Abstract
Background and Aims:
A meta-analysis was performed to explore the role of the D-dimer in the development of portal vein thrombosis (PVT) in liver cirrhosis.
Methods:
All papers were searched via PubMed, EMBASE, China National Knowledge Infrastructure, Wan Fang, and VIP databases. A standardized mean difference (SMD) with 95% confidence interval (CI) was pooled.
Results:
Overall, 284 studies were initially identified, of which 21 were included. Cirrhotic patients with PVT had a significantly higher D-dimer concentration than those without PVT (pooled SMD = 1.249, 95%CI = 0.740–1.758). After the portal hypertension-related surgery, cirrhotic patients with PVT had a similar preoperative D-dimer concentration to those without PVT (pooled SMD = 0.820, 95%CI = −0.122–0.286), but a higher postoperative value of D-dimer concentration than those without PVT (pooled SMD = 2.505, 95%CI = 0.975–4.036). Notably, the D-dimer concentration at the 1st postoperative day was similar between cirrhotic patients with and without PVT (pooled SMD = 0.137, 95%CI = −0.827–1.101), but that at the 7th post-operative day was higher in cirrhotic patients with PVT than in those without PVT (pooled SMD = 1.224, 95%CI = 0.277–2.171).
Conclusion:
D-dimer might be regarded as a diagnostic marker for PVT in liver cirrhosis. In addition, postoperative D-dimer testing is worthwhile for the diagnosis of PVT after portal hypertension-related surgery.
Keywords: Diagnosis, etiology, predict, portal hypertension
Portal vein thrombosis (PVT) is one of the severe complications of liver cirrhosis.[1,2,3] It is defined as the formation of a thrombus within the portal vein trunk and intrahepatic portal branches. The reported prevalence of PVT ranges from 0.6% to 26% in liver cirrhosis.[1,2,3,4,5,6,7,8] PVT deteriorates the liver dysfunction, increases the risk of bleeding, and influences the prognosis of patients with liver cirrhosis.[1,2,3] Development of PVT is associated with systemic prothrombotic factors and local risk factors.[9,10,11] Systemic risk factors include factor V Leiden mutation, G20210A prothrombin mutation, MTHFR C667T mutation, lupus anticoagulant, decreased level of proteins C and S and antithrombin III, and increased levels of factor VIII. Local factors include inflammatory lesions, pancreatitis, splenomegaly, a large splenic vein diameter, reduced portal flow velocity, and increased flow volume in the largest collateral vessel.[1,2,3,4,5,6,7,8] D-dimer level can reflect the fibrinolytic activity in humans, and is elevated in patients with deep vein thrombosis and pulmonary embolism due to the activation of fibrinolysis system. Recently, one study by Zhang et al. has also shown that D-dimer testing with a cutoff value of 0.24 mg/L has a high sensitivity of 100% and a negative predictive value of 100% in excluding a diagnosis of PVT.[12] But the specificity and predictive value are low (30.7% and 16.7%).[12] In addition, the association between D-dimer level and the presence of PVT was not supported by some studies.[13] Herein, we do a meta-analysis to detect the role of D-dimer in the development of PVT in liver cirrhosis.
MATERIALS AND METHODS
Search strategy
Studies were identified using a search strategy in PubMed database, EMBASE database, China National Knowledge Infrastructure, Wan Fang, and VIP databases. Search items were listed as follows: (“D-dimer”[All Fields]) AND (“liver cirrhosis” [All Fields]) AND (“portal vein thrombosis” [All Fields]). The last search was performed on July 20, 2014. When the same data were reported in more than one publication, only the studies with more complete data and more extensive interval of enrolment were included in the meta-analysis.
Data extraction
A data extraction sheet included authors, publication year, country where the study was conducted, period of enrolment, study design, inclusion and exclusion criteria, type of diseases (liver cirrhosis or cirrhotic portal hypertension after surgery), total sample size, demographic data (age and gender), Child–Pugh Score or Class, and number of patients with and without PVT.
Inclusion criteria
The participants of any age with diagnosis of liver cirrhosis
All observational studies, including cohort and case–control studies, regardless of the retrospective or prospective nature of the study
No publication date or publication status restrictions
No language restrictions
D-dimer levels were measured.
Exclusion criteria
Case reports
Animal studies
Reviews or comments on the significance of liver cirrhosis
Contents or indexes
Patients with hepatocellular carcinoma or other malignancy, cholestatic liver diseases, Budd–Chiari syndrome, pancreatitis, preoperative had thrombosis, the use of anticoagulation or antiplatelet drugs, and recent abdominal trauma
Noncomparative studies
Studies which did not report the D-dimer levels
Studies in which the data were not expressed as the mean value and standard deviation
Duplicate studies.
