Abstract
Glomerulonephritis (GN) due to infective endocarditis (IE) is well documented, but most available data are based on old autopsy series. To update information, we now present the largest biopsy-based clinicopathologic series on IE-associated GN. The study group included 49 patients (male-to-female ratio of 3.5:1) with a mean age of 48 years. The most common presenting feature was acute kidney injury. Over half of the patients had no known prior cardiac abnormality. However, the most common comorbidities were cardiac valve disease (30%), intravenous drug use (29%), hepatitis C (20%), and diabetes (18%). The cardiac valve infected was tricuspid in 43%, mitral in 33%, and aortic in 29% of patients. The two most common infective bacteria were Staphylococcus (53%) and Streptococcus (23%). Hypocomplementemia was found in 56% of patients tested and ANCA antibody in 28%. The most common biopsy finding was necrotizing and crescentic GN (53%), followed by endocapillary proliferative GN (37%). C3 deposition was prominent in all cases, whereas IgG deposition was seen in <30% of cases. Most patients had immune deposits detectable by electron microscopy. Thus, IE-associated GN most commonly presents with AKI and complicates staphylococcal tricuspid valve infection. Contrary to infection-associated glomerulonephritis in general, the most common pattern of glomerular injury in IE-associated glomerulonephritis was necrotizing and crescentic glomerulonephritis.
Keywords: crescentic glomerulonephritis, infection-related glomerulonephritis, infective endocarditis, renal biopsy
Renal disease due to infective endocarditis (IE) is well established, with the earliest reports of glomerular lesions published over 100 years ago.1, 2, 3 Although initially believed to be primarily embolic,1, 2, 3 it later became clear that over 80% of cases represented focal, segmental, or diffuse proliferative glomerulonephritis (GN) with prominent endocapillary proliferation and occasional infiltrating leukocytes.4, 5, 6 However, the literature describing nephritis associated with IE still relies heavily on autopsy studies conducted in the pre- and early postantibiotic era or small renal biopsy studies from the 1970s.
Several reviews have emphasized the evolution occurring in recent decades in renal complications of infectious diseases in general, with particular emphasis on the change in demographics from younger to older patients, the frequency of comorbidities such as diabetes and HIV, and the change in predominance of infectious agents from primarily streptococcal to a broader array of organisms with predominance of Staphylococci.7, 8, 9, 10
IE occurs in 30 to 60% of patients with Staphylococcus aureus bacteremia and carries a mortality rate of 40–50%.11 Over the past decades, IE outcomes have not improved, and infection rates are steadily increasing.11 Recent case series and reviews of IE have compared findings from current and previous eras, confirmed similar changes in the demographics of the disease, and updated the clinical and pathologic features in both adults and children.5, 12 However, few of these recent reports have focused primarily on IE-related renal lesions, and much of the data currently available still include predominately autopsy-derived information.5, 13
Based on all of the above, we investigated the clinicopathologic characteristics of a large cohort of patients with IE-associated GN diagnosed by kidney biopsy between 2001 and 2011 in two large nephropathology laboratories. Our data indicate that IE-associated GN in the new era has significantly different clinical and pathologic changes from those described historically.
RESULTS
Clinical features
The clinical characteristics of 49 patients undergoing a renal biopsy with documented IE are detailed in Table 1. Features of note include a male predominance (3.5:1) with a mean age at biopsy of 48 years. Two patients (4%) were children <18 years, and 30% of patients were elderly (≥60 years of age). Acute renal failure was the most common presenting condition (79%), with hematuria present in almost all cases (97%), yet typical acute nephritic syndrome in only <10% of cases. Conditions favoring endocarditis were noted in 29 patients including intravenous drug use (29%), prosthetic valves (18%), and prior valvular disease (12%). However, over 50% of patients did not have known prior cardiac disease. Associated comorbid conditions were noted in a minority of patients, the most common being hepatitis C infection (20%) and diabetes mellitus (18%) (Table 1).
Table 1. Demographics and clinical characteristics.
| Gender/age | |
| Male:female, n/n (%/%) | 38/11 (78/22) |
| Age (years), mean (range) | 48 (3–84) |
| n (%) | |
| Clinical syndrome n=47 with data (%) | |
| Acute renal failure | 37 (79) |
| Acute nephritic syndrome | 4 (9) |
| Rapidly progressive glomerulonephritis | 3 (6) |
| Nephrotic syndrome | 3 (6) |
| Predisposing statesa | |
| Intravenous drug abuse | 14 (29) |
| Prosthetic cardiac valve | 9 (18) |
| Cardiac valve disease/Intracardiac shunt | 6 (12) |
| Associated conditions | |
| Hepatitis C | 10 (20) |
| Diabetes mellitus | 9 (18) |
| Coronary artery disease | 3 (6) |
| Chronic obstructive pulmonary disease | 2 (4) |
| Congestive heart failure | 1 (2) |
| Systemic lupus erythematosus | 1 (2) |
| Recent surgery | 1 (2) |
| Prostate cancer | 1 (2) |
| Median (range) | |
| Laboratory data | |
| Serum creatinine at biopsy (mg/dl), n=45 | 3.8 (1.0–12.0) |
| Proteinuria (g per day), n=18 | 1.8 (0.5–15) |
| n (%) | |
| Hematuria, n=37 | 36 (97) |
| ANA, n=26 | |
| Positive | 4 (15) |
| ANCA, n=29b | |
| Positive | 8 (28) |
| C3/C4, n=32 | |
| Low C3 only | 12 (37) |
| Low C4 only | 1 (3) |
| Low C3 and C4 | 5 (16) |
| Normal C3 and C4 | 14 (44) |
Abbreviations: ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic antibody; C3, complement component 3; C4, complement component.
One patient with a prosthetic valve and one patient with tricuspid insufficiency were also intravenous drug users.
ANCA data was obtained in 43/49 patients (88%), although testing was only carried out in 29/43 patients.
