Abstract
Background and objectives
Cinacalcet and vitamin D are often combined to treat secondary hyperparathyroidism (SHPT) in patients on dialysis. Independent effects on fibroblast growth factor-23 (FGF-23) concentrations in patients on hemodialysis administered cinacalcet or vitamin D analogs as monotherapies during treatment of SHPT are evaluated.
Design, setting, participants, & measurements
A multicenter, randomized, open-label study to compare the efficacy of cinacalcet versus traditional vitamin D therapy for management of secondary hyperparathyroidism among subjects undergoing hemodialysis (PARADIGM) was a prospective, phase 4, multicenter, randomized, open-label study conducted globally. Participants (n=312) were randomized 1:1 to cinacalcet (n=155) or vitamin D analog (n=157) for 52 weeks. Levels of FGF-23 were measured at baseline and weeks 20 and 52. The absolute and percentage changes from baseline in plasma FGF-23, parathyroid hormone (PTH), calcium (Ca), phosphorus (P), and calcium-phosphorus product (Ca×P) were assessed. Correlations and logistic regression were used to explore relationships between changes in FGF-23 and changes in PTH, Ca, P, and Ca×P from baseline to week 52 by treatment arm.
Results
Median (quartiles 1, 3) decrease in FGF-23 concentrations was observed in the cinacalcet arm (−40%; −63%, 16%) compared with median increase in the vitamin D analog arm (47%; 0%, 132%) at week 52 (P<0.001). Changes in FGF-23 in both arms were unrelated to changes in PTH (cinacalcet: r=0.17, P=0.11; vitamin D analog: r=−0.04, P=0.70). Changes in FGF-23 in the vitamin D analog but not the cinacalcet arm were correlated with changes in Ca (cinacalcet: r=0.11, P=0.30; vitamin D analog: r=0.32, P<0.01) and P (cinacalcet: r=0.19, P=0.07; vitamin D analog: r=0.49, P<0.001). Changes in FGF-23 were correlated with changes in Ca×P in both arms (cinacalcet: r=0.26, P=0.01; vitamin D analog: r=0.57, P<0.001). Independent of treatment arm, participants with reductions in P or Ca×P were significantly more likely to show reductions in FGF-23.
Conclusions
During treatment of SHPT, cinacalcet use was associated with a decrease in FGF-23 concentrations, whereas vitamin D analogs were associated with an increase. The divergent effects of these treatments on FGF-23 seem to be independent of modification of PTH. It is possible that effects of cinacalcet and vitamin D analogs on FGF-23 may be mediated indirectly by other effects on bone and mineral metabolism.
Keywords: hyperparathyroidism, ESRD, dialysis, clinical nephrology
Introduction
Elevated concentrations of fibroblast growth factor-23 (FGF-23) are commonly observed in patients with advanced renal failure, and these elevations have been associated with increased risk of cardiovascular morbidity and mortality (1,2). The factors that contribute to increases in FGF-23 in renal failure are complex and not completely understood (3). Both phosphate retention and end organ resistance to the actions of FGF-23 (attributable to decreased expression of the FGF receptor and Klotho in target tissues) are likely partially responsible (4,5). Also, elevated concentrations of parathyroid hormone (PTH) may have direct stimulatory effects on FGF-23 production (6–10). FGF-23 may be more than a marker of adverse outcomes. Recent data show direct effects on cardiac myocytes, which suggest that FGF-23 may be a mediator of cardiac disease in patients with advanced renal failure (11).
Treatments for secondary hyperparathyroidism (SHPT) and disordered mineral metabolism commonly used in ESRD are hypothesized to have an effect on FGF-23 concentrations. Oral phosphate binders, vitamin D analogs, and the calcimimetic cinacalcet (Sensipar/Mimpara; Amgen Inc., Thousand Oaks, California) may have indirect effects on FGF-23 mediated by changes in PTH, calcium (Ca), and phosphorus (P) concentrations. In addition, therapies for SHPT may directly affect FGF-23 independent of their effect on PTH and mineral metabolism; 1,25-dihydroxy-vitamin D3 (calcitriol) is an important direct regulator of FGF-23, likely through a vitamin D response element (VDRE) in the promoter region of the FGF-23 gene (12,13). Additionally, the reported presence of the Ca-sensing receptor on the surface of bone cells suggests that calcimimetics might directly modulate FGF-23 production (14,15).
Studies have shown that treatment of SHPT with vitamin D analogs is associated with increases in FGF-23 concentrations, whereas use of cinacalcet is associated with a reduction in FGF-23 (16–22). However, the majority of these trials were single-arm studies, and in those that used cinacalcet, it was added to standard-of-care treatment regimens that generally included vitamin D analogs. As such, it has been difficult to make direct comparisons of the independent effects of these therapies on FGF-23 concentrations.
In contrast, a multicenter, randomized, open-label study to compare the efficacy of cinacalcet versus traditional vitamin D therapy for management of secondary hyperparathyroidism among subjects undergoing hemodialysis (PARADIGM) Trial was designed to directly compare cinacalcet and vitamin D analogs as monotherapy for the treatment of SHPT in patients with ESRD. This analysis of 312 participants from the PARADIGM Trial provides a unique opportunity to assess the independent effect of vitamin D analogs and cinacalcet on FGF-23 concentrations and their relationship to changes in PTH, Ca, P, and calcium-phosphorus product (Ca×P) in a large cohort of patients with ESRD treated for SHPT.
Materials and Methods
PARADIGM was a prospective, multicenter, phase 4, randomized, open-label study conducted globally. Adult participants (≥18 years old) on three times per week hemodialysis with PTH>450 pg/ml were randomized 1:1 to 12 months of treatment with either cinacalcet or vitamin D analogs to evaluate the mean percentage change in plasma PTH levels (primary end point) and proportion of participants achieving plasma PTH <300 pg/ml or a ≥30% decrease in PTH (secondary end points). The primary results of PARADIGM have been reported separately. Herein, we report the changes in FGF-23 concentrations during 12 months of treatment for SHPT, which was a preplanned exploratory analysis of the PARADIGM Trial.
