-199b-5p is associated with metastatic spread of medulloblastoma cells.[44] This microRNA suppresses Notch signaling, which reduces the number of medulloblastoma stem cells. Notch signaling plays a major role in maintaining glioma stem cell proliferation.
THE CENTRAL ROLE OF NUCLEAR FACTOR KAPPAB AND STAT3 IN TUMOR INFLAMMATION AND BIOLOGY
The central activating molecular processes in tumor initiation, invasion, and metastasis
Activation of NFκB is central to regulation of the inflammatory state of the tumor cells themselves and plays a major role in tumor biology.[64,78,156] It also plays a major role in development and maintenance of the inflammatory microenvironment of the tumor, both during inflammatory activation within cancer stem cells and invading immune cells, in particular macrophages (TAMS) and lymphocytes. NFκB is a cell transcription factor that when activated translocates to the nucleus where it activates a number of genes controlling proinflammatory cytokines, chemokines, angiogenesis factors, cell cycling factors (cyclin D1), antiapoptosis factors (Bcl-2), COX-2 enzymes, and matrix metalloproteinases (MMPs).[64] These NFκB controlled processes in turn drive proliferation, invasion, metastasis, angiogenesis, immune evasion, prostaglandin E2 (PGE2) generation, and resistance to apoptosis.
Most cancers demonstrate increased NFκB activation.[64,78] Higher levels of activation are associated with increased tumor size and vascularization, and hence metastasis.[68] Several studies have shown that inhibition of NFκB reduced tumor incidence in cancer models and in breast cancer animal models inhibition of NFκB reduces metastasis.[57,84,104] It should be appreciated that NFκB activation is not always pro-carcinogenic, and in certain situations activation can have anticarcinogenic effects.[129] In most instances, NFκB activation in tumor cells and inflammatory immune cells in the microenvironment promote cancer growth, invasion and metastasis. With both tumor cells and immune cells activation of NFκB occurs by way of stimulation of toll-like receptors (TLRs), as we see with the wide variety of toxic agents and conditions known to trigger oncogenesis.[118]
Hypoxia link to inflammation and cancer
Hypoxia, which plays a major role in tumor induction as well as maintenance, activates hypoxia inducible factor-1α (HIF-1α), which in turn activates NFκB.[96,121] With tumors, hypoxia can occur as a result of inflammation itself, hypoxia from medical conditions and when the tumor outgrows its blood supply. The release of HIF-1α, in turn, increases the release of the proinflammatory cytokines and growth factors from tumors cells and immune cells such as TNF-α, and promotes the insertion of CXCR4 in the membranes of tumor cells, a chemokine receptor associated with increased invasion and metastasis of cancers.[10,121] HIF-1α also increases angiogenesis through these same cell-signaling pathways. What is occurring is that during conditions of hypoxia, no matter the initial cause, HIF-1α is released into the microenvironment and this cell-signaling factor further enhances inflammation in the microenvironment by both attracting additional inflammatory immune cells and by stimulating the release of proinflammatory cytokines from both tumor cells and the invading immune cells.
The role played by STAT3
STAT3, another inflammation controlling transcription factor, also plays an essential role in the tumor inflammatory microenvironment and therefore tumor behavior.[71] Like NFκB, STAT3 is activated by a number of cell signaling systems and in turn activates an assortment of genes controlling inflammation and immune evasion within immune cells. It is a point of convergence for numerous oncogenic signaling pathways controlling such things as antitumor immunity by inhibiting maturation of dendritic cells, thus suppressing macrophage and cytotoxic T-cell reactivity against tumor cells.[72]
Once a condition of protumor immunity is activated, the tumor, by switching Th1 cytotoxic immunity to Th2 type immunity (an immune suppressing phenotype) in the immune cells, allows the cancer to grow unimpeded by the immune cytotoxic system. A key element in this immune suppression is the generation of large numbers of interleukin-10 (IL-10) producing immune cells.[36] IL-10 suppresses both the adaptive and innate immune systems.
Proinflammatory cytokines, such as IL-1ß, IL-2, IL-6, IL-17, and IL-23, can act through the STAT3 signaling system. For example, IL-6 is a growth stimulating cytokine that is associated with rapid growth and invasion of a number of cancers, including ovarian cancer, prostate cancer, nonsmall cell lung cancer, squamous cell carcinoma of the head and neck, lymphomas and gastric carcinomas induced by H. pylori.[25,81] IL-6's main interaction is with STAT3, which promotes tumor growth, invasion, and metastasis.
INVASION AND METASTASIS: MOLECULAR BASIS OF METASTASIS
Chemokines
Of particular interest is the strong link between inflammation and invasion and metastasis of cancers. In some animal studies, inflammation was necessary for a cancer to metastasize.[9,57] One consistently demonstrated example of this association has been the finding that elevated IL-6 levels, a proinflammatory cytokine, are associated with decreased survival and a shortened disease-free recurrence time for breast, pancreatic, gastric, prostate, and lung cancers.[39,41,79,112]
A strong relationship also exists between the presence of chemokine receptors and metastasis.[8,96] These receptors, in conjunction with their ligands, not only attract increasing numbers of immune cells to the microenvironment, but also when appearing on the cancer cells themselves stimulate mobility of these malignant cells. This has been shown to drive metastasis to distant sites and that the specific sites of metastasis are also chosen based on the presence of these chemokine receptors.[26,63,115]
One of the better-studied chemokine receptors includes CXCR4 and its ligand CXCL12, which is frequently expressed by malignant cells. Studies have shown that the amount of CXCR4 receptor expressed by primary tumors correlates with the extent to which metastasis to regional lymph nodes occurs. This is has been demonstrated for breast, colorectal, liver, and esophageal cancers.[63,115]
Other chemokine receptors expressed by malignant cells include CX3CR1, CCR1, CCR7, CCR9, CCR10, CXCR1, CXCR2, CXCR3, CXCR5, and CXCR7. Interestingly, malignant melanomas express a number of chemokine receptors and may explain its high propensity to metastasize to a number of sites.[103,116] The CCR9 chemokine receptor, as an example, attracts melanomas to the small intestine and CCR7 correlates with lymph node metastasis.
