Abstract
Objective:
A two-way relationship between diabetes and periodontal disease has been suggested; whereas obesity and impaired lipid profile are risk factors for type-2 diabetes mellitus. This study examined the relationship between lipid profile, oral glucose tolerance test (OGTT) with periodontal health/disease dependent variables in healthy, diabetic and impaired glucose tolerance subjects.
Materials and Methods:
120 patients were selected for the study and were determined to be periodontally healthy or diseased. All these patients underwent biochemical tests for OGTT and Lipid profile analysis and data was compared using Z-test.
Results:
The OGTT results deteriorated with deteriorating periodontal condition. A similar correlation was also observed between worsening lipid profile test values, OGTT score, and periodontal condition.
Conclusion:
This study indicates that hyperlipidemia may be one of the factors associated with periodontitis and that periodontitis may itself lead to abnormal serum lipid levels. Therefore, in addition to effects on diabetes, periodontitis may contribute to elevated serum lipid levels and therefore potentially to systemic disease arising from chronic hyperlipidemia.
Keywords: Oral glucose tolerance test, diabetes mellitus, high-density lipoprotein cholesterol, impaired glucose tolerance, low-density lipoprotein cholesterol, normal glucose tolerance, periodontitis (inflammatory), total serum cholesterol, triglycerides
INTRODUCTION
Advanced chronic periodontitis often coexists with poorly controlled diabetes, such that diabetes is considered to be a risk factor for periodontitis.[1,2] Many studies conducted among adults have reported a significant positive two-way association between diabetes (both type-1 and type-2) and periodontal disease that is, diabetes increases risk of developing periodontitis and worsens preexisting periodontitis and periodontitis may raise the risk of diabetes.[3,4,5,6,7] Periodontal infection can increase systemic inflammation, which in turn may induce a chronic state of insulin resistance, contributing to the cycle of hyperglycemia and advanced glycation end product-protein binding accumulation. Therefore, it can amplify the classical pathway of connective tissue degradation, destruction and proliferation in diabetes.[8] Moreover, studies have also shown that treating periodontitis in diabetic patients has a beneficial effect on their blood glucose control.[9,10] On the other hand, poorly controlled diabetes is an important risk factor for periodontitis, and gingivitis, and sometimes periodontitis is the first sign that a diabetic patient presents with.[11] The impact of periodontitis on the diabetes-related inflammatory status has also been studied. These results support considering severe periodontitis as a risk factor for poor glycemic control.[12] It has been indicated that the cytokine – induced inflammatory state in periodontitis can contribute to the overall low – grade inflammation that occurs in diabetes.[13] A recent study investigated the relationship between chronic periodontitis, impaired fasting glucose (IFG), and diabetes in the US population and found chronic periodontitis to be positively associated in a linear relation with IFG and diabetes in US adults.[14] Studies evaluating the relationship between impaired glucose tolerance (IGT) and periodontal disease among Japanese population suggest that periodontal disease is positively associated with IGT, but other studies found no such association.[15,16,17]
Obesity and impaired lipid profile are other strong risk factors for type-2 diabetes, which in turn is a risk factor for periodontal disease.[18] The extent of coronary atherosclerosis is positively correlated with pro-atherogenic lipids, that is, total cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides (TG), and negatively correlated with anti-atherogenic High density lipoprotein (HDL) cholesterol.[19] The high prevalence of cardiovascular disease and periodontitis in individuals with diabetes may be attributed to an increased inflammatory response leading to atherosclerosis as compared to those without diabetes.[20]
Though there are many studies that have evaluated the interrelationship between the diabetic status of an individual with his/her periodontal status; the authors however could not find studies/research reports that evaluated relationship between various biochemical parameters like lipid profile and oral glucose tolerance test (OGTT) with periodontal health/disease status, in both healthy and diabetic patients. Hence, this study was designed to investigate the association between chronic periodontitis and diabetes by taking dental plaque index (DPI) and community periodontal index (CPI) score as periodontal health/disease dependent variables and lipid profile and OGTT as biochemical parameters in healthy, diabetic and IGT subjects.
MATERIALS AND METHODS
This study began in 2008 in the Department of Biochemistry and the Department of Periodontics at Baba Jaswant Singh Dental College Hospital and Research Institute, Ludhiana with the subjects reporting to the OPD of the Department of Periodontics. All patients reporting to the department of periodontics were interviewed and questioned about their diabetic/Lipid profile status. Any patient reporting a known self or family history of deranged lipid/diabetic profile was provisionally selected for this study. Of these, a group of 120 patients who were willing to form a part of the study were finally selected and were sent for blood and periodontal examination. The subjects undertaken also fulfilled the following criteria - community periodontal index (CPI) score of 2 or more [Table 1] and CPI loss of attachment score of 0 and above [Table 2].
Table 1.
Community periodontal index

Table 2.