Serologic studies
Serologic studies are summarized in Table 1. While 53% of the 32 patients tested for serum complement had reduced C3 (complement component 3) levels only a minority of patients (19%) in whom it was tested had reductions in C4, suggesting that most had activation of the alternative complement pathway. Anti-neutrophil cytoplasmic antibody (ANCA) data was obtained in 43/49 patients (88%), although testing was not carried out in 14/43 patients. Of the 29/43 patients with ANCA serologies drawn, 21 were negative (72%) and 8 were positive (28%). ANCA specificities of these 8 patients include 3 pANCA (one with positive MPO), 3 cANCA (two with positive PR3), 1 positive ANCA of unspecified type, and 1 with dual-positive MPO and PR3. Anti-nuclear antibody (ANA) was positive in 4/26 patients tested (15%). One patient with a positive ANA had history of systemic lupus erythematosus (SLE), although renal biopsy was without significant immune complex (IC) deposition. In the other three patients, the positive ANA was an isolated finding, with none having clinical evidence of SLE.
Cardiac involvement
Details of the IE are shown in Table 2. Cardiac infections most commonly involved the tricuspid valve (43%), followed by the mitral (33%), aortic (29%), and pulmonic (5%) valves. Five patients (12%) had involvement of two cardiac valves. One patient with tricuspid valve endocarditis also had a ventricular atrial shunt infection. Echocardiogram vegetations were noted in greater than two-thirds of patients. The most commonly noted sign of cardiac involvement in patients without vegetations on echocardiogram was new valvular regurgitation/murmur; the most common other criteria for diagnosis of IE in these patients included fever, septic pulmonary emboli, and predisposing heart condition or injection drug use. The most common vascular phenomena in the entire cohort was septic pulmonary infarcts, with only a minority of patients with intracranial hemorrhage, and rare patients with conjunctival hemorrhages, nail splinter hemorrhages, or evidence of mycotic aneurysm.
Table 2. Cardiac and bacterial characteristics.
| n (%) | |
|---|---|
| Valve/locationa | |
| Tricuspid | 18 (43) |
| Mitral | 14 (33) |
| Aortic | 12 (29) |
| Pulmonic | 2 (5) |
| Chordae tendinae | 1 (2) |
| Culture results | |
| Positive | 44 (90) |
| Negativeb | 4 (8) |
| Unknown | 1 (2) |
| Bacterial agentc | |
| Staphylococcus | 25 (53) |
| Streptococcus | 11 (23) |
| Bartonella henselaeb | 4 (8) |
| Coxiella burnetii | 2 (4) |
| Cardiobacterium hominis | 1 (2) |
| Gemella | 1 (2) |
|
Location |
|||||
|---|---|---|---|---|---|
| Agent | Tricuspid (%) | Mitral (%) | Aortic (%) | Pulmonic (%) | Chordae (%) |
| Staphylococcus | 76 | 31 | 50 | 50 | 0 |
| Streptococcus | 12 | 46 | 17 | 0 | 0 |
| Otherd | 12 | 23 | 33 | 50 | 100 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MRSS, methicillin-sensitive Staphylococcus aureus.
Two patients had aortic and mitral valve involvement, two had tricuspid and mitral valve involvement, and one had tricuspid and pulmonic valve involvement.
Three patients had blood culture positive for Bartonella, and one patient with negative blood cultures showed serologic evidence of active Bartonella infection.
MRSA, n=14; MRSS, n=9; Staphylococcus not further classified as methicillin-sensitive or methicillin-resistant, n=2; Streptococcus viridans, n=3; Streptococcus agalactiae, n=1; Streptococcus mitis, n=1; Streptococcus sanguinis, n=1; Enterococcus faecalis, n=3; Streptococcus not further specified, n=2; culture result was unavailable in two cases.
Other includes Bartonella henselae, Coxiella, Cardiobacterium hominis, Gemella, and culture-negative cases.
Infectious agents
The most common infectious agent found on blood culture was S. aureus (53%), with methicillin resistance in 56% (Table 2). Streptococcus species were the second most common pathogens found (23%). Less common causes of endocarditis were Bartonella henselae in four patients, Coxiella burnetii in two, Cardiobacterium hominis in one, and Gemella species in one. Four patients (9%) had culture-negative endocarditis, similar to findings in other series.14, 15 Staphylococcal infection was the most common cause of endocarditis in patients with a history of intravenous drug abuse (77%), with the tricuspid valve or tricuspid and pulmonic valves (in one patient) affected in 83% and mitral or aortic valves in 17%. There were no significant associations noted between individual bacteria and the light microscopic appearance of GN, except that 6/7 cases with Bartonella, Coxiella, Cardiobacterium, or Gemella had crescentic GN, and 3/4 cases of culture-negative endocarditis had crescentic GN. Eighty-two percent of patients had community-acquired IE in native valves, 92% of which had positive blood cultures versus 89% positive in patients with prosthetic valves.
Renal pathology
Histologic findings are summarized in Table 3 and illustrated in Figures 1, 2, 3. Light microscopy (LM) samples had a mean of 15 glomeruli (range 2–43). Global sclerosis varied from 0 to 16, with a median of only 1. Overall, crescentic GN was the most common pattern seen in our series (26/49, 53%). In a majority of patients, the glomerular inflammatory changes were diffuse (16/26, 62%), and most patients also had focal necrotizing lesions (20/26, 77%). Necrotizing areas were not seen in biopsies without crescents. By definition, glomeruli and portions of the glomerular tufts uninvolved by necrosis or crescent formation in this group did not show proliferative changes.
