Abstract
Background and objectives
A direct association between low triiodothyronine (T3) syndrome and cardiovascular (CV) mortality has been reported in hemodialysis patients. However, the implications of this syndrome in peritoneal dialysis (PD) patients have not been properly investigated. This study examined the association between low T3 syndrome and CV mortality including sudden death in a large cohort of incident PD patients.
Design, setting, participants, & measurements
This prospective observational study included 447 euthyroid patients who started PD between January 2000 and December 2009. Measurement of thyroid hormones was performed at baseline. All-cause and cause-specific deaths were registered during the median 46 months of follow-up. The survival rate was compared among three groups based on tertile of T3 levels.
Results
In Kaplan–Meyer analysis, patients with the lowest tertile were significantly associated with higher risk of all-cause and CV mortality including sudden death (P<0.001 for trend). In Cox analyses, T3 level was a significant predictor of all-cause mortality (per 10-unit increase, adjusted hazard ratio [HR], 0.86; 95% confidence interval [95% CI], 0.78 to 0.94; P=0.002), CV death (per 10-unit increase, adjusted HR, 0.84; 95% CI, 0.75 to 0.98; P=0.01), and sudden death (per 10-unit increase, adjusted HR, 0.69; 95% CI, 0.56 to 0.86; P=0.001) after adjusting for well known risk factors including inflammation and malnutrition. The higher T3 level was also independently associated with lower risk for sudden death (per 10-unit increase, adjusted HR, 0.71; 95% CI, 0.56 to 0.90; P=0.01) even when accounting for competing risks of death from other causes.
Conclusions
T3 level at the initiation of PD was a strong independent predictor of long-term CV mortality, particularly sudden death, even after adjusting well known risk factors. Low T3 syndrome might represent a factor directly implicated in cardiac complications in PD patients.
Keywords: peritoneal dialysis, mortality, cardiovascular
Introduction
CKD is a frequent cause of alterations in thyroid hormones without any underlying intrinsic thyroid disorder known as the syndrome of nonthyroidal illness. The most common alteration is low triiodothyronine (T3) syndrome, which is characterized by low total T3 and free T3 with normal or slightly decreased thyroid-stimulating hormone (TSH) and thyroxine (T4) concentration (1,2). The underlying mechanism of this derangement is explained by multifactorial causes including central hypothyroidism, reduction in the hormone binding capacity, modified entry of thyroid hormone into tissue, modified expression of iodothyronine deiodinases, and changes in thyroid hormone receptor expression or function (3). CKD affects both the hypothalamus–pituitary–thyroid axis and thyroid hormone peripheral metabolism (4). According to a recent study, low levels of T3 were highly prevalent in patients with CKD, even early in the disease course, and were positively related to eGFR independent of age and serum albumin (5). Several studies have demonstrated that low T3 syndrome is associated with nutritional status, inflammation, and cardiomyopathy (6–8) as well as aortic stiffness in dialysis patients (9,10). In addition, a direct association between low T3 syndrome and all-cause and cardiovascular (CV) mortality has been reported in patients with CKD (11) and hemodialysis (HD) patients (8,12,13).
However, the significance of low T3 syndrome in peritoneal dialysis (PD) patients is poorly studied, even though PD patients frequently display low T3 levels as well (14). Only one small, short-term study of prevalent PD patients suggested the association between low T3 syndrome and all-cause mortality, but not CV outcomes (15). Because risk factors for CV outcome in PD patients do not coincide with those in HD patients, it is needed to confirm the effects of low T3 syndrome on long-term CV outcome in a large cohort of PD patients.
Sudden death is the largest cause of cardiac mortality in dialysis patients by comparison with myocardial infarction (MI) in the general population. Indeed, when taken as a single cause, sudden death accounts for one-quarter of all dialysis patients’ deaths (16). Left ventricular hypertrophy (LVH), left ventricular systolic dysfunction, and aortic stiffness are known to be risk factors for sudden death (16–18), and are also significantly associated with low T3 syndrome (7,9,10) in dialysis patients. Considerable evidence has raised the possibility that low T3 syndrome may be a predisposing factor for sudden death, but the relationship between the two has not yet been determined in PD patients.