Participants not receiving cinacalcet or vitamin D analogs within 60 days before enrollment were eligible if they had a plasma PTH ≥450 pg/ml and a total corrected serum Ca ≥8.4 and <10.2 mg/dl; participants receiving cinacalcet and/or vitamin D analogs were eligible if they met these requirements after a 4- to 5-week washout period. Parathyroidectomy in the 12 weeks before the date of informed consent was an exclusion. All participants provided written informed consent approved by the site’s Institutional Review Board or Ethics Committee.
Participants randomized to cinacalcet were initiated at a dose of 30 mg/d and titrated incrementally every 4 weeks as needed to 60, 90, 120, or 180 mg/d (maximum) on the basis of central laboratory assessments of plasma PTH and total corrected serum Ca to achieve plasma PTH <300 pg/ml. Participants treated with cinacalcet could receive nutritional vitamin D supplementation (ergocalciferol or cholecalciferol). Participants randomized to vitamin D analog therapy were given an initial dose approximately equivalent to an intravenous (iv) dose of 2 μg paricalcitol three times per week, and dosage was titrated to achieve plasma PTH <300 pg/ml. Type and route of vitamin D analog (iv or oral) were at the discretion of the site investigator. Most of the study participants were in the United States (83.3%). As such, the most commonly used vitamin D analogs were iv paricalcitol and iv doxercalciferol. Calcitriol and alfacalcidiol were frequently used in non–United States sites. Doses of vitamin D analogs are, therefore, expressed as paricalcitol equivalents per week, with 2.0 μg paricalcitol considered to be equivalent to 0.5 µg iv calcitriol, 1 µg iv doxercalciferol or alfacalcidol 3, 0.25 µg/d oral calcitriol, or 0.5 µg/d oral alfacalcidol. Nutritional vitamin D supplementation was prohibited in participants randomized to vitamin D analog therapy. Type and dose of phosphate binder prescribed were at the discretion of the investigator.
FGF-23 (intact, measured by a two-site ELISA; Immutopics Inc., San Clemente, California), PTH (intact, measured by a two-site sandwich immunoassay; ADVIA Centaur; Siemens/Bayer Healthcare Diagnostics, Tarrytown, New York), Ca, P, and Ca×P concentrations were determined at baseline and weeks 20 and 52, with all analyses performed at a central laboratory.
Statistical Analyses
Absolute and percentage changes in plasma FGF-23, PTH, Ca, P, and Ca×P concentrations from baseline to weeks 20 and 52 were summarized using descriptive statistics for each treatment arm. The correlation of change from baseline to week 52 between each of PTH, Ca, P, and Ca×P versus FGF-23 was determined with the Pearson correlation coefficient. The treatment group difference in mean change between baseline and week 52 for each of Ca, P, Ca×P, and FGF-23 was evaluated with a t test; the treatment effect for change in PTH was estimated with a nonparametric Wilcoxon test statistic because of non-normal data. Each laboratory measurement was also categorized according to whether the change from baseline to week 52 was a decrease (week 52: baseline ≤0) or an increase (week 52: baseline >0). A logistic regression model was performed to estimate the association of treatment with the decrease/increase change categories for each analyte against the decrease/increase change categories for FGF-23. A scatterplot divided into quadrants was used to illustrate the pairs (by participant) of decrease/increase change for each analyte with the corresponding change point for FGF-23. For each analyte/FGF-23 combination, subsequent tests of homogeneity (chi-squared tests) evaluated the distribution within each quadrant with respect to treatment arm. Multiplicity was not adjusted for, and therefore, all P values <0.05 are considered nominally significant. Analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
Demographic and baseline clinical characteristics of participants in the PARADIGM Study randomized to cinacalcet (n=155) or vitamin D analogs (n=157) were similar across treatment arms and are shown in Table 1. Of participants randomized to treatment, 65% in the cinacalcet arm and 61% in the vitamin D analog arm completed the study; 87.7% of participants randomized to cinacalcet and 92.9% of participants randomized to vitamin D analogs were on previous treatment with cinacalcet and/or vitamin D analogs and therefore, required washout. Baseline results were obtained after a 3- to 5-week washout period. Mean baseline values for PTH, FGF-23, Ca, P, Ca×P, and 25(OH)D3 levels were similar in both treatment arms (Table 2).
Table 1.
Participant demographics and baseline characteristics
| Characteristica | Cinacalcet | Vitamin D Analog |
|---|---|---|
| n | 155 | 157 |
| Age, yr | ||
| Median (minimum–maximum) | 53 (21–81) | 55 (22–86) |
| <65 | 120 (77) | 119 (76) |
| ≥65 | 35 (23) | 38 (24) |
| ≥75 | 12 (8) | 11 (7) |
| Sex | ||
| Men | 93 (60) | 95 (61) |
| Race | ||
| Asian | 8 (5) | 4 (3) |
| Black | 76 (49) | 60 (38) |
| White | 66 (43) | 86 (55) |
| Other | 5 (3) | 7 (4) |
| Ethnicity | ||
| Not Hispanic/Latino | 123 (79) | 110 (70) |
| Primary cause of ESRD | ||
| Diabetes | 62 (40) | 58 (37) |
| Hypertension | 40 (26) | 44 (28) |
| GN | 19 (12) | 18 (11) |
| Polycystic kidney disease | 9 (6) | 12 (8) |
| Urologic | 3 (2) | 1 (1) |
| Other | 18 (12) | 19 (12) |
| Unknown | 4 (3) | 5 (3) |
| Dialysis vintage, mo | ||
| Median (minimum–maximum) | 32.9 (4.3–216.7) | 38.1 (4.5–308.2) |
| Type of vascular access | ||
| Natural fistula | 98 (63) | 111 (71) |
| Graft | 37 (24) | 29 (18) |
| Permanent catheter | 17 (11) | 14 (9) |
| Other | 3 (2) | 3 (2) |
| Dialysate calcium,b mEq/L | ||
| Median (quartile 1, quartile 3) | 2.50 (2.00, 2.50) | 2.50 (2.25, 2.50) |
| History of kidney transplant | ||
| None reported | 147 (95) | 149 (95) |
| 1 | 3 (2) | 5 (3) |
| ≥2 | 5 (3) | 3 (1) |
All data are shown as n (%) unless otherwise stated.