Table 3. Pathologic features.
| Mean (range) | |
|---|---|
| Light microscopy | |
| Total glomeruli | 15 (2–43) |
| Sclerotic glomeruli | 2 (0–16), 11% (0–87%) |
| n (%) | |
| Glomerular pattern | |
| Crescentic | 26 (53) |
| Focal | 10 (20) |
| Diffuse | 16 (33) |
| Necrotizing foci | 20 of 26 (77) |
| Proliferative | 18 (37) |
| Focal | 2 (4) |
| Diffuse | 16 (33) |
| Mesangial proliferative | 5 (10) |
| Immunofluorescence microscopy | |
| Staining pattern | |
| Negative | 3 (6) |
| Mesangial alone | 20 (41) |
| Capillary wall alone | 2 (4) |
| Capillary wall and mesangial | 24 (49) |
| Immunoreactant | IgG | IgM | IgA | C3 |
|---|---|---|---|---|
| n (%) | 13 (27%) | 18 (37) | 14 (29) | 46 (94) |
| C3 only or C3+ single Ig | C3+IgG | C3+IgM | C3+IgA | C3 Only |
| n (%) | 2 (4%) | 8 (16%) | 3 (6%) | 18 (37) |
| Combined Igs | IgG IgM | IgG IgA | IgM IgA | IgG IgM IgA |
| n (%) | 3 (6%) | 6 (12%) | 3 (6%) | 2 (4) |
| Negative for all | Negative | |||
| n (%) | 3 (6) | |||
| n (%) | ||||
| Electron microscopy | ||||
| Mesangial deposits | 41 (84) | |||
| Subendothelial deposits | 22 (45) | |||
| Subepithelial deposits | 17 (35) | |||
| Subepithelial ‘humps' | 7 (14) | |||
| No deposits | 5 (10) | |||
Abbreviations: C3, complement component 3; Ig, immunoglobulin.
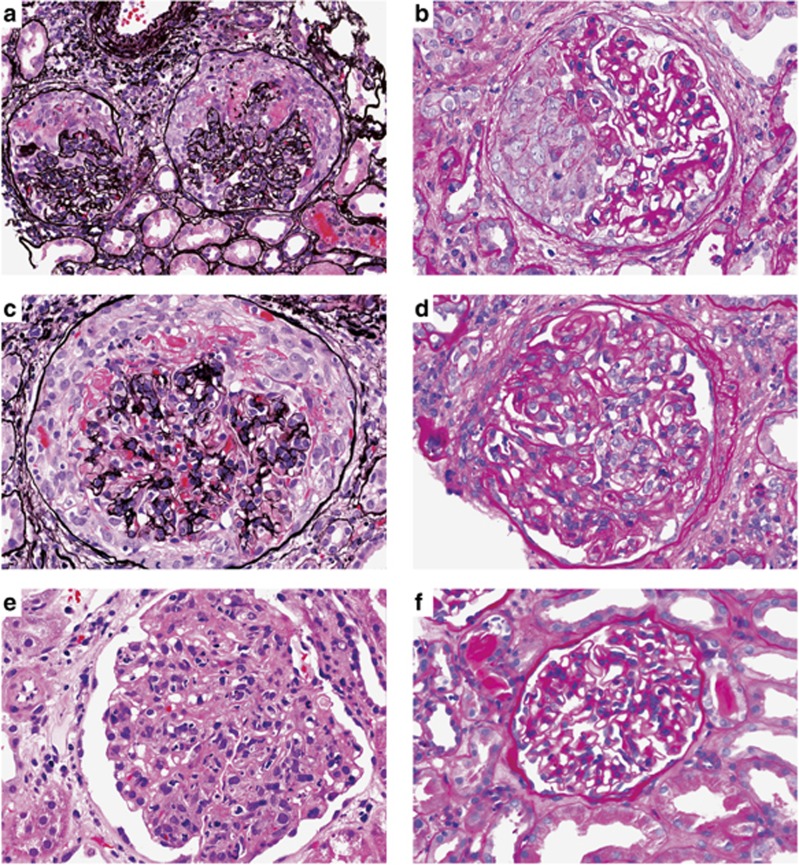
Figure 1.
Light microscopy findings in endocarditis-associated glomerulonephritis. (a) Cellular crescents with necrotizing foci (Jones methenamine silver; original magnification × 400). (b) Segmental cellular crescent with no underlying proliferation (periodic acid–Schiff; original magnification × 400). (c) Diffuse crescentic glomerulonephritis (Jones methenamine silver; original magnification × 400). (d) Acute focal proliferative glomerulonephritis (hematoxylin and eosin; original magnification × 400). (e) Diffuse proliferative glomerulonephritis (periodic acid–Schiff; original magnification × 400). (f) Mesangial proliferative glomerulonephritis (periodic acid–Schiff; original magnification × 400).
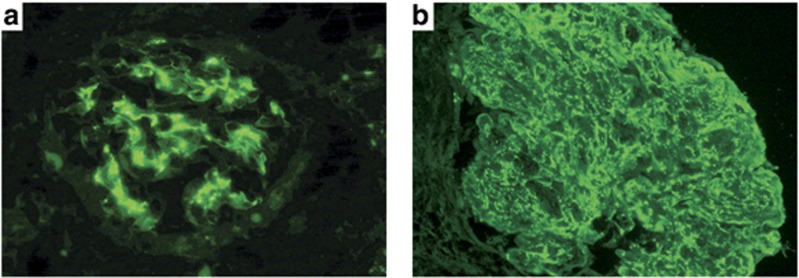
Figure 2.
Immunofluorescence microscopy findings in endocarditis-associated glomerulonephritis. (a) Glomerulus with predominantly mesangial staining by C3 (fluorescein-conjugated anti-human C3; original magnification × 400). (b) Glomerulus with mesangial and capillary wall reaction with C3 (fluorescein-conjugated anti-human C3; original magnification × 400).
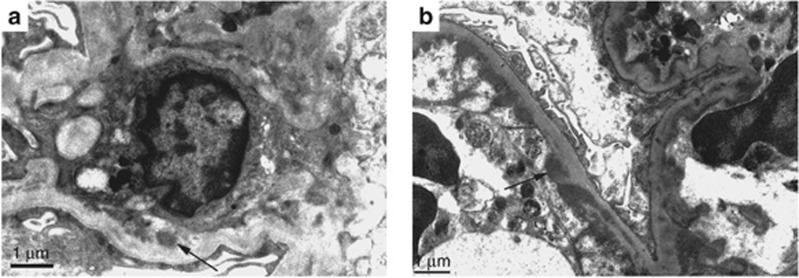
Figure 3.
Electron microscopy findings in endocarditis-associated glomerulonephritis. (a) Mesangial electron-dense deposits (arrow) in diffuse crescentic glomerulonephritis (original magnification × 12,000). (b) Subendothelial electron-dense deposits (arrow) in focal proliferative glomerulonephritis (original magnification × 12,000).
Diffuse proliferative GN was the second most common pattern (16/49, 33%) (Table 3 and Figure 1). Only two cases had the classically described pattern of focal proliferative GN without crescents or necrosis (4%) (Figure 1). Of the 18 total patients with proliferative GN, seven also had focal crescent formation.