To address this existing gap in knowledge, we examined the association between low T3 syndrome and long-term CV mortality including sudden death in a large cohort of incident PD patients.
Materials and Methods
Study Patients
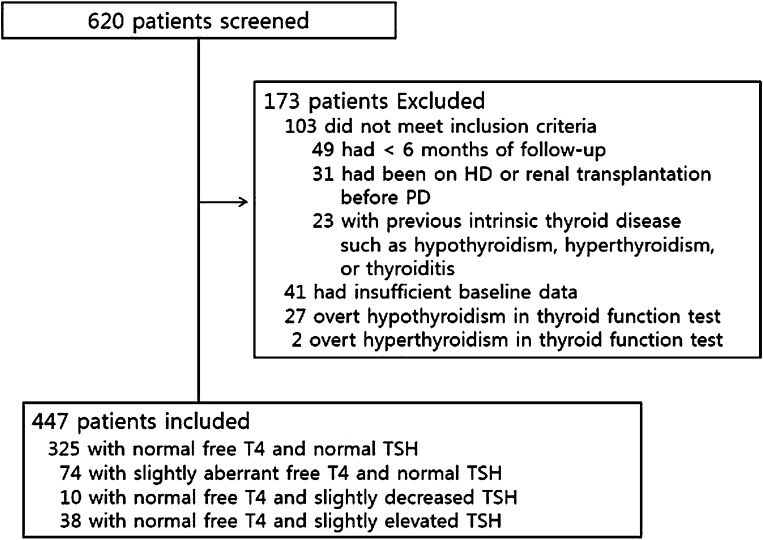
We considered all 620 patients who started PD at National Health Insurance Service Ilsan Hospital between January 2000 and December 2009. All patients underwent urea kinetic studies including residual renal function (RRF) within 3 months of PD initiation. We excluded 49 patients that had <6 months of follow-up due to death or transfer to another modality after the initiation of PD, 31 patients that had been on HD or received a kidney transplant before the initiation of PD, 41 patients with insufficient baseline data, and 23 patients with previous intrinsic thyroid disease such as hypothyroidism, hyperthyroidism, or thyroiditis. We also excluded 27 overt hypothyroid patients and two hyperthyroid patients in the baseline thyroid function test. Finally, 173 patients were excluded and a total of 447 incident patients were included for analysis (Figure 1). No patient was receiving drugs altering thyroid function such as lithium, amiodarone, or propranolol. Patients were treated with three or four 2-liter exchanges per day using standard dialysates containing glucose. Dialysis prescription aimed at obtaining a total Kt/V urea of at least 1.7 per week. There were 392 patients treated with various antihypertensive drugs (374 were treated with calcium channel blockers, 178 with β-blockers, 360 with angiotensin-converting enzyme inhibitors or angiotensin type 1 antagonists, and 84 with α-blockers). The patients were followed for 7–142 months until December 2011, with a median follow-up time of 46 months. The study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Ilsan Hospital Clinical Trial Center. We obtained informed written consent from all participants involved in our study.
Figure 1.
Enrollment of study patients. HD, hemodialysis; PD, peritoneal dialysis; T4, thyroxine; TSH, thyroid-stimulating hormone.
Data Collection and Laboratory Measurements
Demographic and clinical data were collected at the beginning of PD. Previous CV events were defined as electrocardiography-documented angina and MI, stroke, cerebral hemorrhage, heart failure, arrhythmias, or peripheral vascular disease.
Laboratory data obtained at the time of dialysis adequacy measurement within 3 months of PD initiation were considered baseline values. Adequacy of PD was determined by measurement of Kt/V, and RRF was estimated by the average renal clearance of urea and creatinine. For evaluation of nutritional status, dietary protein intake was estimated from the normalized protein catabolic rate (nPCR) and lean body mass was estimated by creatinine kinetics (LBMCr). Hemoglobin, lipids, albumin, calcium, phosphorus, and C-reactive protein (CRP) were measured. Serum T3 (59–150 ng/dl), free T4 (0.89–1.76 ng/dl), and TSH (0.31–5.31 mIU/L) were determined by chemiluminescent immunoassay (Centaur XP; Siemens Healthcare Diagnostics Inc, Tarrytown, NY).
