Abstract
177Lu-ethylenediaminetetramethylene phosphonic acid (EDTMP) is presently suggested as an excellent bone seeking radionuclide for developing metastatic bone pain (MBP) palliation agent owing to its suitable nuclear decay characteristics. To find the exact dosage and its efficiency, this clinical study was performed on the human being, using 177Lu-EDTMP for MBP palliation. 177Lu-EDTMP was prepared by Iran, atomic energy organization. Thirty consecutive patients with determined tumors, incontrollable MBP, and positive bone scan at 4 weeks before the beginning of the study participated in this study in the nuclear medicine ward. 177Lu-EDTMP in the form of sterile slow IV injection was administered with a dose of 29.6 MBq/kg. Short form of brief pain inventory questionnaire was used to evaluate the efficiency of the intervention. Questionnaires were filled out by an expert nuclear physician every 2 weeks while the cell blood count was also checked every 2 weeks up to 12 weeks for evaluation of bone marrow suppression and hematological toxicity. Furthermore, whole body scan was done at days 1, 3, and 7. Twenty-five patients showed a significant pain relief since 2 weeks after the injection, and continued until the end of the follow up period (12 weeks). There were no significant early complications such as bone marrow suppression, hematological toxicity, and no systemic adverse effects. No complication was observed in renal function. Twenty one patients showed flare phenomenon that was started after the 12.2 ± 1.78 h lasting for 38.4 ± 23.08. Sixteen patients (53%) were completely treated; nine patients (30%) showed a partial response, and five patients (17%) had no response to treatment. Total response to treatment was achieved in 25 patients (83%). At the end of the evaluation, no bone marrow suppression or hematologic toxicity was observed. 177Lu-EDTMP has shown suitable physical and biological properties with good results in long term bone pain relief for patients with bone metastasis.
Keywords: 177Lu-ethylenediaminetetramethylene phosphonic acid, bone pain palliation, metastasis, nuclear medicine, targeted radionuclide therapy
Introduction
Metastatic bone pain (MBP) is the most common form of pain syndrome among patients with breast, lung and prostate cancers.[1,2] MBP often leads to other related symptoms such as lack of mobility, neurological deficits, fear of death, anxiety and depression, so it could significantly affect the quality of life (QoL).[3,4] MBP control is an essential step in all cancer management programs; although conventional treatment modalities such as external beam radiotherapy and analgesics are prevalent practices, they have many disadvantages and also multiple side effects.
Systemic administration of beta emitting radionuclides has been introduced in 1940s; it was demonstrated as an effective treatment modality, with lesser side effects while improving the QoL of the patients significantly.[5,6,7] The main goals in MBP palliation using beta emitters are maximizing the radiation dose to the bone lesion with simultaneous minimization of the radiation dose in the bone marrow.[8,9] Systemic radioisotope therapy has many advantages including alleviating pain, improving QoL, reducing the request for analgesics, radiotherapy, and chemotherapy, and improving patients' prognosis and survival. It also decreases the overall cost of MBP palliation in addition to applicability in an outpatient setting without the need for expensive high-technology equipment. More than half of the patients treated experience relief of pain, which may be achieved within 2–7 days depending on the agent and may last for several months after a single injection.[10]
A variety of radioinulclides are used in MBP palliation.[11] In this modality of treatment, radiation from radio-drug doesn't target the tumor itself, but the main target is tumor's surrounding bone, so the delivered dose varies depending on physical properties of radionuclide and tumor.
Physical characteristics such as half-life and the ability of radioisotope to simultaneously emitgamma ray while beta radiate is very important in successful MBP palliation therapy. For example, while phosphorous-32 (32P) and strontium-89 (89Sr) have longer half-lives, 12 and 50 days, samarium-153 (153Sm) or rhenium-186 have a short half-life of 2 and 4 days, respectively. To study the distribution of the radioisotope among different tissues, gamma ray emitting is beneficial.32P phosphate doesn't emit gamma rays; therefore, their distribution and uptake are not detectable through gamma cameras.