Mild mesangial hypercellularity was the third major finding after crescentic and proliferative GN, and it accounted for 5/49 cases (10%) (Table 3). All of these cases showed only mild focal and segmental mesangial hypercellularity without endocapillary proliferation or crescent formation (Figure 1).
No other forms of GN were identified. Specifically, no cases of membranoproliferative GN with or without cryoglobulinemic features or cases of thrombotic microangiopathy were found.
Acute tubular injury was present in the majority of cases (86%). Histological red blood cell casts were noted in more than half of the cases (64%). Almost all cases (88%) had interstitial inflammation, which was most often focal (64%), but abundant interstitial neutrophils were present in only seven cases (14%). No examples of eosinophilic allergic interstitial nephritis were seen. No micro-abscesses or cortical necrosis were present. The degree of tubular atrophy and interstitial fibrosis present was most often mild (43%) or absent (37%). Moderate chronic change was present in 16% of the cases, and severe chronic change was present in 4% of the cases. Similarly, arteriosclerosis and arteriolar hyalinosis were most often absent (39%) or mild (31%). Moderate vascular disease was seen in 27%, with severe vascular disease in only two cases (4%). No histologic evidence of extraglomerular vasculitis was seen.
The immunofluorescence (IF) findings are presented in Tables 3 and 4, and illustrated in Figure 2. Overall, C3 was present in 94% of cases, whereas IgM was found in 37%, IgA in 29% and IgG in 27% of cases. Six cases had IgA-dominant staining and an additional two were codominant for IgA and IgG. Three patients had no significant deposition of immunoglobulin or C3. Twenty-two patients (44%) met the criteria for pauci-immune IF staining,16 of whom 11 patients had crescentic GN by LM. Of these 11 patients, ANCA was positive in 3, negative in 4, and not done in 4. Of interest, only 50% of cases with a proliferative pattern by LM had positive IgG, and biopsies with a crescentic pattern had IgG in only 15% of cases. In some cases, staining was positive for more than one immunoglobulin, but a ‘full house' pattern with IgG, IgM, and IgA positivity was seen in only two cases (Table 4). C3 was positive in all cases in which immunoglobulins were found. C3-only staining was present in 18 (37%) cases. Although there was no statistical difference between the occurrence of staphylococcal or streptococcal species in blood cultures between the pauci-immune cases and those with IC deposition, all cases with Bartonella, Coxiella, Cardiobacterium, or Gemella had immunoglobulin and C3 deposition.
Table 4. Immunofluorescence findings by light microscopy pattern.
| Crescentic (n=26), n (%) | Acute proliferative (n=18), n (%) | Mesangial proliferative (n=5), n (%) | |
|---|---|---|---|
| Staining pattern | |||
| None | 2 (8) | 0 | 1 (20) |
| Mesangial-only | 13 (50) | 3 (17) | 4 (80) |
| Capillary wall and mesangium | 11 (42) | 13 (72) | 0 |
| Capillary wall-only | 0 | 2 (11) | 0 |
| % Positive (mean intensity) | % Positive (mean intensity) | % Positive (mean intensity) | |
| Immunoreactant | |||
| IgG | 15 (1.8) | 50 (1.9) | 0 |
| IgM | 58 (2.1) | 17 (1.7) | 0 |
| IgA | 12 (1.7) | 61 (2.3) | 0 |
| C3 | 92 (2.5) | 100 (2.8) | 100 (2.8) |
| n (%) | n (%) | n (%) | |
| Pauci-immune | 11 (42) | 6 (33) | 5 (100) |
Abbreviations: C3, complement component 3; Ig, immunoglobulin.
The presence and location of deposits by electron microscopy (EM) is detailed in Table 3 and shown in Figure 3. Deposits were present in 44/49 cases (90%). Only seven (14%) showed classic infection-related subepithelial hump–like deposits. Of these seven cases, five were diffuse proliferative and two showed mesangial proliferation, the latter with only C3 deposits. Subendothelial deposits were found in 45% of biopsies. The most common site for deposits was the mesangial area (84%). The degree of foot process effacement was variable, with approximately equal proportions of none plus mild (45%) and moderate plus severe (55%).
Treatment
Treatment data was obtained in 86% of patients, and is presented in Table 5. Treatments used consisted of antibiotics in 28/42 patients (67%) and antibiotics plus immunosuppressive therapy in 14/42 (33%). Specifically, immunosuppressive treatments used in the 14 patients included prednisone/methylprednisone in 10 patients, Cytoxan in 1 patient, and both prednisone/Cytoxan in 3 patients. One of the patients treated with antibiotics plus prednisone received plasma exchange. Eight patients (19%) were treated surgically, including 6 with valve replacement and 2 with valve repair.
Table 5. Patient characteristics and biopsy findings as related to outcome.