Information missing for one participant in the vitamin D analog arm and two participants in the cinacalcet arm.
Table 2.
Summary of differences between cinacalcet and vitamin D at baseline
| Parameter | Vitamin D Analog Mean (SD) | Cinacalcet Mean (SD) | Difference of Cinacalcet − Vitamin D Analog (SD) | 95% CI for Difference | P Value |
|---|---|---|---|---|---|
| Calcium | 9.49 (0.54) | 9.55 (0.46) | 0.06 (0.50) | −0.05 to 0.17 | 0.30 |
| Phosphorus | 5.77 (1.49) | 5.73 (1.62) | −0.04 (1.55) | −0.39 to 0.30 | 0.80 |
| PTH | 815.7 (427.9) | 845.7 (431.3) | 30.02 (429.6) | −65.69 to 125.7 | 0.79a |
| Ca×P | 54.58 (14.16) | 54.81 (15.86) | 0.24 (15.03) | −3.14 to 3.61 | 0.89 |
| FGF-23 | 348.2 (282.0) | 336.0 (300.2) | −12.23 (291.4) | −82.67 to 58.20 | 0.73 |
| Vitamin D (25-hydroxy) | 26.13 (14.12) | 25.59 (13.97) | −0.55 (14.04) | −3.72 to 2.62 | 0.73 |
PTH, parathyroid hormone; Ca×P, calcium-phosphorus product; FGF-23, fibroblast growth factor-23; 95% CI, 95% confidence interval.
P value from nonparametric Wilcoxon test =0.79; nonparametric is more appropriate because of the known skewness of PTH data.
The primary end point of PARADIGM was the percentage change in PTH from baseline to the efficacy assessment period (weeks 40–52). Results showed a similar modest reduction in both treatment arms (mean; 95% confidence interval [95% CI] PTH change was −12%; −20% to −4% and −7%; −15% to 1% for cinacalcet and vitamin D analogs, respectively; P=0.35).
At week 52, the mean (SEM) dose of cinacalcet was 85.6 (5.4) mg/d in participants randomized to cinacalcet, and the mean (SEM) doses of vitamin D analogs were 21.4 (1.5) μg/wk (iv treatment) and 20.9 (4.1) μg/wk (oral treatment) in participants randomized to vitamin D analogs. Nutritional vitamin D was received by 20.0% of participants treated with cinacalcet and 3.4% of participants treated with vitamin D analogs. (Note that use of nutritional vitamin D in the vitamin D analog arm was prohibited by the study protocol.) Virtually all participants received phosphate binders at some point during the study, with Ca-based binders being more commonly used in participants treated with cinacalcet (49.7% in the cinacalcet arm and 31.8% in the vitamin D arm between weeks 40 and 52). Conversely, non-Ca–containing binder use was more prevalent in the vitamin D analog treatment arm (sevelamer at 43.3% and lanthanum carbonate at 8.9% in the vitamin D analog arm; sevelamer at 27.7% and lanthanum carbonate at 7.1% in the cinacalcet arm).
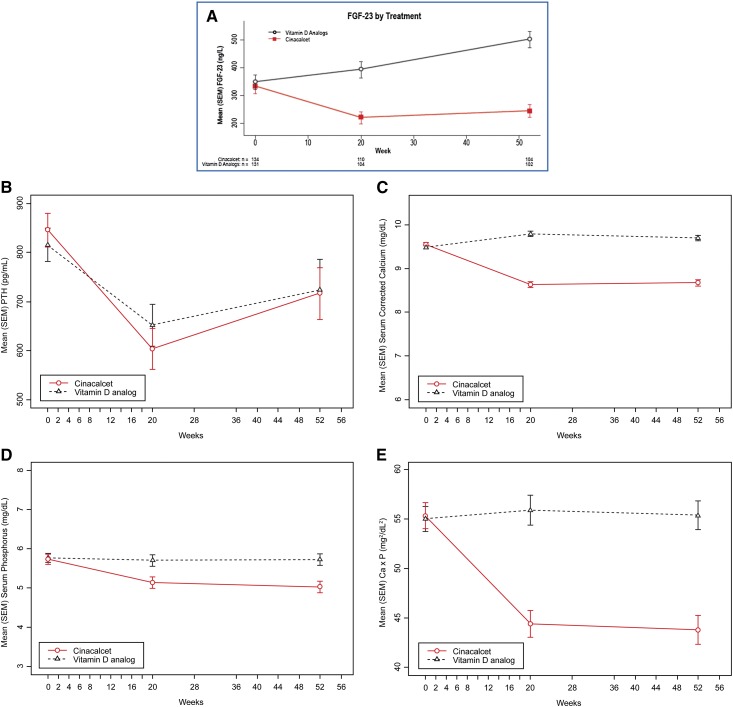
The mean (SEM) absolute values of FGF-23 (nanograms per liter) over time are depicted in Figure 1A. In the cinacalcet arm, a median (quartile 1 [Q1], Q3) change in FGF-23 concentrations of −41% (−64%, 0%) was observed at week 20, with a sustained decrease of −40% (−63%, 16%) at week 52. In contrast, for participants treated with vitamin D analogs, the median (Q1, Q3) percentage change in FGF-23 was 0% (−26%, 89%) between baseline and week 20, whereas a median (Q1, Q3) percentage increase of 47% (0%, 132%) was observed at week 52. Table 3 shows the values for FGF-23, PTH, Ca, P, and Ca×P at week 52 for both treatment arms. The mean (SEM) concentration of FGF-23 in the cinacalcet arm (225.6 ng/L [21.5 ng/L]) at week 52 was lower than the concentration in the vitamin D analog arm (480.5 ng/L [28.7 ng/L]; nominal P<0.001); 71% of the participants treated with cinacalcet showed a reduction in FGF-23 concentrations at week 52, whereas 65% of participants treated with vitamin D analog showed an increase in FGF-23 concentrations at week 52. Figure 1, B–E shows the mean (SEM) concentrations of PTH (picograms per milliliter), Ca (milligrams per deciliter), P (milligrams per deciliter), and Ca×P (milligrams2 per deciliter2) by treatment arm over time. There was no difference in the PTH levels between treatment arms at week 52; however, concentrations of Ca, P, and Ca×P were significantly lower in participants treated with cinacalcet than participants treated with vitamin D analog at week 52 (Table 3). In addition, cinacalcet treatment was associated with significantly greater reductions in FGF-23, Ca, P, and Ca×P than those observed in participants treated with vitamin D over the 52-week duration of the trial (Table 4).