177Lu, a beta emitter lanthanide discovered in 1907, is currently used for somatostatin receptor radiotherapy,[12] radio-immunotherapy,[13] bone palliation therapy,[14] and radiosynovectomy.[15,16] Due to its 6.7-day half-life, maximum beta energy of 497 keV, and reliable gamma emission (Eγ = 112 keV [6.4%], 208 keV [11%]) for imaging [Figure 1], 177Lu has been considered for a number of therapeutic roles. Preclinical studies of 177Lu-ethylenediaminetetramethylene phosphonic acid (177Lu-EDTMP) have shown a specific accumulation within the bones of rats.[17,18] 177Lu-EDTMP use has been reported in humans for imaging purposes.[19]
Figure 1.
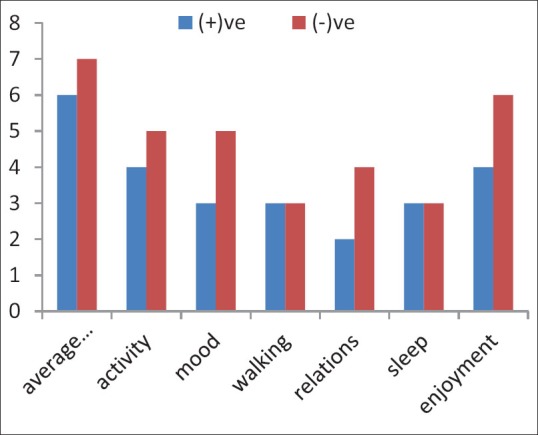
Correlation between positive or negative flare phenomenon and mean scores of routine activities in brief pain inventory form of the patients after the injection of 177Lu-ethylenediaminetetramethylene phosphonic acid (lower score equals to better response of items to treatment)
177Lu has a longer half-life, in comparison to 153Sm, presenting a radiopharmaceutical with better shipment quality and longer shelf-life. Large-scale production in adequate specific activity and radionuclide purity using a moderate flux reactor make it a promising radionuclide to make compounds for MBP palliation drugs.
The purpose of this study was to determine the feasibility and efficacy of 177Lu-EDTMP for palliative treatment of MBP. This is the primary report of clinical trial of 177Lu-EDTMP for MBP palliation.
Materials and Methods
In this randomized controlled trial (RCT) study, 30 eligible patients had clinically confirmed multiple bone metastases, recent positive whole body bone scan (during past 4 weeks) presented by intolerable bone pain to the radiotherapy department of Namazee Hospital. They didn't respond to routine antianalgesic drugs. They referred to nuclear medicine department of the hospital by the radiotherapists. These patients had following criteria: Oncology group performance status 0–2; life expectancy of longer than 3 months; adequate hematological counts (neutrophils ≥1.5 × 109/L; platelets ≥100 × 109/L; haemoglobin [Hb] >100 g/L); normal renal function tests (creatinine <1.5 × upper limit of normal); and normal hepatic function tests (normal bilirubin [within institutional limits], aspartate aminotransferase and alanine aminotransferase <2.5 × upper limit of normal).
The exclusion criteria were: The patients with pregnancy, breastfeeding, other active malignant disease, acute compression fracture, those received chemotherapy, immunotherapy, or external-beam radiotherapy within the past 6 weeks; treatment by bisphosphonates within 3 months; any previous systemic radiotherapy with radioactive strontium, samarium, or rhenium. The clinical trial was approved by Ethics committee of Shiraz University of Medical Sciences. All the patients gave verbal and also written informed consent.
177Lu-ethylenediaminetetramethylene phosphonic acid was prepared by Atomic Energy Organization of Iran (AEOI) regarding international standards.[20] Quality control and sterility tests were also performed in AEOI for the first human clinical study in the Shiraz University of Medical Sciences.[21]
The form of brief pain inventory (BPI)[22] has been validated for use in patients suffering from advanced cancer. BPI is a self-reporting pain score system between 0 and 10, 0 for no pain and 10 for the worst pain ever experienced by the patient. In addition to pain assessment, functional interference resulting from BMP was also estimated, such as a general activity, mood, sleep, ability to walk, and relationship with others.[23]
Persian validated brief BPI questionnaire[24] was filled out just before starting and also every 2 weeks after the injection of the drug by a nuclear physician. Higher scores show that the activities are more impaired, and lower score is representative for better response to treatment. According to an animal study, intravenous injection of 9.25 through 37 MBq/kg body weight of 177Lu-EDTMP was suggested for MBP palliation.[18] Another human study suggested 29.4 ± 12.5 MBq/kg for imaging purpose.[19] Hence, slow intravenous injection of 29.6 MBq/kg was selected for this study.