Normally, tissues such as epithelial cells and mesenchymal cells do not express chemokine receptors but the appearance of these chemokine receptors occurs early with malignant transformation.[96,115] The invasive capacity of cancer cells increase in the presence of proinflammatory cytokines and part of this effect may be that cytokines, such as TNF-α, upregulate the expression of chemokine receptors.[74]
Suppression of chemokines and effect on tumor cell invasion
Suppressing inflammatory cell signaling has been shown to significantly reduce metastatic spread in animal models of prostate cancer, for example.[84] Specifically involved in the metastatic process are macrophages. Using a genetic model of breast cancer, researchers found that macrophage-deficient mice developed the tumor normally, but it would not metastasize to the lung.[29] TAMs appear to be major players in controlling tumor biology, including angiogenesis, invasion, and metastasis.[87] TAMs are attracted to the tumor, beginning at the earliest stages of carcinogenesis, by chemokines.
MACROPHAGE CONVERSION AIDING TUMOR CELL INVASION
Switching from the antitumor M1 phenotype macrophage to the M2 protumor mode is accomplished by activation of NFκB and this promotes proliferations, invasion, and metastasis of the tumor.[70,164,165] TAMs promote angiogenesis and lymphangiogenesis as well as promoting immune escape and therefore increase the likelihood of metastasis.[60] A recent study found the presence of M2 macrophages in the tumor stroma, but not tumor nest, was a strong marker for tumor size, invasion risk, and as an independent prognostic factor for reducing breast cancer survival.[90]
Important in the switching process of macrophages from M1 to M2 phenotype is TGF-ß, an inflammation-triggered mediator of immune suppression as well as the generation and release of MMPs enzymes by cancer cells. MMPs promote tumor invasion and high levels are an independent risk factor for a poor prognosis.[73]
Tumor microenvironment: Special characteristics
An inflammatory microenvironment plays a key role in this conversion of stem cells into cancer stem cells, and newer research is finding that cells in the stroma have a major influence on stem cell behavior. For example, Rao et al. found that endothelial cells play a critical role in the development and behavior of glioblastoma multiforme tumors by regulating the release of the chemokine CXCL12.[107] The influence of stroma cells in this process has been demonstrated for other cancers as well.[89]
A critically important aspect of tumor microenvironment is hypoxia, as mentioned above, especially cyclic hypoxia. It has been shown that hypoxia can predict the likelihood of tumor aggressiveness, invasion, metastasis, tumor recurrence, resistance to chemotherapy and radiotherapy, and patient survival.[51,55,59]
Hypoxia, by increasing the release of HIF-1α in the microenvironment, induces the expression of the chemokine receptor CXCR4 on the membrane surface of stem cells, which is responsible for migration and metastasis of cancer stem cells.[86] One way hypoxia increases stem cell aggressiveness is by activating NADPH oxidase within tumor cells, which has been demonstrated in glioblastoma tumor cells.[56] This leads to the production of high levels of ROS – principally the superoxide radical, which rapidly reacts with NO to form the powerful radical peroxynitrite.[56] This radical powerfully inhibits mitochondrial function leading to the production of a whole array of ROS.
Another characteristic of cancer stem cells is their resistance to chemotherapy and radiotherapy. Currently used chemotherapeutic drugs can often dramatically shrink metastatic tumors, but these effects are usually quite transient and do not significantly extend the life of the patient. In essence, the chemotherapy drugs are killing only daughter cells and not cancer stem cells.[140] This resistance to treatment appears to be based on the high level of antiapoptotic proteins or ABC transporters such as the MDR gene produced by the cancer stem cell.[167] In essence, these cell mechanisms are escorting chemotherapeutic drugs from the cancer stem cell and in combination with overexpression of antiapoptotic proteins, such as bcl-2, cell death is prevented. Of real importance is the observation that following treatment with chemotherapy and radiotherapy, regrowth of the tumor produces a much more aggressive tumor.[42,106]
NATURAL MOLECULAR AGENTS AND THEIR POTENTIAL EFFECT ON CANCER
Natural molecular agents
For example, resveratrol, curcumin, quercetin, hesperidin, luteolin, apigenin, naringenin, urolic acid, and silymarin have all been shown to have powerful inhibitory effects on tumor mechanism without toxicity to normal cells.[18,22,99,102,108,124,159] Curcumin, which alters a great number of inflammatory cell signaling mechanisms, is one of several compounds found thus far that suppresses NFκB and STAT3.[3,157] Curcumin has also been shown to inhibit MDSCs and prevented their interaction with tumor cells; MDSCs promote tumor growth, angiogenesis, and tumor progression.[146] The beauty of these natural compounds is that they do not affect physiological mechanisms in normal cells utilizing these cell signaling pathways.
