Abstract
Objective:
To evaluate the antimicrobial activity of different concentrations of Matricaria chamomilla and Chlorhexidine gel against Candida albicans and Enterococcus faecalis.
Materials and Methods:
The agar diffusion test was used to evaluate the antimicrobial activity of 15%, 25% Matricaria chamomilla in aq. base and 2% chlorhexidine gel against C. albicans (ATCC 24433) and E. faecalis (ATCC 24212) strains. Vancomycin was used as the positive control for E. faecalis and fluconazole for C. albicans . The agar plates were incubated at 37°C for 48 h after which the zone of inhibition were measured separately for each material. Data thus obtained were statistically analyzed using the Wilcoxon rank–order test.
Results:
2% chlorhexidine showed maximum inhibitory zone for C. albicans (33.26 mm) and E. faecalis (24.54 mm). 25% Matricaria showed zones of 24.16 mm and 20.62 mm for C. albicans and E. faecalis, respectively. 15% Matricaria did not show any antimicrobial activity (0 mm).
Conclusion:
The results of the current in vitro study suggest that 25% Matricaria can be used as an antimicrobial agent, but it is less effective than 2% chlorhexidine gluconate gel against C. albicans and E. faecalis. Matricaria at a lesser concentration of 15% aq. base is ineffective against both the microorganisms.
Keywords: Agar diffusion test, Candida albicans, chlorhexidine, Enterococcus faecalis, Matricaria chamomilla
INTRODUCTION
Debridement of root canal space is essential for the success of endodontic treatment. The microbes most commonly found in failed endodontic treatment cases are Enterococcus faecalis and Candida albicans.[1,2]
E. faecalis has demonstrated a high resistance[3] and ability to inactivate antimicrobial agents,[4] survival capacity in harsh environments, with scarce nutrient supply and extreme alkaline pH,[5] and the capacity for growth as a biofilm on root canal walls.[6]
There are evidences that indicate the presence of fungi in the root canal system. Fungi, especially C. albicans, have been demonstrated in the pulp space and periapical area through light electron microscopy and culture techniques, where they have been associated with persistent infections.[7,8] C. albicans releases collagenolytic enzymes that make it possible to use dentin as a nutrient source.[9]
According to various studies, both C. albicans and E. faecalis are resistant to the antimicrobial action of calcium hydroxide, a commonly used intracanal medicament, but are sensitive to the antimicrobial action of chlorhexidine gluconate (CHX).[10,11] To achieve long-term substantive antimicrobial effects, the infected root dentin must be exposed to CHX for a longer time than that afforded by irrigation.[12]
Antibiotics are most commonly used for eradicating infectious disease. But, nowadays, some microbial infectious agents have become resistant against relevant antibiotics as a result of irregular use of drugs by people, which results in failure of antibiotic therapy.[13,14] However, man has found that the therapeutic effects of herbal extracts, unlike many chemical drugs, have no side-effects.[15,16] Some commonly used herbs in modern medicine are Glycyrrhiza glabra, Commiphora mukul, Plantago ovata, Aloe barbadensis and Azadirachta indica. The plants Glycyrrhiza glabra, Piper longum, Adhatoda vasica, Withania somnifera, Cyperus rotundus, Tinospora cordifolia, Berberis aristata, Tribulus terristris, Holarrhena antidysenterica and Boerhavia diffusa have been used in 52–141 herbal formulations and triphala (Terminalia chebula, Terminalia belerica and Embelica officinalis) alone has been used in 219 formulations.[17] Among these plants, Matricaria chamomilla can be mentioned for its remedial features.
Chamomile is a widely recognized herb in Western culture. It is a common ingredient today in herbal teas because of its calming, carminative and spasmolytic properties. It is also a popular ingredient in topical health and beauty products for its soothing and anti-inflammatory effects on skin.[18,19] It also has a role in the treatment of recurrent apthous stomatitis, mucositis and oral ulcers.[20] The aim of this in vitro study is to comparatively evaluate the antibacterial and antifungal activities of 15%, 25% Matricaria chamomilla in aq. base and 2% chlorhexidine gel against C. albicans and E. faecalis.
MATERIALS AND METHODS
The materials evaluated for antimicrobial activity were 15%, 25% Matricaria chamomilla in aq. base and 2% CHX gel (Endogel; Itapetininga, SP, Brazil) against C. albicans (ATCC 24433) and E. faecalis (ATCC 24212) strains. Vancomycin (antibacterial) was used as a positive control for E. faecalis and fluconazole (antifungal) was used for C. albicans . Plant extracts of chamomile were obtained from the National Botanical and Research Institute, Lucknow.