This is the phase I clinical trial for 177Lu-EDTMP, so the safety of this radionuclide compound was the most important task. Patients were under close observation by the medical staff for either hypersensitivity, skin reactions or any early side effect of the drug for 6 h after injection of radionuclide in nuclear medicine ward. Blood and urine samples were collected for dosimetric study and to confirm bio-distribution of radionuclide in the body 8, 24, and 72 h after the injection. Whole body scan was also performed 24, 72 h and also 7 days after the injection using single photon emission computed tomography/computed tomography infinia hawkeye4 GE gamma camera with low-energy high-resolution collimator at 108 keV peak gamma energy and windows of 15%.[19] Flare phenomenon was considered for intensifying bone pain after the injection of the drug. At this time, the patients were asked about flare phenomenon and the data were recorded including the start time of the flare phenomenon and its long-time.
Renal function test was evaluated during 1 week before the injection of the drug and 4 weeks after that and blood samples were taken for complete blood count measurements just before the injection and every 2 weeks after the injection up to 12 weeks for evaluation of bone marrow suppression and hematological toxicity. Complete palliative pain response was achieved when visual analog pain score (VAS) was about 0–3.When ΔVAS (change in the amount of pain palliation/VAS) was 0–2 (VAS = 8–10), no response was achieved. Other amounts of ΔVAS were considered as a partial response to palliative treatment. VAS data were collected by BPI form.
Statistical methods
The data were analyzed in statistical software statistical package for the social sciences, version 20.0, using student t-test and one way analysis of variance; P value < 0.05 was considered significant.
Results and Discussion
Results
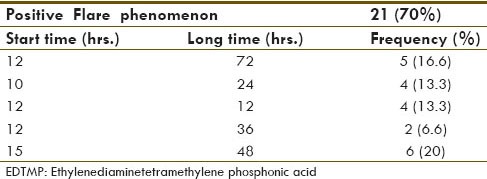
Thirty patients, 8 (26%) males and 22 (74%) females, with the age of 41.7 ± 12.3 (mean ± standard deviation) participated in this study. Demographic data are shown in Table 1. According to the results, no skin reaction or systemic adverse effects were observed in the patients after the injection of the drug. Flare phenomenon was observed in 21 (70%) patients while 9 patients (30%) did not show this phenomenon. Start time of the flare phenomenon was 12.2 ± 1.78 h and its long time was 38.4 ± 23.08 h [Table 2]. Routine activities of the subjects were evaluated considering flare phenomenon; the patients that had positive flare phenomenon had more pleasure in most of their activities [Figure 1].
Table 1.
Demographic data of the patients with bone metastasis eligible for treatment with 177Lu-EDTMP
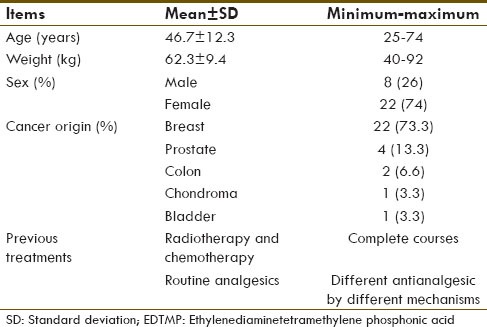
Table 2.
Flare phenomenon in patients after injection of 177Lu-EDTMP
Whole body scan 24, 72 h and also 7 days after the injection of radiotracer showed high bone to soft tissue ratio of radiotracer uptake with more concentration of radiotracer in metastatic bone lesions. Corresponding to bone scan findings, no significant differences were seen. The number of defects was the same in the serial taken scan scintigraphies. The other interesting result was that no radiotracer uptake was seen in the lung or liver in any patient [Figure 2].
Figure 2.