The natural compounds, including flavonoids, special molecules, and certain vitamins and minerals, have also been shown to reverse MDR and radioresistance in tumors.[34,31,128,131,152] Several of these natural compounds can re-activate p53 activity, the suicide gene that prevents the conversion of damaged cells into cancer cells.
The alteration of the immune system in the response to cancer by natural molecular agents
Increasing evidence indicates that the immune system, especially cellular immunity, is a major barrier to successful tumor growth and persistence.[52,69] One of the problems in cancer treatment is that stem cells release factors that inhibit antitumor immunity and essentially cloak the cancer, making it invisible to the immune system. Activation of NFκB plays a major role in tumor-induced immune evasion. Some natural products can increase the levels of STAT1, which stimulates macrophage antitumor activity (M1 macrophages) and also by suppressing STAT3 they remove the immune cloaking by the stem cells.[91,150] Inhibiting NFκB activation has been shown to promote switching from the M2 protumor (immune suppressing) phenotype to an antitumor M1 macrophage phenotype.[70] A number of the flavonoids inhibit NFκB, such as curcumin, quercetin, baicalein, silibinin, silymarin, hesperidin, luteolin, procyanidins, and catechins. Flavonoids can also inhibit STAT3 and include quercetin, epigallocatechin-3-gallate (EGCG), naringenin, Kaempferol, resveratrol, and apigenin.[21,80,125,155,161]
Natural molecular agents and their influence in reversing the resistance to radiation and chemotherapy
Several studies have shown that a number of natural products can enhance the cancer cell killing effects of conventional treatments, including radiotherapy, and at the same time protect normal cells from damage by these treatments – the best of all worlds.[75,143,149,150]
A number of natural products have shown an ability to reverse chemotherapy drug resistance, including an ability to restore apoptotic mechanisms such as p53 activity.[43,47,153,158] Of great importance is the discovery that several natural products can kill cancer stem cells.[67,126] It may be that some of these natural products may also promote the conversion of cancer stem cells back to normal somatic stem cells. Yet, despite the compelling evidence that a number of natural products have powerful anticancer effects on a great number of types of cancer, even the most resistant forms of cancer, oncologists continue to warn their patients not to take antioxidant's natural products. Some patients are even told not to eat vegetables as they interfere with cancer treatments.
Low toxicity of natural molecular agents
When I practiced neurosurgery, I gave all of my cancer patients selected anticancer natural products, primarily curcumin, quercetin, mixed tocopherols, vitamin C, and resveratrol and have never observed interference with conventional treatments. Dr Jerome Block, former chief of the Division of Medical Oncology/Hematology at Harbor UCLA Medical Center not only used complementary nutraceuticals in his cancer patients, but also taught visiting doctors on their use.[13] In a conversation, Dr Block told me that in 30 years of practicing oncology he had never observed interference with conventional treatments when using selected nutriceuticals, most of which had powerful antioxidant/antiinflammatory activity. I emphasize the word “selected” as some antioxidants can interfere with specific chemotherpeutic agents. The flavonoids, mixed tocopherols/tocotrienols, vitamin C, docosahexaenoic acid (DHA), selenium, magnesium, and biacalein do not interfere with these treatments, rather they enhance their tumor killing ability.
Why the traditional Western diet may be proinflammatory and procarcinogenic
Physicians treating cancer should be aware of the fact that most of the omega-6 oils, such as corn, safflower, sunflower, peanut, and soybean oils, promote tumor proliferation, invasion, and metastasis. The main reason is that these oils are proinflammatory. Americans eating the typical Western diet consume 50-fold higher levels of the oils than are needed for good health. High sugar intake also promotes inflammation and cancer growth and invasion. Finally, glutamate and the other excitatory amino acids (aspartate, homocysteic acid, and cysteic acid) also promote tumor proliferation, invasion, and metastasis. Many hospital feeding formula contain high levels of glutamate and rarely are cancer patients told to avoid glutamate additives, aspartame, or foods naturally high in glutamate.
CONCLUSIONS
In this Hypothesis on the Genesis of Cancer paper, what we have learned after 43 years of the war on cancer is that we managed to overlook a critical mechanism that was correctly recognized over 150 years ago, mainly that inflammation is at the center of the cancer process. Our investment also allowed us to correct our having overlooked another important piece of the puzzle – that not all cells can become cancer, rather stem cells appear to be the major cell type involved.
These two changes in our thinking may well lead to a dramatic reduction in cancer development and may change the way we treat established cancers by changing our targets. Over the past 40 years, we have learned an enormous amount about cell signaling and how it is altered in cancer cells. Two of the most important systems are transcription control mechanisms of gene activation known as NFκB and STAT3. It is through these transcription pathways that cancers are formed, proliferate, develop a blood supply (angiogenesis), invade surrounding tissues (including blood and lymphatic vessels), and metastasized to distant sites.
Nonsteroidal antiinflammatory medications and aspirin have shown a significant ability to suppress inflammation and thereby alter the cancer process. A growing number of natural molecular products and their extracts are showing an ability to suppress multiple pathways involved in the cancer process, including suppression of NFκB and STAT3 in the plant and animal kingdom.
Presently, human trials and responses to these natural substances have been hampered by poor absorption and bioavailability of these extracts but newer techniques, such as nanosizing and microencapsulation with phospholipids, have greatly improved both gut absorption and bioavailability. When combined with dietary programs designed to utilize what we now know about the anticancer effects of various foods can greatly improve the prevention and treatment of cancers.
