Abstract
The ANZDATA Registry includes all patients treated with renal replacement therapy (RRT) throughout Australia and New Zealand. Funding is predominantly from government sources, together with the non-government organization Kidney Health Australia. Registry operations are overseen by an Executive committee, and a Steering Committee with wide representation. Data is collected from renal units throughout Australia and New Zealand on a regular basis, and forwarded to the Registry. Areas covered include demographic details, primary renal disease, type of renal replacement therapy, process measures, and a variety of outcomes. From this data collection a number of themes of work are produced. These include production of Registry reports with an extensive range of national and regional data, a suite of quality assurance reports, key process indicator (KPI) reports, and data sets for a variety of audit and research purposes. The various types of information from the ANZDATA Registry are used in a wide variety of areas, including health services planning, safety and quality programs, and clinical research projects.
Keywords: dialysis, end-stage kidney disease, incidence, prevalence, registry
Registries have a long history in the area of kidney disease; the uptake and outcome of therapies (in particular dialysis and transplantation) has been documented far better than that of many other diseases or therapies.
There have, however, been important changes in the role of registries in renal disease. From an initial role in collecting the incidence and outcomes of rare diseases and exotic treatments, increases in incidence rates and availability of dialysis treatment has driven a steady progression toward a role incorporating provision of information to support health service development and quality activities.
The Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry is a registry based in Adelaide, Australia, which covers renal dialysis and kidney transplantation. It includes all patients treated with renal replacement therapy (RRT) throughout Australia and New Zealand. Formed in 1975 by the merger of separate dialysis and transplant registries in Australia, it has national coverage across both countries of all people treated with these therapies since 1963. In this article, the operations and breadth of output of the Registry are described and discussed.
MATERIALS AND METHODS
Data collection and analysis
Data is collected by the registry from renal units throughout Australia and New Zealand, on all patients receiving chronic dialysis or kidney transplantation. The key inclusion criterion is that RRT is commenced with the intention of chronic RRT; unlike some Registries ANZDATA does not impose a criterion based on the time of dialysis but uses an intent-based definition of ‘chronic RRT'.
There are two basic streams of data collection: the registry asks to be notified in ‘real-time' (in actuality, within 30 days) of key events (dialysis, transplantation, death, and loss of transplant function). In addition, a cross-sectional survey is conducted of all patients at 31 December each year. The survey includes substantial amounts of process information, depending on the treatment modality. For those receiving haemodialysis, this includes dialyser type, dialysis prescription, dry weight, and type of dialysis access; for peritoneal dialysis patients, episodes of peritonitis are collected as are PET results and fluids used. Basic biochemistry (haemoglobin, calcium, and phosphate) are collected for all dialysis patients. For transplant recipients graft function, rejection episodes and immunosuppressive drug use and dosage are recorded.
Details of the data collection items are available at anzdata.org.au. ‘Real-time' data is submitted via a secure web-based portal or on paper. The year-end survey is currently paper based, with pre-printed forms distributed from the office at the end of each year. Trials of electronic data entry from some units with computerized patient management systems are underway. Within individual renal units, approaches vary to the actual collection of data. In some cases, nephrologists fill out the bulk of the information. In other units, substantial amounts are performed by administrative staff, with clinical staff adding key components (e.g., comorbidity prevalence).
Like all health information, privacy and confidentiality of data collection and storage are important. This is now governed by clear principles and guidelines agreed at a national level.1, 2
Funding and governance
Funding for ANZDATA is provided by the Australia Organ and Tissue Donation and Transplantation Authority, the New Zealand Ministry of Health and Kidney Health Australia. This funding covers the expenses of the core office staff. The costs of data collection are borne by the individual renal units from within their own budgets.
The central office is conducted at the Royal Adelaide Hospital, co-located with the Australia and New Zealand Organ Donation Registry. Day-to-day operations are overseen by an Executive Group, and strategic directions are overseen by a Steering Committee. This committee is responsible both to the Australia and New Zealand Society of Nephrology and to Kidney Health Australia, and has wide representation from the nephrology community in Australia and New Zealand, and nursing and consumer representation.
RESULTS
Data utilization
From this data collection a number of themes of work are produced. Annual Reports (with an extensive range of national and regional data) are produced and distributed via the website as well as print. A variety of other reports are produced for various groups on a regular basis, including various quality assurance reports, key process indicator (KPI) reports and interim data summaries.