Evaluation of study quality
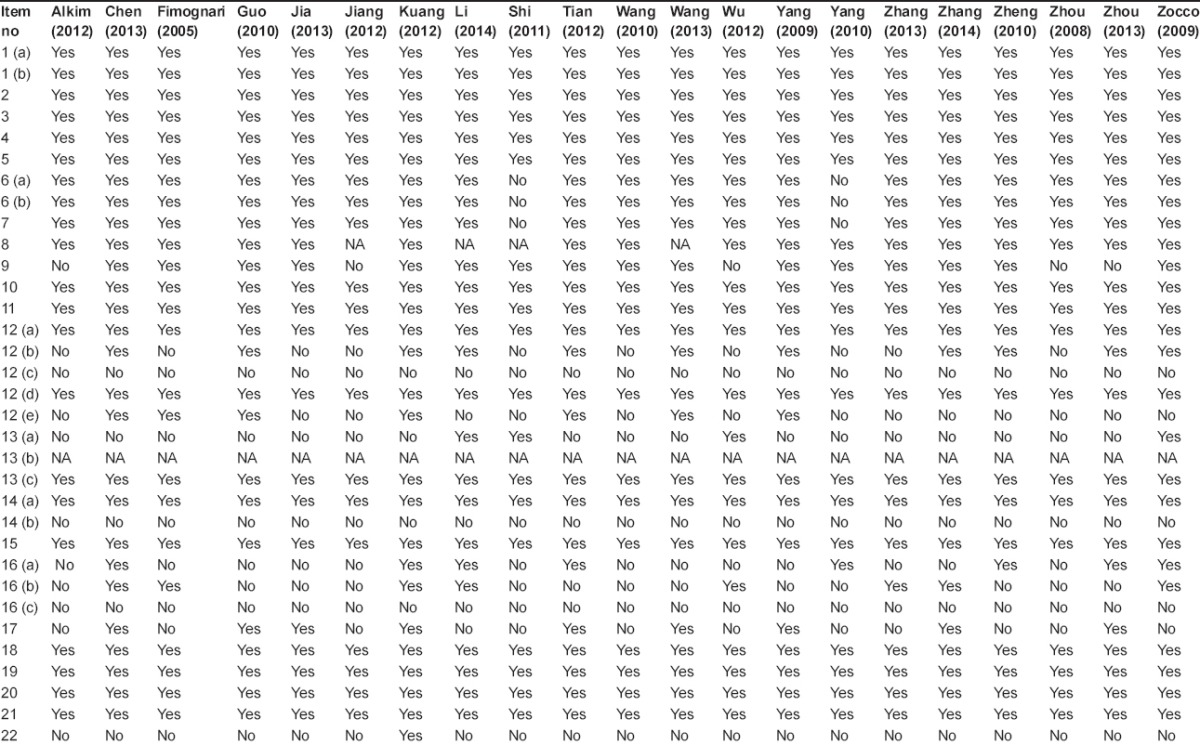
The quality of included studies was evaluated according to the STROBE statement checklists (www.strobe-statement.org). It included 22 items to evaluate the title and abstracts, introduction, methods, results, discussion, and funding [Supplementary Table 1].
Supplementary Table 1.
Study quality STROBE checklists

Data synthesis
The D-dimer levels were collected in cirrhotic patients with and without PVT. The difference of D-dimer levels between them was calculated as mean difference. A standardized mean difference (SMD) with 95% confidence interval (CI) was used to evaluate the association between D-dimer concentration and PVT in liver cirrhosis. A standardized value was employed, primarily because D-dimer level was measured by different units and methods in different studies. Then, the SMD of each study was combined to give a pooled SMD. An SMD >0 favored the effect of increased D-dimer concentration on PVT, and a P < 0.05 was considered statistically significant. Data were pooled using random-effect models. Heterogeneity between studies was assessed by using the I2 statistic (I2 > 50% was considered as having substantial heterogeneity) and the χ2 test (P < 0.10 was considered to represent statistically significant heterogeneity). The Egger test was performed to evaluate the presence of publication bias for all pooled values with 95%CI. All analyses were conducted using Stats Direct statistical software version 2.7.8 (Stats Direct Ltd, Sale, Cheshire, UK).
