Abstract
Extrahepatic portal vein obstruction is a vascular disorder of liver, which results in obstruction and cavernomatous transformation of portal vein with or without the involvement of intrahepatic portal vein, splenic vein, or superior mesenteric vein. Portal vein obstruction due to chronic liver disease, neoplasm, or postsurgery is a separate entity and is not the same as extrahepatic portal vein obstruction. Patients with extrahepatic portal vein obstruction are generally young and belong mostly to Asian countries. It is therefore very important to define portal vein thrombosis as acute or chronic from management point of view. Portal vein thrombosis in certain situations such as liver transplant and postsurgical/liver transplant period is an evolving area and needs extensive research. There is a need for a new classification, which includes all areas of the entity. In the current review, the most recent literature of extrahepatic portal vein obstruction is reviewed and summarized.
Keywords: Cirrhosis, portal biliopathy, portal vein thrombosis
Noncirrhotic portal hypertension (NCPH), as it generally is termed, is a heterogeneous group of diseases that is due to intrahepatic or extrahepatic etiologies. In general, the lesions in NCPH are vascular in nature and can be classified based on the site of resistance to blood flow as “prehepatic,” “hepatic,” and “posthepatic.” The “hepatic” causes of NCPH can be subdivided into “presinusoidal,” “sinusoidal,” and “postsinusoidal [Table 1]”.
Table 1.
Classification and causes of noncirrhotic portal hypertension

Portal vein thrombosis was first seen by Stewart and Balfour in the late 1860s in a patient with splenomegaly, ascites, and variceal dilatation. Kobrich coined the term cavernoma to describe spongy appearance of portal vein (PV).[1] Generally a hypercoagulable state, intra-abdominal infection/peritonitis, and PV anomaly (PV stenosis and atresia) are considered important predisposing factors of EHPVO; however, vast majority of cases are due to primary thrombosis of the PV and often with more than one cause.
Accurate epidemiological data on PVT is difficult to obtain. Prevalence of autopsy research in the United States and Japan ranges from 0.05% to 0.5% population prevalence of portal vein thrombosis (PVT) studied by Ogran et al. seen on autopsy series is 1%.[2] Thus PVT is responsible for 5%–10% of all cases of portal hypertension in western countries. Of all cases of portal hypertension (PHT) in developing countries, 40% are attributed to PVT. In children, EHPVO accounts for 80% cases of PHT.[3] Incidence of PVT among liver cirrhotics ranges from 0.6% to 64.1%.[4] After cirrhosis, EHPVO is the most common cause of portal hypertension globally. In the Indian subcontinent, 20%–30% of all variceal bleeds are due to EHPVO. In Japan, 10%–20% of variceal bleeds and in the west, 2%–5% of variceal bleeds are due to EHPVO.
Clinical presentation of PVT is different in acute and chronic thrombosis. This depends on development and extent of collateral circulation. Intestinal congestion and ischemia with abdominal pain, fever, diarrhea, rectal bleeding, distension, sepsis, and lactic acidosis with or without splenomegaly are common features of acute PVT. In contrast, chronic PVT can be asymptomatic or could be characterized by splenomegaly, pancytopenia, varices, and rarely ascites.
PATHOPHYSIOLOGY
Portal venous obstruction is usually well tolerated and patients are often asymptomatic. There are two important mechanisms which play a role in portal venous obstruction.
Arterial vasodilation or arterial rescue, which can preserve the liver function in acute settings, also allows a second mechanism to operate the venous rescue. Venous rescue allows several collaterals to develop, which try to bypass portal vein obstruction. This neovascularization or neoangiogenesis takes around 4–6 weeks,[5,6] and an obstructed portal vein is replaced by collateral network called cavernoma. The portal cavernoma bypasses the obstructed portal vein and thus a thrombosed portal vein turns into a fibrotic cord.[7,8] The network is seen around structures near the obstructed portal vein such as the bile duct, gall bladder, pancreas, gastric antrum, and duodenum. The bile duct may be difficult to locate within the network of collaterals on abdominal ultrasonography.
Biopsy of liver is usually normal except hemosiderosis related to porto systemic shunt. In much advanced states of PVT, hypoperfused cells of the liver die by apoptosis as a result of increased apoptotic signals and enhanced mitotic activity in normally perfused cells. This process finally leads to reduced synthetic function of the liver in later stages of EHPVO.[9]
EPIDEMIOLOGY
Cohen et al.[10] reported that most patients with PVT are cirrhotics with primary or metastatic cancer; however, nontumoral and noncirrhotic PVT is the second most frequent cause of portal hypertension, worldwide. This constitutes approximately 5%–10% in the western world and 40% in the Indian subcontinent.[11] Some important epidemiological statistical facts are given below[12]:
Acute PVT: 10%–25% of all PVTs
Chronic PVT/EHPVO: 75%–90% of all PVTs
Overall EHPVO: 5%–10% of portal hypertension
In developing countries EHPVO: 35%–40% of portal hypertension
In the Indian subcontinent: 20%–30% of all variceal bleeds are due to PVT
In Japan: 10%–20% of all variceal bleeds are due to PVT
In the West: 2%–5% of all variceal bleeds are due to PVT
In children, 70% of all variceal bleeds are due to EHPVO from the Indian subcontinent.