Registry reports
Each year the Registry produces a report with an extensive range of data on incidence and management of end-stage kidney disease in Australia and New Zealand.
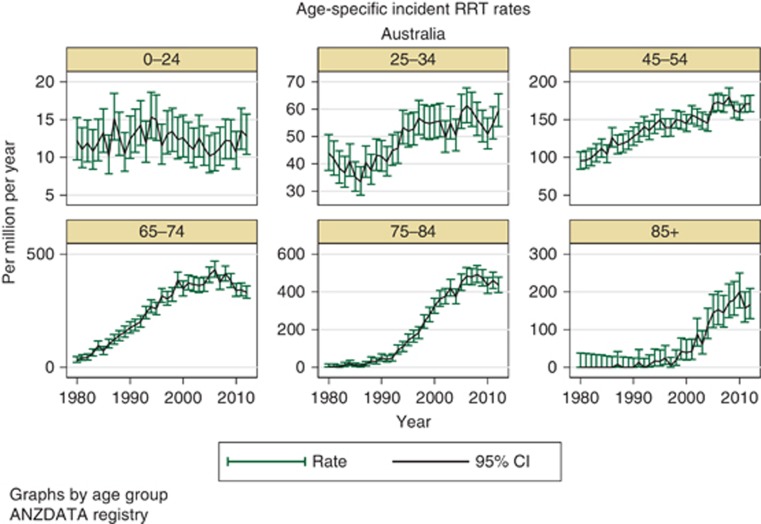
Rates of incident dialysis have stabilized over the last 5 years or so, in both Australia and New Zealand (Table 1). However, the changes in incidence rates have not affected all age groups equally, with differing trends over time seen among younger versus older people (Figure 1). The most common diagnosis for primary renal disease is now diabetic nephropathy, which overtook glomerulonephritis some years ago.
Table 1. Rates of new renal replacement therapy (dialysis and transplantation) per million population per year for Australia and New Zealand.
| Year | Australia | New Zealand |
|---|---|---|
| 2012 | 112 | 116 |
| 2011 | 112 | 110 |
| 2010 | 106 | 18 |
| 2009 | 112 | 135 |
| 2008 | 119 | 116 |
Figure 1.
Age-specific incidence rates for renal replacement therapy in Australia.
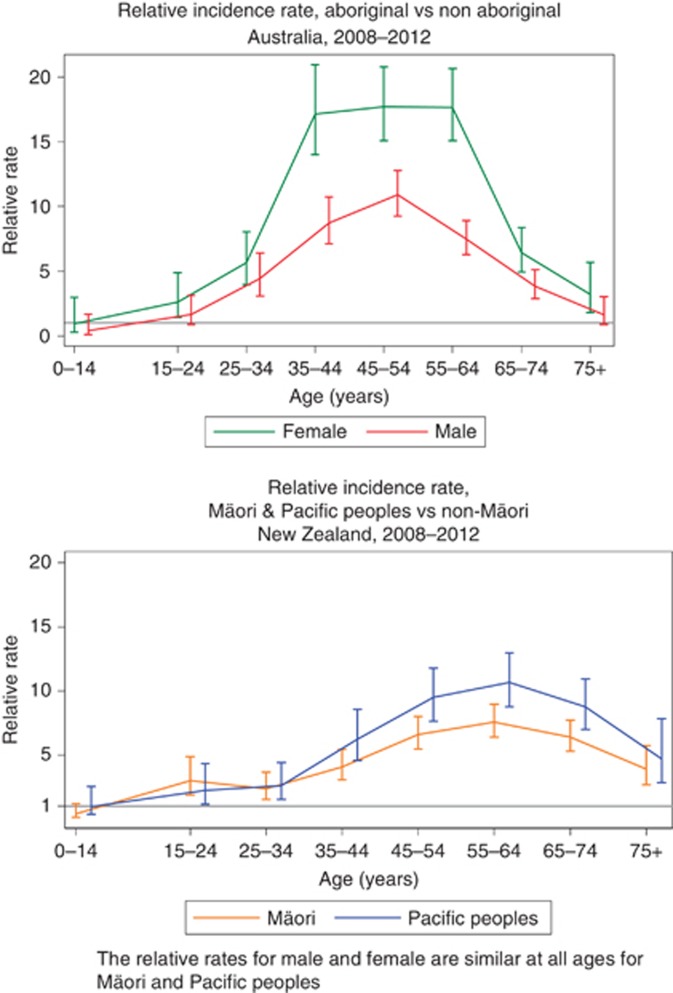
Aboriginal and Torres Strait Islander people (the indigenous people of Australia) and Maori (the indigenous people of New Zealand) both suffer from substantially increased rates of kidney disease, as do ‘Pacific Peoples' in New Zealand. There is considerable literature describing the origins and background to this, with high rates of albuminuria at all ages attributed to multiple causes.3 Risk of end-stage kidney disease varies with age, with a particular increase among those aged 30–60 years (Figure 2).
