Abstract
Human papillomaviruses (HPVs) are epitheliotropic viruses with an affinity for keratinocytes and are principally found in the anogenital tract, urethra, skin, larynx, tracheobronchial and oral mucosa. On the basis of high, but variable frequency of HPV in oral squamous cell carcinoma (OSCC), malignant potential of HPV infection has been hypothesized but not definitely confirmed. The aim of this review was to highlight the genomic structure and possible mechanism of infection and carcinogenesis by HPV in the oral mucosa and to review the frequency of HPV prevalence in OSCC and oral potentially malignant disorders. A computer database search was performed through the use of PubMed from 1994 to 2014. Search keywords used were: HPV and oral cancer, HPV and oral leukoplakia, HPV and oral lichen planus, HPV and OSCC, HPV and verrucous carcinoma, HPV and proliferative verrucous leukoplakia, HPV and oral papilloma.
Keywords: Human papillomavirus, oral squamous cell carcinoma, oral potentially malignant disorder
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the sixth most common cancer worldwide. Several risk factors are related to OSCC, with the main being tobacco use, alcohol consumption, and infection by high-risk genotypes of human papillomavirus (HPV).[1] The specific role of HPV in the development of OSCC is still under debate despite its well-established role in the vast majority of squamous cell carcinoma of the cervix.[2] The relationship between HPV and OSCC was first suggested in 1983, but the presence of viral DNA was only confirmed 2 years later, by means of in situ hybridization (ISH).[1]
In this article, we are reviewing the genomic structure of HPV, its transmission, life cycle, mechanism of carcinogenesis with review of the studies on prevalence of HPV in OSCC and oral potentially malignant disorders (OPMDs) from 1994 to 2014.
HUMAN PAPILLOMAVIRUS GENOME
Human papillomavirus is a small, nonenveloped, double-stranded, circular DNA virus with a diameter of 52–55 nm. The genome contains a double-stranded DNA molecule that is, bound to cellular histones and contained in a protein capsid without envelope.[2,3,4] The HPV-DNA genome encodes approximately eight open reading frames (ORFs). The ORF is divided into 3 functional parts: The early (E) region comprising 45% of genome, the late (L) region extending for 40% of genome and a long control region (LCR).[2,3,4]
Early ORFs encode for E1, E2, E4, E5, E6, and E7 proteins which are necessary for replication, cellular transformation, and the control of viral transcription. E1 and E2 maintain viral DNA in an episomal form and facilitate the segregation of the viral genome during cell division. During productive infection, E6 and E7 stimulate cell cycle progression. E1, E2, E4, and E5 are required for and expressed during viral DNA amplification which occurs in differentiated cells in upper epithelial layers. Late region encodes the structural proteins or capsid proteins that take part in virion assembly. L1 ORF encodes for major capsid protein and L2 ORF for minor capsid protein. Noncoding upstream regulatory region encompassing the origin of replication, the E6/E7 gene promoter, and the enhancers and silencers, is located between the early and the late regions. LCR is necessary for viral DNA replication and transcription.[2,3,4]
The different HPV types are characterized by genotypic variations in the DNA base sequences of E6 and E7. It is these genotypic differences that permit stratification of the virus oncogenic phenotype into high and low-risk type. High risk includes HPV-16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59 and low risk are HPV 6, 11, 42, 43, 44.[2,3,4]
TRANSMISSION
Prevalence sites of HPVs include the epithelium of the vagina, vulva, penis, anal canal, cervix, perianal region, crypts of the tonsil, and oropharynx. The normal oral mucosa may act as a reservoir for new HPV infections and/or as a source of recurring HPV-associated lesions. The prevalence of HPV in normal oral mucosa range from 0.6% to 81%.[5,6] Multiple pathways for HPV transmission to the oral cavity can exist. These include sexual transmission, autoinfection, and rarely through perinatal transmission of the neonate during its passage through an infected birth canal of the mother.[5,7] Oral HPV acquisition was found to be more positively associated with number of recent oral sex and open mouth kissing partners than with the number of vaginal sex partners.[5,6]
MECHANISM OF INFECTION BY HUMAN PAPILLOMAVIRUS
Human papillomaviruses are characterized by a special tropism for squamous epithelial cells, keratinocytes. The synthesis of viral DNA and the expression of viral genes are linked to the keratinocyte level of differentiation.[8,9]
During the initial phase of infection, the viral genome undergoes episomal replication, and few copies of viral DNA per host cell are present. The episomal form acts as a reservoir of infected cells and is responsible for the latent state of infection.[8]