Figure 1.
Change in concentrations of FGF-23, PTH, Ca, P, and Ca x P by treatment arm over time. (A) Mean (SEM) fibroblast growth factor-23 (FGF-23; nanograms per liter) concentrations by treatment arm over time. (B) Mean (SEM) parathyroid hormone (PTH; picograms per milliliter) concentrations by treatment arm over time. (Cinacalcet: n=155, 129, and 111 at weeks 0, 20, and 52, respectively; vitamin D analog: n=157, 127, and 111 at weeks 0, 20, and 52, respectively.) (C) Mean (SEM) serum calcium (Ca; milligrams per deciliter) by treatment arm over time. (Cinacalcet: n=155, 130, and 111 at weeks 0, 20, and 52, respectively; vitamin D analog: n=157, 124, and 112 at weeks 0, 20, and 52, respectively.) (D) Mean (SEM) serum phosphorus (milligrams per deciliter) by treatment arm over time. (Cinacalcet: n=155, 141, and 109 at weeks 0, 20, and 52, respectively; vitamin D analog: n=157, 135, and 110 at weeks 0, 20, and 52, respectively.) (E) Mean (SEM) serum calcium-phosphorus product (Ca×P; milligrams2 per deciliter2) by treatment arm over time. (Cinacalcet: n=146, 129, and 108 at weeks 0, 20, and 52, respectively; vitamin D analog: n=150, 124, and 110 at weeks 0, 20, and 52, respectively.)
Table 3.
Summary of differences in laboratory parameters between cinacalcet and vitamin D analogs at week 52
| Parameter | Vitamin D Analog Mean (SEM) | Cinacalcet Mean (SEM) | Difference (Cinacalcet − Vitamin D Analogs) Mean (SEM) | 95% CI for Mean Difference | P Value |
|---|---|---|---|---|---|
| FGF-23 (ng/L) | 480.5 (28.7) | 225.6 (21.5) | −255.0 (35.8) | −325.5 to −184.2 | <0.001 |
| PTH (pg/ml) | 724.5 (60.9) | 716.9 (52.7) | −7.59 (80.49) | −166.2 to 151.0 | 0.87a |
| Calcium (mg/dl) | 9.7 (0.1) | 8.7 (0.1) | −1.0 (0.1) | −1.2 to −0.8 | <0.001 |
| Phosphorus (mg/dl) | 5.7 (0.2) | 5.0 (0.2) | −0.7 (0.2) | −1.1 to −0.3 | 0.001 |
| Ca×P (mg2/dl2) | 55.4 (1.5) | 43.8 (1.5) | −11.6 (2.1) | −15.6 to −7.5 | <0.001 |
P value from nonparametric Wilcoxon; known skewness of PTH data.
Table 4.
Summary of treatment difference in change between baseline and week 52 for laboratory parameters
| Parameter | Vitamin D Analog Change Mean (SEM) | Cinacalcet Change Mean (SEM) | Difference (Cinacalcet − Vitamin D Analogs) Mean (SEM) | 95% CI for Mean Difference | P Value |
|---|---|---|---|---|---|
| FGF-23 (ng/L) | 138.9 (27.8) | −107.5 (26.8) | −246.3 (38.7) | −322.6 to −170.0 | <0.001a |
| PTH (pg/ml) | −78.9 (42.6) | −147.3 (47.2) | −68.4 (63.6) | −193.6 to 56.90 | 0.28 |
| Calcium (mg/dl) | 0.20 (0.06) | −0.86 (0.08) | −1.06 (0.10) | −1.26 to −0.87 | <0.001 |
| Phosphorus (mg/dl) | 0.005 (0.160) | −0.617 (0.170) | −0.622 (0.234) | −1.08 to −0.16 | <0.001 |
| Ca×P (mg2/dl2) | 1.26 (1.51) | −10.26 (1.55) | −11.52 (2.16) | −15.78 to −7.27 | <0.001 |
Median, quartile 1, quartile 3, and interquartile range for change from baseline: cinacalcet: −49.5, −183.8, 17.3, and 201.1; vitamin D analogs: 70.3, 0, 307.8, and 307.8.
Wilcoxon rank sum test (nonparametric): P value <0.001.