Figure 2.
Relative rates of incident renal replacement therapy (RRT) among Aboriginal and Torres Strait Islander people in Australia, and among Maori and Pacific Islanders in New Zealand.
Health service planning
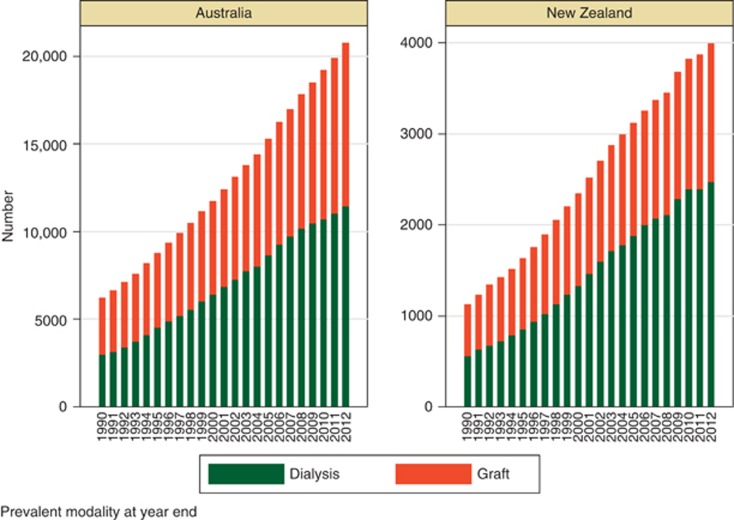
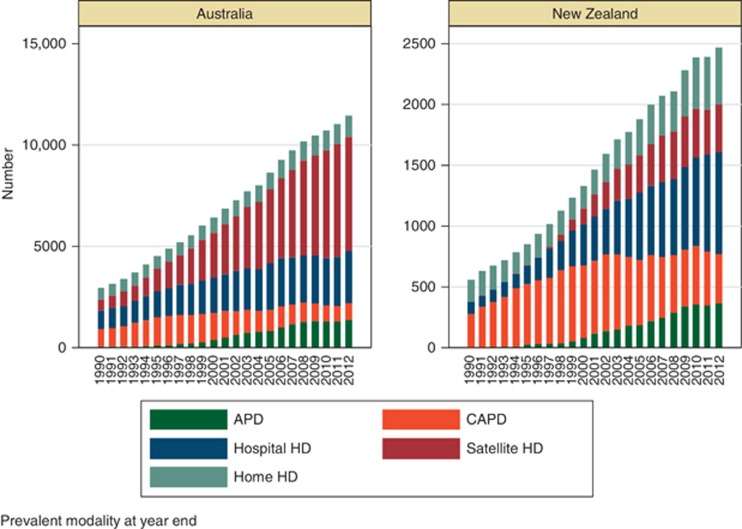
One of the key roles of a registry is to provide information to assist appropriate planning of health services. Data from the ANZDATA registry has been provided to a variety of jurisdictions over time, to support work analyzing and projecting demand for various types of RRT. Although incidence rates have stabilized, the number of prevalent patients has progressively increased over time, with an increasing proportion of dialysis patients Figure 3. Within the dialysis population, the overall proportion of peritoneal dialysis patients is falling in both Australia and New Zealand with a progressive increase in the proportion of satellite and hospital haemodialysis-treated patients Figure 4.
Figure 3.
Number of prevalent dialysis and transplant patients in Australia and New Zealand by year.
Figure 4.
Numbers of prevalent dialysis patient at 31 December by modality.