Outcomes Assessment
The primary outcome parameters were all-cause and cause-specific mortality. Patients were censored at transplantation or when completing the follow-up period. Within the follow-up periods, 162 patients died and 35 patients underwent transplantation. Combined CV death, sudden death, and infection-related death were considered as separate outcomes of cause-specific mortality. Combined CV deaths included cardiac death from coronary artery disease (MI or angina), congestive heart failure (CHF), and pulmonary edema, death from cerebrovascular disease (ischemic stroke or cerebral hemorrhage) or peripheral vascular disease, and sudden death. Sudden death was defined as an unexpected out-of-hospital death occurring within 1 hour of onset of symptoms (16). Deaths during sleep and unwitnessed deaths occurring at home were considered sudden deaths.
Covariate Selection
Multiple confounding factors previously reported to be associated with CV outcomes and mortality in patients with ESRD were considered as covariates (19). Demographic and clinical factors analyzed include age, sex, previous CV events, comorbidities including Charlson comorbidity index, and diabetic status. Dialysis-related factors included Kt/V, LBMCr, nPCR, and RRF. Laboratory values included hemoglobin, albumin, bicarbonate, CRP, calcium, and phosphorus.
Statistical Analyses
Baseline demographics and laboratory test results were described as the mean±SD, median and interquartile range, or frequency distribution for categorical variables. The Shapiro–Wilk test was used to determine the normality of the distribution of parameters.
Patients were divided into three groups based on tertiles of T3 levels. Statistical significance of differences between groups was tested using one-way ANOVA for continuous variables and the chi-squared test for categorical variables. The relationships between serum T3 and continuous variables were examined by Pearson’s correlation coefficient, and categorical variables were examined using Spearman’s R test. Multiple linear regression analysis was performed to identify the determinants of T3 levels. We selected baseline variables with P<0.10 in univariate analysis for inclusion in multivariate regression models, which we built with a stepwise selection procedure after forcing in age and sex.
The survival rate was compared among groups based on serum T3 tertile using the Kaplan–Meier method and the log-rank test. In the patient survival analysis, data for patients transferred to renal transplantation or HD or lost to follow-up were censored. We investigated the relationship between T3 and all-cause and cause-specific mortality using Cox proportional hazards regression. Under conditions of competing risk, traditional Cox regression models can overestimate results; therefore, corresponding competing risk methods using those of Fine and Gray were also performed (20). The results are expressed as hazard ratios (HRs) and 95% confidence intervals (95% CIs). Statistical analyses were performed using SPSS for Windows (version 13.0; SPSS Inc., Chicago, IL) and the R software package (version 3.0.2). The level of significance was set at 0.05 for all tests.