Recent data suggests a prevalence of approximately 0.6%–26% of PVT in cirrhotics[4,13,14] and highest in orthoptic liver transplant patients. A 6.5% PVT is seen in patients with hepatocellular carcinoma (HCC) at the time of diagnosis, which increases in later stages of HCC. The etiology of liver disease has an influence on prevalence of PVT according to a study of 885 liver transplant patients, being 3.6% in primary sclerosing cholangitis (PSC), 8% in primary biliary cirrhosis (PBC), 16% each in acute liver disease (ALD), and hepatitis B virus (HBV)-related cirrhosis, and 35% in HCC.[15] Finally, the risk of PVT is independently associated with severity of cirrhosis.[16] In a cohort of 251 patients, with cirrhosis listed for liver transplantation, 7.4% developed de novo PVT after mean 12 months follow up,[17] whereas an incidence of 16% was reported in a recent Italian study with 12 months prospective follow up.[18]
ETIOLOGY WITH ETIO PATHOGENIC FACTS
Thrombophilic conditions are seen in 60% of PVT patients while local predisposing factors account for 30%.[19,20,21,22,23] Usually there is more than one factor responsible for PVT.[24,25,26] Despite an intensive workup, idiopathic groups still form 30% of PVT. Among the thrombophilic states, primary myeloproliferative disorders are more common causes of PVT.[27] One should be aware of occult myeloproliferative disorders (MPD). A study of prospective evaluation of primary MPD revealed that half of the patients of presumed idiopathic PVT suffered from primary MPD. This was because it was not detected from conventional testing but by detection of spontaneous formation of erythroid colonies in bone marrow culture.[28]
An acquired mutation (JAK 2/V617F) testing associated with presence of MPD yields a higher rate of diagnosis. JAK2 gene mutation is presently considered among the major criteria in MPD.[29] Genetic variations in thrombin activatable fibrinolysis inhibitor (TAFI) gene, has been recently described as a risk factor for PVT.[30] Much less commonly acquired disorders are antiphospholipid syndrome and paroxysmal nocturnal hemoglobinuria (PNH). Inherited and acquired prothrombotic states responsible for PVT are given below.
Acquired
MPD
Antiphospholipid syndrome
PNH
Oral contraceptives and pregnancy
Hyperhomocysteinemia.
Inherited
Factor V Leiden mutation
Prothrombin mutation
Protein S (PS) and Protein C (PC) deficiency.
Factor V Leiden mutation is the most common thrombophilia predisposing factor to PVT followed by PC deficiency.[31] The role of protein S and antithrombin (AT) III deficiency has not yet been confirmed in PVT.
A simple method of screening deficiency of natural anticoagulants in patients with liver disease comprises of the ratio of PS or PC or AT to [(Factor II + Factor X/2)]. If the result is less than 70%, a genetic cause needs to be evaluated.[32]
To evaluate the role of thrombophilia, laboratory screening should include functional tests for activated PC resistance, genotyping of Factor V to search for G1691A mutation and prothrombin gene to search for G20210A mutation. In addition, Factor VIII and antiphospholipid assays should also be done. Screening should include measurements of naturally occurring anticoagulant proteins such as AT, PC, and PS.[33] Individuals with occult MPD are very often younger than those with full blown MPD who conversely have a relatively low incidence of splanchnic vein thrombosis in their postdiagnosis follow up. These findings reflect that MPD presenting as splanchnic thrombosis has often atypical phenotype, that is, juvenile disorder with high thrombotic risk and typical MPD is less likely to develop in these patients. The diagnosis of occult MPD is not straight forward and requires endogenous erythroid colony assessment.[34,35,36] Endogenous erythroid colony assessment (EEC) is spontaneous growth of erythroid colonies in cultures of bone marrow in absence of added erythropoietin, which has been demonstrated in up to 78% of patients of Budd–Chiari syndrome and 48% of patients with EHPVO.[37,38]
Bone marrow morphology is currently included in WHO criteria of MPD and clusters of enlarged, mature, and pleiomorphic megakaryocytes is considered to be a diagnostic hallmark of Philadelphia negative MPD. By using bone marrow, occurrence of underlying MPD has been demonstrated in 30% of EHPVO.[39] Molecular markers of clonal disease are useful in the diagnostic workup of MPD such as JAK2 (V617F), which is rare (<1%) in general population.[40] The local and systemic risk factors are mentioned below.
Any abdominal cancer
-
Focal inflammatory lesions
- Diverticulitis
- Pancreatititis
- Tuberculus lymphadenitis
- Inflammatory bowel disease (IBD)
- Cytomegalovirus (CMV) infection
-
Injuries to a portal venous system by
- Surgery
- Transplantation
- Trauma
- Vascular procedures/tips.
Cirrhosis
-
Systemic risks factors
-
Factor V Leiden mutation
- 6%–32%
-
Protein C/S deficiency
- C- 0%–26%
- S- 2%–30%
-
Factor II mutation
- 14%–40%
-
Antithrombin deficiency
- 0%–26%.
-
Acquired
-
MPD
- 30%–40%
-
Antiphospholipid antibody syndrome (APLA-S)
- 6%–19%
- PNH
-
Hyperhomocystinemia
- 12%–22%.
-
-
Sarin and Agarwal[41] compared seven studies that assessed etiology of PVT in infants and children. In most cases the cause could not be identified and where it could be, the majority of cases showed direct injury to the umbilical vascular system (oomphalites, umbilical vein catheterization) or intrabdominal and umbilical sepsis. However, there seemed to be a relationship between various causes suggesting a coexistent prothrombotic state. With regard to umbilical catheterization, the predisposing factors are later insertion, catheter dwell time over 3 days, catheter misplacement, trauma on catheter insertion site, and type of solution infused.[41]
Abdominal sepsis has been identified as a risk factor in 11% of PVT in some large prospective studies. The apparent strong association between Bacteroides fragilis infection with PVT leads to a concept of ruling out PVT in B. fragilis bacteremia, of unknown origin.[42] The transient development of anticardiolipin antibodies has been suggested as a pathophysiological link between this infection and PVT.[42]
CLINICAL FEATURES
Sarin et al. has seen in a large Indian series, over 800 patients of venous inflow tract disease (1983 till date) in which over 500 were EHPVO. The clinical and demographic profile of these patients is shown in Table 2.[43,44]
Table 2.