CPI loss of attachment score

Criteria for selection of patients
Community periodontal index CPI ≥2
Subjects with community periodontal index loss of attachment score ≥0 underwent a periodontal examination to determine mean periodontal pocket depth and attachment loss. For the biochemical analyses, the reports of OGTT and lipid profile tests were analyzed as per WHO criteria for the diagnosis of diabetes[21] that is, normal glucose tolerance (NGT; fasting and 2 h postchallenge plasma glucose levels <110 mg/dl and <140 mg/dl, respectively), diabetes (fasting or 2 h postchallenge plasma glucose levels ≥126 mg/dl or ≥200 mg/dl, respectively) and IGT (IGT; all others with some glucose tolerance impairment including Impaired Fasting Glucose (IFG), that is, with one of the two glucose tolerance levels between normal and diabetic values and the other below the diabetic level). The samples of venous blood were taken in the fasting state for OGTT and lipid profile analysis. Immediately after, 75 g glucose dissolved in 300 ml water was given to be ingested in about 5 min and sample of blood was again collected at an interval of 30 min for a period of 3 h for Glucose Tolerance Test (GTT) analysis. Data obtained was tabulated, compared and statistically analyzed using Z-test to know the correlation between lipid profile test values, type 2 diabetes mellitus and periodontitis.
RESULTS
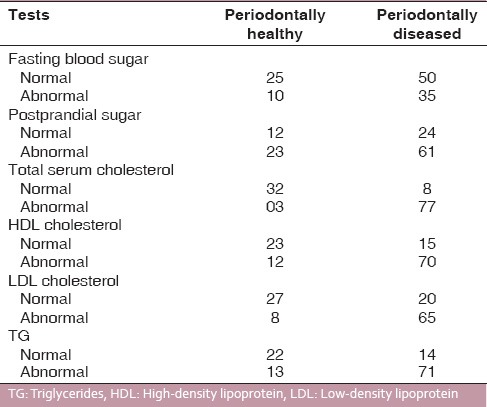
Of 120 subjects undertaken for study, 35 patients were found to be periodontally healthy and 85 periodontally diseased; 48 subjects had IGT, and 17 had diabetes and the rest of the subjects that is, 55 had NGT. All subjects were assessed for their periodontal condition by taking DPI [Table 3] and CPI scoreas parameters. When DPI score (suggesting periodontal condition of the patient) and OGTT results were compared and studied, it was observed that DPI for NGT, IGT and diabetic patients were 1.05 ± 0.5, 1.16 ± 0.7, and 1.65 ± 0.8, respectively [Table 4]. When CPI score (suggesting periodontal condition of the patient) and OGTT results were studied, it was observed that CPI score for NGT, IGT, and diabetics were 1.33 ± 0.4, 1.67 ± 0.5, and 2.33 ± 0.8, respectively [Table 4]. This showed that OGTT results deteriorated with deteriorating periodontal condition. A similar correlation was found between LDL levels and OGTT results, that is, LDL levels in patients with NGT and IGT were within normal range, while it was higher than normal in patients with diabetes [Table 5]. Mean total cholesterol and mean HDL values were within normal range for all OGTT patient types [Table 5]. In a correlation table of various biochemical analyses and periodontal parameters, [Table 6] it is evident that out of 85 periodontally diseased patients 35 showed abnormal fasting blood sugar levels and 61 showed abnormal post prandial blood sugar levels which potrays a non-significant co-relation. Of the 85 periodontally diseased patients 77 patients showed abnormal total serum cholesterol level, 65 showed abnormal LDL cholesterol level and 71 showed abnormal TG levels. These findings suggest that worsening lipid profile test values can be positively correlated with increased severity of periodontal disease [highly significant P < 0.001].
Table 3.
Dental plaque index (Sillness and Loe, 1964)
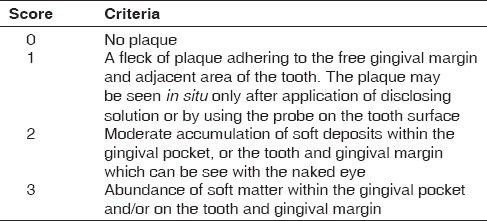
Table 4.
Periodontal condition of subjects in relation to their biochemical status
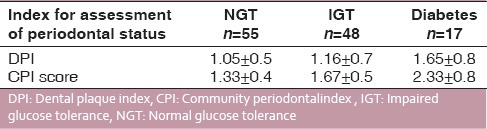
Table 5.
Association of participants’ diabetic status OGTT and lipid profile tests
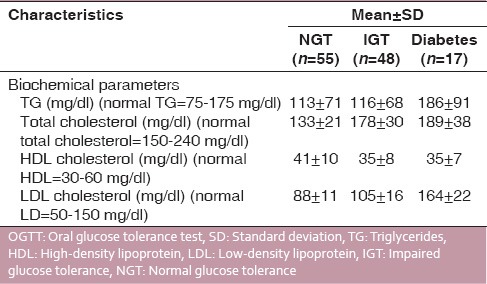
Table 6.