Results
Clinical and Biochemical Data in Study Patients According to T3 Tertiles
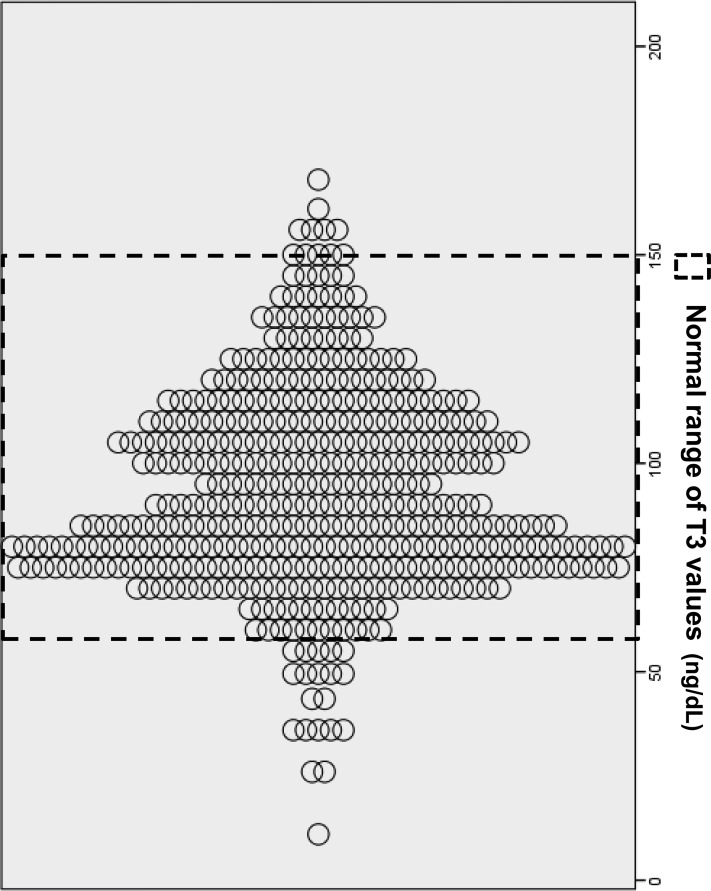
Table 1 details clinical and biochemical characteristics of 447 patients categorized by tertiles of T3 level. The mean age was 58 years (range, 22–85 years), 54.3% of patients were men, and patients were on PD for a median duration of 46 months (range, 7–142 months). The prevalence of diabetes and previous CV events was 50.2% and 30.7%, respectively. The mean T3 level was 97.6±27.2 ng/dl (median, 97 ng/dl; interquartile range, 83–115 ng/dl). Figure 2 shows the scatter plot of T3 values in study patients. Patients with a lower T3 level were significantly older and more likely to have diabetes or previous CV events. Hemoglobin, albumin, RRF, nPCR, and LBMCr were significantly lower at the lower T3 level, whereas the CRP level was significantly higher at the lower T3 level (Table 1). The correlations between T3 and several variables were statistically significant but the strength of the correlation was not very strong, with r coefficients ranging from 0.15 to 0.29 (Table 1). Multivariate linear regression analysis revealed that previous CV events (β=−8.95 ng/dl if previous CV events; P=0.01), hemoglobin (β=2.87 ng/dl per g/dl; P=0.04), and RRF (β=0.88 ng/dl per ml/min per 1.73 m2; P=0.03) were independently associated with serum T3 level after adjusting for age, sex, diabetic status, albumin, CRP, LBMCr, and nPCR.
Table 1.
Baseline demographic, clinical, and biochemical data of study patients
| Characteristic | T3 Tertile | P Value for Trend | T3 versus Variables | |||
|---|---|---|---|---|---|---|
| I (<85; n=151) | II (85 ≤ T3 < 107; n=146) | III (≥107; n=150) | r Value | P Value | ||
| Age (yr) | 60.8±13.6 | 57.7±14.9 | 57.5±13.8 | 0.04 | −0.19 | 0.001 |
| Men (%) | 81 (53.6) | 69 (47.3) | 91 (60.7) | 0.2 | 0.05 | 0.31 |
| Diabetes (%) | 84 (55.6) | 75 (51.3) | 65 (43.3) | 0.02 | −0.16 | 0.004 |
| Previous CV events (%) | 57 (37.5) | 48 (33.6) | 32 (21.2) | 0.002 | 0.22 | <0.001 |
| CCI | 3.1±1.11 | 3.1±1.09 | 2.9±0.99 | 0.4 | −0.07 | 0.16 |
| BMI (kg/m2) | 22.3±2.95 | 22.5±3.79 | 23.1±2.89 | 0.1 | −0.60 | 0.19 |
| Laboratory findings | ||||||
| Hemoglobin (g/dl) | 10.7±1.6 | 10.7±1.4 | 11.3±1.5 | 0.003 | 0.19 | 0.001 |
| Albumin (g/dl) | 3.1±0.6 | 3.2±0.5 | 3.3±0.5 | 0.004 | 0.16 | 0.004 |
| Bicarbonate (mEq/L) | 25.7±3.5 | 25.3±3.8 | 25.9±3.4 | 0.4 | 0.10 | 0.15 |
| Total cholesterol (mg/dl) | 184.5±55.4 | 183.4±41.1 | 183.6±47.1 | 0.7 | −0.01 | 0.94 |