When the infection becomes productive, the viral genes are expressed sequentially from early genes to late genes, following the epithelial squamous differentiation, starting from basal and parabasal cells, where early portions of the viral genome are more active and proceeding to higher epithelial layers along with the formation of complete virion.[8,10] In HPV-infected basal cells, E1 and E2 proteins are expressed, and they regulate early viral DNA transcription. When expression of E2 is more pronounced, E2 represses viral DNA replication by blocking cellular transcription factors, thus controlling the number of HPV DNA copies in the basal cell by a process analogous to negative feedback.[11,12] In cases of high-risk HPV infection, E6 and E7 may also be expressed in HPV-infected basal cells, and the epithelium may then enter a proliferative phase characterized by an increasing number of HPV-infected basal cells, culminating in intraepithelial or invasive neoplasm.[11,13] As the basal cells divide, E2 mediates distribution of some HPV DNA copies to daughter cells, while some copies remain in the progenitor cells, in both cases, as episomes.[11,14] As the epithelial cells mature, HPV cycle progresses to productive replication.[11,13] In HPV infected epithelium, the matured epithelial cells express HPV E6 and E7 proteins in the suprabasal layers. E6 prevents apoptosis and E7 activates the cellular DNA replication mechanism allowing matured epithelial cells to re-enter the S-phase of the cell cycle, and makes the cellular replication machinery available for viral DNA replication.[11,12,14,15] Specific cellular factors associated with epithelial cell maturation activate late viral promoter located within E7 ORF, which activate late viral gene expression. Eventually, mediated by L1 and L2 proteins, the virus escapes from the shedding epithelial cells.[11,13]
MECHANISM OF HUMAN PAPILLOMAVIRUS INDUCED CARCINOGENESIS
The possibility of evolving into direction of malignancy depends on the type of virus, the synergic action with different physical, chemical, and biological agents, the genetic constitution, and the immune defense mechanisms of the host, all of which are able to modify the course of HPV infection. In the case of high-risk HPV infection and under favorable conditions, the viral genome is integrated into the host genome, which is the necessary event for the keratinocytes immortality.[3]
During this process of integration, the circular form of viral genome breaks at the level of the E1 and E2 regions. The loss of E2 during this process of integration produces the loss of E6 and E7 control.[2,3,4] Therefore, the sequences E6 and E7 are directly involved in the cellular cycle by inhibiting the normal functions of p53 and pRb, respectively.
The most manifest function of the E6 protein is to promote the degradation of p53 through its interaction with a cellular protein, E6 associated protein (E6AP). The p53 tumor suppressor gene itself regulates growth arrest and apoptosis after DNA damage. In addition, E6 interferes with other pro-apoptotic proteins, Bak, and procaspase 8, to comprehensively prevent apoptosis.[16,17,18] Recently, the product of the notch1 gene has been identified as a novel target of p53.[16,19,20]
Over the past dozen years or so, an increasing number of other proteins have also been revealed to be target proteins of E6 that might contribute to cellular transformation, with telomerase as one probable important example.[16]
E7 is known to bind to the retinoblastoma tumor suppressor gene product, pRb, and its family members, p107 and p130. In the hypophosphorylated state, pRb family proteins can bind to transcription factors such as E2F family members and repress the transcription of particular genes involved in DNA synthesis and cell cycle progression.[16,21] Because E7 is able to bind to unphosphorylated pRb, it may prematurely induce cells to enter the S phase by disrupting pRb–E2F complexes. The E7 protein function enables HPV replication in the upper layers of the epithelium where uninfected daughter cells normally differentiate and completely exit the cell cycle. P16INK4a, which prevents the phosphorylation of pRb family members, is overexpressed when pRb is inactivated by HPV E7. Thus, overexpression of p16INK4a is suggested to be a useful biomarker for evaluating HPV pathogenic activity.[16,22]
E6 and E7 can cooperate with cellular oncoproteins such as ras and myc, which enables the virus to act at the level of growth factors and cellular and nuclear metabolism producing oncogenic cells.[16]
HUMAN PAPILLOMAVIRUS AND ITS ASSOCIATION WITH ORAL SQUAMOUS CELL CARCINOMA AND ORAL POTENTIALLY MALIGNANT DISORDERS WITH REVIEW OF LITERATURE
Low-risk HPV mainly HPV-6 and 11 appears to be closely associated with a range of oral benign papillomatous lesions including oral squamous papilloma, oral verruca vulgaris, oral condyloma accuminatum and focal epithelial hyperplasia. High-risk HPV, that is, HPV-16, 18 are in turn associated with OPMDs and OSCC.[3] HPV-16 and 18 has been found to be associated with OSCC and HPV-16 with oral leukoplakia (OL) including proliferative verrucous leukoplakia (PVL). The reported rates of HPV DNA detection in OPMDs and OSCC range from 0% to 100%. This extreme variation is owing to difference in ethnicity, geographic locations to variations in methods used for detection of HPV.[8]
We carried out PubMed search for prevalence of HPV in oral lesions including Oral Lichen Planus, lichen planus, OSCC, oral papilloma, verrucous carcinoma (VC), and PVL during period of 1994–2014. The results are presented in Tables 1 and 2.