Projecting the likely direction of these trends into the future is important both to understanding the demands for service provision, and also to inform policy developments. At a national level, major work has recently been undertaken by the Australian Institute of Health and Welfare4 with ongoing increase in dialysis numbers expected. At a state and territory, more detailed work has been undertaken examining the current and projected demand for various dialysis modalities. Examples of recent policies in Australia to encourage greater uptake of home-based modalities include the introduction of a national scheme to reimburse costs of living kidney donors, and a number of state-based schemes to encourage uptake of home-based dialysis modalities by providing compensation for patients' water and electricity charges, and aligning hospital funding incentives with the proportion of home dialysis haemodialysis (HD) and peritoneal dialysis (PD) patients.
Individual hospital reports and KPIs
For many years, ANZDATA has produced yearly individual hospital reports describing the outcomes at an individual hospital level. From origins with simple unadjusted analyses, these have evolved into more complex documents with comparisons of observed outcomes with those expected on the basis of an individual centre's patient mix.
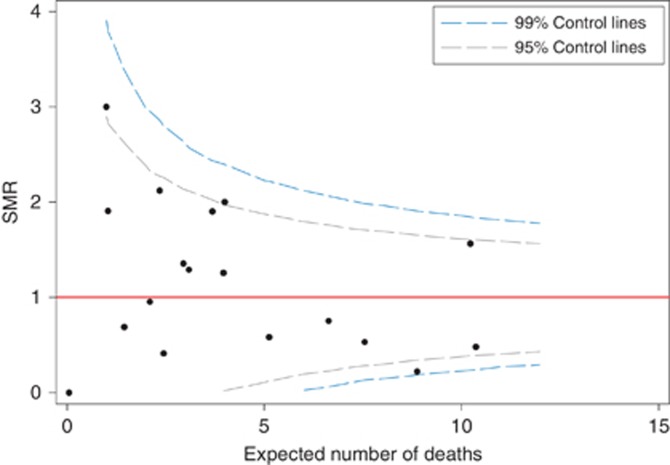
An example of one such analysis is shown in Figure 5. This is a funnel plot; it illustrates the ratio between observed and expected numbers of deaths within 1 year of transplantation together with the statistical confidence limits assuming the actual (true) performance of a centre is at the national average (i.e., underlying ratio of observed/expected events=1.0). The graph illustrates some important issues surrounding these types of graphs related to the calculation of expected numbers from multivariate analysis. This particular illustration uses expected numbers based on a fixed effects logistic regression for survival at 1 year post transplantation. The presence of more points outside the 95 and 99% confidence intervals suggests the presence of greater variation than that seen simply due to random change, consistent with unobserved confounding factors. There are a number of analytic techniques which address this such as hierarchical and random effects analyses and other techniques of ‘shrinkage' of variance.
Figure 5.
Funnel plot with ratio of observed/expected patient deaths by 1 year post transplantation. Centre analyzed as fixed effect.
Currently the ANZDATA Registry produces yearly reports for each hospital covering the outcomes of dialysis patients and transplant patients cared for within that hospital over a 5-year period using a random effects model; for transplanting hospitals, a separate report is produced. For dialysis patients, these reports include mortality as well as peritonitis rates (for PD patients) and technique survival (for PD and home HD patients) and dialysis access (for HD patients).
Although these reports contain a host of detailed information, maintaining a quality improvement cycle requires more contemporary feedback of results. To address this need, in 2011 the Registry began to produce reports on a quarterly basis addressing to KPIs for dialysis patients—access in use for patients newly starting haemodialysis, and the peritonitis rate among prevalent PD patients. The data in these reports is based on the ‘real time' data submitted, and is distributed at the end of each quarter, comparing the outcomes for each centre over the preceding 3 months with the other centres throughout Australia and New Zealand.