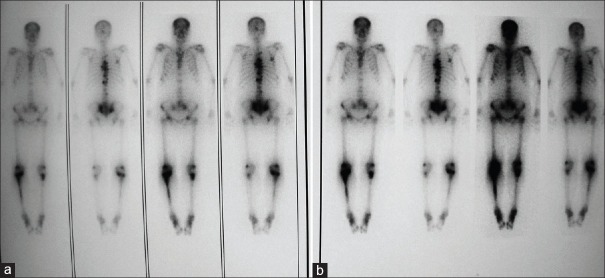
Whole body scans with 177Lu-ethylenediaminetetramethylene phosphonic acid from a patient at the day 1 (a) and 3 (b) after the injection
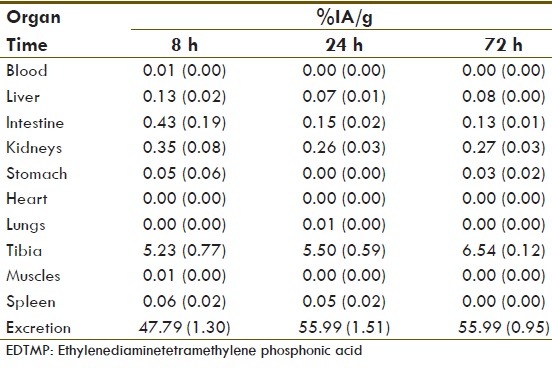
Biokinetics study using the collected blood and urine samples 8, 24 and 72 h after the injection of the drug shows that the biologic half-life of Lu-EDTMP for an average person with normal renal function test were in the range of 3.8 ± 1.7 days. The remaining radioactive material reduced to <37 kBq/ml in blood samples and <740 kBq/ml in the urine samples after 72 h [Table 3].
Table 3.
Biokinetic study of various organs in the patients 8 h, 24 h and 72 h after the injection of 177Lu-EDTMP
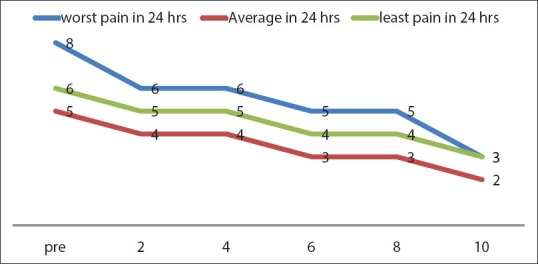
Pain relief began 2.53 ± 2.08 weeks after the injection, and the response lasted for 4.38 ± 3.34 weeks. Among the 30 patients treated with 177Lu-EDTMP, 16 patients (53%) showed complete palliative pain response; 9 patients (30%) revealed partial response and 5 (17%) showed no response to treatment. Total response to treatment was achieved in 25 patients (83%) [Figure 3].
Figure 3.
Mean pain assessment by brief pain inventory scoring in the patients before the injection of the 177Lu-ethylenediaminetetramethylene phosphonic acid and every 2 weeks up to 12 weeks
Results of BPI questionnaire are summarized in Table 2. According to short form of BPI among the studied criteria including walking ability, ability for doing normal daily work, mood, and sleep were promoted respectively more than the other criteria, where almost all of the cases reported that pain didn't interfere in these aspects of their life during the follow up period [Table 4 and Figure 4].
Table 4.
Mean response to treatment by patients with bone metastasis treated with 177Lu-EDTMP
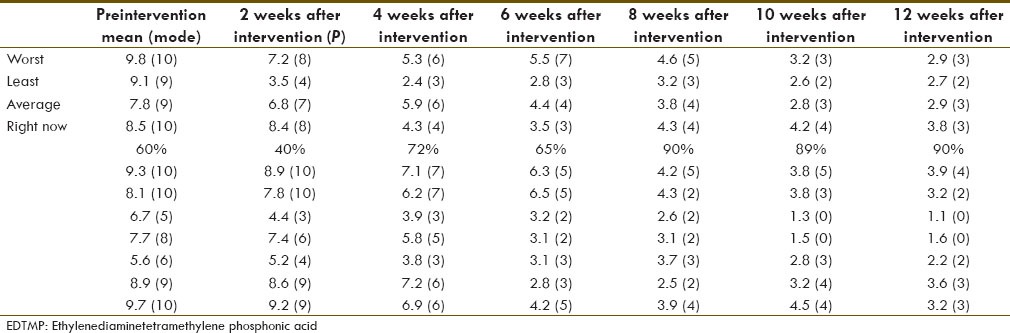
Figure 4.