Table 1.
Review of the studies on the prevalence of HPV in OSCC and OPMDs (2014-2008)
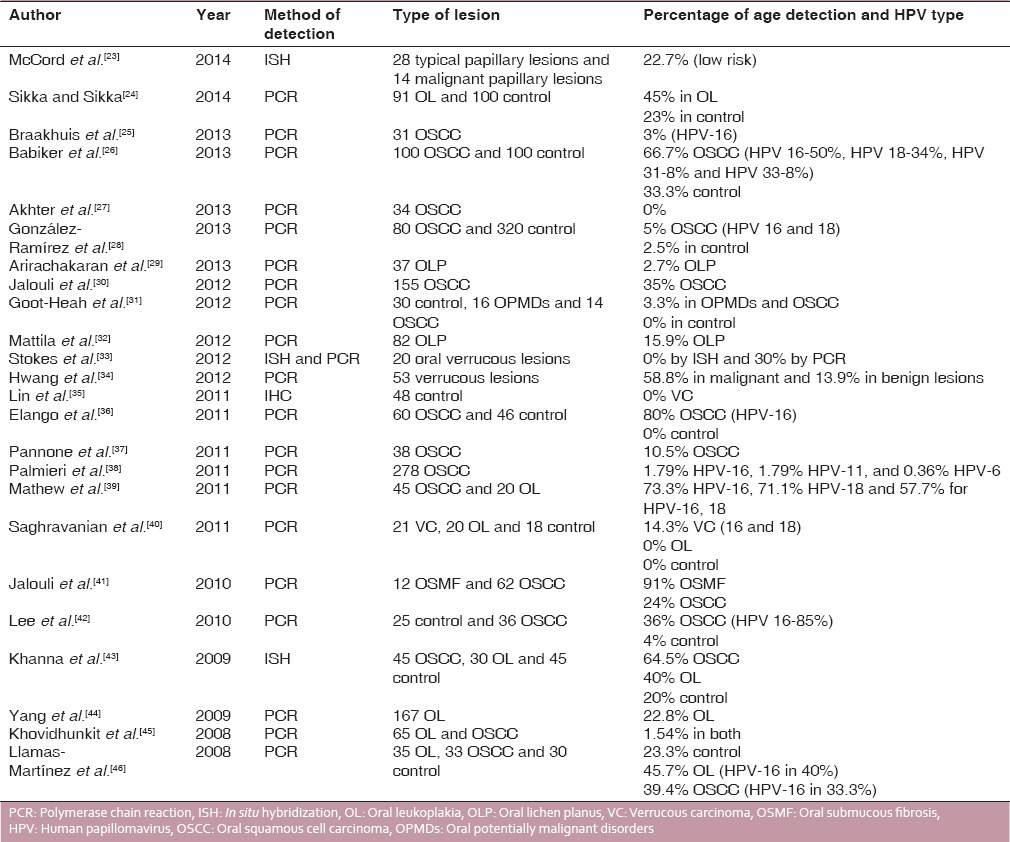
Table 2.