| Death | End-stage renal disease | Persistent renal dysfunction | Complete recovery | |
|---|---|---|---|---|
| No. of patients, n (% out of 38 with follow-up) | 8 (21) | 4 (10) | 14 (37) | 12 (32) |
| Gender/age | ||||
| Male:female, n/n | 7/1 | 2/2 | 12/2 | 7/5 |
| Age (years), mean (range) | 57 (3–79) | 56 (48–66) | 50 (16–84) | 45 (31–) |
| Average creatinine (range) | ||||
| At biopsy | 2.6 (1.0–4.7) | 5.6 (3.4–6.7) | 3.6 (2.6–5.8) | 3.6 (1.0–7.0) |
| At follow-up | 4.0a (1.7–7.6) | n/ab | 2.0 (1.2–3.1) | 1.0 (0.8–1.3) |
| ANCA | ||||
| Unknown/not done | 0/2 | 1/2 | 0/4 | 0/4 |
| Positive | 1 | 0 | 5 | 1 |
| Negative | 5 | 1 | 5 | 7 |
| Agent on culture, n (%) | ||||
| Staphylococcus | 4 (50) | 3 (75) | 4 (28) | 8 (66) |
| Streptococcus | 0 | 1 (25) | 5 (36) | 2 (17) |
| Otherc | 4 (50) | 0 | 5 (36) | 2 (17) |
| Pattern of glomerular injury, n (%) | ||||
| Mesangial proliferative | 0 | 1 (25) | 2 (14) | 0 |
| Diffuse proliferative | 1 (13) | 2 (50) | 4 (29) | 7 (58) |
| Focal crescentic | 3 (37) | 0 | 3 (21) | 2 (17) |
| Diffuse crescentic | 4 (50) | 1 (25) | 5 (36) | 3 (25) |
| Other renal biopsy findings | ||||
| % Of globally sclerotic glomeruli, average n (range) | 7 (0–29) | 17 (8–28) | 14 (0–53) | 5 (0–18) |
| Interstitial fibrosis, n (%) | ||||
| None or mild | 7 (88) | 1 (25) | 11 (79) | 11 (92) |
| Moderate | 1 (12) | 3 (75) | 2 (14) | 1 (8) |
| Severe | 0 | 0 | 1 (7) | 0 |
| Acute tubular injury, n (%) | 7 (88) | 3 (75) | 12 (86) | 8 (67) |
| Interstitial inflammation, n (%) | ||||
| None or focal | 5 (63) | 3 (75) | 11 (79) | 9 (75) |
| Diffuse | 3 (37) | 1 (25) | 3 (21) | 3 (25) |
| Immune deposition, n (%) | ||||
| Negative | 0 | 1 (25) | 0 | 1 (9) |
| C3 only | 4 (50) | 3 (75) | 9 (64) | 4 (33) |
| C3 and immunoglobulin(s) | 4 (50) | 0 | 5 (36) | 7 (58) |
| Treatment, n (%) | ||||
| Antibiotics | 4 (50) | 3 (75) | 8 (57) | 8 (67) |
| Antibiotics and immunosuppression | 4 (50) | 1 (25) | 6 (43) | 4 (33) |
| Valve replacement or repair | 0 | 1 (25) | 4 (29) | 3 (25) |
Abbreviations: ANCA, anti-neutrophil cytoplasmic antibody; C3, complement component 3; Cr, creatinine.
Excluding follow-up of Cr values from patients on hemodialysis at the time of follow-up/death;
Patients on hemodialysis at the time of follow-up.
Other includes Bartonella henselae, Coxiella, Cardiobacterium hominis, Gemella and culture-negative cases.
Follow-up and outcomes
Follow-up outcome was available in 78% of patients, with an average follow-up term of 25 months (range 0.5–84 months). Patient characteristics and biopsy findings related to outcome are presented in Table 5. Of the 38 patients with follow-up, eight died (21%); of the surviving patients, 4 progressed to end-stage renal disease (10%), 14 had persistent renal dysfunction (37%), and 12 had complete renal recovery (32%).
The eight patients who died included the 3-year-old and seven adults (age range 47–79, mean 64 years). Three of these patients had a prosthetic cardiac valve. All patients who died had fevers and vegetations by echocardiogram, as well as a combination of various other clinical findings. The organisms on culture included one patient each with Coxilla burnetii, Gemella species, and Bartonella, with the remainder staphylococcal species. Half of the patients were treated with antibiotics and half of them were treated with antibiotics and steroids, including one with Cytoxan. None were treated surgically.
Of the eight deaths, four deaths occurred within 2 months of biopsy: one died 3 days after biopsy owing to sepsis, one remained on hemodialysis for 1.5 months and died owing to disseminated intravascular coagulation, one died owing to cerebral hemorrhage 1 month after biopsy, and another died 1.5 months after biopsy from endocarditis/pulmonary hemorrhage. The four patients with later deaths included one with persistent renal dysfunction and then death 90 months later of unknown cause, and three with end-stage renal disease and deaths occurring 18, 23, and 78 months after the biopsy.
There were no clinicopathologic trends useful in differentiating the patients who died from surviving patients (Table 5). In comparison of renal biopsy findings among surviving patients, those with higher percentages of globally sclerotic glomeruli and more interstitial fibrosis had worst outcomes. The group that progressed to end-stage renal disease had a higher average serum creatinine at biopsy (5.6), compared with both groups with persistent renal dysfunction and complete recovery (3.6).
DISCUSSION
Our study provides data from the current era (2001–11) on the clinical and pathologic features of IE-related GN in 49 patients who underwent renal biopsies during the active phase of their illnesses, and represents the largest study of this entity with both clinical and complete renal biopsy data available.
In keeping with the overall trends in infection-related GN, our findings in IE in this predominately adult series include a 3.5:1 male predominance, older mean age (48 years) with over 30% elderly patients, increased prevalence of staphylococcal rather than streptococcal infection, and the disease was usually ongoing at the time of biopsy rather than being postinfectious such that the finding of GN prompted investigations that led to a diagnosis of IE. The observation that the most common presentation was acute renal failure rather than nephritic syndrome differs from overall findings in infection-related GN7 and may be unique to this patient population with compromised cardiac function.
