Abstract
Background/Aims:
Fetuin-A, a glycoprotein with anti-inflammatory properties, plays an important role in counter-regulating inflammatory responses. It has also been associated with insulin resistance and metabolic syndrome. We aimed to investigate circulating concentrations of fetuin-A and its possible association with hepatic and systemic inflammation in nondiabetic subjects with nonalcoholic fatty liver disease (NAFLD).
Patients and Methods:
We included 105 nondiabetic male subjects with NAFLD [nonalcoholic steatohepatitis (NASH, n = 86) and simple steatosis (SS, n = 19)]. Plasma levels of fetuin-A and markers of inflammation [high-sensitive C reactive protein (hsCRP), tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and adiponectin] were measured by enzyme-linked immunosorbent assay method. Insulin sensitivity was determined by homeostasis model assessment of insulin resistance (HOMA-IR) index.
Results:
Fetuin-A was negatively correlated with age (r = −0.27, P = 0.006), however there was no association between fetuin-A and body mass index, waist circumference (WC), glucose, insulin, HOMA-IR, lipid parameters, and inflammatory markers. In addition, no significant association was observed between fetuin-A and histological findings including liver fibrosis.
Conclusion:
This study demonstrated that plasma fetuin-A levels are not correlated with the hepatic histology and systemic markers of inflammation in nondiabetic subjects with NAFLD. Our data also suggested that age is significantly associated with fetuin-A in this clinically relevant condition.
Keywords: Adiponectin, fetuin-A, inflammation, nonalcoholic fatty liver disease
Obesity-related nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in both the developed and the developing countries. It has a broad spectrum of manifestations ranging from simple steatosis (SS) to nonalcoholic steatohepatitis (NASH) and cirrhosis.[1] Obesity, hyperglycemia, type 2 diabetes (T2D), and hypertriglyceridemia are well-known risk factors for the development of NAFLD. NAFLD is therefore regarded as a manifestation of the metabolic (or insulin resistance) syndrome (MetS).[2,3]
Fetuin-A is a 64-kDa glycoprotein produced exclusively by the liver and secreted into blood where it is found in relatively high concentrations in humans.[4,5] It binds and inhibits the insulin receptor tyrosine kinase in skeletal muscle and hepatocytes, inhibiting insulin signal transduction and resulting in insulin resistance in these target tissues.[6,7] In the general population, fetuin-A is associated with insulin resistance and obesity,[5,8] and predicts the future risk of T2D.[9] Higher fetuin-A levels are also associated with NAFLD, and short-term diet and exercise interventions results in decline in fetuin-A serum levels commensurate with improvement in NAFLD.[8,9] We earlier reported that circulating fetuin-A is independently associated with endothelial dysfunction and subclinical atherosclerosis in patients with NAFLD.[10]
Current evidence indicates a pro-inflammatory role of fetuin-A.[11] It was reported to be downregulated during inflammation and inversely correlated with C-reactive protein (CRP) level, a well-known marker of systemic inflammation.[12,13] Therefore, in the present study, we aimed to investigate the association of circulating fetuin-A with liver histology and markers of systemic inflammation in subjects with NAFLD. To prevent any interference of confounding factors for inflammation, we studied a specifically selected group having no additional disorders such as hypertension, T2D, or morbid obesity.
PATIENTS AND METHODS
Study design and population
In this retrospective study, a total of 105 male subjects with NAFLD (NASH, n = 86 and SS, n = 19) were enrolled. Subjects were recruited from individuals attending outpatient clinic of the Gastroenterology Department, Gulhane School of Medicine, Ankara, Turkey. The study was approved by the local ethics committee of Gulhane School of Medicine (2013/15), which was conducted according to the Helsinki Declaration.
Inclusion criteria were persistently (at least for 6 months) elevated aminotransferases, ultrasonographic presence of bright liver without any other liver or biliary tract disease, and liver histology compatible with a diagnosis of NAFLD. Subjects with the following conditions or diseases were excluded; alcohol consumption ≥20 g/day in the previous year, a positive test for hepatitis B surface antigen, hepatitis C antibody, and other causes of liver disease, serum creatinine >133 μmol/L, presence of T2D, hypertension, morbid obesity [body mass index (BMI) ≥40 kg/m2], any acute or chronic inflammatory disease as determined by a leukocyte count >10,000/mm3 or clinical signs of infection, and history of major diseases such as generalized inflammation or advanced malignant diseases, exposure to occupational hepatotoxins or drugs known to be steatogenic or to affect glucose, and lipid metabolism.