Review of the studies on the prevalence of HPV in OSCC and OPMDs (2007-1994)
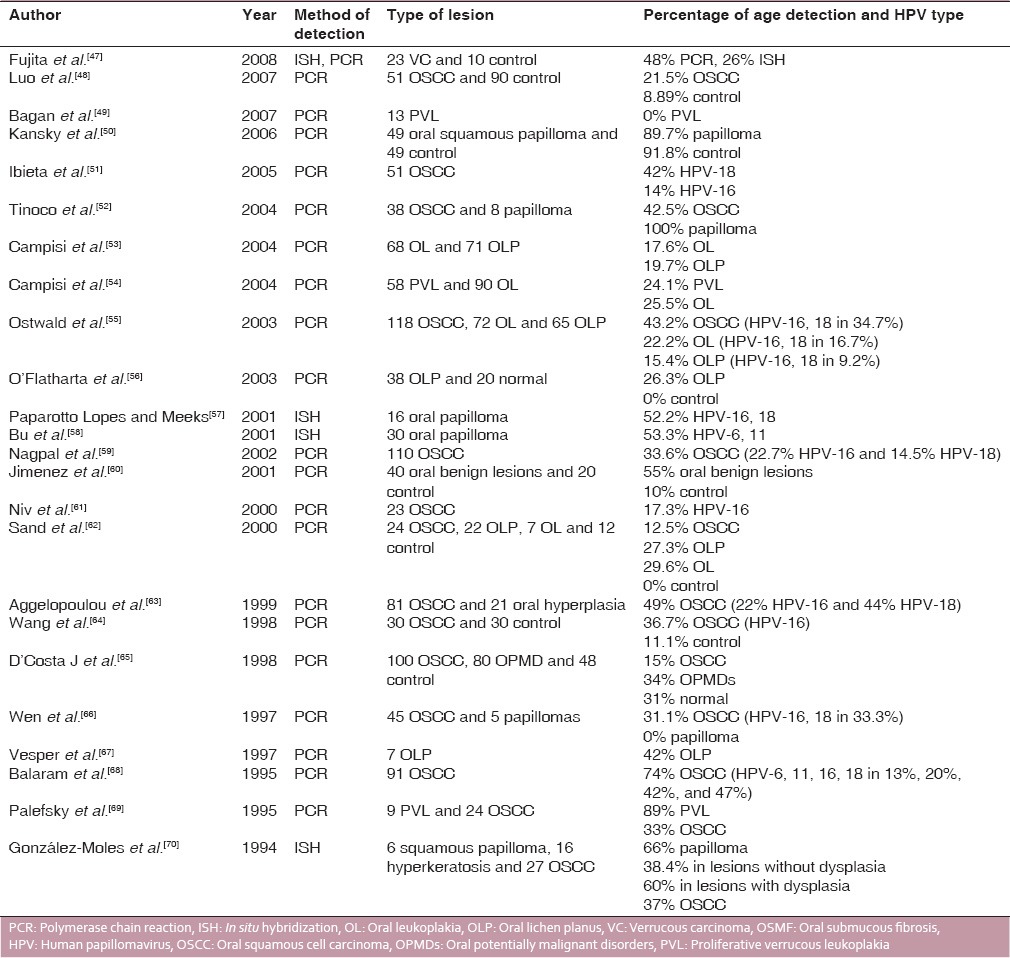
A total of 50 studies are included in this review including frequency of HPV in OSCC, OL, oral lichen planus, VC, PVL, and benign and malignant papillary lesions. Except for 8 studies which utilized In situ hybridization (ISH) as the assay for detection, most of the authors quantified HPV-DNA using polymerase chain reaction (PCR). The frequency of HPV in OSCC varied from 0% to 80%. The HPV type most commonly detected in OSCC and OPMDs was HPV-16, 18 with HPV-6, 11 found in only a few studies. Whereas, HPV type found in oral benign lesions and papilloma was HPV-6 and 11.
Miller and Johnston in a meta-analysis of OSCC observed that HPV may be a significant and independent risk factor. The prevalence of HPV in OSCC varies depending on several parameters such as geographic differences in population, type of specimen, selection of preparation method, and use of HPV detection method.[71] A brief review of the association of various parameters and OSCC based on these studies is presented as follows:
Molecular factors
Human papillomavirus-positive OSCCs seem to have a different molecular profile compared with that of HPV-negative cancer. In addition, HPV-positive cancers share some similarities with cervical carcinoma. By immunohistochemistry (IHC), most HPV-positive tumors show p16 overexpression. The expression of p53 and bcl-2 is not associated with HPV-positive OSCC and mutations in p53 are rarely seen in HPV-positive tumors compared with HPV-negative tumors.[72] Genetic signatures of HPV-positive OSCC have been shown to be different from those of HPV-negative OSCC.[72]
Influence of method used for analysis
There is a wide array of assays used for detection of HPV in sample including PCR, ISH, IHC, and Western blot analysis with PCR being the most widely used to estimate the HPV-DNA in samples. Besides the method used, different results are obtained when using fresh frozen or formalin-fixed paraffin-embedded material.[73] HPV in saliva and oral exfoliated cells has been detected in some recent studies, but the sensitivity and specificity are too low, and the role of HPV detection in saliva is still uncertain.[74]
Other risk factors and human papillomavirus
No correlation between HPV-positive OSCC and tobacco or alcohol consumption has been found. A strong association has been found between sexual behavior and risk of HPV infection.[75] In India, HPV-DNA was detected less frequently in tumor specimens from tobacco chewers than in those from nonchewers.