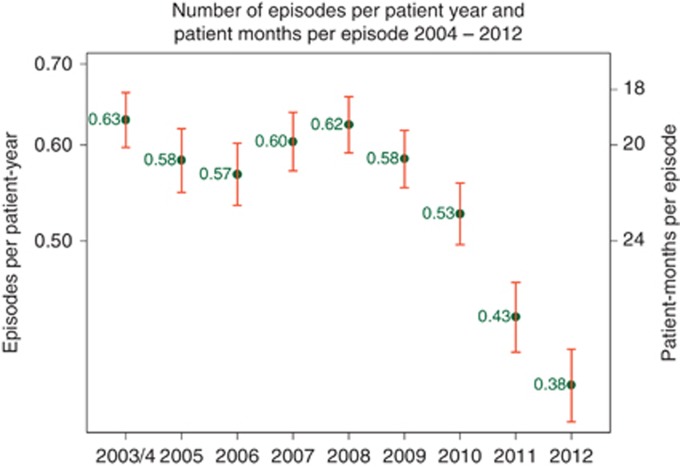
These two indicators were chosen as they are of particular concern to the nephrological community in Australia and New Zealand. Although Australia and New Zealand have historically had low rates of central venous catheter use among prevalent dialysis patients, the rate among incident patients has remained high (above 50%) for some time. Rates of peritonitis have also been high by international standards. This has led to a concerted series of actions, including updating of guidelines, the introduction of the KPI reporting, and a publicity campaign to heighten awareness of the issue.5 To date, results have been encouraging with a progressive fall in peritonitis rates (Figure 6).
Figure 6.
Rates of peritonitis among Australian peritoneal dialysis patients by year.
Research
In addition to use for health service planning and quality assurance, the large longitudinal observational data set held within the Registry is a valuable resource for addressing many clinical nephrological research questions. This has been an area of strategic development over the period from 2001 for the Registry, with a deliberate approach of increasing the breadth and depth of analyses of the database. This has been achieved by increasing the analytic resources available within the Registry, and also by building collaborations with contributors throughout Australia and New Zealand. As a part of this process, there has been a progressive increase in the number of nephrologists with expertise in analyses of large data sets. The process has been facilitated by the creation of a series of working groups (haemodialysis, peritoneal dialysis, transplantation, paediatrics, cancer, and indigenous kidney disease) with explicit mandate to foster the development of expertise and analyses within their particular area.
Registry data sets offer the ability to address areas that cannot be examined well with conventional approaches of randomized controlled trials and smaller observational studies. Inclusion of whole regions/countries minimizes selection bias and allows ascertainment of rare outcomes. ANZDATA has collected data for over 40 years, allowing the examination of long-term outcomes. Examples from the ANZDATA Registry of use of Registry data to address questions difficult to address by other means include the following:
Long-term outcomes of children with end-stage kidney disease.6
Risks of cancer associated with dialysis and with transplantation.7
Definition of the subgroups that are associated with benefits from peritoneal versus haemodialysis.8
Associations of dialysis session length with mortality.9
Associations of home therapies (particularly home haemodialysis) with differential outcomes.10
Investigation of the proportion of people with ‘end-stage kidney disease' that actually receive treatment with dialysis or transplantation.11
Variation in the incidence rates12 and treatment patterns13, 14 of end-stage kidney disease associated with socio-economic status.
Outcomes of pregnancy among patients with people with end-stage kidney disease.15
DISCUSSION
The ANZDATA registry is an example of a long established ‘mature' disease registry. Although similar registries now exist for a variety of disciplines and procedures, nephrology can take pride in its role in developing Registries. It fulfils a variety of roles for a variety of groups, which have evolved over time. In earlier stages, the focus was on documenting the incidence and outcomes of an emerging therapy applied to a rare disease. In contemporary times, Registries have extended become important resources for health departments; numbers of people receiving dialysis and transplantation have risen to a point where such treatments are common place. Tracking the rates and outcomes (and costs) of renal replacement therapies has become increasingly important. This has led to involvement of registries as key participants in the quality and safety sector, where their value has become increasingly recognized.16 The role of Registries in supporting research is also becoming increasingly important; e.g., they offer a cost-effective way to conduct follow-up for the longer period of time than the conventional randomized controlled trial.17
A hallmark of the ANZDATA Registry has been the strong participation by the nephrology community in Australia and New Zealand. This is reflected in the governance structure, ongoing provision of data by renal units, and also the strong involvement in Registry activities. This strength also creates a challenge, as the burden of data collection for units is large and onerous; there are ongoing discussions about methods to alleviate this and speed data returns. Like many cooperative ventures, the ANZDATA Registry relies on an enormous amount of goodwill and unpaid contributions from many people. This is both a strength and a weakness, as the ability of individuals to contribute is largely determined by factors outside ANZDATA's control.