Clinical and laboratory data
All participants provided a medical history and underwent a clinical examination. Body weight and height were measured with a calibrated scale. Waist circumference (WC) measurement was performed at the end of normal expiration in duplicate on bare skin midway between the lower rib margin and the anterior superior iliac crest. BMI (kg/m2) was calculated as weight (kg) divided by height (m) squared.
All subjects were screened with a standard 75-g oral glucose tolerance test after a 10- to 12-h overnight fast and provided the other conditions to perform the test (normal diet for 3 days before the test, abstention from smoking for > 24 h). Glucose tolerance status was determined according to the classification of the American Diabetes Association in which fasting plasma glucose (FPG) levels up to 99 mg/dL are considered normal and T2D is defined by a FPG level of 126 mg/dL or greater, or 2-h plasma glucose levels of 200 mg/dL or greater.[14]
Fasting venous blood samples were collected into tubes with anticoagulant (ethylene diamine tetra-acetate), centrifuged at 3000g for 10 min at room temperature and then stored at −80°C until the time of the assay. All samples were assayed in duplicate. FPG, total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels were measured by the enzymatic colorimetric methods with Olympus AU2700 (Beckman Coulter, USA) autoanalyzer by using commercially available reagents. Low-density lipoprotein cholesterol (LDL-C) was calculated by Fridewald's formula.[15] The serum basal insulin levels were measured by an ADVIA Centaur assay (Siemens Medical Solutions Diagnostics, Tokyo, Japan). Homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated according to the formula: FPG (mg/dL) × fasting insulin (μU/mL)/405. HOMA-IR index, which was originally reported by Matthews et al.,[16] has been shown to be well correlated with the results of the euglycemic–hyperinsulinemic clamp method to determine insulin resistance.
High-sensitive CRP (hsCRP) level was determined in serum by immune turbidimetric fixed rate method by Olympus AU-2700 autoanalyzer (Hamburg, Germany). Intra-assay and interassay CV were 5.8% and 3.1%, respectively. The minimum detectable concentration for hsCRP was 0.07 mg/L. Plasma fetuin-A levels were determined by enzyme-linked immunosorbent assay (ELISA) (Human fetuin-A ELISA Kit, Epitope Diagnostics, Inc, San Diego, CA, USA). Minimum detectable concentration for fetuin-A was 5 ng/mL. Intra-assay CV ranged from 4.8% to 5.5%, whereas interassay CV ranged from 5.7% to 6.8%. Plasma adiponectin levels were determined by Total Human Adiponectin ELISA Kit (TECOmedical AG, Sissach, Switzerland). Plasma levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were also determined by Human TNF-α High Sensitivity ELISA and Human IL-6 High Sensitivity ELISA kits (both from eBioscience, Vienna, Austria). The minimum detectable concentration for TNF-α and IL-6 were 0.13 pg/mL and 0.03 pg/mL, respectively. The calculated overall intra-assay CV for TNF-α and IL-6 were 8.5% and 4.9%, whereas the calculated overall interassay CV for TNF-α and IL-6 were 9.8% and 6.0%, respectively. Measurements were carried out using ELISA plate reader Bio-Tek Synergy HT (Biotek Instruments Inc., Winooski, VT, USA).
Liver histology
An experienced hepatopathologist blinded to subjects’ details scored liver biopsy specimens using the semi-quantitative classification of Kleiner et al.[17]; SS (steatosis in the absence of inflammation and ballooning hepatocyte degeneration), borderline NASH (steatosis with minimal inflammation and hepatocyte ballooning), and NASH (steatosis with inflammation and hepatocyte ballooning, often with fibrosis). In brief, the degree of steatosis, liver injury, and inflammatory activity were scored using an 8-point scale (steatosis 0–3; lobular inflammation 0–3; ballooning hepatocyte degeneration 0–2). The NAFLD activity score (NAS) is the unweighted sum of steatosis, lobular inflammation, and hepatocellular ballooning scores. The stage of fibrosis was scored using a 6-point scale (1a, b = mild (1a)/moderate (1b) zone 3 perisinusoidal fibrosis; 1c = portal fibrosis only; 2 = zone 3 and portal/periportal fibrosis; 3 = bridging fibrosis; 4 = cirrhosis).