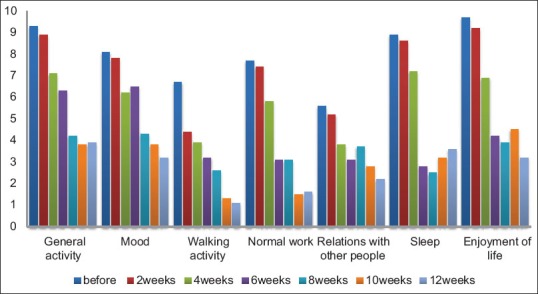
Response to treatment by 177Lu-ethylenediaminetetramethylene phosphonic acid of the patients with bone metastasis
Comparison of alkaline phosphatase before (419.4 ± 270.46) and after (434.7 ± 162.35) the injection of the drug showed that the amount of the enzyme decreased after the injection, but there was no significant difference before and after the treatment (P = 0.19). Moreover, white blood cells (WBC) dropped earlier than Hb and platelet count (PLT). A significant reduction of Hb and PLT occurred 4 weeks post-injection (P = 0.031), but not in WBC count 4 weeks after the injection of the drug (P = 0.01 and P = 0.024). Furthermore, about WBC, after a slow decrease of counts at 2 weeks post-injection, a slow increase of counts occurred and then its levels reached the normal levels. Blood parameters in eight patients at 4–6th week's post-injection were too low, and they were treated by subcutaneous injection of granulocyte colony stimulating factor (GCSF). Finally, no significant hematologic defects were observed, and blood parameters were normal at the end of the study [Figure 5 and Table 5]. Furthermore, no disorder was seen in renal function test in any patient [Figure 6].
Figure 5.
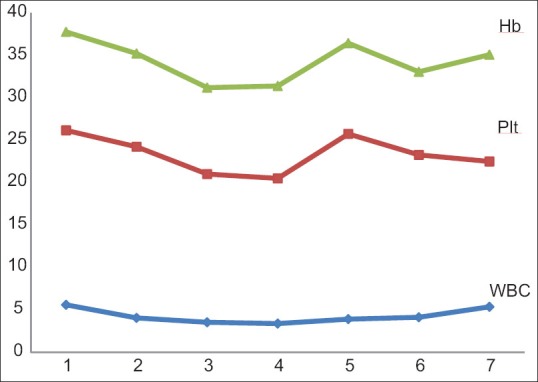
Mean changes in hematologic parameters in the patients after the injection of 177Lu-ethylenediaminetetramethylene phosphonic acid
Table 5.
Mean amount of blood parameters before and after the injection of the drug (mean±SD)
Figure 6.
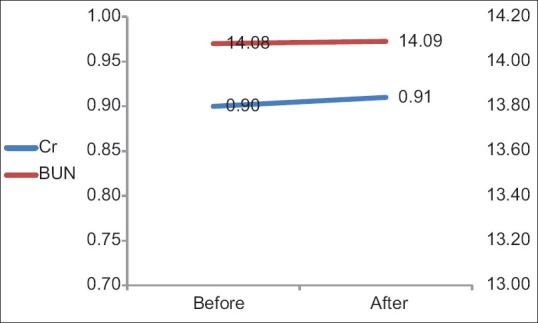
The results of renal function tests before and 3 weeks after the injection of 177Lu-ethylenediaminetetramethylene phosphonic acid
Discussion
Selecting a suitable radiopharmaceutical for bone pain metastasis (BPM) palliation is a multi-factorial question which needs awareness not only about physical properties of radioisotope, but also about biological properties like bio-distribution, biological half-life and mechanism of targeting through the tissue. Among the rare earth elements 177Lu seems to have good physical properties because of long half-life, better logistic from reactor to hospital, longer effect with single administration, adequate beta emission to kill the adjacent cells, and good gamma photon for imaging in regard to checking the bio-distribution and metastatic lesion follow up.