Patient factors
Patients with HPV-positive OSCC usually are younger and more often present at a higher stage and with large metastatic lymph nodes.[76]
Prognostic factors
Many studies have now confirmed that HPV-positive tumors in head and neck area have a better prognosis compared with those that are HPV-negative. The better prognosis is independent of the treatment given. The positive prognosis is also more pronounced in HPV-positive patients who are also p16 positive.[77]
CONCLUSION
Risk factors mainly responsible for OSCC include tobacco, alcohol, ultraviolet rays but many cases have none of these identifiable risk factors. On the basis of high frequency of HPV in some types of OSCC and OPMDs, an oral malignant potential of HPV infection in oropharyngeal carcinoma is likely. Of particular significance is the association of high frequency of HPV in oral cancers involving base of the tongue, in those occurring in younger patients and without the prior history of exposure to the usual risk factors. Still further research is needed in order to standardize a particular protocol for screening of patients with OSCC and OMPDs for HPV as well as to determine a specific and universal method/assay for analysis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lima LA, Silva CG, Rabenhorst SH. Association between human papilloma virus and oral squamous cell carcinoma: A systematic review. J Bras Patol Med Lab. 2014;50:75–84. [Google Scholar]
- 2.Syrjänen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 3.Prabhu SR, Wilson DF. Human papillomavirus and oral disease – Emerging evidence: A review. Aust Dent J. 2013;58:2–10. doi: 10.1111/adj.12020. [DOI] [PubMed] [Google Scholar]
- 4.de Villiers EM, Gunst K. Characterization of seven novel human papillomavirus types isolated from cutaneous tissue, but also present in mucosal lesions. J Gen Virol. 2009;90:1999–2004. doi: 10.1099/vir.0.011478-0. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–9. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragin C, Edwards R, Larkins-Pettigrew M, Taioli E, Eckstein S, Thurman N, et al. Oral HPV infection and sexuality: A cross-sectional study in women. Int J Mol Sci. 2011;12:3928–40. doi: 10.3390/ijms12063928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189:686–98. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 8.Campisi G, Panzarella V, Giuliani M, Lajolo C, Di Fede O, Falaschini S, et al. Human papillomavirus: Its identity and controversial role in oral oncogenesis, premalignant and malignant lesions (review) Int J Oncol. 2007;30:813–23. [PubMed] [Google Scholar]
- 9.Femiano F. Papilloma virus. Review of the literature. Note II. Diagnosis and treatment Minerva Stomatol. 2000;49:179–86. [PubMed] [Google Scholar]
- 10.Santoro V, Pozzuoli ML, Colella G. Role of human papilloma virus in precancerous and cancerous lesions of the oral cavity. Review of the literature. Minerva Stomatol. 1997;46:595–601. [PubMed] [Google Scholar]
- 11.Feller L, Khammissa RA, Wood NH, Lemmer J. Epithelial maturation and molecular biology of oral HPV. Infect Agent Cancer. 2009;4:16. doi: 10.1186/1750-9378-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68:362–72. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32(Suppl 1):S7–15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 14.von Knebel Doeberitz M. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer. 2002;38:2229–42. doi: 10.1016/s0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 15.Elgui de Oliveira D. DNA viruses in human cancer: An integrated overview on fundamental mechanisms of viral carcinogenesis. Cancer Lett. 2007;247:182–96. doi: 10.1016/j.canlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007;98:1505–11. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol. 1999;80(Pt 6):1513–7. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 18.Garnett TO, Filippova M, Duerksen-Hughes PJ. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006;13:1915–26. doi: 10.1038/sj.cdd.4401886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21:562–77. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T. Regulation of Notch1 gene expression by p53 in epithelial cells. Mol Cell Biol. 2007;27:3732–42. doi: 10.1128/MCB.02119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–62. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa M, Fujii T, Saito M, Nindl I, Ono A, Kubushiro K, et al. Overexpression of p16 INK4a as an indicator for human papillomavirus oncogenic activity in cervical squamous neoplasia. Int J Gynecol Cancer. 