Statistical analysis
Results are reported as the mean ± standard deviation (SD) and median (min–max). Differences between groups were tested for significance by independent samples t-test, Chi-square test and Mann–Whitney U test. The relationship between variables was analyzed by Spearman's and Pearson's correlation coefficient. Variables that were significantly different between two groups were analyzed on multivariate analysis. In addition, multivariate linear regression analysis was used to assess the association between fetuin-A, adiponectin, TNF-α, IL-6, and hsCRP. The statistical analysis was carried out by using Statistical Package for the Social Science (SPSS), version 15.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
RESULTS
The clinical and laboratory characteristics of the study population are presented in Table 1. The average age of the study participants was 31.8 years, and all of them were male. The mean plasma level of fetuin-A was 74.41 μg/mL and showed a normal distribution. The distribution of histopathological parameters is shown in Table 2.
Table 1.
Anthropometric and metabolic characteristics of the study participants
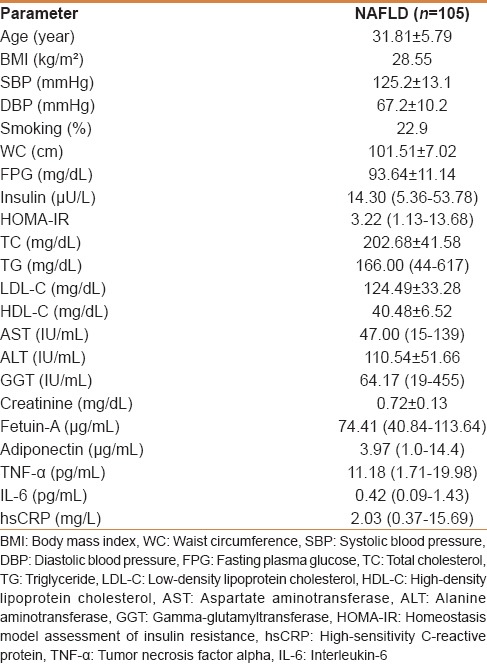
Table 2.
Distribution of histopathological findings in subjects with NAFLD (n=105)

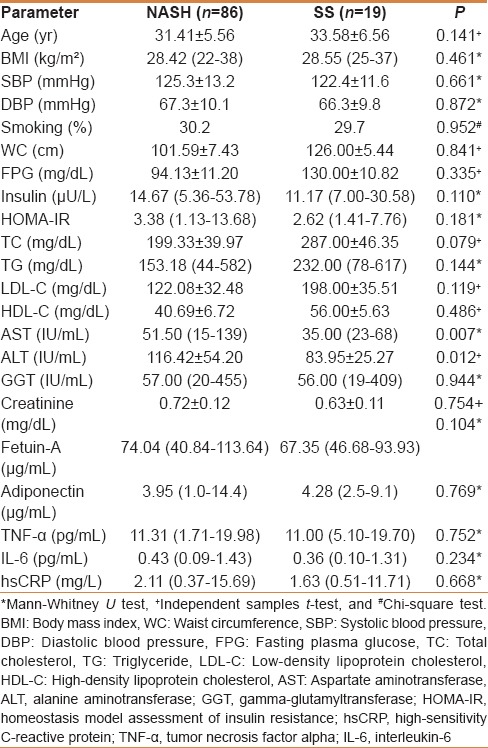
The clinical and laboratory features of the patients with NASH and SS are shown in Table 3. There were no significant differences in fetuin-A levels between patients with NASH compared with those with SS. Significant differences were found in AST (P = 0.007) and ALT levels (P = 0.012) between two groups. However, no differences were found for age, BMI, WC, glucose, insulin, HOMA-IR index, lipid parameters, and inflammatory markers among the groups.
Table 3.