Dosimetric and imaging studies show the possibility of using this radioisotope for BPM palliation therapy in terms of physical properties; in contrast to other studies using 177Lu just for imaging of metastatic bone lesions, we found that for using this radioisotope therapeutically with the dose of 29.6 MBq/Kg, the best time for imaging is 24 h.
Biological specifications of 177Lu-EDTMP is also studied, showing that it has a good affinity to metastatic bone lesions while the lung and liver uptake is low enough although effective half-life of 177Lu-EDTMP seems to be <4 days in normal patients.[25,26]
Routine activities were more pleasurable in patients with positive flare phenomenon; it may be because of less pain after the injection of the drug in such patients. Furthermore, MBP decreased dramatically after this intervention; however, there were not any side effect reported according to our early close observation or 12 week follow up. Routine activities were improved after treatment with 177Lu-EDTMP, especially in patients with positive flare phenomenon.
In one study, Samarium-EDTMP therapy with 36.5% complete response rate was reported to be an effective and safe solution for MBP palliation. Their study showed a significant reduction in all three types of blood cell counts (WBC, red blood cell [RBC] and PLT) and grade 3 bone marrow toxicity was not observed for RBCs.[27] Two other retrospective studies on the clinical role of 153Sm-EDTMP on a large group of patients with different kinds of malignancies showed 21% and 40% complete response, respectively.[28,29] In another study, 177Lu-EDTMP and 177Lu-tetramethylene-phosphonic acid (DOTMP) were evaluated as potential agents for palliative radiotherapy of bone metastasis, revealing that 177Lu-EDTMP has marginally higher skeletal accumulation in comparison to that of 177Lu-DOTMP, while the latter has slightly faster blood clearance along with lower retention in the liver and kidneys in animal models.[17] Furthermore another study reported that the treatment with [177Lu-DOTA0, Tyr3] Octreotate has few adverse effects.[30]
Treatment with 177Lu-octreotate is also reported to result in tumor remission in a high percentage of patients with gastroenteropancreatic tumors.[31]. 89Sr with response rates ranging from 60% to 84%,[32] but no survival benefit, even when a dose of 399.6 MBq (10.8 mCi) is used[33,34] and 153Sm with pain palliation rates of 62%–74%[35,36,37] that may have myelotoxicity are comparable with 177Lu –EDTMP in bone pain palliation.
Hematological factors, such as WBC or PLT drop which was seen in the case, in Nadir period could be managed by subcutaneous injection of stimulating growth factor such as GCSF.
It is recommended that another study should be conducted with longer follow up times (6 months) while performing RCT among cases and control groups and also quantitative dosimetric calculation using acquired images.
Because of better physical properties of 177Lu compared to 153Sm and acceptable bio-distribution results of the compound, 177Lu-EDTMP seem to be an interesting new candidate for clinical trials for a bone pain palliation therapy.[38]
Conclusion
This clinical trial of 177Lu-EDTMP for MBP palliation therapy shows good performance in pain relief with the dose of 29.6 MBq/Kg in the long term follow up. At the end of the evaluation, no bone marrow suppression or hematologic toxicity was observed.