RESULTS
Description of the included studies
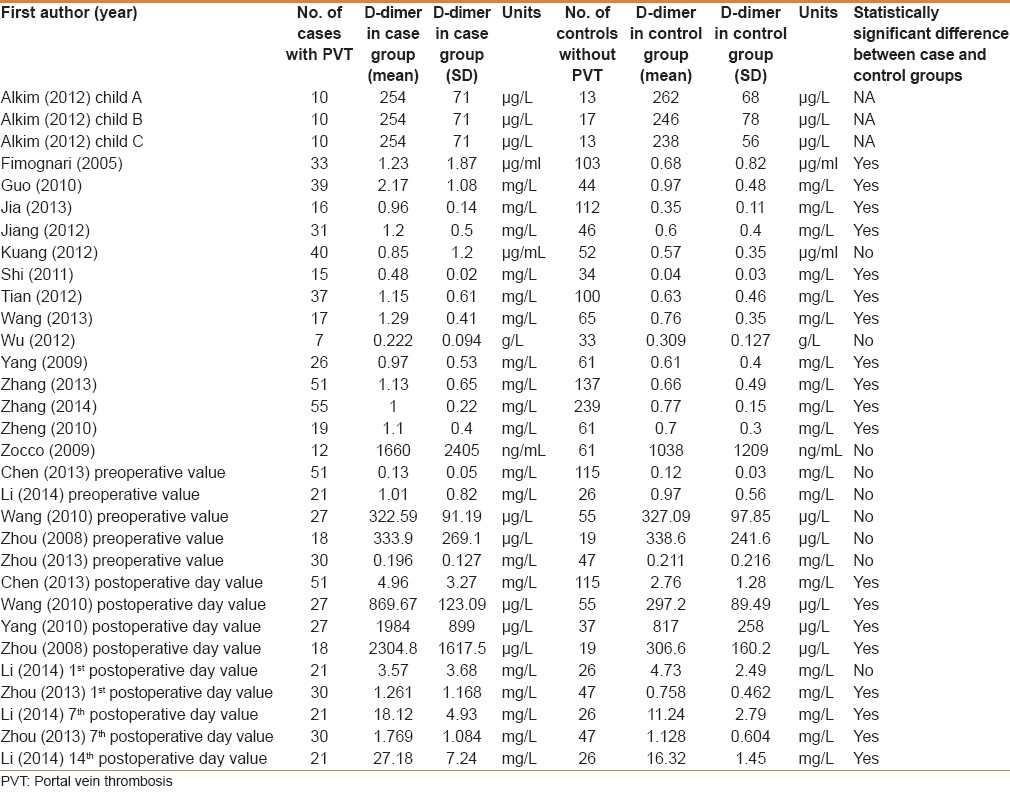
A total of 284 studies were initially identified by the search strategy. Among them, 21 studies involving 602 cirrhotic patients with PVT and 1490 cirrhotic patients without PVT were eligible in the meta-analyses [Figure 1].[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] Baseline characteristics are summarized in Table 1 and D-dimer concentration is summarized in Table 2. The quality of the included studies are demonstrated in Supplementary Table 2.
Figure 1.
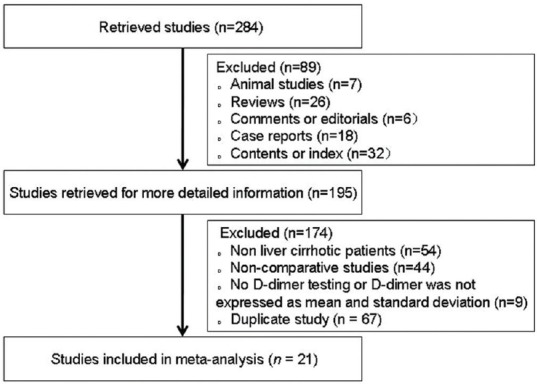
Study selection
Table 1.
Study characteristics: An overview
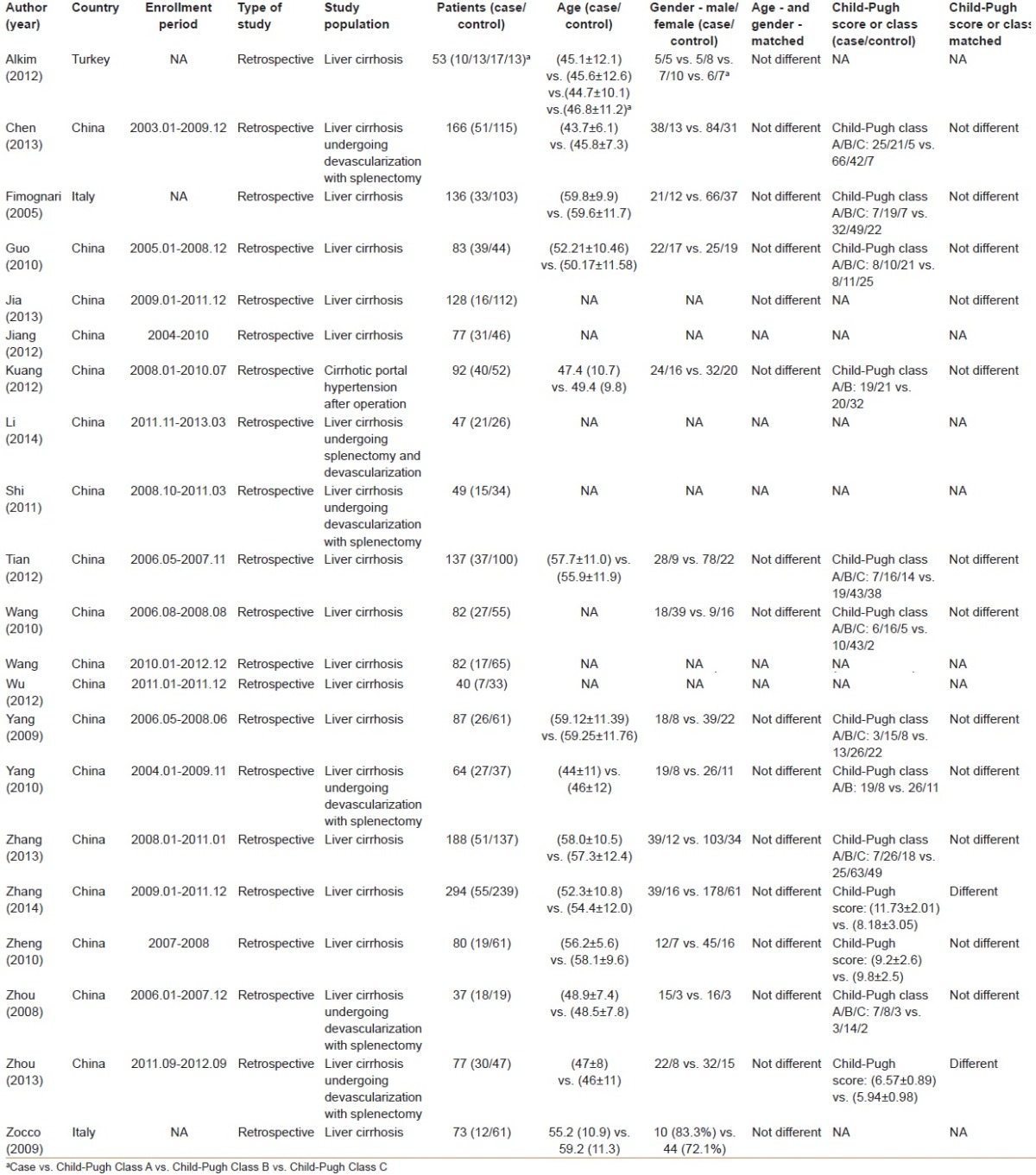
Table 2.
Comparison of D-dimer concentration between cirrhotic patients with and without PVT: An overview
Supplementary Table 2.
Results of study quality
Association between D-dimer concentrations and PVT in liver cirrhosis without surgery
D-dimer concentration between the PVT group and the non-PVT group was similar in three studies[13,19,25] and significantly different in 11 studies.[12,15,16,17,18,21,22,24,26,28,29] Additionally, the difference between the two groups was unclear in one study.