The biopsy findings in our study also differ from many of those previously reported. Both renal medicine and pathology textbooks17, 18, 19, 20, 21, 22 report that more than 80% of cases of IE with GN have a focal, segmental, or diffuse proliferative GN with endocapillary proliferation and occasional infiltrating leukocytes. These descriptions are based primarily on autopsy studies often from the pre-antibiotic era. In contrast, we found crescentic GN to be the most common finding, with over 50% of patients exhibiting extensive crescent formation and a majority having diffuse rather than focal pattern of injury. Crescentic GN has been previously described in IE, primarily in case reports. Kannan and Mattoo23 identified 11 cases described before 2000 but identified crescents as a rare finding. However, Neugarten and Baldwin6 reported focal or diffuse proliferative GN in 22% of biopsied and autopsied patients with IE, of whom 50% had crescents in glomeruli, and Majumdar et al.13 identified 16 cases of GN out of 62 IE patients studied by biopsy (20) or autopsy (42), of whom 11 had crescents. Our findings in a larger cohort studied exclusively by renal biopsy suggest a similar predominance of crescentic lesions, an observation that differs from the findings in typical infection-related GN where proliferative lesions predominate and extensive crescents are seen in only 5% of cases.7
The immunopathology of IE GN has not been well characterized beyond identification of IgG and C3 deposition in an IC pattern.13, 24, 25, 26, 27 Our findings suggest that more complex pathogenetic mechanisms are involved. Although C3 staining was positive in virtually the entire cohort, staining for IgG was present in only 27% and in fewer than 15% of those with the most severe crescentic lesions. A lack of immunoglobulin staining in crescentic, or vasculitic, IE-associated GN has also been noted previously.13 The finding of prominent C3 staining and the presence of readily detectable immune deposits by EM are more consistent with the C3-dominant pattern of immune deposition commonly seen in infection-related GN in general7 than with typical findings in either IC disease or ‘pauci-immune' ANCA-associated vasculitis. However, some C3 deposition can also be seen in ANCA-associated vasculitis.28, 29, 30
Our findings raise several questions regarding the pathogenesis of the GN in patients with IE. Although initially believed to be embolic in nature, an underlying IC pathogenesis has been assumed for years based on findings of granular IgG and C3 deposits by IF.24, 25, 26, 27 Our findings support a primary IC mechanism in only a minority of patients (27%), a conclusion also reached by others.13 When glomerular IC formation does occur, it could result from passive trapping of ICs from the circulation, from the formation of ICs in situ following prior localization of exogenous cationic bacterial antigens, or from reactivity of IgG antibody with endogenous components of the glomerulus itself, as occurs in membranous nephropathy or anti-glomerular basement membrane disease.31 In the latter case, molecular mimicry between bacterial and glomerular constituents would likely be involved, making the process autoimmune in nature.8 However, our findings, including the paucity of IgG deposition in severe cases and the probable alternate pathway mechanism of complement activation, suggest that in a majority of cases IC formation in glomeruli is not the principal pathogenic event.
Several alternative mechanisms could explain how glomerular injury occurs in IE without IgG deposition. Some bacterial antigens, for example, the streptococcal pyogenic exotoxin B antigen incriminated in poststreptococcal GN, can localize in glomeruli independently of antibody, causing injury through activation of the plasmin system and direct activation of the alternate complement pathway via mannose-binding lectin, thus producing a C3-dominant nephropathy.8, 32 Staphylococcal super-antigens are also capable of causing direct tissue injury, especially to endothelial cells, in the absence of immune deposits.33 No studies of localization or biologic activity of bacterial antigenic proteins in IE have yet been performed. Another potential mechanism could be the associated ANCA antibody (28%), a finding also reported by others.34, 35 Bacterial infections are well known to lead to ANCA-positive serology including suppurative lung disease, and infections with Pseudomonas, Klebsiella, E. coli, and Ross River virus.28, 36, 37, 38 Infectious agents such as Staphylococci can induce antibodies to complementary peptides of the target antigen (autoantigen complementarity), leading to anti-idiotypic antibodies that react with self-proteins such as proteinase-3 to produce autoimmune tissue injury without depositing in glomeruli.39 If ANCA antibodies are pathogenic rather than secondary phenomena in these patients, they are currently believed to damage glomeruli indirectly by activating neutrophils in the microvasculature that release complement-activating factors leading to alternative pathway activation involving the C5a receptor.36, 37, 40 Finally, the recent explosion of interest in nephropathies with a dominance of C3 deposition has clarified the role of inherited and acquired abnormalities in complement-regulatory proteins such as complement factor H in contributing to unregulated activation of the alternative complement pathway leading to deposition of complement proteins in glomeruli and GN in diseases such as dense deposit disease.41 Initiation of complement activation by infections in the presence of inherited or acquired abnormalities in complement regulation has been documented to lead to persistent, chronic C3 nephropathies with similar pathologic appearances to many of the IE patients reported here.42 Thus, some of the lesions seen in IE-related GN could reflect complement regulatory protein dysfunction.
The findings in this study update and expand current understanding of both the clinical and pathologic spectrum of GN in IE and add to the knowledge of infection-related GN in general. Appreciation of both the clinical and pathologic manifestations of IE GN in the current era, including the frequency of AKI and of crescentic GN, should contribute to earlier diagnosis and more effective treatment. In one series, IE was unrecognized in almost 20% of cases at the time of nephrology consult,13 and in another series it was not recognized until autopsy in 38% of cases.5 These observations suggest that the immunologic mechanisms that underlie GN in patients with IE are more complex than previously appreciated.
MATERIALS AND METHODS
By retrospective review, 49 patients with a clinical diagnosis of IE that fulfilled the modified Duke criteria43 who underwent native renal biopsy between 2001 and 2011 were identified at Mayo Clinic, Rochester (18), and at Nephropath, Little Rock (31). These biopsies were received from multiple medical centers across the United States, with various nephrology practice settings represented.
All 49 biopsies were processed by standard techniques for LM, IF, and EM.44, 45, 46 Briefly, LM samples were fixed in formalin, processed into paraffin, cut at 3 μm, and stained with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, and periodic acid–Schiff reagent. IF sections were snap-frozen, cut at 4–5 μm in a cryostat, and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgA, IgM, C3, C1q, fibrinogen, and κ- and λ-light chains (Dako, Carpenteria, CA) for 1 h; next, they were rinsed and a coverslip was placed using aqueous mounting medium. IF intensity was scored on a 0–3+ scale. Samples for EM were processed into plastic and thin sections were made, and electron photomicrographs were routinely taken at × 4000, × 12,000, and × 20,000.
Clinical data including demographic information, medical history, presenting clinical and laboratory findings, endocarditis valve involvement, culture data, treatment, and follow-up data were obtained from the patient's medical record/treating physicians. The following clinical definitions were applied: acute renal failure—doubling of the serum creatinine; chronic renal failure—progressive decline in glomerular filtration rate over months; rapidly progressive renal failure—renal failure developing over weeks to months; acute nephritic syndrome—red blood cell casts, hematuria, hypertension, and renal failure; nephrotic syndrome—nephrotic range proteinuria (>3.5 g per day) with hypoalbuminemia (serum albumin <3 g per dl) and peripheral edema; isolated hematuria—≥5 red blood cells per high power field in the urinary sediment. Quantification of proteinuria was performed by 24-h urine collection or by spot urine protein to creatinine ratio when quantification was not available.