The vast majority of the natural products found to have powerful and versatile anticancer effects have shown a very wide margin of safety. Curcumin, for example, in extremely high doses is nontoxic to normal cells and tissues. One can appreciate the careful testing of manufactured drugs, as most have extremely high toxicity and treatment concentrations are often close to fatal systemic toxic effects.
The fact that these natural compounds are powerful anticancer agents when used alone and significantly improve conventional chemotherapy and radiation therapy treatments, plus the fact that they protect normal tissues and cells, is reason enough to begin use of these valuable agents now, both as cancer preventatives and in the treatment of established cancers. The patients with advanced cancers or high aggressive cancers cannot afford to wait another 10 years while these safe compounds are tested for years as if they were dangerous drugs.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/1/92/157890
REFERENCES
- 1.Acikalin MF, Oner U, Topeu I, Yasar B, Kiper H, Colak E. Tumor angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Live Dis. 2005;37:162–9. doi: 10.1016/j.dld.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Alberti C, Pinciroli P, Valeri B, Ferri R, Ditto A, Umezawa K, et al. Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFκB pathway in advanced-stage epithelial ovarian cancer. Oncogene. 2012;31:4139–49. doi: 10.1038/onc.2011.572. [DOI] [PubMed] [Google Scholar]
- 3.Alexandrow MG, Song LJ, Altiok S, Gray J, Haura EB, Kumar NB. Curcumin: A novel STAT3 pathway inhibitor for chemoprevention of lung cancer. Eur J Cancer Prev. 2012;21:407–12. doi: 10.1097/CEJ.0b013e32834ef194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernendez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Ambs S, Glynn SA. Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer. Cell Cycle. 2011;10:619–24. doi: 10.4161/cc.10.4.14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annemijn M, Algra B, Rothwell P. effects of regular aspirin n long-term cancer incidence and metastasis: A systematic comparison if evidence from observational studies versus randomized trails. Lancet. 2012;13:518–27. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F. Tumor necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 10.Balkwill F, Charles KA, Montovani A. Smoldering and polarizing inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 12.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trail of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 13.Block JB. Interview with Jerome B Block, MD. J Am Nutraceutical Assoc. 1999;2:57. [Google Scholar]
- 14.Boccardo E, Lepique AP, Vilia LL. The role if inflammation in HPV carcinogenesis. Carcinogenesis. 2010;31:1905–12. doi: 10.1093/carcin/bgq176. [DOI] [PubMed] [Google Scholar]
- 15.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neuronal crest nerve growth factor receptor CD271. Nature. 2010;466:133–7. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Aging Dev. 2004;125:811–26. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Bourillot PY, Aksoy I, Schreiber V, Wianny F, Schultz H, Hummel O, et al. Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells. 2009;27:1760–71. doi: 10.1002/stem.110. [DOI] [PubMed] [Google Scholar]
- 18.Bruning A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anticancer Agents Med Chem. 2013;13:1025–31. doi: 10.2174/18715206113139990114. [DOI] [PubMed] [Google Scholar]
- 19.Burleigh AR. Of germs cells, trophoblast, and cancer stem cells. 2008;7:276–81. doi: 10.1177/1534735408326454. [DOI] [PubMed] [Google Scholar]
- 20.Cabrera MC, Hollingsworth RE, Hurt EM. Cancer stem cell plasticity and tumor hierarchy. World J Stem Cells. 2015;7:27–36. doi: 10.4252/wjsc.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao HH, Tse AK, Kwan HY, Yu H, Chen CY, Su T, et al. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem Pharmacol. 2014;87:424–34. doi: 10.1016/j.bcp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Carocho M, Ferreira IC. The role of phenolic compounds in the fight against cancer - a review. Anticancer Agents Med Chem. 2013;13:1236–58. doi: 10.2174/18715206113139990301. [DOI] [PubMed] [Google Scholar]
- 23.Chan A, Ogino S, Fuch C. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–58. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60:2132–5. [PubMed] [Google Scholar]
- 26.Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2:1124–31. doi: 10.1158/2326-6066.CIR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clement V, Sanchez P, de Tribolet N, Taobovanic I, Ruizialtaba A. Hedgehog-GL13 signaling regulates human glioma growth, cancer stem cells self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 29.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Correa GT, Bandeira GA, Cavalcanti BG, de Carvalho Fraga CA, dos Santos EP, Silva TF, et al. Association of – 308 TNF-alpha promoter polymorphism with clinical aggressiveness in patients with head and neck squamous cell carcinoma. Oral Oncol. 2011;47:888–94. doi: 10.1016/j.oraloncology.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Crawford S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: A new therapeutic approach to disease progression and recurrence. Ther Adv Med Oncol. 2014;6:52–68. doi: 10.1177/1758834014521111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawford S. Is it time for a new paradigm for systemic cancer treatment? Lessons from a century of cancer chemotherapy. Front Pharmacol. 2012;4:68. doi: 10.3389/fphar.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dannenmann SR, Thielicke J, Stokli M, Matter C, von Boehmer L, Cecconi V, et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562. doi: 10.4161/onci.