Aqueous extraction (cold water)
The method of Al-Magboul et al., as modified by Okıgbo and Omodamıro, was used.[21,22] The extract was filtered with sterile filter paper (labline filter paper) inserted in a funnel and the filtrate was evaporated in a water bath at 100°C to dryness. The standard extracts obtained were stored in a refrigerator at 4°C until required for use.
Aqueous extraction (hot water)
Fifteen and 25 g of the weighed plant material were soaked in 100 mL of hot water boiled for 30 min in a conical flask for 24 h. The solution was filtered using filter paper and evaporated.
Plant extract disc preparation
The plant extract discs were prepared from labline filter paper by punching with a cork borer of 6 mm diameter. Discs with concentration of 1.5 mg (15%), 2.5 mg (25%) were prepared for M. chamomilla. The discs were autoclaved at 121°C for 15 min. The plant extract discs were dried in an oven and stored in a refrigerator until required for use.
Agar diffusion test
C. albicans (ATCC 24433) and E. faecalis (ATCC 24212) strains were cultured on Sabouraud's dextrose agar (Difco; BD Diagnostic Systems, Denmark, US) and blood agar, respectively. The organisms were incubated under aerobic condition. The agar plates were prepared in sterile glass Petri dishes and kept overnight for sterility at 37°C. After ensuring sterility, inoculae of the strains were prepared with sterile saline and the turbidity was compared using McFarland's turbidity standard tube No. 0.5. This results in 1-2 x 108 CFU/mL of E. faecalis and 1-5 x 106 CFU/mL of C. albicans . These inoculae were used to make the lawn culture of the organism using sterile cotton swabs on Sabouraud's agar and blood agar. Wells of 4 mm deep and 6 mm wide in diameter were then punched in the agar plates with a sterile punch for 2% chlorhexidine. The wells in the plates were filled with 2% CHX gel. The plant extract discs were placed in the cultured plates using a sterile forceps. The discs were placed far from each other to avoid overlap of zone of inhibition.
Sensitivity to all these drugs was seen on the Muller–Hilton Agar (MHA) (Oxoid, Cambridge, UK) plate for E. faecalis and MHA with 2% glucose and 0.5 μg methylene blue for C. albicans . Media were lawn cultured with the respective organism. The agar plates were then incubated at 37°C for 24 h, after which time the zone of inhibition was measured using a plastic ruler and was recorded for each material. The results thus obtained were statistically analyzed using the Wilcoxon rank–order test.
RESULTS
The mean values of microbial growth inhibition produced by M. chamomilla and CHX gel are shown in Tables 1 and 2. All the test groups were statistically different (P < 0.005) for both organisms tested.
Table 1.
Antifungal activities of materials used against C. albicans
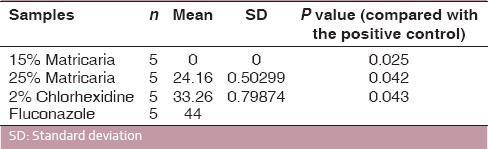
Table 2.
Antibacterial activities of materials used against E. faecalis
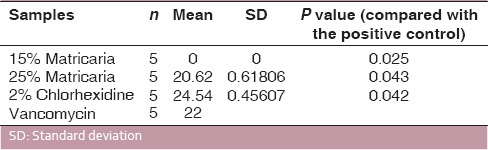
2% CHX gel showed the strongest antimicrobial action, producing the largest zones of inhibition, followed by 25% Matricaria, and 15% Matricaria did not show any antimicrobial activity [Figure 1].
Figure 1.