Biopsies were designated as having one of five patterns of glomerular injury: mesangial proliferative, focal necrotizing and crescentic, diffuse necrotizing and crescentic, focal proliferative, or diffuse proliferative. Glomeruli with an increase in mesangial matrix and cells without closure of capillary lumens were included in the mesangial proliferative group. Designation as focal versus diffuse was made by applying a cutoff value of 50%, with focal <50% of intact glomeruli involved and diffuse ≥50% of intact glomeruli involved.47 Proliferation in biopsies with focal or diffuse proliferative patterns was defined as endocapillary hypercellularity and occlusion of capillary lumens by endothelial cells, mesangial cells, and/or white blood cells from circulation. Proliferation in biopsies with the mesangial proliferative pattern of glomerular injury was defined as ≥4 cells per mesangial region in more than 50% of glomeruli without occlusion of capillary loops.48 Biopsies in the crescentic pattern of injury showed cellular proliferation within Bowman's space, with or without necrosis of the glomerular tuft, without endocapillary hypercellularity. Interstitial fibrosis was defined by the percentage of cortical involvement: mild, <25% moderate, 25–50% and severe, >50%.
IF was given a score of 0 if negative, and a score of 1, 2, or 3+ for positive staining with increasing levels of intensity. Pauci-immune was defined as 0–2+ intensity for immunoglobulins (IgG, IgM, and IgA). This cutoff was based on the definition provided by Jennette et al.16 as 2+ or less staining for immunoglobulins on a scale of 0 to 4+. Of note, as the definition of pauci-immune disease is defined by immunoglobulin staining only, we did not use an intensity cutoff for complement staining in glomeruli. This also seems prudent given that large case series have shown that glomerular C3 deposition is not uncommon in pauci-immune, ANCA-associated GN.29, 30
For outcome analysis, end-stage renal disease was defined as requiring renal replacement therapy, persistent renal dysfunction was defined by elevation of serum creatinine 0.2 mg/dl above baseline levels or follow-up creatinine >1.2 mg/dl (for those in whom baseline levels were unavailable), and complete recovery was defined as normalization of serum creatinine to baseline levels or to creatinine ≤1.2 mg/dl (for those patients in whom baseline creatinine values were unavailable).
The Institutional Review Board of the Mayo Clinic Foundation and, for Nephropath, the Institutional Review Board of Schulman Associates approved this study.
Acknowledgments
We are grateful to the following physicians for providing clinical information on their patients: Istvan Bognar, Christine Chen, Domenick Cover, Clinton Edwards, Humam Humeda, Nizar Iskander, Siva Jagarlapudi, Robert Mathias, Jim Mertz, Muhammad Mustafa, Sailesh Nayar, Benjamin Pflederer, Mark Saddler, Raed Sulaiman, Alexander Vitievsky, John Wu, and Juan Zeik. We thank the following physicians and their staff for providing clinical information: Thelmo Barrantes-Ramirez, Richard Blaszak, Michael Haderlie, Robert Kossmann, Sandeep Munjal, Brian Pavey, Mohammad Qamar, Naeem Rahim, David Reynolds, Denise Rivers, and Lawrence Wong. We also thank I-Shin Wen for her assistance.
All the authors declared no competing interests.
References
- Lohlein M. Ueber hāmorrhagische nierenaffektioned bei chronischer ulzerözer endokarditis. Med Klin. 1910;6:375–379. [Google Scholar]
- Baehr G. Glomerular lesions of subacute bacterial endocarditis. J Exp Med. 1912;15:330–347. doi: 10.1084/jem.15.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ET. Glomerular lesions associated with endocarditis. Am J Pathol. 1932;8:639–669. [PMC free article] [PubMed] [Google Scholar]
- Eknoyan G, Lister BJ, Kim HS, et al. Renal complications of bacterial endocarditis. Am J Nephrol. 1985;5:457–469. doi: 10.1159/000166984. [DOI] [PubMed] [Google Scholar]
- Fernandez Guerrero ML, Alvarez B, Manzarbeitia F, et al. Infective endocarditis at autopsy: a review of pathologic manifestations and clinical correlates. Medicine (Baltimore, MD) 2012;91:152–164. doi: 10.1097/MD.0b013e31825631ea. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Baldwin DS. Glomerulonephritis in bacterial endocarditis. Am J Med. 1984;77:297–304. doi: 10.1016/0002-9343(84)90706-x. [DOI] [PubMed] [Google Scholar]
- Nasr SH, Radhakrishnan J, D'Agati VD. Bacterial infection–related glomerulonephritis in adults. Kidney Int. 2013;83:792–803. doi: 10.1038/ki.2012.407. [DOI] [PubMed] [Google Scholar]
- Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 2014;86:905–914. doi: 10.1038/ki.2014.49. [DOI] [PubMed] [Google Scholar]
- Nasr SH, Fidler ME, Valeri AM, et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol. 2011;22:187–195. doi: 10.1681/ASN.2010060611. [DOI] [PubMed] [Google Scholar]
- Nadasdy T, Hebert LA. Infection-related glomerulonephritis: understanding mechanisms. Semin Nephrol. 2011;31:369–375. doi: 10.1016/j.semnephrol.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Bor DH, Woolhandler S, Nardin R, et al. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS One. 2013;8:e60033. doi: 10.1371/journal.pone.0060033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Boyce TG, Cetta F, et al. Infective endocarditis in the pediatric patient: a 60-year single-institution review. Mayo Clin Proc. 2012;87:629–635. doi: 10.1016/j.mayocp.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Chowdhary S, Ferreira MA, et al. Renal pathological findings in infective endocarditis. Nephrol Dial Trans. 2000;15:1782–1787. doi: 10.1093/ndt/15.11.1782. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis. 2010;51:131–140. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- Hoen B, Selton-Suty C, Lacassin F, et al. Infective endocarditis in patients with negative blood cultures: analysis of 88 cases from a one-year nationwide survey in France. Clin Infect Dis. 1995;20:501–506. doi: 10.1093/clinids/20.3.501. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135:921–930. [PMC free article] [PubMed] [Google Scholar]