| Triglyceride (mg/dl) | 140.7±77.2 | 142.1±86.2 | 146.9±79.8 | 0.8 | −0.02 | 0.74 |
| Calcium (mg/dl) | 8.7±0.8 | 8.8±0.9 | 8.7±0.8 | 0.5 | 0.04 | 0.37 |
| Phosphorus (mg/dl) | 3.9±1.1 | 4.1±1.4 | 4.1±0.9 | 0.8 | −0.05 | 0.27 |
| CRP (mg/dl)a | 0.10 (0.02–0.59) | 0.10 (0.04–0.50) | 0.06 (0.02–0.37) | 0.04 | −0.15 | 0.02 |
| T3 (ng/dl) | 73.4±12.4 | 98.4±5.7 | 125.4±15.8 | <0.001 | ||
| fT4 (ng/dl) | 1.10 (1.00–1.10) | 1.10 (0.97–1.20) | 1.10 (1.00–1.20) | 0.06 | 0.29 | 0.001 |
| TSH (mIU/L) | 2.20 (1.33–3.57) | 2.01(1.22–3.21) | 2.24 (1.63–3.05) | 0.7 | −0.01 | 0.93 |
| Kt/V urea | 2.5±0.9 | 2.8±1.0 | 2.7±0.9 | 0.2 | 0.08 | 0.11 |
| Peritoneal Kt/V urea | 1.5±0.6 | 1.6±0.5 | 1.3±0.5 | 0.001 | −0.12 | 0.02 |
| RRF (ml/min per 1.73 m2) | 5.4±4.6 | 5.8±4.1 | 7.0±3.8 | 0.02 | 0.17 | 0.003 |
| nPCR (g/kg per day) | 0.9±0.2 | 1.0±0.3 | 1.0±0.3 | 0.04 | 0.10 | 0.03 |
| LBMCr | 36.8±13.5 | 37.8±11.8 | 42.5±11.3 | 0.001 | 0.18 | 0.001 |
Continuous variables are expressed as means±SD or medians (interquartile range). Categorical variables are expressed as n (%). CV, cardiovascular; CCI, Charlson comorbidity index; BMI, body mass index; CRP, C-reactive protein; T3, triiodothyronine; fT4, free thyroxine; TSH, thyroid-stimulating hormone; RRF, residual renal function; nPCR, normalized protein catabolic rate; LBMCr, lean body mass estimated by creatinine kinetics.
Median and interquartile range are reported; however, the P value represents analysis of log-transformed variables.
Figure 2.
Scatterplots of T3 values (in nanograms per decaliter). T3, triiodothyronine.
Association of T3 and Long-Term Outcome
During the follow-up period, 162 deaths were recorded. CV disease (102 deaths, 63.0%) was the most common cause of death in this study, followed by infection (51 deaths, 31.5%) and other causes (nine deaths, 5.6%). There were 42 sudden deaths, which is 25.9% of total mortality and 41.2% of CV mortality. A Kaplan–Meier plot showed the stepwise increase in risks of all-cause, combined CV, and sudden death from the third (highest) to the first (lowest) tertile of T3 level (Figure 3).
Figure 3.
In Kaplan–Meyer survival curves, patients with the lowest tertile were significantly associated with higher risk of all-cause and CV mortality including sudden death. Kaplan–Meier plots for all-cause mortality (P<0.001) (A), combined cardiovascular death (P=0.001) (B), and sudden death (P<0.001) (C) according to tertiles of T3. T3, triiodothyronine.