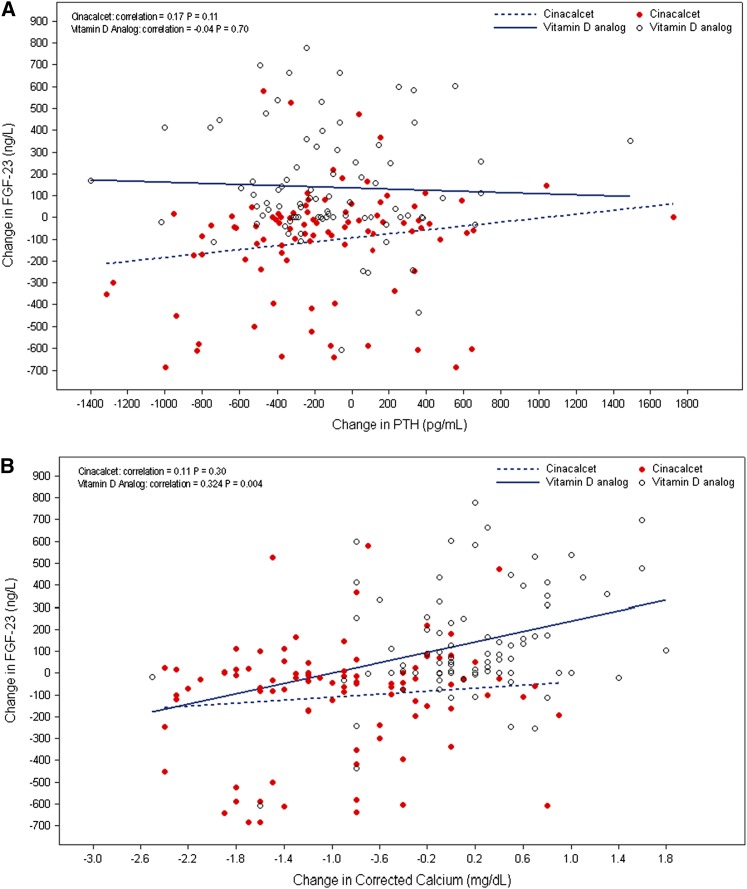
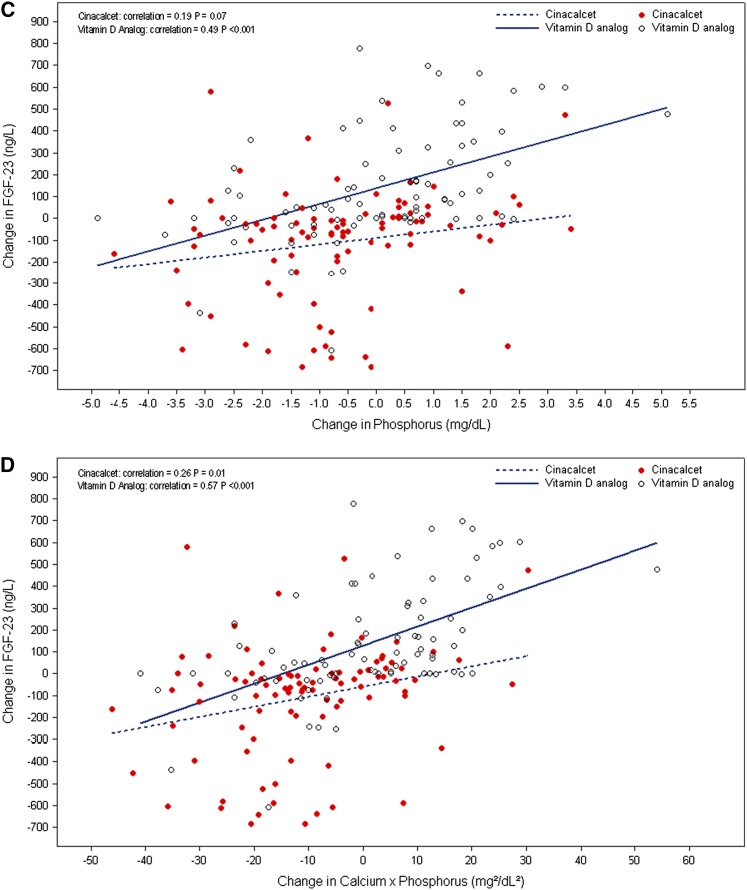
Changes in FGF-23 in the cinacalcet arm were not correlated with changes in PTH, Ca, or P, but a weak correlation with changes in Ca×P (correlation=0.26, P=0.01) was found (Figure 2). Changes in FGF-23 concentrations in participants treated with vitamin D analog were correlated with changes in Ca (correlation=0.32, P=0.004), P (correlation=0.49, P<0.001), and Ca×P (correlation=0.57, P<0.001) (Figure 2, B–D).
Figure 2.
Correlation between FGF-23 and PTH, Ca, P, or Ca x P by treatment arm. (A) Change from baseline in FGF-23 (nanograms per liter) versus change from baseline in PTH (picograms per milliliter) at week 52. (B) Change from baseline in FGF-23 (nanograms per liter) versus change from baseline in Ca (milligrams per deciliter) at week 52. (C) Change from baseline in FGF-23 (nanograms per liter) versus change from baseline in phosphorus (P;milligrams per deciliter) at week 52. (D) Change from baseline in FGF-23 (nanograms per liter) versus change from baseline in Ca×P (milligrams2 per deciliter2) at week 52.