Clinical and Demographic Features in EHPVO patients

EHPVO can present as early as 6 weeks after birth as well as manifest in adulthood. Clinical presentation depends on recent or chronic onset of clinical disease and age of presentation. The most common clinical features are hemetemesis; often massive and usually not associated with hepatocellular dysfunction. Gastrointestinal bleed is usually recurrent before a patient seeks medical attention. There is no firm data to support that recurrence of variceal bleeding decreases after puberty. Patients can present with hemetemesis and malena from conventional esophageal gastric varices and can also bleed from ectopic varices or may present with obscure GI bleeding or bleeding from the biliary tract.[45]
Anemia and splenomegaly are other common features of EHPVO with reduction in cell lines, but hypersplenism is only in 5%–10% of patients. Massive splenomegaly can give a dragging sensation or left upper quadrant pain due to splenic infarct or perisplenitis.
Ascites can present transiently in 10%–20% of children following surgery or GI bleed.[46] It is also seen more frequently in adult patients with long-standing disease and declining liver function.
Jaundice may result from bile duct compression because of dilated venous collaterals due to portal biliopathy. Portal biliopathy refers to abnormalities of extrahepatic and intrahepatic bile ducts with or without abnormalities of the GB wall. This change includes indentation of paracholedochal collaterals on bile ducts, strictures, angulations, focal narrowing, stones, and irregular walls. GB varices are common.[47] However, GB contractibility remains intact. Frequency of cholelithiasis is higher in EHPVO.[48] Biliopathy is recognized in 90%–100% of the cases; however, only a few patients are symptomatic, usually in the adult age groups and reflects advance disease. Biliopathy is complicated by gall stones, CBD stones, cholangitis, secondary biliary cirrhosis, and hemobilia.
Decreased growth velocity and short stature is another complication of EHPVO and may be due to declining hepatotropic growth factors and growth spurts usually observed after shunt surgery.[49,50] Growth retardation has been seen in 51% of children in the authors’ group of 500 patients, whereas other studies showed this association with decreased levels of IGF-1 and IGF BP-3.
Immunological defects seen in EHPVO patients are usually cell-mediated defects because of sequestration of T cells by spleen and from unknown factors, which regulate lymphocytosis.
Clinical features can be summarized as under.
Recent PVT
Asymptomatic
-
Symptomatic
- Severe nonalcoholic abdominal pain, distention, fever, systemic inflammatory (SIRS)
- Persistent pain, ascites; ileus should raise suspicion of intestinal infarction
- Portal pylephlebitis should be suspected if spiky fevers, tenderness, shock, and sepsis-related cholestasis is seen
- Associated thrombosis in other regions to be investigated.
Chronic PVT/EHPVO
With portal hypertension
-
Variceal bleed well tolerated
- Splenomegaly moderate
- Hypersplenism.
Growth retardation in children
Jaundice, biliopathy, mild hepatic dysfunction.
EHPVO DIAGNOSIS
Liver function test (LFT): Normal
Endoscopy: Esophageal varices, gastric varices, anorectal varices
Doppler: PVT and portal vein cavernoma
CECT and CT angiocollaterals
Liver biopsy: Normal but not mandatory.
EHPVO imaging characteristic and pattern of obstruction
Color Doppler ultrasound
Recent: No color flow or Doppler signal within portal vein, distention of portal vein, absence of cavernoma. Contrast EUS is useful to confirm portal vein thrombosis
Chronic: No color flow in portal vein and hepatopetal signal within the cavernoma or varices at gall bladder wall and signs of portal hypertension.
Contrast-enhanced CT/MR
Recent
Nonenhancing material within portal vein and increased hepatic enhancement in arterial phase. Enhancement of thrombus suggests malignant thrombus. CT/MR angiography are more useful.
Chronic
Cavernomatus transformation of portal vein with splenomegaly, collaterals, and/or no opacification of intrahepatic portal vein.
Chronic venous thrombus can manifest as linear areas of calcification within thrombus. Rim enhancement of vessel wall may also be seen and is presumed to be due to normal flow in vasa vasorum. Care must be taken to avoid confusion between “pseudo thrombus image” with true portal vein thrombus. Pseudothrombus appearance occurs during HAP in main portal vein lumen and is due to mixed flow from enhanced splenic vein return and nonenhanced superior mesentric vein return.
Newly proposed Baveno 5 classification (yet to be published) for EHPVO; Site of PVT (TYPE 1, 2a, 2b, 3) [Figure 1a and 1b].
Figure 1.
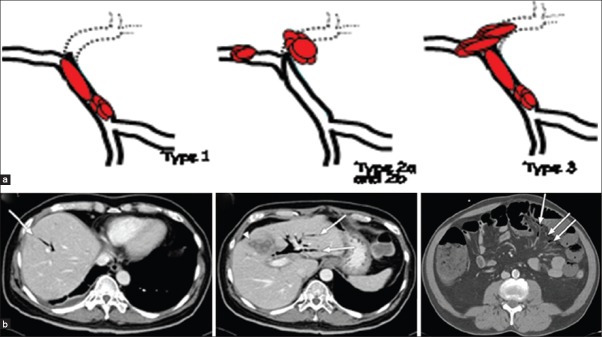
(a) Different types of PVT as per site; (b) CT abdomen showing different types of PVT as per site
TYPE 1: Only trunk.
TYPE 2: Only branch 2a (one), 2b (both branches).
TYPE 3: Trunk and branches.
PRESENTATION
R (Recent)
Ch (Chronic) with portal cavernoma and PHT
TYPE of underlying liver disease
C: Cirrhotic
N: Noncirrhotic liver disease
H: HCC and local malignancy
L: Post–liver transplant
A: Absence of liver disease.