[32] Using a random-effects model, a meta-analysis demonstrated that the pooled SMD was significant (1.249, 95%CI = 0.740–1.758, P < 0.0001), suggesting a higher D-dimer concentration in cirrhotic patients with PVT than in those without PVT [Figure 2]. The heterogeneity among studies was significant (I2 = 93.6%, P < 0.0001). Egger test implied no proof of publication bias (P = 0.205).
Figure 2.
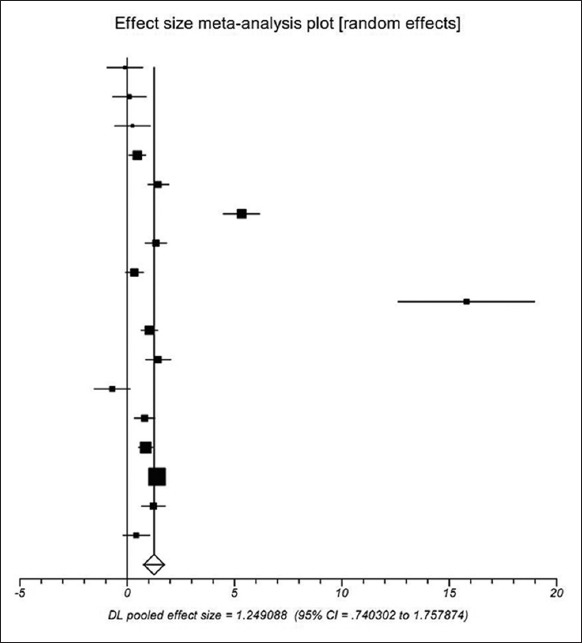
Meta-analysis regarding D-dimer concentration on portal vein thrombosis in liver cirrhosis without surgery
Association between preoperative D-dimer concentration and PVT after the surgery of cirrhotic portal hypertension
Preoperative D-dimer concentration between the PVT group and the non-PVT group was similar in all five studies.[14,20,23,30,31] Using a random-effects model, a meta-analysis demonstrated that the pooled SMD was not significant (0.820, 95%CI = −0.122–0.286, P = 0.431), showing that D-dimer concentration was similar between the PVT group and the non-PVT group [Figure 3]. The heterogeneity among studies was not significant (I2 = 0%, P = 0.721). Egger test implied no proof of publication bias (P = 0.179).
Figure 3.