The clinical and laboratory features of the patients with NASH and SS
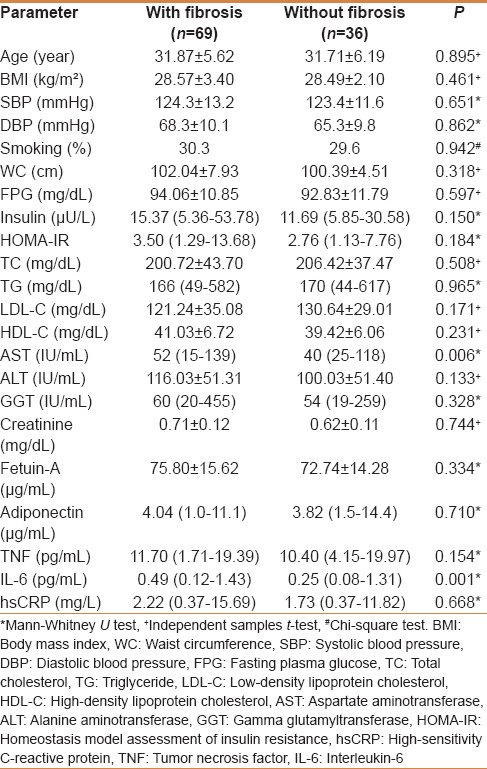
The clinical and laboratory features of patients with (n = 69) and without (n = 36) fibrosis are shown in Table 4. A significantly higher AST (P = 0.006) level was found in subjects with fibrosis in comparison to subjects without fibrosis. On the other hand, there was no significant difference between the two groups regarding the other parameters studied.
Table 4.
The clinical and biochemical characteristics of subjects with and without fibrosis
In correlation analysis, fetuin-A was negatively correlated with age (r = −0.27, P = 0.006). However, there was no association between fetuin-A and glucose, HOMA-IR index, lipid parameters, NAS, and inflammatory markers, including TNF-α, IL-6, hsCRP, and adiponectin. Moreover, analysis of the plasma fetuin-A in relation to the histological findings also showed no association between these parameters.
Because of the well-known relationship of fetuin-A with MetS,[10,18] we also aimed to search the possible relationship of this glycoprotein with MetS in subjects with NAFLD. But, according to the National Cholesterol Education Program (NCEP)[19] only seven of our patients had MetS, we therefore could not analyze this relationship.
DISCUSSION
An increasing body of evidence suggests that fetuin-A expression is significantly increased in subjects with NAFLD.[20,21] In addition, circulating fetuin-A levels were reported to be elevated in subjects with high liver fat and a decrease in liver fat was accompanied by decrease in fetuin-A concentrations.[8] However, there are also contradictory reports in the literature regarding the relationship of circulating fetuin-A with NAFLD. For instance, Yilmaz et al. reported no association between the serum fetuin-A and NASH and this molecule was not different between subjects with NASH and SS. But they found a significant association of fetuin-A with liver fibrosis and insulin resistance as assessed by the HOMA-IR index.[22] On the other hand, in a study by Haukeland et al. there were no significant differences in fetuin-A levels between patients with NASH compared with those with SS, and no significant differences were observed according to fibrosis stage, or according to degree of liver steatosis. Among controls, fetuin-A levels positively correlated with serum ALT but not with other measured variables.[21] In the present study, no difference was observed regarding the fetuin-A plasma levels between NASH and SS groups and also patients with and without fibrosis. Moreover, we did not find any association of circulating fetuin-A with liver histology and insulin resistance in subjects with NAFLD. We suggest that the discrepant findings of the studies mentioned above were likely due to differences in the populations of patients enrolled, as we excluded subjects with T2D, hypertension and morbid obesity. Hence, it is well known that all these metabolic confounders may affect the circulating fetuin-A concentrations.[23,24,25] In light of these data, our findings further support the hypothesis that fetuin-A per se is not associated with NAFLD and insulin resistance is the key factor for the relationship of this protein with this clinically relevant condition.[10,26] In fact, it is still uncertain whether fetuin-A directly contributes to the development of NAFLD, whether elevated blood levels reflect the presence or severity of NAFLD, or whether other unidentified factors simultaneously influence both.