Degree of portal venous system occlusion
Incomplete: Flow visible in PV lumen through imaging
Total: No flow visible in PV lumen on imaging
-
Extent of portal vein system occlusion
- Splenic vein
- Mesentric
- Or both.
TREATMENT
RECENT EHPVO
This rarely resolves spontaneously in noncirrhotic patients with symptomatic recent EHPVO
Low molecular weight heparin should be started immediately followed by oral anticoagulant therapy. In asymptomatic patients, anticoagulation should be considered
Anticoagulation should be given for at least 3 months, unless an underlying persistent pro-thrombotic state has been documented in which case anticoagulation is recommended
Antibiotic therapy should be given if there is any evidence of SIRS or infection.
TREATMENT OF CHRONIC EHPVO
patients with chronic EHPVO there is no consensus on indication for anticoagulant therapy, whereas in patients with a persistent prothrombotic state, anticoagulant therapy can be considered. There is insufficient evidence in favor of interventional therapy such as TIPS or local thromobolysis.
TREATMENT OF BLEEDING
For primary prophylaxis of variceal bleeding, there is insufficient data on whether beta-blocker or endoscopic therapy should be preferred. For control of acute variceal bleed, endoscopic therapy is effective. For secondary prophylaxis, endoscopic therapy is effective and there is preliminary evidence to suggest that beta-blockers are as effective as EVL.
Decompressive surgery or interventional radiological treatment should be considered for patients with failure of endoscopic therapy. Mesenteric left portal vein bypass (REX Shunt) is preferred in managing bleeding from pediatric patients with chronic EHPVO if feasible.
PORTAL BILIOPATHY
Asymptomatic: No treatment.
Symptomatic –
Stones need endoscopic treatment
-
CBD stricture
- Endoscopic stenting should be considered whenever possible if not treated by the above treatment, hepaticojejunostomy is preferred.
ERCP is only recommended if therapeutic is contemplated, otherwise MRCP is the first line of investigation [Figure 2].
Figure 2.
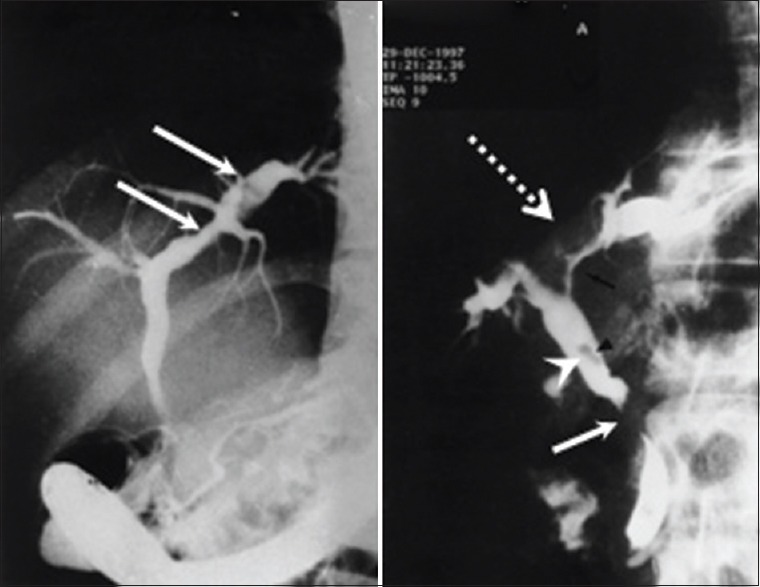
Cholangiogram revealing portal biliopathy in the form of indentations, irregularity of walls, strictures and dilatations, angulation, displacement, and stones
CHRONIC EHPVO IN CHILDREN
Mesentric: Left portal vein bypass (REX Shunt) should be considered in all children with complications of chronic EHPVO
SHUNT SURGERIES IN EHPVO
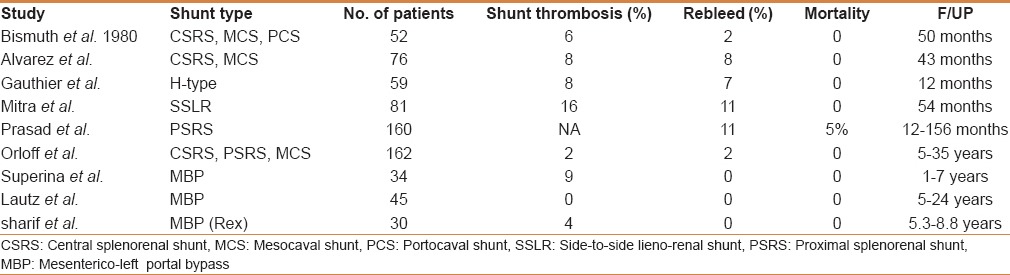
Conventionally, medical and endoscopic management is usually recommended for EHPVO, and various surgical shunts are used for refractory or complicated cases, surgery is primarily indicated when endotherapy fails to control bleeding, in the presence of gastric or ectopic varices not amenable to endoscopic management and with delayed sequelae such as portal biliopathy and rectal varices. Emergency shunt surgeries have become a rarity in the era of endoscopic management. Other indications of shunt surgery include symptomatic hypersplenism, growth retardation, portal biliopathy, massive splenomegaly affecting the quality of life, rare blood group, and remote area of residence. Various studies have shown shunt patency in 85%–98% patients with long-term survival in >95% patients with conventional portosystemic shunts, proximal splenorenal shunt, central splenorenal shunt, side to side lienorenal shunt, and mesocaval shunt. Comparison of various studies is drawn in the following table [Table 3].
Table 3.
A Comparative analysis of shunt surgeries in EHPVO
INDICATIONS/CONTRAINDICATIONS TO TREATMENT
The indication of medical or surgical treatment in EHPVO patient, especially garden variety group idiopathic EHPVO in Asian children without underlying liver disease or HCC is not well settled.