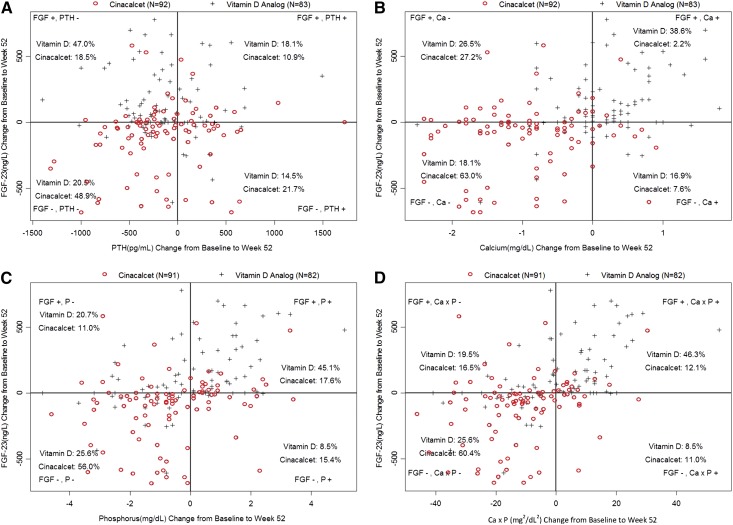
Quadrant Analyses
The quadrant analysis (Figure 3) explores differences in the patterns of the most commonly observed concurrent laboratory changes in the two treatment arms. In the cinacalcet arm, 49% of participants had concurrent decreases in PTH and FGF-23, 63% showed concurrent decreases in Ca and FGF-23, 56% had concurrent decreases in P and FGF-23, and 60% had concurrent decreases in Ca×P and FGF-23. Conversely, in the vitamin D analog arm, 47% showed a decrease in PTH and an increase in FGF-23, 39% had an increase in both Ca and FGF-23, 45% showed an increase in P and an increase in FGF-23, and 46% had concurrent increases in Ca×P and FGF-23. A test of homogeneity for the distribution of increase/decrease pairs for FGF-23 versus PTH, Ca, P, or Ca×P by treatment arm had a P<0.001, which even in the absence of multiplicity control, indicates a nonhomogenous distribution.
Figure 3.
Quadrant analysis showing the differences in the patterns of the most commonly observed concurrent laboratory changes by treatment arm. (A) Quadrant analysis—FGF-23 (nanograms per liter) versus PTH (picograms per milliliter) decrease/increase from baseline to week 52. FGF+, PTH−: cinacalcet, 18.5%; vitamin D analogs, 47.0%; FGF+, PTH+: cinacalcet, 10.9%; vitamin D analogs, 18.1%; FGF−, PTH−: cinacalcet, 48.9%; vitamin D analogs, 20.9%; FGF−, PTH+: cinacalcet, 21.7%; vitamin D analogs, 14.5%. (B) Quadrant analysis—FGF-23 (nanograms per liter) versus Ca (milligrams per deciliter) decrease/increase from baseline to week 52. FGF+, Ca−: cinacalcet, 27.2%; vitamin D analogs, 26.5%; FGF+, Ca+: cinacalcet, 2.2%; vitamin D analogs, 38.6%; FGF−, Ca−: cinacalcet, 63.0%; vitamin D analogs, 18.1%; FGF−, Ca+: cinacalcet, 7.6%; vitamin D analogs, 16.9%. (C) Quadrant analysis—FGF-23 (nanograms per liter) versus P (milligrams per deciliter) decrease/increase from baseline to week 52. FGF+, P−: cinacalcet, 11.0%; vitamin D analogs, 20.7%; FGF+, P+: cinacalcet, 17.6%; vitamin D analogs, 45.1%; FGF−, P−: cinacalcet, 56.0%; vitamin D analogs, 25.6%; FGF−, P+: cinacalcet, 15.4%; vitamin D analogs, 8.5%. (D) Quadrant analysis—FGF-23 (nanograms per liter) versus Ca×P (milligrams2 per deciliter2) decrease/increase from baseline to week 52. FGF+, Ca×P−: cinacalcet, 16.5%; vitamin D analogs, 19.5%; FGF+, Ca×P+: cinacalcet, 12.1%; vitamin D analogs, 46.3%; FGF−, Ca×P−: cinacalcet, 60.4%; vitamin D analogs, 25.6%; FGF−, Ca×P+: cinacalcet, 11.0%; vitamin D analogs, 8.5%.
The logistic regression models were adjusted for treatment of the decrease/increase in Ca or PTH concentration from baseline; neither Ca nor PTH change category was significantly associated with the change category for FGF-23 independent of treatment assignment with cinacalcet or vitamin D analogs. However, independent of treatment arm, the odds of a decrease from baseline in FGF-23 were significantly greater in participants who had a decrease from baseline in either P or Ca×P levels (P: odds ratio, 6.2; 95% CI, 3.0 to 12.5; Ca×P: odds ratio, 5.4; 95% CI, 2.6 to 11.1) (Table 5).
Table 5.
Summary of the odds ratio (logistic regression) for the decrease in FGF-23 by analyte
| Odds Ratio | PTH | Phosphorus | Calcium | Ca×P |
|---|---|---|---|---|
| OR (95% CI) for decrease in FGF-23 | 0.85 (0.43 to 1.68) | 6.16 (3.02 to 12.54) | 1.25 (0.58 to 2.70) | 5.39 (2.62 to 11.10) |
| P value | 0.64 | <0.001 | 0.57 | <0.001 |
OR, odds ratio.
Decreases in FGF-23 are defined as having change from baseline ≤0.
Discussion
This study showed that, when used as monotherapy for 52 weeks for the treatment of SHPT in participants on hemodialysis, treatment with cinacalcet was associated with reductions in FGF-23 concentrations, whereas treatment with vitamin D analogs was associated with increased concentrations compared with baseline. These divergent effects on FGF-23 occurred despite comparable reductions in PTH concentrations; there was no relationship between the change in PTH and the change in FGF-23 in either treatment arm. This would indicate that the differential effects of cinacalcet and vitamin D analogs on FGF-23 are not likely mediated by their effects on PTH but rather, may be mediated by direct effects on bone cells and/or other indirect effects on mineral metabolism, such as changes in Ca and/or P concentrations. Although previous studies have also noted these opposite effects of vitamin D analogs and cinacalcet on FGF-23 concentrations (16–22), this trial is unique in that participants were randomized to treatment with either cinacalcet or vitamin D analogs as monotherapy, thereby permitting a direct comparison of their effects.
Both animal and human studies have shown that PTH administration increases FGF-23 (6–10), supporting the hypothesis that PTH is an important regulator of FGF-23. Reductions in PTH after parathyroidectomy have been associated with reductions in FGF-23 in animals with experimental renal failure and patients with ESRD (7,23,24). Therefore, it is plausible that reducing PTH concentrations with medical intervention in patients with SHPT might directly result in reductions in FGF-23 concentrations.