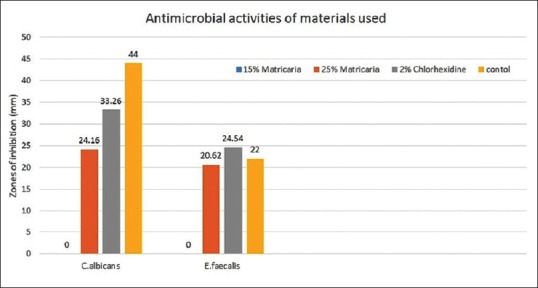
Antimicrobial activities of 15% Matricaria, 25% Matricaria, 2% chlorhexidine and control (fluconazole for C. albicans and vancomycin for E. faecalis) against C. albicans and E. faecalis
DISCUSSION
The tested microorganisms were selected because they represent bacteria and fungus commonly isolated from necrotic canals. E. faecalis is associated with persistent apical inflammation and, in clinical situations, it is difficult to eliminate from the root canal system, and therefore selected as a test organism in this study.[23,24] C. albicans is more often isolated from infected root canals and is one of the common microorganisms that survive chemical-mechanical procedures and the application of root canal medicaments.[10,11] The agar diffusion method was used to test antimicrobial activity because of its simplicity and rapidity. The advantage of this method is that it allows direct comparison of materials against the organism, indicating the potential of the test material to eliminate bacteria in the local microenvironment of the root canal system. However, the disadvantage of this method is that the result does not only depend on the toxicity of material for a particular organism but is also highly influenced by the ability of the material to diffuse across the medium.[25,26]
Chlorhexidine in gel formulation was chosen for this study because of its low toxicity on the periapical tissues, solubility in water as well as viscosity that keeps the active agent in contact with the root canal walls and dentinal tubules.[27,28] The antimicrobial property of CHX is due to its permeability to the cell wall or outer membrane of bacteria and ability to attack the bacterial cytoplasmic membrane and inner plasma membrane of the yeast. In high concentrations, CHX causes coagulation of intracellular components.[29] This could be the reason for its strong antimicrobial action against microbes.
German chamomile is one of the oldest favorites among garden herbs, and its reputation as a medicinal plant shows little signs of abatement. It is especially suitable for children with teething problems and in those who have been in a highly emotional state over a long period of time.[30] The herb kills certain bacteria and can be used as a mouth wash for dental abscesses and tonsillitis;[31] it is excellent in treating any type of inflammation, whether internal or external.[32]
While for German Chamomile extract, the antimicrobial effects are primarily the result of the active components α-bisabolol and azulenes, which have an anti-inflammatory activity.[33] This activity has been demonstrated not only by long empirical use but also by a number of different laboratory models.[34] More than 120 chemical constituents have been identified in the chamomile flower as secondary metabolites,[35,36] including 28 terpenoids, 36 flavonoids[37,38,39] and 52 additional compounds with potential pharmacological activity.[40] Components such as α-bisabolol and cyclic ethers are antimicrobial,[41,42] umbelliferone is fungistatic, whereas chamazulene and α-bisabolol are antiseptic.[43] The chamomile was found to have the most effective antileishmanial activity.[42] But, the total anti-inflammatory effect of whole chamomile depends on the presence of flavonoids such as apignine and luteoline.[44,45]
Although chamomile oil, at a concentration of 25 mg/mL, demonstrated antibacterial activity against Gram positive bacteria such as Bacillus subtilis, Staphylococcus aureus, Streptococcus mutans and Streptococcus salivarius in previous studies performed by Berry et al., 1995 and Cinco et al., 1972.[16,46,47] It showed no microbial action against E. faecalis and C. albicans at the concentration of 150 mg/mL in the present study.[48] However, at a higher concentration of 250 mg/mL, it is effective against both microorganisms, but the efficacy is not more than 2% CHX. These concentrations were used to determine the effect of the commercial preparations available in the market for dental uses.
A natural substance MC was compared with a synthetic substance (CHX) because of the known side-effects of CHX like brown discolorations of teeth, some restorative materials and the dorsum of tongue, taste alterations, oral mucosa erosion, unilateral or bilateral parotid swelling (extremely rare occurrence), enhanced supragingival calculus and bitter taste,[49] whereas herbal medicines are free from side-effects, easy to obtain, considered healthy and easily accepted by the host and have a lot of useful pharmacological actions comparable to synthetic drugs.[44]
A herbal approach MC can be used as an alternate to drugs available as antimicrobial agents for dental uses if used in proper concentrations, although CHX still represents a satisfactory result against microbes used in the study even at lower concentrations. However, further studies using the same medicament in failed root canal cases in vivo have to be conducted.