- Rodriquez-Iturbe B, Burdmann EA, Barsoum RS.Glomerular diseases associated with infectionIn: Floege J, Johnson RJ, Feehally J (eds)Comprehensive Clinical Nephrology4th edn.Elsevier Saunders: St Louis, MO; 2010662–674. [Google Scholar]
- Appel GB, Radhakrishnan J, D'Agati VD.Secondary glomerular diseaseIn: Taal MW, Chertow GM, Marsden PA, Skorecki K et al. (eds)Brenner & Rector's The Kidney9th ednvol. 1Elsevier: Philadelphia, PA; 20121246–1247. [Google Scholar]
- Rogers TE, Rakheja D, Zhou XJ.Glomerular diseases associated with nephritic syndrome and/or rapidly progressive glomerulonephritisIn: Zhou XJ, Laszik Z, Nadasdy T et al. (eds)Silva's Diagnostic Renal Pathology Cambridge University Press: New York, NY; 2009178–228. [Google Scholar]
- D'Agati VD, Jennette JC, Silva FG. Non-Neoplastic Kidney Diseases. American Registry of Pathology: Silver Spring, MD; 2005. [Google Scholar]
- Farris BA., IIIEndocarditisIn: Colvin RB (ed)Diagnostic Pathology: Kidney Diseases Section 2Amirsys: Manitoba, Canada; 2011108–113. [Google Scholar]
- Nadasdy T, Silva FG.Acute post-infectious glomerulonephritis and glomerulonephritis caused by persistent bacterial infectionIn: Jennette JC, Olson JL, Schwartz MM, Silva FG (eds)Heptinstall's Pathology of the Kidney6th ednvol. 1Lippincott Williams & Wilkins: Philadelphia, PA; 2007372–380. [Google Scholar]
- Kannan S, Mattoo TK. Diffuse crescentic glomerulonephritis in bacterial endocarditis. Pediatr Nephrol. 2001;16:423–428. doi: 10.1007/s004670000550. [DOI] [PubMed] [Google Scholar]
- Cordeiro A, Costa H, Laginha F. Immunologic phase of subacute bacterial endocarditis a new concept and general considerations. Am J Cardiol. 1965;16:477–481. doi: 10.1016/0002-9149(65)90022-6. [DOI] [PubMed] [Google Scholar]
- Bayer AS, Theofilopoulos AN. Immunopathogenetic aspects of infective endocarditis. Chest. 1990;97:204–212. doi: 10.1378/chest.97.1.204. [DOI] [PubMed] [Google Scholar]
- Gutman RA, Striker GE, Gilliland BC, et al. The immune complex glomerulonephritis of bacterial endocarditis. Medicine (Baltimore, MD) 1972;51:1–25. doi: 10.1097/00005792-197201000-00001. [DOI] [PubMed] [Google Scholar]
- Keslin MH, Messner RP, Williams RC. Glomerulonephritis with subacute bacterial endocarditis. Immunofluorescent studies. Arch Intern Med. 1973;132:578–581. [PubMed] [Google Scholar]
- Kambham N. Crescentic glomerulonephritis: an update on pauci-immune and anti-GBM diseases. Adv Anat Pathol. 2012;19:111–124. doi: 10.1097/PAP.0b013e318248b7a1. [DOI] [PubMed] [Google Scholar]
- Vizjak A, Rott T, Koselj-Kajtna M, et al. Histologic and immunohistologic study and clinical presentation of ANCA-associated glomerulonephritis with correlation to ANCA antigen specificity. Am J Kidney Dis. 2003;41:539–549. doi: 10.1053/ajkd.2003.50142. [DOI] [PubMed] [Google Scholar]
- Chen M, Xing G-Q, Yu F, et al. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol Dial Transplant. 2009;24:1247–1252. doi: 10.1093/ndt/gfn586. [DOI] [PubMed] [Google Scholar]
- Couser WG. Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol. 2012;23:381–399. doi: 10.1681/ASN.2011030304. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B, Musser JM. The current state of poststreptococcal glomerulonephritis. J Am Soc Nephrol. 2008;19:1855–1864. doi: 10.1681/ASN.2008010092. [DOI] [PubMed] [Google Scholar]
- Salgado-Pabon W, Breshears L, Spaulding AR, et al. Superantigens are critical for Staphylococcus aureus Infective endocarditis, sepsis, and acute kidney injury mBio 20134e00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos JA, Corrales-Medina VF, Garcia S, et al. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol. 2007;26:590–595. doi: 10.1007/s10067-005-0176-z. [DOI] [PubMed] [Google Scholar]
- Tiliakos AM, Tiliakos NA. Dual ANCA positivity in subacute bacterial endocarditis. J Clin Rheum. 2008;14:38–40. doi: 10.1097/RHU.0b013e318164187a. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. ANCA disease: Where is the field heading. J Am Soc Nephrol. 2010;21:745–752. doi: 10.1681/ASN.2009121238. [DOI] [PubMed] [Google Scholar]
- Cartin-Ceba R, Peikert T, Specks U. Pathogenesis of ANCA-associated vasculitis. Curr Rheumatol Rep. 2012;14:481–493. doi: 10.1007/s11926-012-0286-y. [DOI] [PubMed] [Google Scholar]
- Savige J, Pollock W, Trevisin M. What do antineutrophil cytoplasmic antibodies (ANCA) tell us. Best Pract Res Clin Rheumatol. 2005;19:263–276. doi: 10.1016/j.berh.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pendergraft WF, III, Preston GA, Shah RR, et al. Autoimmunity is triggered by cPR-3(105–201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10:72–79. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Falk RJ, Gasim AH. Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens. 2011;20:263–270. doi: 10.1097/MNH.0b013e3283456731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomback AS, Appel GB. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat Rev Nephrol. 2012;8:634–642. doi: 10.1038/nrneph.2012.213. [DOI] [PubMed] [Google Scholar]
- Sethi S, Fervenza FC, Zhang Y, et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83:293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- Larsen CP, Messias NC, Silva FG, et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor (PLA2R1) staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- Walker PD. The renal biopsy. Arch Pathol Lab Med. 2009;133:181–188. doi: 10.5858/133.2.181. [DOI] [PubMed] [Google Scholar]
- Walker PD, Cavallo T, Bonsib SM. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]
- Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- Roberts ISD, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]