Regarding medical treatment of patients who have no varices but have EHPVO on imaging, there is no consensus regarding use of beta-blockers for prevention of variceal formation as per current literature and guidelines. Patients who have nonbleeding varices but have EHPVO there is only limited evidence of treating them with beta-blockers and guidelines do not recommend the same.
Only few studies have used sclerotherapy or ligation for prevention of bleeding in EHPVO patients. However, current guidelines justify use of such modalities for large and high-risk varices, especially for patients who belong to far flung areas but for others such modality is not indicated.
Patients who are not bleeders and who have no symptoms of hypersplenism, growth retardation, decreased quality of life with massive splenomegaly, and who have only asymptomatic biliopathy should not go for shunt surgery as shunt surgery has its own problems such as postoperative complications, shunt thrombosis, and so on.
FOLLOW UP
In children, follow up of growth retardation especially in Indian setting is done every 3–6 months.[51] Endoscopic surveillance should be done 1–2 yearly for varices. In asymptomatic biliopathy, follow-up algorithm is shown as Flowchart 1.[51]
Flowchart 1.
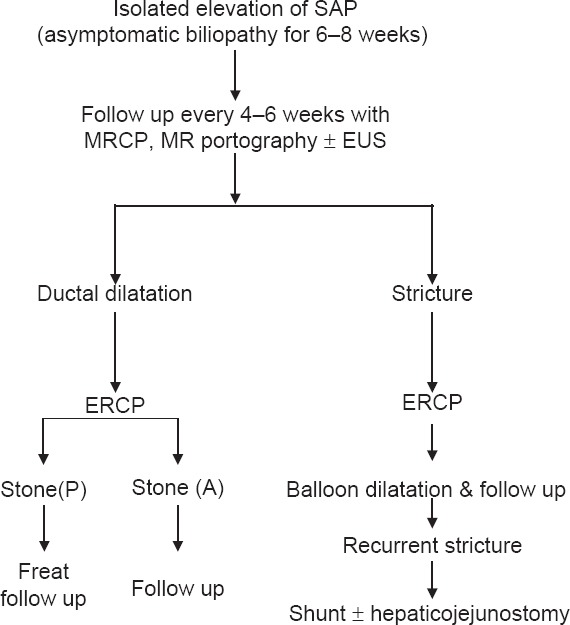
Schematic presentation showing stepwise management of Portal Billiopathy
PORTAL VEIN THROMBOSIS IN SPECIAL SITUATIONS (POSTOPERATIVE/LIVER TRANSPLANTATION)
Smoot et al. reported 5% of acute (less than 30 days) postoperative PVT rate in patients who underwent portal vein reconstruction during pancreaticoduodenectomy. Low incidence of acute vein portal vein thrombosis may be secondary to lack of detection until chronic changes have occurred.[52] Certainly mortality rates are higher in cases with associated mesenteric ischemia. Thus early diagnosis and ability to treat is very important. Use of Doppler ultrasound and CT/MRA helps in reaching diagnosis.
Site-directed thrombolytic therapy can be indirect via SMA catheter placement or directly via catheter in portal vein. Numerous series have demonstrated the excellent response rates ranging from 75% to 100%. Partial/complete recanalization Flowchart 2.[53,54]
Flowchart 2.
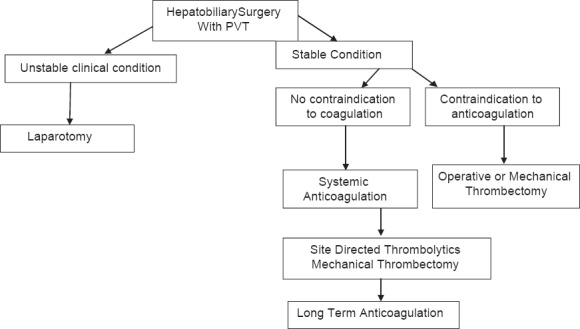
Schematic presentation showing management of patients with Hepatobilliary surgery and PVT
PORTAL VEIN THROMBOSIS IN LIVER TRANSPLANT
As shown by Villa and Sharma from an Italian study, enoxaprin given prophylactically to chronic liver disease patients with a CTP score of 7–10 revealed significantly reduced decompensation events. This was discussed in the International Liver Meet 2011. From this study, there may be the possibility that a liver biopsy showing thrombosis of the small radicles and downregulation of thrombin receptors on hepatic satellite cells may objectively answer the significant decrease in events from the above study.
Due to liver injury in CLD patients, more thrombin is generated, which causes intrahepatic thrombosis. This in turn causes stimulation of PAR-1 on HSC that can lead to further fibrosis.
Portal vein thrombosis is seen in 2%–6%[55,56] post-transplantation though portal vein thrombosis pre-transplant was considered at one time an absolute contraindication for transplant. As a result of tremendous medical care, surgical techniques and radiological interventions, portal vein thrombosis can represent itself as an indication for liver transplant.[57,58] The first successful liver transplant in patients with portal vein thrombosis was reported by Shaw. There are various surgical options depending on the grading of the intraoperative PVT as seen in Yerdel's grading system for postoperative PVT. Terminal to terminal PV anastomosis with or without thrombectomy is usually done in low-grade PVT, that is, less than 50% portal vein occlusion and portocaval hemitransposition, which is mandatory in Grade IV Yerdel's. Transplantation of Grade 1 Yerdel's postoperative PVT is similar to non-PVT patients undergoing transplantation.[59,60,61] The rate of thrombosis recurrence is 9%–-42%;[62,63,64] however, certain series represent lower incidence. After liver transplantation, PVT is rare (1%–2%) in the early period with preferential localization to the anastomotic site: Technical complication, small diameter of the portal vein are risk factors for PVT. A French study used the prophylactic anticoagulants in CLD patients with PVT waiting for transplant and found good results post-transplant.