Acknowledgments
This project was performed with financial support of Shiraz University of Medical Sciences (SUMS 8901012039). Authors would like to thank all the staff of Radiopharmaceutical Research and Development Lab, Nuclear Science and Technology Research Institute for preparing 177Lu-EDTMP. Staff in Nuclear medicine department of Namazee teaching hospital are appreciated for helpful cooperation in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Goeckeler WF, Edwards B, Volkert WA, Holmes RA, Simon J, Wilson D. Skeletal localization of samarium-153 chelates: Potential therapeutic bone agents. J Nucl Med. 1987;28:495–504. [PubMed] [Google Scholar]
- 2.Maini CL, Bergomi S, Romano L, Sciuto R. 153Sm-EDTMP for bone pain palliation in skeletal metastases. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S171–8. doi: 10.1007/s00259-004-1540-y. [DOI] [PubMed] [Google Scholar]
- 3.Twycross RG, Fairfield S. Pain in far-advanced cancer. Pain. 1982;14:303–10. doi: 10.1016/0304-3959(82)90137-3. [DOI] [PubMed] [Google Scholar]
- 4.Pandit-Taskar N, Batraki M, Divgi CR. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med. 2004;45:1358–65. [PubMed] [Google Scholar]
- 5.Lewington VJ. Cancer therapy using bone-seeking isotopes. Phys Med Biol. 1996;41:2027–42. doi: 10.1088/0031-9155/41/10/012. [DOI] [PubMed] [Google Scholar]
- 6.Hoskin PJ. Bisphosphonates and radiation therapy for palliation of metastatic bone disease. Cancer Treat Rev. 2003;29:321–7. doi: 10.1016/s0305-7372(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 7.Lewington VJ. Bone-seeking radionuclides for therapy. J Nucl Med. 2005;46(Suppl 1):38S–47. [PubMed] [Google Scholar]
- 8.Volkert WA, Hoffman TJ. Therapeutic radiopharmaceuticals. Chem Rev. 1999;99:2269–92. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]
- 9.Hosain F, Spencer RP, editors. Radiopharmaceuticals for palliation of metastatic osseous lesions: Biologic and physical background. Semin Nucl Med. 1992;22:11–6. doi: 10.1016/s0001-2998(05)80152-7. [DOI] [PubMed] [Google Scholar]
- 10.Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001;42:895–906. [PubMed] [Google Scholar]
- 11.McEwan AJ, editor. Use of radio nuclides for the palliation of bone metastases. Semin Radiat Oncol. 2000;10:103–14. doi: 10.1016/s1053-4296(00)80047-8. [DOI] [PubMed] [Google Scholar]
- 12.Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierckx RA, et al. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest. 2009;32:360–9. doi: 10.1007/BF03345728. [DOI] [PubMed] [Google Scholar]
- 13.Michel RB, Andrews PM, Rosario AV, Goldenberg DM, Mattes MJ. 177Lu-antibody conjugates for single-cell kill of B-lymphoma cells in vitro and for therapy of micrometastases in vivo. Nucl Med Biol. 2005;32:269–78. doi: 10.1016/j.nucmedbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty S, Das T, Banerjee S, Balogh L, Chaudhari PR, Sarma HD, et al. 177Lu-EDTMP: A viable bone pain palliative in skeletal metastasis. Cancer Biother Radiopharm. 2008;23:202–13. doi: 10.1089/cbr.2007.374. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty S, Das T, Banerjee S, Sarma HD, Venkatesh M. Preparation and preliminary biological evaluation of 177Lu-labelled hydroxyapatite as a promising agent for radiation synovectomy of small joints. Nucl Med Commun. 2006;27:661–8. doi: 10.1097/00006231-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty S, Das T, Sarma HD, Venkatesh M, Banerjee S. Preparation and preliminary studies on 177Lu-labeled hydroxyapatite particles for possible use in the therapy of liver cancer. Nucl Med Biol. 2008;35:589–97. doi: 10.1016/j.nucmedbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty S, Das T, Sarma HD, Venkatesh M, Banerjee S. Comparative studies of 177Lu-EDTMP and 177Lu-DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl Radiat Isot. 2008;66:1196–205. doi: 10.1016/j.apradiso.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 18.Máthé D, Balogh L, Polyák A, Király R, Márián T, Pawlak D, et al. Multispecies animal investigation on biodistribution, pharmacokinetics and toxicity of 177Lu-EDTMP, a potential bone pain palliation agent. Nucl Med Biol. 2010;37:215–26. doi: 10.1016/j.nucmedbio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Brašic JR, Liu X, Li H, Xiang X, Luo Z, et al. Timing and optimized acquisition parameters for the whole-body imaging of 177Lu-EDTMP toward performing bone pain palliation treatment. Nucl Med Commun. 2012;33:90–6. doi: 10.1097/MNM.0b013e32834d3c13. [DOI] [PubMed] [Google Scholar]