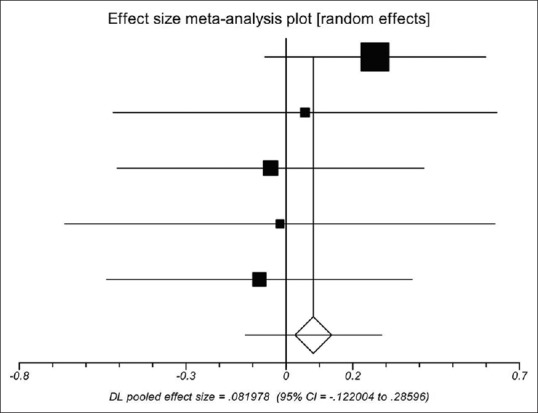
Meta-analysis regarding preoperative D-dimer concentration on portal vein thrombosis after the surgery of cirrhotic portal hypertension
Association between postoperative D-dimer concentration and PVT after the surgery of cirrhotic portal hypertension
Postoperative D-dimer concentration between the PVT group and the non-PVT group was significantly different in all four studies.[14,23,27,30] Using a random-effects model, a meta-analysis demonstrated that the pooled SMD was significant (2.505, 95%CI = 0.975–4.036, P = 0.0013), showing that D-dimer concentration was significantly higher in the PVT group than in the non-PVT group [Figure 4]. The heterogeneity among studies was significant (I2 = 96%, P < 0.0001). Egger test implied no proof of publication bias (P = 0.153).
Figure 4.
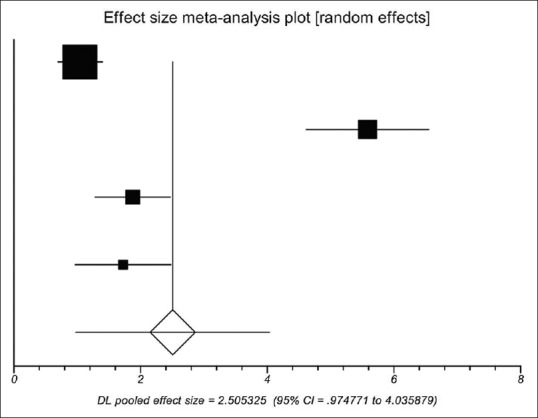
Meta-analysis regarding postoperative D-dimer concentration on portal vein thrombosis after the surgery of cirrhotic portal hypertension
Association between 1st postoperative D-dimer concentration and PVT after the surgery of cirrhotic portal hypertension
First postoperative D-dimer concentration between the PVT group and the non-PVT group was similar in one study[20] and significantly different in the other study.[31] Using a random-effects model, a meta-analysis demonstrated that the pooled SMD was not significant (0.137, 95%CI = −0.827–1.101, P = 0.781), showing that D-dimer concentration was similar between the PVT group and the non-PVT group [Figure 5]. The heterogeneity among studies was significant (P = 0.0096). Egger test could not evaluate the publication bias.
Figure 5.
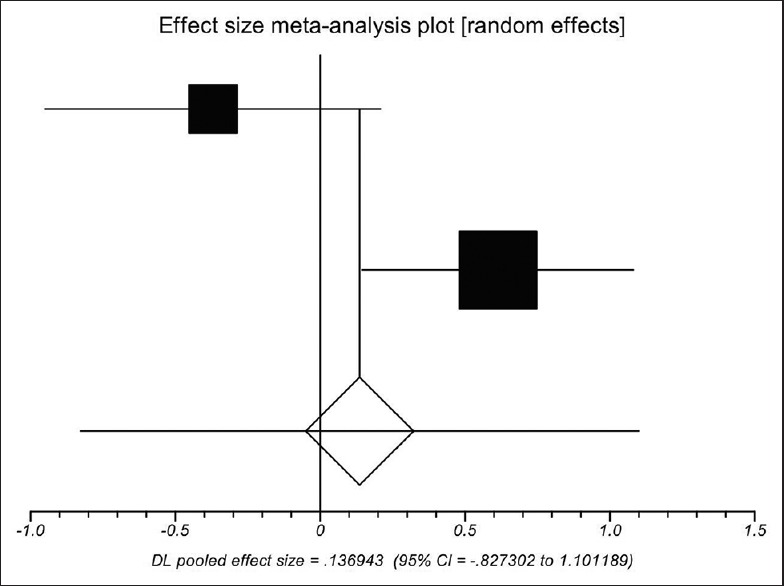
Meta-analysis regarding 1st postoperative D-dimer concentration on portal vein thrombosis after the surgery of cirrhotic portal hypertension
Association between 7th postoperative D-dimer concentration and PVT after the surgery of cirrhotic portal hypertension
Seventh postoperative D-dimer concentration between the PVT group and the non-PVT group was significantly different in the two studies.[20,31] Using a random-effects model, a meta-analysis demonstrated that the pooled SMD was significant (1.224, 95%CI = 0.277–2.171, P = 0.0113), suggesting a higher D-dimer concentration in cirrhotic patients with PVT than in those without PVT [Figure 6]. The heterogeneity among studies was significant (P = 0.0212). Egger test could not evaluate the publication bias.