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das M, Sahoo SK. Folate decorated dual drug loaded nanoparticle: role of curcumin in enhancing therapeutic potential of nutlin-3a by reversing multidrug resistance. PloS One. 2012;7:e32920. doi: 10.1371/journal.pone.0032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, et al. Cancer and inflammation: Promise for biological therapy. J Immunother. 2010;33:335–51. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol. 2013;25:637–45. doi: 10.1097/CCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–38. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 38.Dressens G, Beck B, Caawe A, Simons BD, Blanpain C. Defining the mode of tumor growth by clonal analysis. Nature. 2012;488:527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: Correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–36. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 40.Elgert K, Alleva D, Mullens D. Tumor-induced immune dysfunction: The macrophage connection. J Leukoc Biol. 1998;64:275–90. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- 41.Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G, et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prov. 2009;18:215–22. doi: 10.1158/1055-9965.EPI-08-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem cell-like neuronal precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 43.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–47. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 44.Garzia L, Andolfo I, Cusanelli E, Marino N, Petrpsino G, De Martino D, et al. MicroRNA-199b-5p impairs cancer stem cells through negative regulation if HES1 in medulloblastoma. PLoS One. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gat U, Das Gupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 46.Gilmore TD. The Rel/NF-κB/IκB signal transduction pathway and cancer. Cancer Treat Res. 2003;115:241–65. [PubMed] [Google Scholar]
- 47.Gu Q, Hu C, Chen Q, Xia Y. Tea polyphenols prevent lung from preneoplastic lesions and effect p53 and bcl-2 expression in rat lung tissues. Int J Clin Exp Pathol. 2013;6:1523–31. [PMC free article] [PubMed] [Google Scholar]
- 48.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Gurchot C. The trophoblastic theory of cancer (John Beard, 1857-1924) revisted. Oncology. 1975;31:310–33. doi: 10.1159/000225037. [DOI] [PubMed] [Google Scholar]
- 50.Hamberger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–3. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto O, Shimizu K, Semba S, Ciba S, Ku Y, Yokozaki H, et al. Hypoxia induces tumor aggressiveness and the expansion of CD133-positive cells in a hypoxia-inducible factor-1α-dependent manner in pancreatic cancer cells. Pathobiology. 2011;76:181–92. doi: 10.1159/000325538. [DOI] [PubMed] [Google Scholar]
- 52.Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–9. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellegass JM, Shukla A, Lathrop SA, MacPherson MB, Beuschel SL, Butnor KJ, et al. Inflammation precedes the development f human malignant mesotheliomas in a SCID mouse xenograft model. Ann NY Acad Sci. 2010:1203–14. doi: 10.1111/j.1749-6632.2010.05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmes M, Chen W, Li L, Hertzmark E, Spiegelman D, Hankinson S. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–72. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh CH, Lee CH, Liang JA, Yu CY, Shyu WC. Cycling hypoxia increases U87 glioma cell radioresistance via ROS induced higher and long-term HIF-1 signaling. Oncol Rep. 2010;24:1629–36. doi: 10.3892/or_00001027. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh CH, Shyu WC, Chiang CY, Kuo JW, Shen WC, Liu RS. NADPH oxidase subunit 4-mediated reactive oxygen species contribute to cycling hypoxia-promoted tumor progression in glioblastoma multiforme. PLoS One. 2011;6:e23945. doi: 10.1371/journal.pone.0023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huber MA, Azoitel N, Baumann B, Grunert S, Sommer A, Pehamberger H, et al. NF-kappaB is essential for epithelial-mesenchyme transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ibrahim EE, Babael-Jadidi R, Saadeddin A, Spencer-Dene B, Houssaini S, Abuzinadah M, et al. Embryonic NANOG activity define colorectal cancer stem cells and modulates through AP1-and TCF-dependent mechanisms. Stem Cells. 2012;30:2076–87. doi: 10.1002/stem.1182. [DOI] [PubMed] [Google Scholar]
- 59.Jensen RL. Hypoxia in the tumorigenesis of gliomas and as a potential target for therapeutic measures. Neurosurg Focus. 2006;20:E24. doi: 10.3171/foc.2006.20.4.16. [DOI] [PubMed] [Google Scholar]
- 60.Ji RC. Macrophages are important mediators of either tumor-or-inflammation-induced lymphangiogenesis. Cell Mol Life Sci. 2012;69:897–914. doi: 10.1007/s00018-011-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson TS, Munn DH. Host indolamine 2,3 dioxygenase: contribution to systemic acquired tumor tolerance. Immunol Invest. 2012;41:765–97. doi: 10.3109/08820139.2012.689405. [DOI] [PubMed] [Google Scholar]
- 62.Kagoya Y, Yoshimi A, Kataoka K, Nakagawa M, Kumano K, Arai S, et al. Positive feedback between NF-κB and TNF-α promotes leukemia-initiating cell capacity. J Clin Invest. 2014;124:528–42. doi: 10.1172/JCI68101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, et al. Tumor-cell homing lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Nat Cancer Inst. 2005;97:1840–7. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- 64.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 65.Katz JB, Muller AJ, Pendergast GC. Indolamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–21. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 66.Keibel A, Singh V, Sharma MC. Inflammation, microevironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15:1949–55. doi: 10.2174/138161209788453167. [DOI] [PubMed] [Google Scholar]