Our results, which show a dissociation between PTH changes and changes in FGF-23, are consistent with recently reported data from the Improved Management of Intact Parathyroid Hormone with Paricalcitol-Centered Therapy Versus Cinacalcet Therapy with Low-Dose Vitamin D in Hemodialysis Patients with SHPT Study (16,25). Cozzolino et al. (16) showed that a mean PTH reduction of 56% in the paricalcitol arm was accompanied by an 800%–1500% increase in FGF-23 concentrations, whereas the 38% reduction in PTH observed in the cinacalcet arm was associated with a 25%–32% reduction in FGF-23 concentrations.
Changes in Ca and/or P concentrations likely affected the changes in FGF-23 observed during the treatment of SHPT in this trial, most notably in the vitamin D arm. Ca and P have been shown to independently and synergistically stimulate FGF-23 (26). Thus, therapies for SHPT may indirectly affect FGF-23 production mediated by their effects on Ca and P metabolism.
In this trial, treatment with cinacalcet resulted in lower concentrations of serum Ca and P compared with treatment with vitamin D analogs. However, there were no observed relationships between the changes in FGF-23 in the cinacalcet arm and the changes in Ca or P. Conversely, changes in FGF-23 concentrations in the vitamin D analog arm were related to changes in concentrations of Ca and P. In participants treated with vitamin D analog, changes in FGF-23 were most strongly related to changes in Ca×P, and there was a weak relationship between Ca×P and FGF-23 in the cinacalcet arm. This apparent synergistic effect of Ca and P raises the possibility that bone cells respond to the combined circulating concentration of bone minerals, which was first suggested by Quinn et al. (26).
Several studies have evaluated the relationship of FGF-23 changes induced by cinacalcet and vitamin D and the associated changes in Ca and P concentrations. Hansen et al. (17) noted that both paricalcitol and alfacalcidol induced comparable increases in FGF-23, which were correlated with increases in Ca and P as well as cumulative vitamin D dosage. Koizumi et al. (20) noted FGF-23 reductions with cinacalcet treatment that were correlated with changes in Ca or P but not with changes in PTH. Conversely, Kim et al. (19) described FGF-23 reductions with cinacalcet that were unrelated to any other changes in mineral metabolism. A post hoc analysis of the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events Trial showed that the addition of cinacalcet to baseline standard of care, which could include vitamin D analogs and/or phosphate binders, resulted in a mean 25% reduction in FGF-23 concentrations after 20 weeks of treatment compared with a mean 31% increase in the placebo arm. The cinacalcet-induced reductions in FGF-23 were not related to changes in PTH but were correlated with changes in Ca and P (27).
Our data also support probable direct effects of SHPT therapy on FGF-23 independent of changes in Ca and P metabolism, especially in those treated with cinacalcet. The vitamin D receptor and the Ca-sensing receptor are both expressed in bone cells, and the FGF-23 gene has a promoter region VDRE (13,15). Vitamin D analogs have been shown to increase FGF-23 concentrations as well as levels of FGF-23 mRNA in intact animals and isolated osteoblast-like cells (13), and presence of the VDRE is essential for this response. Finch et al. (28) showed that administration of cinacalcet to uremic rats decreased FGF-23, despite a concomitant increase in serum P, which purports an effect independent of P reduction. In the same study, paricalcitol administration increased FGF-23 concentrations, despite no substantial change in Ca or P concentrations, suggesting a direct effect of paricalcitol on FGF-23 (28).
Both dietary phosphate restriction and phosphate binder treatment have been hypothesized to effect FGF-23 levels. The effect of phosphate binders on FGF-23 has been investigated in both patients with CKD and patients on maintenance dialysis (29–34). Overall, these data suggest that the use of non-Ca–based binders seems to lower FGF-23 levels, whereas the use of Ca-containing binders either minimally affects or actually raises FGF-23 levels. This effect of Ca-containing binders is consistent with the known ability of Ca to stimulate FGF-23 (35).
In this trial, dietary phosphate intake and phosphate binder used were not controlled. Consistent with previous findings, treatment with cinacalcet was associated with increased use of Ca-containing binders, possibly as a response to reductions in serum Ca, whereas participants on vitamin D analogs were more likely to use non-Ca–containing binders (36). However, it is unlikely that the observed differences in binder use would have affected our study results. On the basis of the reported effects of Ca- and non-Ca–containing binders on FGF-23 levels, the pattern of binder use in our study would have tended to offset the observed differences in FGF-23 between treatment arms.
Limitations
This trial has several limitations. First, the upper limit of quantification for the assay used to measure FGF-23 was 800 ng/L, which could have affected study results. However, this would not have influenced the observed divergent effects of vitamin D analogs and cinacalcet on FGF-23 concentrations. Second, phosphate binder treatment can affect FGF-23 production, and the type and dose of phosphate binders were not controlled in this study. Third, specific markers of bone turnover were not measured in this study; such an analysis might provide insight into mechanisms by which cinacalcet and vitamin D analog treatment directly affect bone cells. Fourth, various vitamin D analogs were used in the study, which could possibly have had differential effects on PTH, Ca, P, and FGF-23.
In summary, the divergent effects of cinacalcet or vitamin D analogs on FGF-23, when used as monotherapy for SHPT, are unrelated to their effect on PTH levels. The observed changes in FGF-23 may be mediated by both direct effects on bone cells and other indirect effects on mineral metabolism. Future trials should prospectively address the effects of different SHPT treatments on FGF-23 levels and whether such treatment-mediated changes affect clinical outcomes.
Disclosures
This study was funded and supported by Amgen Inc. S.M.S. has received grant support and/or consulting fees from Amgen Inc., AbbVie, Cytochroma, Deltanoid, Shire, and Vifor. J.B.W. served on the Amgen Speaker’s Bureau for 1 year up to October of 2013.