2006;16:347–53. doi: 10.1111/j.1525-1438.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 23.McCord C, Xu J, Xu W, Qiu X, Muhanna N, Irish J, et al. Association of human papilloma virus with atypical and malignant oral papillary lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:722–32. doi: 10.1016/j.oooo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Sikka S, Sikka P. Association of human papilloma virus 16 infection and p53 polymorphism among tobacco using oral leukoplakia patients: A clinicopathologic and genotypic study. Int J Prev Med. 2014;5:430–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Braakhuis BJ, Rietbergen MM, Buijze M, Snijders PJ, Bloemena E, Brakenhoff RH, et al. TP53 mutation and human papilloma virus status of oral squamous cell carcinomas in young adult patients. Oral Dis. 2014;20:602–8. doi: 10.1111/odi.12178. [DOI] [PubMed] [Google Scholar]
- 26.Babiker AY, Eltom FM, Abdalaziz MS, Rahmani A, Abusail S, Ahmed HG. Screening for high risk human papilloma virus (HR-HPV) subtypes, among Sudanese patients with oral lesions. Int J Clin Exp Med. 2013;6:275–81. [PMC free article] [PubMed] [Google Scholar]
- 27.Akhter M, Ali L, Hassan Z, Khan I. Association of human papilloma virus infection and oral squamous cell carcinoma in Bangladesh. J Health Popul Nutr. 2013;31:65–9. doi: 10.3329/jhpn.v31i1.14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González-Ramírez I, Irigoyen-Camacho ME, Ramírez-Amador V, Lizano-Soberón M, Carrillo-García A, García-Carrancá A, et al. Association between age and high-risk human papilloma virus in Mexican oral cancer patients. Oral Dis. 2013;19:796–804. doi: 10.1111/odi.12071. [DOI] [PubMed] [Google Scholar]
- 29.Arirachakaran P, Chansaengroj J, Lurchachaiwong W, Kanjanabud P, Thongprasom K, Poovorawan Y. Oral lichen planus in thai patients has a low prevalence of human papillomavirus. ISRN Dent 2013. 2013 doi: 10.1155/2013/362750. 362750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jalouli J, Jalouli MM, Sapkota D, Ibrahim SO, Larsson PA, Sand L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res. 2012;32:571–80. [PubMed] [Google Scholar]
- 31.Goot-Heah K, Kwai-Lin T, Froemming GR, Abraham MT, Nik Mohd Rosdy NM, Zain RB. Human papilloma virus 18 detection in oral squamous cell carcinoma and potentially malignant lesions using saliva samples. Asian Pac J Cancer Prev. 2012;13:6109–13. doi: 10.7314/apjcp.2012.13.12.6109. [DOI] [PubMed] [Google Scholar]
- 32.Mattila R, Rautava J, Syrjänen S. Human papillomavirus in oral atrophic lichen planus lesions. Oral Oncol. 2012;48:980–4. doi: 10.1016/j.oraloncology.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Stokes A, Guerra E, Bible J, Halligan E, Orchard G, Odell E, et al. Human papillomavirus detection in dysplastic and malignant oral verrucous lesions. J Clin Pathol. 2012;65:283–6. doi: 10.1136/jclinpath-2011-200454. [DOI] [PubMed] [Google Scholar]
- 34.Hwang CF, Huang CC, Chien CY, Huang SC, Yang CH, Su CY. Human papillomavirus infection in oral papillary and verrucous lesions is a prognostic indicator of malignant transformation. Cancer Epidemiol. 2012;36:e122–7. doi: 10.1016/j.canep.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Lin HP, Wang YP, Chiang CP. Expression of p53, MDM2, p21, heat shock protein 70, and HPV 16/18 E6 proteins in oral verrucous carcinoma and oral verrucous hyperplasia. Head Neck. 2011;33:334–40. doi: 10.1002/hed.21452. [DOI] [PubMed] [Google Scholar]
- 36.Elango KJ, Suresh A, Erode EM, Subhadradevi L, Ravindran HK, Iyer SK, et al. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev. 2011;12:889–96. [PubMed] [Google Scholar]
- 37.Pannone G, Santoro A, Carinci F, Bufo P, Papagerakis SM, Rubini C, et al. Double demonstration of oncogenic high risk human papilloma virus DNA and HPV-E7 protein in oral cancers. Int J Immunopathol Pharmacol. 2011;24:95–101. doi: 10.1177/03946320110240S217. [DOI] [PubMed] [Google Scholar]
- 38.Palmieri A, Scapoli L, Martinelli M, Pezzetti F, Girardi A, Spinelli G, et al. Incidence of low risk human papillomavirus in oral cancer: A real time PCR study on 278 patients. Int J Immunopathol Pharmacol. 2011;24:83–7. doi: 10.1177/03946320110240S215. [DOI] [PubMed] [Google Scholar]
- 39.Mathew A, Mody RN, Patait MR, Razooki AA, Varghese NT, Saraf K. Prevalence and relationship of human papilloma virus type 16 and type 18 with oral squamous cell carcinoma and oral leukoplakia in fresh scrappings: A PCR study. Indian J Med Sci. 2011;65:212–21. [PubMed] [Google Scholar]