- 67.Kim YS, Farrar W, Colburn NH, Milner JA. Cancer stem cells: potential target for bioactive food components. J Nutr Biochem. 2012;23:691–8. doi: 10.1016/j.jnutbio.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kisseleva T, Song L, Voromtchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–63. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 70.Kono Y, Kawakami S, Higuchi Y, Yasashita F, Hashida M. In vitro evaluation of inhibitory effects of nuclear factor-kappaB activity by small interfering RNA on pro-tumor characteristics of M2-like macrophages. Biol Pharm Bull. 2014;37:137–44. doi: 10.1248/bpb.b13-00659. [DOI] [PubMed] [Google Scholar]
- 71.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–27. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 72.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting STAT3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 73.Krstic J, Santibanez JF. Transforming growth factor-beta and matric metalloprotenases: Functional interactions in tumor stroma-infiltrating myeloid cells. Scientific World Journal 2014. 2014 doi: 10.1155/2014/521754. 521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355–62. doi: 10.1158/0008-5472.CAN-05-0957. [DOI] [PubMed] [Google Scholar]
- 75.Kunnummakara AB, Guha S, Krishnan S, Diagaradjiane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 76.Lee M, Nam EJ, Kim SW, Kim S, Kim JH, Kim YT. Prognostic impact of the cancer stem cell-related marker NANOG in ovarian serious carcinoma. Int J Gynecol Cancer. 2012;22:1489–96. doi: 10.1097/IGJ.0b013e3182738307. [DOI] [PubMed] [Google Scholar]
- 77.Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936–41. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-κB is the lynchpin. Trends Immunol. 2005;26:318–25. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Liao WC, Lin JT, Wu CY, Huang SP, Lin MT, Wu As, et al. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin Cancer Res. 2008;14:428–34. doi: 10.1158/1078-0432.CCR-07-1032. [DOI] [PubMed] [Google Scholar]
- 80.Liao Y, Shen W, Kong G, Lv H, Tao W, Bo P. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol Cell Biochem. 2012;366:319–34. doi: 10.1007/s11010-012-1310-2. [DOI] [PubMed] [Google Scholar]
- 81.Lin L, Hutzen B, Li PK, Ball S, Zuo M, DeAngelis S, et al. A novel small molecule, LLL12 inhibits STAT3 phosphorylation and activities and exhibits potent growth-suppressive activity in human cancer cells. Neoplasia. 2010;12:39–50. doi: 10.1593/neo.91196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44 doi: 10.3109/10715761003667554. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu H, Quyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:1–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 84.Luo JL, Tan W, Ricino JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–4. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 85.Malhotra GK, Zhao X, Band H, Band V. Shared signaling pathways in normal and breast cancer stem cells. J Carcinog. 2011;10:38. doi: 10.4103/1477-3163.91413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malianna SK, Rizzino A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev Biol. 2010;344:16–25. doi: 10.1016/j.ydbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mantovani A, Allaventa P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 88.Martin-Green M, Boudreau N, Bissel MJ. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994;54:4334–41. [PubMed] [Google Scholar]
- 89.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–26. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 90.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Menegazzi M, Mariotto S, Dal Bosco M, Darra E, Vaiana N, Shiji K, et al. Direct interaction of natural and synthetic catechins with signal transducer activator of transcription 1 affects both its phosphorylation and activity. FEBS J. 2014:281724–38. doi: 10.1111/febs.12618. [DOI] [PubMed] [Google Scholar]
- 92.Moss RW. An annotated bibliography of works by John Beard. Integ Cancer Ther. 2008;7:317–21. doi: 10.1177/1534735408326754. [DOI] [PubMed] [Google Scholar]
- 93.Munn DH. Indolamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–5. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Murley JS, Kataoka Y, Cao D, Li JJ, Oberley LW, Grdina DJ. Delayed radioprotection by Nf kappaB-mediated induction of Sod2 (MnSOD) in Sa-NH tumor cells after exposure to clinically used thiol-containing drugs. Radiat Res. 2004;162:536–46. doi: 10.1667/rr3256. [DOI] [PubMed] [Google Scholar]
- 95.Nagata T, Shimada Y, Sekine S, Hori R, Matsui K, Okumura T, et al. Prognostic significance of NANOG and KLF4 for breast cancer. Breast Cancer. 2012;21:96–101. doi: 10.1007/s12282-012-0357-y. [DOI] [PubMed] [Google Scholar]
- 96.Oh YS, Kim HY, Song IC, Yun HJ, Jo DY, Kim S, et al. Hypoxia induces CXCR4 expression and biological activity in gastric cancer cells through activation of hypoxia-inducible factor-1α. Oncol Rep. 2012;28:2239–46. doi: 10.3892/or.2012.2063. [DOI] [PubMed] [Google Scholar]
- 97.Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M, et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev 2013. 2013 doi: 10.1155/2013/387014. 387014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okada F. Inflammation and free radicals tumor development and progression. Redox Rep. 2002;7:375–68. doi: 10.1179/135100002125001135. [DOI] [PubMed] [Google Scholar]
- 99.Orr WS, Denbo JW, Saab KR, Ng CY, Wu J, Li K, et al. Curcumin potentiates rhabdomyosarcoma radiosensitivity by suppressing NF-κB activity. PloS One. 2013;8:e51309. doi: 10.1371/journal.pone.0051309. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Ott G. Impact of MYC on malignant behavior. Hematology Am Soc Hematol Educ Program 2014. 2014:100–6. doi: 10.1182/asheducation-2014.1.100. [DOI] [PubMed] [Google Scholar]
- 101.Park CH, Bergaagel DE, McCulloch EA. Mouse myloma stem cells: A primary culture assay. J Natl Cancer Inst. 1971;46:411–22. [PubMed] [Google Scholar]