Support for the ANZDATA registry comes from a number of sources. Financial support comes predominantly from government sources, with contribution from the non-government (philanthropic) sources. In the Australian and New Zealand context, there is an enormous unfunded contribution by clinicians in renal units throughout both countries in collecting and forwarding data. Inevitably, there are tensions at times between the dictates and demands from these various groups. Timely return of complete and accurate data is often one area of such tension—e.g., some users of the data need contemporary information to guide and influence policy and health services; however, there is no direct incentive for contributors to address this issue.
The research output from ANZDATA has been a successful program, both in terms of the number of publications and involvement of a broad range of people. However, the nature of this involvement must continue to evolve. There have been important differences between the approach taken by the ANZDATA Registry to others. One of these is a strong emphasis on involvement of contributors in projects, recognizing the source of the data. Future plans include information technology developments to facilitate online interrogation of the data set for simpler queries, and a focus on data linkage to facilitate projects on issues beyond those areas where ANZDATA hold data.
Acknowledgments
The ANZDATA Registry is funded by the Australian Organ and Tissue Donation and Transplantation Authority, the New Zealand Ministry of Health and Kidney Health Australia. The ANZDATA Registry could not exist without the untold hours of contributions of clinical and administrative staff from renal unit throughout Australia and New Zealand. This supplement was supported by a grant from the 59th Annual Meeting of the JSDT.
The author has received consulting fees from Baxter Healthcare and grant support from AMGEN Australia. The author declared no competing interests.
References
- Australian Commission on Safety and Quality in Health Care . Strategic and Operating Principles for a National Approach to Australian Clinical Quality Registries. ACSQHC: Sydney; 2010. [Google Scholar]
- Australian Commission on Safety and Quality in Health Care . Framework for Australian Clinical Quality Registries. ACSQHC: Sydney; 2014. [Google Scholar]
- Hoy WE, Mathews JD, McCredie DA, et al. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int. 1998;54:1296–1304. doi: 10.1046/j.1523-1755.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare Projections of the Prevalence of Treated End-Stage Kidney Disease in Australia 2012–2020Cat. no. PHE 176. AIHW: Canberra; 2014 [Google Scholar]
- Jose MD, Johnson DW, Mudge DW, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology. 2011;16:19–29. doi: 10.1111/j.1440-1797.2010.01390.x. [DOI] [PubMed] [Google Scholar]
- McDonald SP, Craig JC. Long term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- Vajdic CM, McDonald SP, McCredie MRE, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- McDonald SP, Marshall MR, Johnson DW, et al. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20:155–163. doi: 10.1681/ASN.2007111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MR, Byrne BG, Kerr PG, et al. Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int. 2006;69:1229–1236. doi: 10.1038/sj.ki.5000188. [DOI] [PubMed] [Google Scholar]
- Marshall MR, Hawley CM, Kerr PG, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis. 2011;58:782–793. doi: 10.1053/j.ajkd.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Sparke C, Moon L, Green F, et al. Estimating the total incidence of kidney failure in Australia including individuals who are not treated by dialysis or transplantation. Am J Kidney Dis. 2013;61:413–419. doi: 10.1053/j.ajkd.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Grace BS, Clayton P, Cass A, et al. Socio-economic status and incidence of renal replacement therapy: a registry study of Australian patients. Nephrol Dial Transplant. 2012;27:4173–4180. doi: 10.1093/ndt/gfs361. [DOI] [PubMed] [Google Scholar]
- Grace BS, Clayton PA, Cass A, et al. Transplantation rates for living- but not deceased-donor kidneys vary with socioeconomic status in Australia. Kidney Int. 2013;83:138–145. doi: 10.1038/ki.2012.304. [DOI] [PubMed] [Google Scholar]
- Grace BS, Clayton PA, Gray NA, et al. Socioeconomic differences in the uptake of home dialysis. Clin J Am Soc Nephrol. 2014;9:929–935. doi: 10.2215/CJN.08770813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesudason S, Grace BS, McDonald SP. Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin J Am Soc Nephrol. 2014;9:143–149. doi: 10.2215/CJN.03560413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S, Lawyer P, Garellick G, et al. Use of 13 disease registries in 5 countries demonstrates the potential to use outcome data to improve health care's value. Health Aff. 2012;31:220–227. doi: 10.1377/hlthaff.2011.0762. [DOI] [PubMed] [Google Scholar]
- Lauer MS, D'Agostino RB. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med. 2013;369:1579–1581. doi: 10.1056/NEJMp1310102. [DOI] [PubMed] [Google Scholar]