Correlation between periodontal condition and various biochemical parameters
DISCUSSION
The results of this study show that in patients having poor periodontal condition poor OGTT score was observed. It is also clear that as the periodontal condition and OGTT scores worsen, the TG levels and LDL levels also worsen. It has been shown that diabetes is one of the predisposing factors for the development of periodontal disease.[22] The inter relationships between periodontitis and diabetes provide an example of systemic disease predisposing to oral infection, and once that infection is established, oral infection exacerbates the systemic disease.[23] It has been shown that diabetic patients are prone to elevated LDL cholesterol and TGs even when blood glucose levels were well controlled.[24] This is significant; as our study indicated that hyperlipidemia may be one of the factors associated with periodontitis. The results of the study suggest that periodontitis itself may lead to elevated LDL/TG levels. Within this context, it may be put forth that in addition to effects of diabetes, periodontitis may contribute to elevated serum lipids and potentially to systemic disease arising from chronic hyperlipidemia. In a recent study in women subjects, periodontal disease was shown to be associated with later development of impaired glucose metabolism with a prior history of gestational diabetes.[25] Therefore, treating Periodontal disease may in addition to controlling and or improving the diabetic status of the patient may also improve the deranged lipid profile in a patient. When seen in this context, treating periodontal disease may also have a significant impact on improving the systemic health of the patient as both diabetes and a deranged lipid profile are known risk factors for several life-threatening diseases and conditions. As this study has demonstrated that the lipid profile can be a determinant of diabetes and periodontitis and vice versa, the clinicians must determine its relevance to patient care. Many cases of diabetes and deranged lipid profile may remain undiagnosed, and screening of patients in dental clinics may lead to a diagnosis of these in some cases.
CONCLUSION
It is evident from this study that abnormal levels of total serum cholesterol, LDL cholesterol, and TGs can be positively correlated with periodontally diseased conditions. As evidence of close link between periodontal disease, diabetes and deranged lipid profile is seen, treating and preventing recurrence of periodontal disease in patients with diabetes and deranged lipid profile values should be considered an important component of the treatment and management of patients suffering from diabetes and deranged lipid profile. The results of this study need to be further verified by large sample studies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 2.Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 3.Papapanou PN. Periodontal diseases: Epidemiology. Ann Periodontol. 1996;1:1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–40. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 5.Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990;121:532–6. doi: 10.14219/jada.archive.1990.0211. [DOI] [PubMed] [Google Scholar]
- 6.Noack B, Jachmann I, Roscher S, Sieber L, Kopprasch S, Lück C, et al. Metabolic diseases and their possible link to risk indicators of periodontitis. J Periodontol. 2000;71:898–903. doi: 10.1902/jop.2000.71.6.898. [DOI] [PubMed] [Google Scholar]
- 7.Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: Results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–9. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–72. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 9.Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713–9. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–10. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch R. Diabetes and periodontitis. Aust Prescr. 2004;27:36–8. [Google Scholar]
- 12.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67:1085–93. doi: 10.1902/jop.1996.67.10s.1085. [DOI] [PubMed] [Google Scholar]
- 13.Santos Tunes R, Foss-Freitas MC, Nogueira-Filho Gda R. Impact of periodontitis on the diabetes-related inflammatory status. J Can Dent Assoc. 2010;76:a35. [PubMed] [Google Scholar]
- 14.Choi YH, McKeown RE, Mayer-Davis EJ, Liese AD, Song KB, Merchant AT. Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care. 2011;34:381–6. doi: 10.2337/dc10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marugame T, Hayasaki H, Lee K, Eguchi H, Matsumoto S. Alveolar bone loss associated with glucose tolerance in Japanese men. Diabet Med. 2003;20:746–51. doi: 10.1046/j.1464-5491.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 16.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: The Hisayama study. J Dent Res. 2004;83:485–90. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Murakami M, Shimazaki Y, Matsumoto S, Yamashita Y. The extent of alveolar bone loss is associated with impaired glucose tolerance in Japanese men. J Periodontol. 2006;77:392–7. doi: 10.1902/jop.2006.050061. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: The Hisayama study. J Periodontal Res. 2005;40:346–53. doi: 10.1111/j.1600-0765.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 19.Tarchalski J, Guzik P, Wysocki H. Correlation between the extent of coronary atherosclerosis and lipid profile. Mol Cell Biochem. 2003;246:25–30. [PubMed] [Google Scholar]
- 20.Sutherland JH, Taylor GW, Offenbacher S. Diabetes and periodontal infection: Making the correction. Clin Diabetes. 2005;23:171–8. [Google Scholar]
- 21.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Hossain MZ, Fageeh HI, Gader FA, Saleem MY. Relationship of blood glucose level and severity of periodontitis in patients attended the outpatient periodntitcs clinic of College of Dentistry, King Khalid University. Saudi Arabia City Dental College J. 2012;9:7–11. [Google Scholar]
- 23.Iacopino AM. Periodontitis and diabetes interrelationships: Role of inflammation. Ann Periodontol. 2001;6:125–37. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 24.Mealey BL, Oates TW. American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 25.Xiong X, Elkind-Hirsch KE, Xie Y, Delarosa R, Maney P, Pridjian G, et al. Periodontal disease as a potential risk factor for the development of diabetes in women with a prior history of gestational diabetes mellitus. J Public Health Dent. 2013;73:41–9. doi: 10.1111/jphd.12004. [DOI] [PubMed] [Google Scholar]