CONCLUSION
It is very important to clinically define PVT as acute or chronic due to variation in management. It is equally important to identify cirrhosis, HCC, or any other malignancy in cases of PVT. In cases of idiopathic MPD, occult MPD should be investigated. Mutations such as JAK2 and TAFI should be ruled out in addition to conventional testing for inherited and acquired coagulation disorders.
In the modern era, CT and MR angiography as tools have made the diagnosis of PVT more accurate. Adopting newer methods of treatment for acute portal vein thrombosis in the setting of postoperative and liver transplantation is rational.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.De Gaetano AM, Lafortune M, Patriquin H, De Franco A, Aubin B, Paradis K. Cavernous transformation of the portal vein: Patterns of intra hepatic and splanchnic collateral circulation of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR Am J Roentgenol. 1995;165:1151–5. doi: 10.2214/ajr.165.5.7572494. [DOI] [PubMed] [Google Scholar]
- 2.Ogren M, Bergqvist D, Wahlander K, Eriksson H, Sternby NH. Trousseau's syndrome-what is the evidence? A population-based autopsy study. Thromb Haemost. 2006;95:541–5. doi: 10.1160/TH05-10-0694. [DOI] [PubMed] [Google Scholar]
- 3.Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: Current concepts and management. J Gastroenterol Hepatol. 2002;17:526–34. doi: 10.1046/j.1440-1746.2002.02764.x. [DOI] [PubMed] [Google Scholar]
- 4.Amitrano L, Guardascione1 MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736–41. doi: 10.1016/j.jhep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi K, Okuda K, Ohtsuki T, Nakayama T, Hiyama Y, Iwama S, et al. Formation of hilar collaterals or cavernous transformation after portal vein obstruction by hepatocellular carcinoma. Observations in ten patients. Gastroenterology. 1984;87:1150–3. [PubMed] [Google Scholar]
- 6.De Gaetano AM, Lafortune M, Patriquin H, De Franco A, Aubin B, Paradis K. Cavernous transformation of the portal vein: Patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR Am J Roentgenol. 1995;165:1151–5. doi: 10.2214/ajr.165.5.7572494. [DOI] [PubMed] [Google Scholar]
- 7.Condat B, Valla D. Nonmalignant portal vein thrombosis in adults. Nat Clin Pract Gastroenterol Hepatol. 2006;3:505–15. doi: 10.1038/ncpgasthep0577. [DOI] [PubMed] [Google Scholar]
- 8.Hoekstra J, Janssen HL. Vascular liver disorders (II): Portal vein thrombosis. Neth J Med. 2009;67:46–53. [PubMed] [Google Scholar]
- 9.Sengupta KP, Malik KC, Maddrey WC, Biswas SK, Pal NC, Das MM, et al. Liver changes in extrahepatic portal venous obstruction. Indian J Med Res. 1968;56:1643–50. [PubMed] [Google Scholar]
- 10.Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: A review. Am J Med. 1992;92:173–82. doi: 10.1016/0002-9343(92)90109-o. [DOI] [PubMed] [Google Scholar]
- 11.Valla DC, Condat B, Lebrec D. Spectrum of portal vein thrombosis in the West. J Gastroenterol Hepatol. 2002;17(Suppl 3):S224–7. doi: 10.1046/j.1440-1746.17.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 12.Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M, et al. Consensus on extra-hepatic portal vein obstruction. Liver Int. 2006;26:512–9. doi: 10.1111/j.1478-3231.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 13.Chawla Y, Duseja A, Dhiman RK. Review article: The modern management of portal vein thrombosis. Aliment Pharmacol Ther. 2009;30:881–94. doi: 10.1111/j.1365-2036.2009.04116.x. [DOI] [PubMed] [Google Scholar]
- 14.Ogren M, Bergqvist D, Bjorck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: Prevalence, patient characteristics and lifetime risk: A population study based on 23,796 consecutive autopsies. World J Gastroenterol. 2006;12:2115–9. doi: 10.3748/wjg.v12.i13.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonami T, Yokoyama I, Iwatsuki S, Starzl TE. The incidence of portal vein thrombosis at liver transplantation. Hepatology. 1992;16:1195–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Amitrano L, Guardascione MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736–41. doi: 10.1016/j.jhep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, et al. Splanchnic vein thrombosis in candidates for liver transplantation: Usefulness of screening and anticoagulation. Gut. 2005;54:691–7. doi: 10.1136/gut.2004.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, et al. Thrombotic risk factors in patients with liver cirrhosis: Correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–9. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Denninger MH, Chait Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, et al. Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors. Hepatology. 2000;31:587–91. doi: 10.1002/hep.510310307. [DOI] [PubMed] [Google Scholar]
- 20.Janssen HL, Meinardi JR, Vleggaar FP, van Uum SH, Haagsma EB, van Der Meer FJ, et al. Factor VLeiden mutation, prothrombin gene mutation, and deficiencies in coagulation inhibitors associated with Budd-Chiari syndrome and portal vein thrombosis: Results of a case-control study. Blood. 2000;96:2364–8. [PubMed] [Google Scholar]