The liver orchestrates a host defense response by altering the synthesis and systemic release of acute phase proteins (APP), such as fetuin-A, also termed the a2-Heremans-Schmid glycoprotein.[27] Fetuin-A has been reported to be an anti-inflammatory molecule that participates in macrophage deactivation, antifibrotic activity, and inhibition of apoptosis of vascular smooth muscle cells.[28,29,30] The expression of fetuin-A was reported to be counter-regulated by proinflammatory cytokines such as TNF-α, IL-1, and IL-6, classifying it as a negative APP.[31,32] In addition, prior studies suggest that levels of circulating fetuin-A fall during inflammation.[33] It has been shown that fetuin-A administration significantly inhibits TNF-α in an experimental model.[34] Recently, Memoli et al. reported that IL-6 induces significant down regulation of fetuin-A expression in human hepatocytes.[35] However, contradictory results have also been published regarding the relationship of fetuin-A with pro- and anti-inflammatory cytokines in clinical studies. A recent study reported that the secreted liver protein fetuin-A induces low-grade inflammation in terms of TNF-α and IL1- mRNA expression and represses adiponectin production in animals and in humans.[11] In another study, a reciprocal change was observed between fetuin-A and adiponectin levels in subjects of different glycemic status. However, further analysis showed no significant association between fetuin-A and adiponectin.[36] Serum fetuin-A concentrations were negatively correlated with hsCRP levels in subjects with coronary artery disease.[37] On the other hand, no significant association was found between fetuin-A and hsCRP levels in other studies.[38,39,40] Moreover, plasma fetuin-A levels were reported to be elevated in patients after ischemic stroke, implying that fetuin-A may also function as a positive APP.[41,42]
In our study, we could not find any association of plasma fetuin-A with adiponectin, TNF-α, IL-6, and hsCRP levels in subjects with NAFLD. Consequently, there were significant differences regarding the relationship of fetuin-A with markers of inflammation in these studies mentioned above. We suggest some possible explanations for the different results of these studies. First, when the above-mentioned studies were analyzed separately, it can be seen that some of the subjects with NAFLD had metabolic confounders such as morbid obesity, T2D, and hypertension. In addition, some of these patients were also using medications related to these metabolic problems.[2,11] It has been reported that circulating levels of inflammatory cytokines may be easily affected by these metabolic risk factors and by the drugs that are commonly prescribed for these metabolic problems.[21,43,44,45] So, previous data regarding the association of fetuin-A with inflammation might be affected by these confounders. For this reason, we excluded these possible metabolic confounders in the present study. Secondly, mean age of the participants in our study was less than that of the subjects in the previous studies. In literature, fetuin-A has been shown to be negatively associated with age.[9,23] Although the mechanism of aging related decrease in fetuin-A is still unknown; our study provides further evidence that age is inversely associated with fetuin-A levels. So, age may be an important determinant for the levels of circulating fetuin-A in NAFLD. Finally, as recently reported, genetic factors may also affect the fetuin-A concentrations independently from inflammation.[46]
Our study has several limitations. First, because of the small number of patients and the strict inclusion criteria, the findings obtained are not representative for all subjects with NAFLD. But we think that the design of our study was a requirement for the goals to achieve. Second, all participants were men, and that it remains to be determined if these results are similar even in women. Last, although it is simple, noninvasive, and known to be correlated well with clamp test, the HOMA-IR formula used to calculate insulin sensitivity in this work is only an estimate and cannot be as accurate as the euglycemic hyperinsulinemic clamp method.