CONCLUSION
The results of the current in vitro study suggest that 25% Matricaria is less effective than 2% CHX gel against C. albicans and E. faecalis. Matricaria at a lower concentration of 15% aq. base is not effective. Therefore, it can be concluded that Matricaria can be used as an antimicrobial agent in various fields of dentistry also because of its advantage of being a natural substance, free from side-effects, easy to obtain, considered healthy and acceptable host response.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Nair PN, Sjogren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy resistant periapical lesions: A long term light and electron microscopic follow up study. J Endod. 1990;16:580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner JC, Watts CM, Xia T. Occurance of Candida albicans in infections of endodontic origin. J Endod. 2000;26:695–8. doi: 10.1097/00004770-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Sedgley CM, Lennan SL, Clewell DB. Prevalence, phenotype and genotype of oral Enterococci. Oral Microbiol Immunol. 2004;19:95–101. doi: 10.1111/j.0902-0055.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- 4.Portenier I, Haapasalo H, Orstavik D, Yamauchi M, Haapasalo M. Inactivation of the antibacterial activity of iodine, potassium iodide and chlorhexidine digluconate against Enterococcus faecalis by dentin, dentin matrix, type-I collagen, and heat-killed microbial whole cells. J Endod. 2002;28:634–7. doi: 10.1097/00004770-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 6.Sedgley C, Nagel A, Dahlén G, Reit C, Molander A. Real-time quantitative polymerase chain reaction and culture analyses of Enterococcus faecalis in root canals. J Endod. 2006;32:173–7. doi: 10.1016/j.joen.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Sen BH, Piskin B, Dimirci T. Observations of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995;11:6–9. doi: 10.1111/j.1600-9657.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 8.Waltimo TM, Siren EK, Orstavik D, Haapasalo MP. Susceptibility of oral candida species to calcium hydroxide in vitro. Int Endod J. 1999;32:94–8. doi: 10.1046/j.1365-2591.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 9.Hagihara Y, Kaminishi H, Cho T, Tanaka M, Kaita H. Degradation of human dentin collagen by an enzyme produced by the yeasts Candida albicans. Arch Oral Biol. 1988;33:617–9. doi: 10.1016/0003-9969(88)90138-0. [DOI] [PubMed] [Google Scholar]
- 10.White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997;23:29–31. doi: 10.1016/S0099-2399(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 11.Heling I, Sommer M, Steinberg D, Friedman M, Sela MN. Microbiological evaluation of the efficacy of chlorhexidine in a sustained release device for dentin sterilization. Int Endod J. 1992;25:15–9. doi: 10.1111/j.1365-2591.1992.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 12.Heling I, Steinberg D, Kenig S, Gavrilovich I, Sela MN, Friedman M. Efficacy of a sustained-release device containing chlorhexidine and calcium hydroxide in preventing secondary infection of dentinal tubules. Int Endod J. 1992;25:20–4. doi: 10.1111/j.1365-2591.1992.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 13.Cawan MM. Plant products as antimicrobial agents. Clin Microbial Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayfetr D, Turgay O. Antimicrobial activities of various medicinal and commercial plants extract. Turk J Biol. 2003;27:157–62. [Google Scholar]
- 15.Mohammad A, Bano Faruqi F, Mustafa J. Edible compounds as antitumor agents. Indian J Sci Technol. 2009;2:62–74. [Google Scholar]
- 16.Carmen C, Reyes A, Rafael G. Beneficial effects of green tea-a review. J AM Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 17.Kamboj VP. Herbal medicines. Curr Sci. 2000;78:35–9. [Google Scholar]
- 18.Talaeipour AR, Sakhtari S, Hadad P. Chamomilla mouthrinse effects on mucositis reduction after radiotherapy. J Dent Tehran Univ Med Sci. 2000;13:8. [Google Scholar]
- 19.Shahraz S, Ghaziani T. 1st ed. Tehran: Iran pharma; 2002. A comprehensive textbook of drug information; pp. 752–3. [Google Scholar]
- 20.Sahba S, Alipour M. Evaluation of the Effects of Chamomill Mouthrinse on Recurrent Aphthous Stomatitis. J Dent Tehran Univ Med Sci. 2005;2:147–51. [Google Scholar]
- 21.Al-Magboul AZ, Bashir AK, Khalid SA, Farouk A. Antihepatotoxic and Antimicrobial Activities of Harunyana madagascariensis Leaf Extracts. Intern J Pharmacogn. 1997;33:129–34. [Google Scholar]