- 21.Bombeli T, Basic A, Fehr J. Prevalence of hereditary thrombophilia in patients with thrombosis in different venous systems. Am J Hematol. 2002;70:126–32. doi: 10.1002/ajh.10103. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya M, Makharia G, Kannan M, Ahmed RP, Gupta PK, Saxena R. Inherited prothrombotic defects in Budd-Chiari syndrome and portal vein thrombosis: A study from North India. Am J Clin Pathol. 2004;121:844–7. doi: 10.1309/F2U1-XBV4-RXYU-AYG0. [DOI] [PubMed] [Google Scholar]
- 23.Primignani M, Martinelli I, Bucciarelli P, Battaglioli T, Reati R, Fabris F, et al. Risk factors for thrombophilia in extrahepatic portal vein obstruction. Hepatology. 2005;41:603–8. doi: 10.1002/hep.20591. [DOI] [PubMed] [Google Scholar]
- 24.Plessier A, Darwish Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Heller J, et al. A prospective multicentric follow-up study on 105 patients with acute portal vein thrombosis (PVT): Results from the European Network for Vascular Disorders of the Liver (EN-VIE) Hepatology. 2007;46:310A. [Google Scholar]
- 25.Rosendaal FR. Venous thrombosis: A multicausal disease. Lancet. 1999;353:1167–73. doi: 10.1016/s0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 26.Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, et al. Extrahepatic portal vein thrombosis: Aetiology and determinants of survival. Gut. 2001;49:720–4. doi: 10.1136/gut.49.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valla D, Casadevall N, Huisse MG, Tulliez M, Grange JD, Muller O, et al. Etiology of portal vein thrombosis in adults. A prospective evaluation of primary myeloproliferative disorders. Gastroenterology. 1988;94:1063–9. doi: 10.1016/0016-5085(88)90567-7. [DOI] [PubMed] [Google Scholar]
- 28.De Stefano V, Teofili L, Leone G, Michiels JJ. Spontaneous erythroid colony formation as the clue to an underlying myeloproliferative disorder in patients with Budd-Chiari syndrome or portal vein thrombosis. Semin Thromb Hemost. 1997;23:411–8. doi: 10.1055/s-2007-996117. [DOI] [PubMed] [Google Scholar]
- 29.Chait Y, Condat B, Cazals-Hatem D, Rufat P, Atmani S, Chaoui D, et al. Relevance of the criteria commonly used to diagnose myeloproliferative disorder in patients with splanchnic vein thrombosis. Br J Haematol. 2005;129:553–60. doi: 10.1111/j.1365-2141.2005.05490.x. [DOI] [PubMed] [Google Scholar]
- 30.de Bruijne EL, Darwish Murad S, de Maat MP, Tanck MW, Haagsma EB, van Hoek B, et al. Genetic variation in thrombin-activatable fibrinolysis inhibitor (TAFI) is associated with the risk of splanchnic vein thrombosis. Thromb Haemost. 2007;97:181–5. [PubMed] [Google Scholar]
- 31.Janssen HL, Meinardi JR, Vleggaar FP, van Uum SH, Haagsma EB, van Der Meer FJ, et al. Factor V Leiden mutation, prothrombin gene mutation, and deficiencies in coagulation inhibitors associated with Budd-Chiari syndrome and portal vein thrombosis: Results of a case-control study. Blood. 2000;96:2364–8. [PubMed] [Google Scholar]
- 32.Amitrano L, Brancaccio V, Guardascione MA, Margaglione M, Iannaccone L, D’Andrea G, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology. 2000;31:345–8. doi: 10.1002/hep.510310213. [DOI] [PubMed] [Google Scholar]
- 33.Dubuisson C, Boyer-Neumann C, Wolf M, Meyer D, Bernard O. Protein C, protein S and antithrombin III in children with portal vein obstruction. J Hepatol. 1997;27:132–5. doi: 10.1016/s0168-8278(97)80292-9. [DOI] [PubMed] [Google Scholar]
- 34.Barosi G, Buratti A, Costa A, Liberato LN, Balduini C, Cazzola M, et al. An atypical myeloproliferative disorder with high thrombotic risk and slow disease progression. Cancer. 1991;68:2310–8. doi: 10.1002/1097-0142(19911115)68:10<2310::aid-cncr2820681034>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Hirshberg B, Shouval D, Fibach E, Friedman G, Ben-Yehuda D. Flow cytometric analysis of autonomous growth of erythroid precursors in liquid culture detects occult polycythemia vera in the Budd-Chiari syndrome. J Hepatol. 2000;32:574–8. doi: 10.1016/s0168-8278(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 36.De Stefano V, Teofili L, Leone G, Michiels JJ. Spontaneous erythroid colony formation as the clue to an underlying myeloproliferative disorder in patients with Budd-Chiari Syndrome or portal vein thrombosis. Semin Thromb Hemost. 1997;23:411–8. doi: 10.1055/s-2007-996117. [DOI] [PubMed] [Google Scholar]
- 37.Dayal S, Pati HP, Pande GK, Sharma MP, Saraya AK. Multilineage hemopoietic stem cell defects in Budd Chiari syndrome. J Hepatol. 1997;26:293–7. doi: 10.1016/s0168-8278(97)80044-x. [DOI] [PubMed] [Google Scholar]
- 38.Pagliuca A, Mufti GJ, Janossa-Tahernia M, Eridani S, Westwood NB, Thumpston J, et al. In vitro colony culture and chromosomal studies in hepatic and portal vein thrombosis. Possible evidence of an occult myeloproliferative state. Q J Med. 1990;76:981–9. [PubMed] [Google Scholar]
- 39.McNamara C, Juneja S, Wolf M, Grigg A. Portal or hepatic vein thrombosis as the first presentation of a myeloproliferative disorder in patients with normal peripheral blood counts. Clin Lab Haematol. 2002;24:239–42. doi: 10.1046/j.1365-2257.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 40.Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91:169–75. [PubMed] [Google Scholar]
- 41.Sarin SK, Agarwal SR. Extrahepatic portal vein obstruction. Semin Liver Dis. 2002;22:43–58. doi: 10.1055/s-2002-23206. [DOI] [PubMed] [Google Scholar]