In conclusion, we demonstrated that plasma fetuin-A levels are not related to the liver histology and systemic markers of inflammation in nondiabetic male subjects with NAFLD. Our data also suggest that age is significantly associated with circulating fetuin-A in this clinically relevant condition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes. 2001;50:1844–50. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 3.Swallow CJ, Partridge EA, Macmillan JC, Tajirian T, Di Guglielmo GM, Hay K, et al. Alpha2HS-glycoprotein, an antagonist of transforming growth factor beta in vivo, inhibits intestinal tumor progression. Cancer Res. 2004;64:6402–9. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- 4.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: Data from the Heart and Soul Study. Circulation. 2006;113:1760–7. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauth G, Pöschke O, Fink E, Eulitz M, Tippmer S, Kellerer M, et al. The nucleotide and partial aminoacid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 1992;204:523–9. doi: 10.1111/j.1432-1033.1992.tb16663.x. [DOI] [PubMed] [Google Scholar]
- 6.Srinivas PR, Wagner AS, Reddy LV, Deutsch DD, Leon MA, Goustin AS, et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol. 1993;7:1445–55. doi: 10.1210/mend.7.11.7906861. [DOI] [PubMed] [Google Scholar]
- 7.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29:853–7. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 8.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Kröber SM, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–7. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93:4479–85. doi: 10.1210/jc.2008-1505. [DOI] [PubMed] [Google Scholar]
- 10.Dogru T, Genc H, Tapan S, Aslan F, Ercin CN, Ors F, et al. Plasma fetuin-A is associated with endothelial dysfunction and subclinical atherosclerosis in subjects with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 2013;78:712–7. doi: 10.1111/j.1365-2265.2012.04460.x. [DOI] [PubMed] [Google Scholar]
- 11.Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Häring HU, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008;12:1765. doi: 10.1371/journal.pone.0001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moe SM, Chen NX. Inflammation and vascular calcification. Blood Purif. 2005;23:64–71. doi: 10.1159/000082013. [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations of gene encoding fetuin. Kidney Int. 2005;67:2283–392. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 14.Standards of medical care in diabetes. American Diabetes Association. Diabetes Care. 2013;36:11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Xu M, Bi Y, Song A, Huang Y, Liu Y, et al. Serum fetuin-A is correlated with metabolic syndrome in middle-aged and elderly Chinese. Atherosclerosis. 2011;216:180–6. doi: 10.1016/j.atherosclerosis.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB. National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–9. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 20.Kahraman A, Sowa JP, Schlattjan M, Sydor S, Pronadl M, Wree A, et al. Fetuin-A mRNA expression is elevated in NASH compared with NAFL patients. Clin Sci (Lond) 2013;125:391–400. doi: 10.1042/CS20120542. [DOI] [PubMed] [Google Scholar]
- 21.Haukeland JW, Dahl TB, Yndestad A, Gladhaug IP, Løberg EM, Haaland T, et al. Fetuin A in nonalcoholic fatty liver disease: In vivo and in vitro studies. Eur J Endocrinol. 2012;166:503–10. doi: 10.1530/EJE-11-0864. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz Y, Yonal O, Kurt R, Ari F, Oral AY, Celikel CA, et al. Serum fetuin A/α2HS-glycoprotein levels in patients with non-alcoholic fatty liver disease: Relation with liver fibrosis. Ann Clin Biochem. 2010;47:549–53. doi: 10.1258/acb.2010.010169. [DOI] [PubMed] [Google Scholar]
- 23.Ou HY, Yang YC, Wu HT, Wu JS, Lu FH, Chang CJ. Serum fetuin-A concentrations are elevated in subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes. Clin Endocrinol (Oxf) 2011;75:450–5. doi: 10.1111/j.1365-2265.2011.04070.x. [DOI] [PubMed] [Google Scholar]
- 24.Vörös K, Gráf L, Jr, Prohászka Z, Gráf L, Szenthe P, Kaszás E, et al. Serum fetuin-A in metabolic and inflammatory pathways in patients with myocardial infarction. Eur J Clin Invest. 2011;41:703–9. doi: 10.1111/j.1365-2362.2010.02456.x. [DOI] [PubMed] [Google Scholar]
- 25.Brix JM, Stingl H, Höllerl F, Schernthaner GH, Kopp HP, Schernthaner G. Elevated Fetuin-A concentrations in morbid obesity decrease after dramatic weight loss. J Clin Endocrinol Metab. 2010;95:4877–81. doi: 10.1210/jc.2010-0148. [DOI] [PubMed] [Google Scholar]