Acknowledgments
The authors thank Holly Tomlin (employee and stockholder, Amgen Inc.) for her medical writing, editing, and journal formatting assistance.
This study was funded and supported by Amgen Inc.
Some parts of these data were presented at American Society of Nephrology Kidney Week 2013, Atlanta, Georgia, November 5–10, 2013.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver J, Naveh-Many T: FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat Rev Nephrol 9: 641–649, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M: FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol 21: 1125–1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zisman AL, Wolf M: Recent advances in the rapidly evolving field of fibroblast growth factor 23 in chronic kidney disease. Curr Opin Nephrol Hypertens 19: 335–342, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ: Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res 24: 1681–1685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T: PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–F889, 2010 [DOI] [PubMed] [Google Scholar]
- 8.López I, Rodríguez-Ortiz ME, Almadén Y, Guerrero F, de Oca AM, Pineda C, Shalhoub V, Rodríguez M, Aguilera-Tejero E: Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int 80: 475–482, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T: Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49: 636–643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wesseling-Perry K, Harkins GC, Wang HJ, Elashoff R, Gales B, Horwitz MJ, Stewart AF, Jüppner H, Salusky IB: The calcemic response to continuous parathyroid hormone (PTH)(1-34) infusion in end-stage kidney disease varies according to bone turnover: A potential role for PTH(7-84). J Clin Endocrinol Metab 95: 2772–2780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, Ramjit NJ, Ryder K, Tabash SP, Herzenberg AM, Epps TM, Petkovich M: Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int 78: 463–472, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD: Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D: Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A 101: 5140–5145, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi T: The calcium-sensing receptor in bone. J Bone Miner Metab 26: 301–311, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Cozzolino M, Ketteler M, Martin KJ, Sharma A, Goldsmith D, Khan S: Paricalcitol- or cinacalcet-centred therapy affects markers of bone mineral disease in patients with secondary hyperparathyroidism receiving haemodialysis: Results of the IMPACT-SHPT study. Nephrol Dial Transplant 29: 899–905, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Hansen D, Rasmussen K, Pedersen SM, Rasmussen LM, Brandi L: Changes in fibroblast growth factor 23 during treatment of secondary hyperparathyroidism with alfacalcidol or paricalcitol. Nephrol Dial Transplant 27: 2263–2269, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Hryszko T, Brzosko S, Rydzewska-Rosolowska A, Koc-Zorawska E, Mysliwiec M: Cinacalcet lowers FGF-23 level together with bone metabolism in hemodialyzed patients with secondary hyperparathyroidism. Int Urol Nephrol 44: 1479–1486, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Kim H, Shin N, Na KY, Kim YL, Kim D, Chang JH, Song YR, Hwang YH, Kim YS, Ahn C, Lee J, Oh KH, Representing the Cinacalcet stUdy for Peritoneal Dialysis Patients In Double Arm on the Lowing Effect Of iPTH Level (CUPID) Study Group : Cinacalcet lowering of serum fibroblast growth factor-23 concentration may be independent from serum Ca, P, PTH and dose of active vitamin D in peritoneal dialysis patients: A randomized controlled study. BMC Nephrol 14: 112, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koizumi M, Komaba H, Nakanishi S, Fujimori A, Fukagawa M: Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant 27: 784–790, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB: Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 79: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD: Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol 5: 110–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, Tominaga Y, Ueki T, Goto N, Matsuoka S, Katayama A, Haba T, Uchida K, Nakanishi S, Kazama JJ, Gejyo F, Yamashita T, Fukagawa M: Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis 44: 481–487, 2004 [PubMed] [Google Scholar]
- 24.Takahashi H, Komaba H, Takahashi Y, Sawada K, Tatsumi R, Kanai G, Suzuki H, Kakuta T, Fukagawa M: Impact of parathyroidectomy on serum FGF23 and soluble Klotho in hemodialysis patients with severe secondary hyperparathyroidism. J Clin Endocrinol Metab 99: E652–E658, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Ketteler M, Martin KJ, Wolf M, Amdahl M, Cozzolino M, Goldsmith D, Sharma A, Marx S, Khan S: Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: Results of the IMPACT SHPT study. Nephrol Dial Transplant 27: 3270–3278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn SJ, Thomsen AR, Pang JL, Kantham L, Bräuner-Osborne H, Pollak M, Goltzman D, Brown EM: Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am J Physiol Endocrinol Metab 304: E310–E320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moe SM: Cinacalcet decreases FGF23 levels in prevalent dialysis patients compared to placebo. Presented at the American Society of Nephrology Kidney Week 2013, Atlanta, GA, November 5–10, 2013 [Google Scholar]
- 28.Finch JL, Tokumoto M, Nakamura H, Yao W, Shahnazari M, Lane N, Slatopolsky E: Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol 298: F1315–F1322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancela AL, Oliveira RB, Graciolli FG, dos Reis LM, Barreto F, Barreto DV, Cuppari L, Jorgetti V, Carvalho AB, Canziani ME, Moysés RM: Fibroblast growth factor 23 in hemodialysis patients: Effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract 117: c74–c82, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Ketteler M, Petermann AT: Phosphate and FGF23 in early CKD: On how to tackle an invisible foe. Nephrol Dial Transplant 26: 2430–2432, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Mallamaci F, Zoccali C: Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: A randomized clinical trial. Am J Kidney Dis 59: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Soriano S, Ojeda R, Rodríguez M, Almadén Y, Rodríguez M, Martín-Malo A, Aljama P: The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol 80: 17–22, 2013 [DOI] [PubMed] [Google Scholar]
- 35.David V, Dai B, Martin A, Huang J, Han X, Quarles LD: Calcium regulates FGF-23 expression in bone. Endocrinology 154: 4469–4482, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messa P, Macário F, Yaqoob M, Bouman K, Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazão JM, Bos WJ, Torregrosa V, Saha H, Reichel H, Wilkie M, Zani VJ, Molemans B, Carter D, Locatelli F: The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 3: 36–45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]