- 22.Okigbo RN, Omodamiro OD. Antimicrobial Effects of Leaf Extracts of Piegon Pea (Cajanus cajan (L.) Millsp.) on Some Human Pathogens. J Herbs Spices Med Plants. 2006;12:117–27. [Google Scholar]
- 23.Spangberg LS, Haapasalo M. Rationale and efficacy of root canal medicaments and root filling materials with emphasis on treatment outcome. Endod Topics. 2002;2:35–58. [Google Scholar]
- 24.Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–34. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 25.Gomes BP, Pedroso JA, Jacinto RC, Vianna ME, Ferraz CC, Zaia AA, et al. In vitro evaluation of antimicrobial activity of five root canal sealers. Braz Dent J. 2004;15:30–5. doi: 10.1590/s0103-64402004000100006. [DOI] [PubMed] [Google Scholar]
- 26.Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CC, Souza Filho FJ. In vitro evaluation of antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:544–50. doi: 10.1016/j.tripleo.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, de Souza Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001;27:452–5. doi: 10.1097/00004770-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FR, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–9. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bown D. London: Dorling Kindersley; 1995. Encyclopedia of herbrs and their uses. ISBN 07513-020-31. [Google Scholar]
- 31.Carl W, Emrich LS. Management of oral mucositis during local radiation and systemic chemotherapy: A study of 98 patients. J Prosthet Dent. 1991;66:361–9. doi: 10.1016/0022-3913(91)90264-w. [DOI] [PubMed] [Google Scholar]
- 32.Abebe W. Herbal medication: Potential for adverse interactions with analgesic drug. J Clin Pharm Therapeut. 2002;27:391–401. doi: 10.1046/j.1365-2710.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 33.Barene I, Daberte I, Zvirgzdina L, Iriste V. The complex technology on products of chamomile. Medicina (Kaunas) 2003;39:127–31. [PubMed] [Google Scholar]
- 34.Budzinski JW, Foster BC, Vandenhoek S, Amason JT. An in vitro evaluation of human cytochrome P4503A4 inhibition by selected commercial herbal extracts and tinctures. Phytomed. 2000;7:273–82. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 35.Pino JA, Bayat F, Marbot R, Aguero J. Essential oil of Chamomilla recutita (L.) Rausch. From Iran. J Essent Oil Res. 2002;14:407–8. [Google Scholar]
- 36.Pirzad A, Alyari MR, Shaliba S, Zehtab-Salmasi, Moammadi A. Essential oil content and composition of German chamomile (Matricaria chamomilla L.) at different irrigation regimes. J Agron. 2006;5:451–5. [Google Scholar]
- 37.Exner J, Reichling J, Becker H. Flavonoid in Matricaria chamomile. Planta Med. 1980;39:219–30. [Google Scholar]
- 38.Kunde R, Isaac O. On the flavones of chamomile (Matricaria chamomilla L.) and a new acetylated apigenin-7-glucoside. Planta Med. 1980;37:124–30. [Google Scholar]
- 39.Isaac O. Therapy with chamomile-experience and verification. Disch Apoth Ztg. 1980;120:567–70. [Google Scholar]
- 40.Manday E, Szoke E, Muskath Z, Lemberkovics E. A study of the production of essential oils in chamomile hairy root cultures. Eur J Drug Metab Pharmacokinet. 1999;24:303–8. doi: 10.1007/BF03190037. [DOI] [PubMed] [Google Scholar]
- 41.Duke JA. 1st ed. Boca Raton: CRC Press; 1985. CRC handbook of medicinal herbs; pp. 297–414. [Google Scholar]
- 42.Shnitzler AC, Nolan LL, Labbe R. Screening of medicinal plants for antileishmanial and antimicrobial activity. Acta Hortic. 1996;426:235–42. [Google Scholar]
- 43.Tyihak E, Sarkany-Kiss J, Verzar-Petri G. Phytochemical investigation of apigenin glycosides of Matricaria chamomilla. Pharmazie. 1962;17:301–4. [Google Scholar]
- 44.Singh O, Khanam Z, Srivastava MK. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011;5:82–95. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eswar KA, Karunakar T, Naresh K, Rohan, Sriram N. Role of flavonoids in stress disorders. Int J Pharm Pract Drug Res. 2013;3:56–62. [Google Scholar]
- 46.Berry M. The chamomiles. Pharm J. 1995;254:191–3. [Google Scholar]
- 47.Cinco M, Banfi E, Tubaro A, Loggia D. A microbiological survey on the activity of a hydroalcoholic extract of camomile. Int J Drug Res. 1983;21:145–51. [Google Scholar]
- 48.Bayoub K, Baibai T, Mountassif D, Retmane A, Soukri A. Antibacterial activities of the crude ethanol extracts of medicinal plants against Listeria monocytogenes and some other pathogenic strains. Af J Biotechnol. 2010;9:4251–8. [Google Scholar]
- 49.Gupta R, Chandavarkar V, Galgali RS, Mishra M. Chlorhexidine, A Medicine for all the Oral Diseases. Glob J Med Pub Health. 2012;1:43–4. [Google Scholar]