Figure 6.
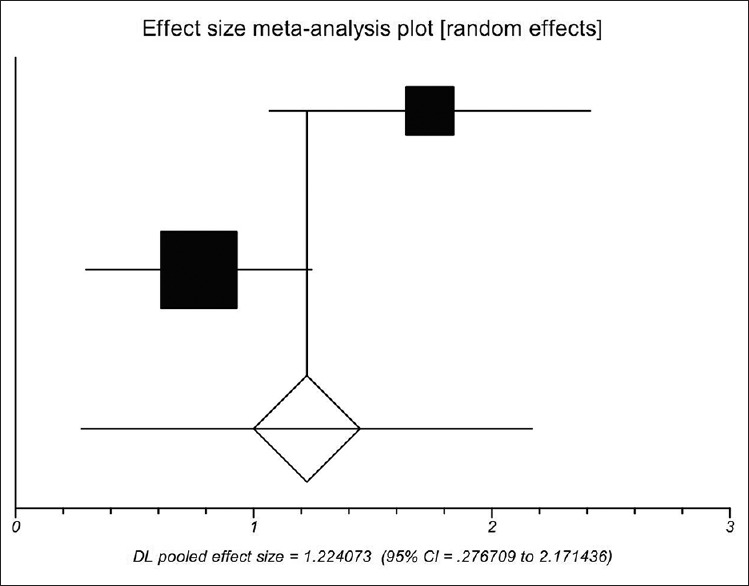
Meta-analysis regarding 7th postoperative D-dimer concentration on portal vein thrombosis after the surgery of cirrhotic portal hypertension
DISCUSSION
D-dimer is produced during the fibrinolysis. D-dimer testing is cheap and readily available, and importantly, it has a high negative predictive value and sensitivity for deep vein thrombosis and pulmonary embolism.[33] At present, it has been widely used for the diagnostic workup of suspected venous thromboembolism in clinical practice. Evidence suggests that a negative D-dimer in the combination with clinical probability tools can effectively rule out the diagnosis of deep vein thrombosis and pulmonary embolism, which reduce the need for ultrasound testing.[34,35] However, the role of D-dimer in the diagnosis of PVT in liver cirrhosis remained unclear. The present study for the first time systematically reviewed the currently available data to clarify the issue. The most important finding was that an increased D-dimer concentration was associated with a higher risk of PVT in liver cirrhosis in the absence of surgery, which potentially suggested that D-dimer should be a diagnostic marker for PVT in cirrhotic patients. However, the accurate cutoffs of D-dimer for predicting the development of PVT were largely lacking. In one included study, Zhang et al. calculated the area under curve (AUC) of D-dimer for the diagnosis of PVT.[28] They found that D-dimer had the largest AUC of 0.868, followed by Child–Pugh Score of 0.823, diameter of main portal vein of 0.810, and portal vein velocity of 0.756. Another included study also demonstrated that the AUC of D-dimer was 0.782 for the diagnosis of PVT in all cirrhotic patients, and the cutoff value was 0.52 mg/dL.[36] The subgroup analyses according to the Child–Pugh class further demonstrated that the AUC of D-dimer was 0.963, 0.75, and 0.698 in Child–Pugh class A, B, and C patients, respectively. The cutoff value was heterogeneous, such as 0.52, 0.56, 1.12 mg/dL in Child–Pugh Class A, B, and C patients, respectively.
Considering a high risk of PVT in cirrhotic patients undergoing the portal hypertension-related surgery, we analyzed the association between D-dimer concentration and PVT in such patients. Ideally, D-dimer should have an early predictive probability of developing PVT. However, we found that the postoperative value of D-dimer, but not preoperative value of D-dimer, could predict the development of PVT after surgery. In addition, the 7th postoperative value of D-dimer, but not 1st postoperative value of D-dimer, was associated with the development of PVT after surgery. Because PVT developed within the 7th postoperative days, these unexpected findings might suggest that the change in the D-dimer concentrations was accompanied by the occurrence of PVT after surgery; however, a simple D-dimer test could not provide any possibilities of early prediction of PVT.
The study had several limitations as follows. First, we could only establish the association between D-dimer and the development of PVT in liver cirrhosis. However, we did not identify the negative predictive value of D-dimer in ruling out PVT. Second, a clinical probability tool could further stratify the risk for the assessment of venous thromboembolism and pulmonary embolism, thereby increasing the negative predictive value of D-dimer tests.[34,37] However, no clinical probability tools were established for the risk of the assessment of PVT. Third, only a few studies explored the association between D-dimer and PVT after portal hypertension-related surgery, especially the 1st and 7th postoperative value of D-dimer. Thus, the conclusions needed to be confirmed. Fourth, a majority of the reviewed studies were of poor quality, which potentially influenced the reliability of these findings.