- 42.Ni YH, Wang NC, Peng MY, Chou YY, Chang FY. Bacteroides fragilis bacteremia associated with portal vein and superior mesentery vein thrombosis secondary to antithrombin III and protein C deficiency: A case report. J Microbiol Immunol Infect. 2002;35:255–8. [PubMed] [Google Scholar]
- 43.Gallego C, Velasco M, Marcuello P, Tejedor D, De Campo L, Friera A. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002;22:141–59. doi: 10.1148/radiographics.22.1.g02ja08141. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A, Sharma BC, Sarin SK. Profile of gastrointestinal hemorrhage in 499 patients of extra-hepatic portal vein obstruction (EHPVO) [abstract] Indian J Gastroenterol. 2005;24(Suppl 1):A69. [Google Scholar]
- 45.Chawla Y, Dilawari JB, Katariya S. Gallbladder varices in portal vein thrombosis. AJR Am J Roentgenol. 1994;162:643–5. doi: 10.2214/ajr.162.3.8109513. [DOI] [PubMed] [Google Scholar]
- 46.Chiu B, Superina R. Extrahepatic portal vein thrombosis is associated with an increased incidence of cholelithiasis. J Pediatr Surg. 2004;39:1059–61. doi: 10.1016/j.jpedsurg.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 47.Khuroo MS, Yattoo GN, Zargar SA, Javid G, Dar MY, Khan BA, et al. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–13. [PubMed] [Google Scholar]
- 48.Bhatia V, Jain AK, Sarin SK. Choledocholithiasis associated with portal biliopathy in patients with extrahepatic portal vein obstruction: Management with endoscopic sphincterotomy. Gastrointest Endosc. 1995;42:178–81. doi: 10.1016/s0016-5107(95)70080-3. [DOI] [PubMed] [Google Scholar]
- 49.Mehrotra RN, Bhatia V, Dabadghao P, Yachha SK. Extrahepatic portal vein obstruction in children: Anthropometry, growth hormone, and insulin-like growth factor I. J Pediatr Gastroenterol Nutr. 1997;25:520–3. doi: 10.1097/00005176-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Kato T, Romero R, Koutouby R, Mittal NK, Thompson JF, Schleien CL, et al. Portosystemic shunting in children during the era of endoscopic therapy: Improved postoperative growth parameters. J Pediatr Gastroenterol Nutr. 2000;30:419–25. doi: 10.1097/00005176-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Khanna R, Sarin SK. Non-cirrhotic portal hypertension–Diagnosis and management. J Hepatol. 2014;60:421–41. doi: 10.1016/j.jhep.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Poplausky MR, Kaufman JA, Geller SC, Waltman AC. Mesenteric venous thrombosis treated with urokinase via the superior mesenteric artery. Gastroenterology. 1996;110:1633–5. doi: 10.1053/gast.1996.v110.pm8613072. [DOI] [PubMed] [Google Scholar]
- 53.Woo DH, Laberge JM, Gordon RL, Wilson MW, Kerlan RK., Jr Management of portal venous complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:233–9. doi: 10.1053/j.tvir.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Wozney P, Zajko AB, Bron KM, Point S, Starzl TE. Vascular complications after liver transplantation: A 5-year experience. AJR Am J Roentgenol. 1986;147:657–63. doi: 10.2214/ajr.147.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobhonslidsuk A, Reddy KR. Portal vein thrombosis: A concise review. Am J Gastroenterol. 2002;97:535–41. doi: 10.1111/j.1572-0241.2002.05527.x. [DOI] [PubMed] [Google Scholar]
- 56.Gao PJ, Zhu JY, Li GM, Leng XS. Liver transplantation for the patients with end stage liver disease and portal vein thrombosis. Beijing Da Xue Xue Bao. 2009;41:558–60. [PubMed] [Google Scholar]
- 57.Shaw BW, Jr, Iwatsuki S, Bron K, Starzl TE. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. 1985;161:66–8. [PMC free article] [PubMed] [Google Scholar]
- 58.Molmenti EP, Roodhouse TW, Molmenti H, Jaiswal K, Jung G, Marubashi S, et al. Thrombendvenectomy for organized portal vein thrombosis at the time of liver transplantation. Ann Surg. 2002;235:292–6. doi: 10.1097/00000658-200202000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seu P, Shackleton CR, Shaked A, Imagawa DK, Olthoff KM, Rudich SR, et al. Improved results of liver transplantation in patients with portal vein thrombosis. Arch Surg. 1996;131:840–4. doi: 10.1001/archsurg.1996.01430200050009. [DOI] [PubMed] [Google Scholar]
- 60.Manzanet G, Sanjuán F, Orbis P, López R, Moya A, Juan M, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl. 2001;7:125–31. doi: 10.1053/jlts.2001.21295. [DOI] [PubMed] [Google Scholar]
- 61.Dumortier J, Czyglik O, Poncet G, Blanchet MC, Boucaud C, Henry L, et al. Eversion thrombectomy for portal vein thrombosis during liver transplantation. Am J Transplant. 2002;2:934–8. doi: 10.1034/j.1600-6143.2002.21009.x. [DOI] [PubMed] [Google Scholar]
- 62.Arcadipane A, Nadalin S, Gruttadauria S, Panarello G, Burgio G, Vizzini G, et al. The recipient with portal thrombosis and/or previous surgery. Transplant Proc. 2008;40:1183–6. doi: 10.1016/j.transproceed.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 63.Stieber AC, Zetti G, Todo S, Tzakis AG, Fung JJ, Marino I, et al. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg. 1991;213:199–206. doi: 10.1097/00000658-199103000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lerut J, Tzakis AG, Bron K, Gordon RD, Iwatsuki S, Esquivel CO, et al. Complications of venous reconstruction in human orthotopic liver transplantation. Ann Surg. 1987;205:404–14. doi: 10.1097/00000658-198704000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