- 20.Kocherov N, McLaughlin P. The international reactor dosimetry file (IAEA-NDS-141 Rev. 1993 Oct. [Google Scholar]
- 21.Bahrami-Samani A, Anvari A, Jalilian AR, Shirvani-Arani S, Yousefnia H, Aghamiri MR, et al. Production, quality control and pharmacokinetic studies of 177lu-edtmp for human bone pain palliation therapy trials. Iran J Pharm Res. 2012;11:137–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 23.Wu JS, Beaton D, Smith PM, Hagen NA. Patterns of pain and interference in patients with painful bone metastases: A brief pain inventory validation study. J Pain Symptom Manage. 2010;39:230–40. doi: 10.1016/j.jpainsymman.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Mirzamani S, Sadidi A, Salimi S, Besharat M. Validation of the persian version of the brief pain inventory. Acta Med Iran. 2005;43:425–8. [Google Scholar]
- 25.Pommé S, Paepen J, Altzitzoglou T, Van Ammel R, Yeltepe E. Measurement of the 177Lu half-life. Appl Radiat Isot. 2011;69:1267–73. doi: 10.1016/j.apradiso.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Garske U, Sandström M, Johansson S, Sundin A, Granberg D, Eriksson B, et al. Minor changes in effective half-life during fractionated 177Lu-octreotate therapy. Acta Oncol. 2012;51:86–96. doi: 10.3109/0284186X.2011.618511. [DOI] [PubMed] [Google Scholar]
- 27.Ayati N, Aryana K, Jalilian A, Hoseinnejad T, Bahrami Samani A, Ayati Z, et al. Treatment efficacy of 153Sm-EDTMP for painful bone metastasis. Asia Oceania J Nucl Med Biol. 2013;1:27–31. doi: 10.7508/aojnmb.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coronado M, Redondo A, Coya J, Espinosa E, Couto RM, Zamora P, et al. Clinical role of Sm-153 EDTMP in the treatment of painful bone metastatic disease. Clin Nucl Med. 2006;31:605–10. doi: 10.1097/01.rlu.0000238304.08812.c1. [DOI] [PubMed] [Google Scholar]
- 29.Baczyk M, Czepczynski R, Milecki P, Pisarek M, Oleksa R, Sowinski J. 89Sr versus 153Sm-EDTMP: Comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nucl Med Commun. 2007;28:245–50. doi: 10.1097/MNM.0b013e32805b72a0. [DOI] [PubMed] [Google Scholar]
- 30.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3] octreotate: Toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 31.Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3] octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–62. doi: 10.1200/JCO.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 32.Sciuto R, Festa A, Pasqualoni R, Semprebene A, Rea S, Bergomi S, et al. Metastatic bone pain palliation with 89-Sr and 186-Re-HEDP in breast cancer patients. Breast Cancer Res Treat. 2001;66:101–9. doi: 10.1023/a:1010658522847. [DOI] [PubMed] [Google Scholar]
- 33.Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25:805–13. doi: 10.1016/0360-3016(93)90309-j. [DOI] [PubMed] [Google Scholar]
- 34.Brundage MD, Crook JM, Lukka H. Use of strontium-89 in endocrine-refractory prostate cancer metastatic to bone. Provincial Genitourinary Cancer Disease Site Group. Cancer Prev Control. 1998;2:79–87. [PubMed] [Google Scholar]
- 35.Eary JF, Collins C, Stabin M, Vernon C, Petersdorf S, Baker M, et al. Samarium-153-EDTMP biodistribution and dosimetry estimation. J Nucl Med. 1993;34:1031–6. [PubMed] [Google Scholar]
- 36.Collins C, Eary JF, Donaldson G, Vernon C, Bush NE, Petersdorf S, et al. Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: A phase I/II trial. J Nucl Med. 1993;34:1839–44. [PubMed] [Google Scholar]
- 37.Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: A double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574–81. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 38.BahramiI SA, Anvari A, Jalilian AR, Shirvani AS, Yousefnia H, Aghamiri MR. Production, quality control and pharmacokinetic studies of 177LU-EDTMP for human bone pain palliation therapy trials. Iran J Pharm Res (IJPR) 2012;11:137. [PMC free article] [PubMed] [Google Scholar]