- 102.Patel N, Joseph C, Corcoran GB, Ray SD. Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol Appl Pharmacol. 2010;245:143–52. doi: 10.1016/j.taap.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 103.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–22. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 104.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumor promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 105.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 106.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rao S, Sengupta R, Choe EJ, Woerner M, Jackson E, Sun T, et al. CXCL12 mediates trophic interactions between endothelial and tumor cells in glioblastoma. PLoS One. 2012;7:e33005. doi: 10.1371/journal.pone.0033005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ravishankar D, Rajora AK, Greco F, Osborn HM. Flavonoids as prospective compounds for anti-cancer therapy. In J Biochem Cell Biol. 2013;45:2821–31. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 109.Read SA, Douglas MW. Virus induced inflammation and cancer development. Cancer Lett. 2014;345:174–81. doi: 10.1016/j.canlet.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 110.Reya T, Morrison SJ, Clarke MF, Weissman IK. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 111.Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, et al. Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: A report from the InterLymph Consortium. Lancet Oncol. 2006;7:27–38. doi: 10.1016/S1470-2045(05)70434-4. [DOI] [PubMed] [Google Scholar]
- 112.Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol. 2003;84:151–9. doi: 10.1002/jso.10305. [DOI] [PubMed] [Google Scholar]
- 113.Sakuma S, Sawamura Y, Tada M, Aida T, Abe H, Suzuki K, et al. Responses of human glioblastoma cells to human tumor necrosis factor-alpha: Susceptibility, mechanism of resistance and cytokine production studies. J Neurooncol. 1993;15:197–208. doi: 10.1007/BF01050066. [DOI] [PubMed] [Google Scholar]
- 114.Salsbury AJ. The significance of the circulating cancer cells. Cancer Treat Rev. 1975;2:55–72. doi: 10.1016/s0305-7372(75)80015-6. [DOI] [PubMed] [Google Scholar]
- 115.Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, et al. The role of CXCR4 receptor in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275–83. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 116.Sarvalya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4:2171–85. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sato A, Okada M, Shibuya K, Watanabe E, Seino S, Narita Y, et al. Pivotal role for ROS activation of p38 MAPK in control of differentiation and tumor initiating capacity of glioma initiating cells. Stem Cell Res. 2014;12:119–31. doi: 10.1016/j.scr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 118.Sato Y, Goto Y, Narita N, Hoon DS. Cancer cells expressing toll-like receptors and the tumor microenvironment. Cancer Microenviron. 2009;2(Suppl 1):205–14. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schepers AG, Snippert HJ, Strange DE, van den Born M, van Es JH, van de Wetering M, et al. Linage tracing reveals Lgr5+stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–5. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 120.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schraivogel D, Weinmann L, Beier D, Tabatabai G, Eichner A, Zhu JY, et al. CAMTA1 is a novel tumor suppressor regulated by miR-9/9 in glioblastoma stem cells. EMBRO J. 2011;30:4309–22. doi: 10.1038/emboj.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 124.Senggunprai L, Kuongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventative effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2013;28:841–8. doi: 10.1002/ptr.5061. [DOI] [PubMed] [Google Scholar]
- 125.Seo HS, Choi HS, Kim SR, Choi YK, Woo SM, Park SY, et al. Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: Effects on inhibition of STAT3 phosphorylation. Life Sci. 2005;78:389–97. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 126.Sethi S, Sarkar FH. Regulating miRNA by natural agents as a new strategy for cancer treatment. Curr Drug Targets. 2013;14:1167–74. doi: 10.2174/13894501113149990189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 2006;20:2527–38. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shehzad A, Park JW, Lee J, Lee YS. Curcumin induces radiosensitivity of in vitro and in vivo cancer models by modulating pre-mRNA processing factor 4 (Prp4) Chem Biol Interact. 2013;206:394–402. doi: 10.1016/j.cbi.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 129.Shen HM, Tergaonkar V. NFkappaB signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis. 2009;14:348–63. doi: 10.1007/s10495-009-0315-0. [DOI] [PubMed] [Google Scholar]
- 130.Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, et al. Role of tumor-associated macrophages ion the progression of hepatocellular carcinoma. Surg Today. 2012;42:1–7. doi: 10.1007/s00595-011-0058-8. [DOI] [PubMed] [Google Scholar]
- 131.Si M, Zhao J, Li X, Tian JG, Li YG, Li JM. Reversion effects of curcumin on multidrug resistance pf MNNG/HOS human osteosarcoma cells in vitro and in vivo through regulation of P-glycoprotein. Chin Med J. 2013;126:4116–23. [PubMed] [Google Scholar]
- 132.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J. Identification of cancer stem cells in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 133.Sinkovics JG. Molecular biology of oncogenic inflammatory processes. I. Non-oncogenic and oncogenic pathogens, intrinsic inflammatory reactions without pathogens, and microRNA/DNA interactions (review) Int J Oncol. 2012;40:305–49. doi: 10.3892/ijo.2011.1248. [DOI] [PubMed] [Google Scholar]
- 134.Siu MK, Wong ES, Kong DS, Chan HY, Jiang L, Wong OG, et al. Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene. 2013;32:3500–9. doi: 10.1038/onc.2012.363. [DOI] [PubMed] [Google Scholar]