In conclusion, D-dimer might be regarded as a diagnostic marker of PVT in liver cirrhosis, but further work should identify the accurate cutoff value of D-dimer for excluding the diagnosis of PVT. In addition, postoperative D-dimer testing is worthwhile for the diagnosis of PVT after portal hypertension-related surgery in liver cirrhosis, but the timing of D-dimer testing after surgery should be further explored.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Congly SE, Lee SS. Portal vein thrombosis: Should anticoagulation be used? Curr Gastroenterol Rep. 2013;15:306–13. doi: 10.1007/s11894-012-0306-0. [DOI] [PubMed] [Google Scholar]
- 2.Ponziani FR, Zocco MA, Garcovich M, D’Aversa F, Roccarina D, Gasbarrini A. What we should know about portal vein thrombosis in cirrhotic patients: A changing perspective. World J Gastroenterol. 2012;18:5014–20. doi: 10.3748/wjg.v18.i36.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Castro KI, Simioni P, Burra P, Senzolo M. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int. 2012;1478:1465–76. doi: 10.1111/j.1478-3231.2012.02839.x. [DOI] [PubMed] [Google Scholar]
- 4.Kinjo N, Kawanaka H, Akahoshi T, Matsumoto Y, Kamori M, Nagao Y, et al. Portal vein thrombosis in liver cirrhosis. World J Hepatol. 2014;6:64–71. doi: 10.4254/wjh.v6.i2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh S, Shah R, Kapoor P. Portal Vein Thrombosis. Am J Med. 2010;123:111–8. doi: 10.1016/j.amjmed.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: Portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31:366–74. doi: 10.1111/j.1365-2036.2009.04182.x. [DOI] [PubMed] [Google Scholar]
- 7.Chawla Y, Duseja A, Dhiman RK. Review article: The modern management of portal vein thrombosis. Aliment Pharmacol Ther. 2009;30:881–94. doi: 10.1111/j.1365-2036.2009.04116.x. [DOI] [PubMed] [Google Scholar]
- 8.Qi XS, Han GH, Fan DM. Management of portal vein thrombosis in liver cirrhosis. Nat Rev Gastroenterol Hepatol. 2014;11:435–46. doi: 10.1038/nrgastro.2014.36. [DOI] [PubMed] [Google Scholar]
- 9.Qi X, Ren W, De Stefano V, Fan D. Associations of Coagulation Factor V Leiden and Prothrombin G20210A Mutations With Budd-Chiari Syndrome and Portal Vein Thrombosis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2014;12:1801–12e7. doi: 10.1016/j.cgh.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Qi X, Chen H, Han G. Effect of antithrombin, protein C and protein S on portal vein thrombosis in liver cirrhosis: A meta-analysis. Am J Med Sci. 2013;346:38–44. doi: 10.1097/MAJ.0b013e31826485fc. [DOI] [PubMed] [Google Scholar]
- 11.Qi X, Yang Z, De Stefano V, Fan D. Methylenetetrahydrofolate reductase C677T gene mutation and hyperhomocysteinemia in Budd-Chiari syndrome and portal vein thrombosis: A systematic review and meta-analysis of observational studies. Hepatol Res. 2014;44:E480–98. doi: 10.1111/hepr.12348. [DOI] [PubMed] [Google Scholar]
- 12.Zhang DL, Hao JY, Yang N. Value of D-dimer and protein S for diagnosis of portal vein thrombosis in patients with liver cirrhosis. J Int Med Res. 2013;41:664–72. doi: 10.1177/0300060513483413. [DOI] [PubMed] [Google Scholar]
- 13.Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, et al. Thrombotic risk factors in patients with liver cirrhosis: Correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–9. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Chen GJ. Portal venous system thrombosis in patients with cirrhotic portal hypertension after surgery: Investigation of risk factors and evaluation of early prevention (Article in Chinese) Zhong Guo Ren Min Jie Fang Jun Zong Yi Yuan. 2013 (Postgraduate dissertation) [Google Scholar]
- 15.Fimognari FL, De Santis A, Piccheri C, Moscatelli R, Gigliotti F, Vestri A, et al. Evaluation of D-dimer and factor VIII in cirrhotic patients with asymptomatic portal venous thrombosis. J Lab Clin Med. 2005;146:238–43. doi: 10.1016/j.lab.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Guo YY. The clinical value of D-dimer in cirrhotic patients with the portal venous thrombosis (Article in Chinese) J Clin Hepatol. 2010;26:304–5. [Google Scholar]
- 17.Jia YR, Ge XS, Zhang SF, Liu XL, Wang HC, Zhang GL. Gan ying hua bing men jing mai xue shuan huan zhe xue jiang xian wei dan bai yuan ji D-dimer shui ping bian hua (Article in Chinese) J Chin Pract Diagn Ther. 2013;27:477–8. [Google Scholar]