- 40.Saghravanian N, Ghazvini K, Babakoohi S, Firooz A, Mohtasham N. Low prevalence of high risk genotypes of human papilloma virus in normal oral mucosa, oral leukoplakia and verrucous carcinoma. Acta Odontol Scand. 2011;69:406–9. doi: 10.3109/00016357.2011.572560. [DOI] [PubMed] [Google Scholar]
- 41.Jalouli J, Ibrahim SO, Mehrotra R, Jalouli MM, Sapkota D, Larsson PA, et al. Prevalence of viral (HPV, EBV, HSV) infections in oral submucous fibrosis and oral cancer from India. Acta Otolaryngol. 2010;130:1306–11. doi: 10.3109/00016481003782041. [DOI] [PubMed] [Google Scholar]
- 42.Lee SY, Cho NH, Choi EC, Baek SJ, Kim WS, Shin DH, et al. Relevance of human papilloma virus (HPV) infection to carcinogenesis of oral tongue cancer. Int J Oral Maxillofac Surg. 2010;39:678–83. doi: 10.1016/j.ijom.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Khanna R, Rao GR, Tiwary SK, Rai A, Khanna S, Khanna AK. Detection of human papilloma virus 16 and 18 DNA sequences by southern blot hybridization in oral leukoplakia and squamous cell carcinoma. Indian J Surg. 2009;71:69–72. doi: 10.1007/s12262-009-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang SW, Lee YS, Chen TA, Wu CJ, Tsai CN. Human papillomavirus in oral leukoplakia is no prognostic indicator of malignant transformation. Cancer Epidemiol. 2009;33:118–22. doi: 10.1016/j.canep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Khovidhunkit SO, Buajeeb W, Sanguansin S, Poomsawat S, Weerapradist W. Detection of human papillomavirus in oral squamous cell carcinoma, leukoplakia and lichen planus in Thai patients. Asian Pac J Cancer Prev. 2008;9:771–5. [PubMed] [Google Scholar]
- 46.Llamas-Martínez S, Esparza-Gómez G, Campo-Trapero J, Cancela-Rodríguez P, Bascones-Martínez A, Moreno-López LA, et al. Genotypic determination by PCR-RFLP of human papillomavirus in normal oral mucosa, oral leukoplakia and oral squamous cell carcinoma samples in Madrid (Spain) Anticancer Res. 2008;28:3733–41. [PubMed] [Google Scholar]
- 47.Fujita S, Senba M, Kumatori A, Hayashi T, Ikeda T, Toriyama K. Human papillomavirus infection in oral verrucous carcinoma: Genotyping analysis and inverse correlation with p53 expression. Pathobiology. 2008;75:257–64. doi: 10.1159/000132387. [DOI] [PubMed] [Google Scholar]
- 48.Luo CW, Roan CH, Liu CJ. Human papillomaviruses in oral squamous cell carcinoma and pre-cancerous lesions detected by PCR-based gene-chip array. Int J Oral Maxillofac Surg. 2007;36:153–8. doi: 10.1016/j.ijom.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Bagan JV, Jimenez Y, Murillo J, Gavaldá C, Poveda R, Scully C, et al. Lack of association between proliferative verrucous leukoplakia and human papillomavirus infection. J Oral Maxillofac Surg. 2007;65:46–9. doi: 10.1016/j.joms.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 50.Kansky AA, Seme K, Maver PJ, Luzar B, Gale N, Poljak M. Human papillomaviruses (HPV) in tissue specimens of oral squamous cell papillomas and normal oral mucosa. Anticancer Res. 2006;26:3197–201. [PubMed] [Google Scholar]
- 51.Ibieta BR, Lizano M, Fras-Mendivil M, Barrera JL, Carrillo A, Ma Ruz-Godoy L, et al. Human papilloma virus in oral squamous cell carcinoma in a Mexican population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:311–5. doi: 10.1016/j.tripleo.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Tinoco JA, Silva AF, Oliveira CA, Rapoport A, Fava AS, Souza RP. Human papillomavirus (HPV) infection and its relation with squamous cell carcinoma of the mouth and oropharynx. Rev Assoc Med Bras. 2004;50:252–6. doi: 10.1590/s0104-42302004000300029. [DOI] [PubMed] [Google Scholar]
- 53.Campisi G, Giovannelli L, Aricò P, Lama A, Di Liberto C, Ammatuna P, et al. HPV DNA in clinically different variants of oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:705–11. doi: 10.1016/j.tripleo.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Campisi G, Giovannelli L, Ammatuna P, Capra G, Colella G, Di Liberto C, et al. Proliferative verrucous vs conventional leukoplakia: No significantly increased risk of HPV infection. Oral Oncol. 2004;40:835–40. doi: 10.1016/j.oraloncology.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Ostwald C, Rutsatz K, Schweder J, Schmidt W, Gundlach K, Barten M. Human papillomavirus 6/11, 16 and 18 in oral carcinomas and benign oral lesions. Med Microbiol Immunol. 2003;192:145–8. doi: 10.1007/s00430-002-0161-y. [DOI] [PubMed] [Google Scholar]