- 135.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsh R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–77. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 136.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Fick HE, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–35. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–8. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 138.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 139.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 140.Stockler M, Wilcken NR, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev. 2000;26:151–68. doi: 10.1053/ctrv.1999.0161. [DOI] [PubMed] [Google Scholar]
- 141.Takahashi RU, Miyazaki H, Ochiya T. The role of microRNAs in the regulation of cancer stem cells. Front Genet. 2014;4:295. doi: 10.3389/fgene.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nature Med. 2002;8:1089–97. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 143.Tian F, Fan T, Zhang Y, Jiang Y, Zhang X. Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous cells through downregulating the activation of NF-κB signaling pathway in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 2012;44:847–55. doi: 10.1093/abbs/gms074. [DOI] [PubMed] [Google Scholar]
- 144.Todd R, Wong DT. Wong. Oncogenes. Anticancer Res. 1999;19:4729–46. [PubMed] [Google Scholar]
- 145.Trabanelli S, Ocadlikova D, Evangelisti C, Parisi S, Curti A. Induction of regulatory T cells by dendritic cells through indolamine 2,3-dioxygenase: A potent mechanism of acquired peripheral tolerance. Curr Med Chem. 2011;18:2234–9. doi: 10.2174/092986711795656054. [DOI] [PubMed] [Google Scholar]
- 146.Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, et al. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res. 2012;5:205–15. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–22. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 148.Wang CY, Cusack JC, Liu R, Baldwin AS. Control of inducible chemoresistance enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–7. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 149.Wang G, Zhang J, Sharma S, Dong O. Quercetin potentiates doxorubicin mediated antitumor effects against liver cancer through p53/Bcl-xl. PloS One. 2012;7:e51764. doi: 10.1371/journal.pone.0051764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang J, Pae M, Meydani SN. Green tea epigallocatechin-3 gallate modulates differentiation of naïve CD4 T cells into specific lineage effector cells. J Mol Med (Berl) 2013;91:485–95. doi: 10.1007/s00109-012-0964-2. [DOI] [PubMed] [Google Scholar]
- 151.Wang ML, Chiou SH, Wu CW. Targeting cancer stem cells: Emerging role of nanog transcription factor. OncoTargets Ther. 2013;6:1207–20. doi: 10.2147/OTT.S38114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Q, Fan H, Liu Y, Yin Z, Cai H, Liu J, et al. Curcumin enhances the radiosensitivity in nasopharyngeal carcinoma cells involving the reversal of differentially expressed long non-coding TNAs. In J Oncol. 2014;44:858–64. doi: 10.3892/ijo.2013.2237. [DOI] [PubMed] [Google Scholar]
- 153.Wang TT, Wang SK, Huang GL, Sun GJ. Luteolin induced-growth inhibition and apoptosis of human esophageal squamous carcinoma cell line Eca109 cells in vitro. Asian Pac J Cancer Prev. 2012;13:5455–61. doi: 10.7314/apjcp.2012.13.11.5455. [DOI] [PubMed] [Google Scholar]
- 154.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea – a paradigm shift. Cancer Res. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 155.Wiejak J, Dunlop J, Mackay SP, Yarwood SJ. Flavonoids induce expression of the suppressor of cytokine signaling 3 (SOCS3) gene and suppress TL-6-activated signal transducer and activator of transcription 3 (STAT3) activation in vascular endothelial cells. Biochem J. 2013;454:283–93. doi: 10.1042/BJ20130481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–44. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, et al. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through janus kinase-STAT3 signaling pathway. PloS One. 2012;7:e37960. doi: 10.1371/journal.pone.0037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ye MX, Zhao YL, Li Y, Miao Q, Li ZK, Ren XL, et al. Curcumin reverses cis-platin resistance and promotes human lung adenocarcinoma A549/DDP cell apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine. 2012;15:779–87. doi: 10.1016/j.phymed.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 159.Yiannakopoulou ECh. Effect of green tea catechins on breast carcinogenesis: A systematic review of in-vitro and in-vivo experimental studies. Eur J Cancer Prev. 2014;23:84–9. doi: 10.1097/CEJ.0b013e328364f23e. [DOI] [PubMed] [Google Scholar]
- 160.Yu L, Baxter PA, Voicu H, Gurusiddappa S, Zhao Y, Adesina A, et al. A clinically relevant orthoptic xenograft model of ependymoma that maintains the genomic signature of the primary tumor and preserves cancer stem cells in vivo . Neuro Oncol. 2010;12:580–94. doi: 10.1093/neuonc/nop056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu L, Chen C, Wang LF, Kuang X, Liu K, Zhang H, et al. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NFκB and STAT3 in transient focal stroke. PloS One. 2013;8:e55839. doi: 10.1371/journal.pone.0055839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer Stem Cells. Int J Biochem Cell Biol. 2012;44:2144–51. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhai K, Ding J, Zhou Y. Different role of tumor necrosis factor-alpha polymorphism in non-Hodgkin lymphomas among Caucasion and Asian populations: A meta-analysis. Int J Mol Sci. 2014;15:7684–98. doi: 10.3390/ijms15057684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang M, He Y, Sun X, Li G, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Y, Sime W, Juhas M, Sjolander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumor cell migration. Eur J Cancer. 2013;49:3320–34. doi: 10.1016/j.ejca.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 166.Zhou D, Bigarella CL, Liang R, GhaffRI S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–18. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]