- 18.Jiang JL, Zhan YT, Li L. The plasma D-dimer levels and t heir clinical significance in liver cirrhosis patients with portal vein thrombosis (Article in Chinese) J Clin Hepatol. 2012;15:26–8. [Google Scholar]
- 19.Kuang J, Yang WP, Chen H, Peng CH, Li HW. Risk factors for portal venous thrombosis after operation of portal hypertension (Article in Chinese) J Surg Concepts Pract. 2012;17:634–8. [Google Scholar]
- 20.Li DW. Significance of D-dimer in predicting portal vein thrombosis after laparoscopic splenectomy and esophagogastric devascularization (Article in Chinese) Chin J Gen Surg. 2014;23:207–11. [Google Scholar]
- 21.Shi DQ, Jiang DQ, Cao Y, Huang JC, Ke QG. Effect of peri-esophagogastric devascularization with splenectomy on portal venous haemorheology in patients with portal hypertension (Article in Chinese) Chin J Gen Pract. 2011;9:854–6. [Google Scholar]
- 22.Tian Y, Chen WM, Zhang DL, Yang N. Multivariate analysis of the portal vein thrombosis in patients with liver cirrhosis (Article in Chinese) Chin J Postgrad Med. 2012;35:21–4. [Google Scholar]
- 23.Wang L, Liu GJ, Chen YX, Dong HP, Zhang YQ, Wang LX. Combined use of D-dimer and P-selectin for the diagnosis of splenic or portal vein thrombosis following splenectomy. Thromb Res. 2010;125:e206–e9. doi: 10.1016/j.thromres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Chen WY, Lan M. Clinical significance of plasma soluble thrombomodulin and D-dimer detection in cirrhosis patients (Article in Chinese) Chin J Integr Trad West Med Dig. 2013;21:625–7. [Google Scholar]
- 25.Wu TT, Chen W, Wu ZY. Risk factors of portal venous thrombosis development in patients with portal hypertension (Article in Chinese) J Surg Concepts Pract. 2012;17:629–33. [Google Scholar]
- 26.Yang N, Zhang DL. A study of coagulation and fibrinolytic system changes in liver cirrhosis patients with and without portal vein thrombosis (Article in Chinese) Chin J Postgrad Med. 2009;32:11–3. [Google Scholar]
- 27.Yang W, Hu YQ, Mo RX. Portal vein thrombosis developed in cirrhotic portal hypertensive patients after spleenectomy and portaazygous devascularization (Article in Chinese) Chin J Gen Surg. 2010;25:710–2. [Google Scholar]
- 28.Zhang L, Wang L, Yang GM. Analysis of risk factors of portal vein thrombosis in liver cirrhosis (Article in Chinese) Chin J Dig. 2014;34:100–4. [Google Scholar]
- 29.Zheng S. Analysis of the risk factors of portal vein thrombosis in liver cirrhosis patients. Hepatol Int. 2010;4:256. [Google Scholar]
- 30.Zhou H. Transformation of D-dimer after devascularization operation, that forming of portal vein thrombus. the meaning of nonage prognosticate in clinic (Article in Chinese) Ji Lin Da Xue. 2008 (university thesis without pages and volumes) [Google Scholar]
- 31.Zhou J, Li XM, Chen HM. Men jing mai gao ya zheng pi qie chu ben men zhou wei xue guan li duan shu hou men jing mai xue shuan xing cheng de xiang guan yin su fen xi (Article in Chinese) Shi Yong Yi Xue Za Zhi. 2013;29:581–3. [Google Scholar]
- 32.Alkim H, Ayaz S, Sasmaz N, Oguz P, Sahin B. Hemostatic abnormalities in cirrhosis and tumor-related portal vein thrombosis. Clin Appl Thromb Hemost. 2012;18:409–15. doi: 10.1177/1076029611427900. [DOI] [PubMed] [Google Scholar]
- 33.DiNiSio M, Squizzato A, Rutjes AW, Bu¨ller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: A systematic review. J Thromb Haemost. 2007;5:296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 34.Fancher TL, White RH, Kravitz RL. Combined use of rapid d-dimer testing and estimation of clinical probability in the diagnosis of deep vein thrombosis: Systematic review. BMJ. 2004;329:821. doi: 10.1136/bmj.38226.719803.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Belle A, Buller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172–9. doi: 10.1001/jama.295.2.172. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol. 2010;25:116–21. doi: 10.1111/j.1440-1746.2009.05921.x. [DOI] [PubMed] [Google Scholar]
- 37.Ghanima W, Almaas V, Aballi S, Dorje C, Nielssen BE, Holmen LO, et al. Management of suspected pulmonary embolism (PE) by D-dimer and multi-slice computed tomography in outpatients: An outcome study. J Thromb Haemost. 2005;3:1926–32. doi: 10.1111/j.1538-7836.2005.01544.x. [DOI] [PubMed] [Google Scholar]