- 26.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: The roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–12. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie DL, Dziegielewska KM, Hill RM, Saunders NR. Fetuin: The bovine homologue of human alpha 2HS glycoprotein. FEBS Lett. 1987;214:45–9. doi: 10.1016/0014-5793(87)80010-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Zhang M, Bianchi M, Sherry B, Sama A, Tracey KJ. Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophage deactivating molecules. Proc Natl Acad Sci USA. 1998;95:14429–34. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–61. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, et al. Multifunctional roles for serum protein fetuin-A in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–30. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 31.Daveau M, Christian-Davrinche, Julen N, Hiron M, Arnaud P, Lebreton JP. The synthesis of human alpha-2-HS glycoprotein is down-regulated by cytokines in hepatoma HepG2 cells. FEBS Lett. 1988;241:191–4. doi: 10.1016/0014-5793(88)81059-7. [DOI] [PubMed] [Google Scholar]
- 32.Ohnishi T, Nakamura O, Ozawa M, Arakaki N, Muramatsu T, Daikuhara Y. Molecular cloning and sequence analysis of cDNA for a 59 kD bone sialoprotein of the rat: Demonstration that it is a counterpart of human alpha 2-HS glycoprotein and bovine fetuin. J Bone Miner Res. 1993;8:367–77. doi: 10.1002/jbmr.5650080314. [DOI] [PubMed] [Google Scholar]
- 33.Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: Evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979;64:1118–29. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ombrellino M, Wang H, Yang H, Zhang M, Vishnubhakat J, Frazier A, et al. Fetuin, a negative acute phase protein. Shock. 2001;15:181–5. doi: 10.1097/00024382-200115030-00004. [DOI] [PubMed] [Google Scholar]
- 35.Memoli B, De Bartolo L, Favia P, Morelli S, Lopez LC, Procino A, et al. Fetuin-A gene expression, synthesis and release in primary human hepatocytes cultured in a galactosylated membrane bioreactor. Biomaterials. 2007;28:4836–44. doi: 10.1016/j.biomaterials.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 36.Tönjes A, Fasshauer M, Kratzsch J, Stumvoll M, Blüher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS One. 2010;9:e13911. doi: 10.1371/journal.pone.0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilgir O, Kebapcilar L, Bilgir F, Bozkaya G, Yildiz Y, Pinar P, et al. Decreased serum fetuin-A levels are associated with coronary artery diseases. Intern Med. 2010;49:1281–5. doi: 10.2169/internalmedicine.49.3223. [DOI] [PubMed] [Google Scholar]
- 38.Fiore CE, Celotta G, Politi GG, Di Pino L, Castelli Z, Mangiafico RA, et al. Association of high alpha2-Heremans–Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195:110–5. doi: 10.1016/j.atherosclerosis.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 39.Pertosa G, Simone S, Ciccone M, Porreca S, Zaza G, Dalfino G, et al. Serum Fetuin A in hemodialysis: A link between derangement of calcium-phosphorus homeostasis and progression of atherosclerosis? Am J Kidney Dis. 2009;53:467–74. doi: 10.1053/j.ajkd.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 40.Szeberin Z, Fehérvári M, Krepuska M, Apor A, Rimely E, Sarkadi H, et al. Fetuin-A serum levels in patients with aortic aneurysms of Marfan syndrome and atherosclerosis. Eur J Clin Invest. 2011;41:176–82. doi: 10.1111/j.1365-2362.2010.02393.x. [DOI] [PubMed] [Google Scholar]
- 41.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–62. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 42.Tuttolomondo A, Di Raimondo D, Di Sciacca R, Casuccio A, Bivona G, Bellia C, et al. Fetuin-A and CD40 L plasma levels in acute ischemic stroke: Differences in relation to TOAST subtype and correlation with clinical and laboratory variables. Atherosclerosis. 2010;208:290–6. doi: 10.1016/j.atherosclerosis.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Emoto M, Mori K, Lee E, Kawano N, Yamazaki Y, Tsuchikura S, et al. Fetuin-A and atherosclerotic calcified plaque in patients with type 2 diabetes mellitus. Metabolism. 2010;59:873–8. doi: 10.1016/j.metabol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Kara M, Genc H, Dogru T, Bagci S. Comparison of the asymmetric dimethyl arginin levels and carotid intima media thickness with hemoglobin levels in patients with non-alcoholic fatty liver disease. Gulhane Med J. 2012;54:40–8. [Google Scholar]
- 45.Mori K, Emoto M, Araki T, Yokoyama H, Lee E, Teramura M, et al. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1248–52. doi: 10.1016/j.metabol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Maréchal C, Schlieper G, Nguyen P, Krüger T, Coche E, Robert A, et al. Serum fetuin-A levels are associated with vascular calcifications and predict cardiovascular events in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6:974–85. doi: 10.2215/CJN.06150710. [DOI] [PMC free article] [PubMed] [Google Scholar]