- 56.O’Flatharta C, Flint SR, Toner M, Butler D, Mabruk MJ. Investigation into a possible association between oral lichen planus, the human herpesviruses, and the human papillomaviruses. Mol Diagn. 2003;7:73–83. doi: 10.1007/BF03260023. [DOI] [PubMed] [Google Scholar]
- 57.Paparotto Lopes SM, Meeks VI. Analysis of HPV 16 and 18 by in situ hybridization in oral papilloma of HIV+patients. Gen Dent. 2001;49:386–9. [PubMed] [Google Scholar]
- 58.Bu J, Pang J, Bu R. Study on the role of human papillomavirus in carcinogenesis of oral papillomas by in situ hybridization. Zhonghua Kou Qiang Yi Xue Za Zhi. 2001;36:34–6. [PubMed] [Google Scholar]
- 59.Nagpal JK, Patnaik S, Das BR. Prevalence of high-risk human papilloma virus types and its association with P53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int J Cancer. 2002;97:649–53. doi: 10.1002/ijc.10112. [DOI] [PubMed] [Google Scholar]
- 60.Jimenez C, Correnti M, Salma N, Cavazza ME, Perrone M. Detection of human papillomavirus DNA in benign oral squamous epithelial lesions in Venezuela. J Oral Pathol Med. 2001;30:385–8. doi: 10.1034/j.1600-0714.2001.300701.x. [DOI] [PubMed] [Google Scholar]
- 61.Niv A, Sion-Vardi N, Gatot A, Nash M, Fliss DM. Identification and typing of human papillomavirus (HPV) in squamous cell carcinoma of the oral cavity and oropharynx. J Laryngol Otol. 2000;114:41–6. doi: 10.1258/0022215001903870. [DOI] [PubMed] [Google Scholar]
- 62.Sand L, Jalouli J, Larsson PA, Hirsch JM. Human papilloma viruses in oral lesions. Anticancer Res. 2000;20:1183–8. [PubMed] [Google Scholar]
- 63.Aggelopoulou EP, Skarlos D, Papadimitriou C, Kittas C, Troungos C. Human papilloma virus DNA detection in oral lesions in the Greek population. Anticancer Res. 1999;19:1391–5. [PubMed] [Google Scholar]
- 64.Wang J, Li J, Huang H, Fu Y. Detection of the E7 transform gene of human papilloma virus type 16 in human oral squamous cell carcinoma. Chin J Dent Res. 1998;1:35–7. [PubMed] [Google Scholar]
- 65.D’Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta AR. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998;34:413–20. doi: 10.1016/s1368-8375(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 66.Wen S, Tsuji T, Li X, Mizugaki Y, Hayatsu Y, Shinozaki F. Detection and analysis of human papillomavirus 16 and 18 homologous DNA sequences in oral lesions. Anticancer Res. 1997;17:307–11. [PubMed] [Google Scholar]
- 67.Arndt O, Johannes A, Zeise K, Brock J. High-risk HPV types in oral and laryngeal papilloma and leukoplakia. Laryngorhinootologie. 1997;76:142–9. doi: 10.1055/s-2007-997403. [DOI] [PubMed] [Google Scholar]
- 68.Balaram P, Nalinakumari KR, Abraham E, Balan A, Hareendran NK, Bernard HU, et al. Human papillomaviruses in 91 oral cancers from Indian betel quid chewers – High prevalence and multiplicity of infections. Int J Cancer. 1995;61:450–4. doi: 10.1002/ijc.2910610403. [DOI] [PubMed] [Google Scholar]
- 69.Palefsky JM, Silverman S, Jr, Abdel-Salaam M, Daniels TE, Greenspan JS. Association between proliferative verrucous leukoplakia and infection with human papillomavirus type 16. J Oral Pathol Med. 1995;24:193–7. doi: 10.1111/j.1600-0714.1995.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 70.González-Moles MA, Ruiz-Avila I, González-Moles S, Martinez I, Ceballos A, Nogales F. Detection of HPV DNA by in situ hybridization in benign, premalignant and malignant lesions of the oral mucosa. Bull Group Int Rech Sci Stomatol Odontol. 1994;37:79–85. [PubMed] [Google Scholar]
- 71.Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: A meta-analysis, 1982-1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:622–35. doi: 10.1067/moe.2001.115392. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira MC, Soares RC, Pinto LP, Souza LB, Medeiros SR, Costa Ade L. High-risk human papillomavirus (HPV) is not associated with p53 and bcl-2 expression in oral squamous cell carcinomas. Auris Nasus Larynx. 2009;36:450–6. doi: 10.1016/j.anl.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Braakhuis BJ, Brakenhoff RH, Meijer CJ, Snijders PJ, Leemans CR. Human papilloma virus in head and neck cancer: The need for a standardised assay to assess the full clinical importance. Eur J Cancer. 2009;45:2935–9. doi: 10.1016/j.ejca.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 74.Smith EM, Ritchie JM, Summersgill KF, Hoffman HT, Wang DH, Haugen TH, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–55. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 75.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 76.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 77.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]