The unadjusted and adjusted associations of T3 levels with all-cause mortality and cause-specific mortality in Cox proportional hazards models are shown in Table 2 along with their 95% CIs and P values. In the unadjusted Cox proportional hazards models, per 10-unit increases in T3 levels were associated with lower risk of all-cause death by 23.4% (P<0.001). After adjusting for demographic risk factors, nutritional markers (albumin, nPCR, and LBMCr), an inflammation marker (log-transformed CRP), and RRF, per 10-unit increases in T3 levels were independently associated with the lower risk of all-cause death by 14.0% (P=0.002).
Table 2.
Association of T3 levels with all-cause and cause-specific mortality in Cox proportional hazards models
| T3 (per 10-ng/dl Increase) | Crude Analyses | Adjusted Analyses | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | (95% CI) | P Value | |
| All-cause death | 0.77 (0.70 to 0.85) | <0.001 | 0.86 (0.78 to 0.94) | 0.002 |
| Combined cardiovascular death | 0.74 (0.66 to 0.83) | <0.001 | 0.84 (0.75 to 0.98) | 0.01 |
| Sudden death | 0.64 (0.53 to 0.78) | <0.001 | 0.69 (0.56 to 0.86) | 0.001 |
| Infection-related death | 0.83 (0.70 to 0.98) | 0.03 | 0.92 (0.78 to 1.11) | 0.40 |
Adjusted for age, sex, presence of diabetes, Charlson comorbidity index, previous cardiovascular events, albumin, normalized protein catabolic rate, lean body mass estimated by creatinine kinetics, log-transformed C-reactive protein, and residual renal function. T3, triiodothyronine; HR, hazard ratio; 95% CI, 95% confidence interval.
In the multivariable analysis, the higher T3 was associated with a significantly lower risk of combined CV death (per 10-unit increase, adjusted HR, 0.84; 95% CI, 0.75 to 0.98; P=0.01) and sudden death (per 10-unit increase, adjusted HR, 0.69; 95% CI, 0.56 to 0.86; P=0.001) after adjusting for age, sex, diabetes, Charlson comorbidity index, previous CV events, albumin, LBMCr, log-transformed CRP, and RRF. However, neither free T4 nor TSH nor the interaction between T3 and free T4 was associated with survival or CV outcome (data not shown).
Association of T3 with Specific Cause of Death Using Competing Risk Analyses
To overcome the shortcomings of conventional Cox models that can overestimate the cumulative mortality probabilities for each of the separate causes of death, we also conducted competing risk analysis (Table 3). In the multivariable analysis, a higher T3 level was associated with a significantly lower risk of sudden death (per 10-unit increase, adjusted HR, 0.71; 95% CI, 0.56 to 0.90; P=0.01). However, there was no significant association of T3 levels with other CV death or infectious death when applying competing risk methods.
Table 3.
Association of T3 levels with cause-specific mortality using competing risk analysis
| T3 (per 10-ng/dl Increase) | Crude Analyses | Adjusted Analyses | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Sudden death | 0.72 (0.60 to 0.85) | <0.001 | 0.71 (0.56 to 0.90) | 0.01 |
| Other cardiovascular death | 0.88 (0.78 to 0.99) | 0.03 | 1.03 (0.90 to 1.17) | 0.68 |
| Infection-related death | 0.90 (0.76 to 1.10) | 0.21 | 1.00 (0.85 to 1.22) | 0.95 |
Adjusted for age, sex, presence of diabetes, Charlson comorbidity index, previous cardiovascular events, albumin, normalized protein catabolic rate, lean body mass estimated by creatinine kinetics, log-transformed C-reactive protein, and residual renal function. T3, triiodothyronine; HR, hazard ratio; 95% CI, 95% confidence interval.
There was also no significant association of the interaction between T3 and free T4 with the different outcomes in competing risk analysis (data not shown).
Discussion
This study clearly demonstrated that T3 levels at the initiation of dialysis were significantly associated with all-cause and CV mortality, including sudden death in PD patients. Although these findings are in accordance with previously published research (7,12,13,15), this is the first investigation to demonstrate that T3, assessed at baseline, was associated with risk of sudden death in dialysis patients during long-term follow-up. The significant association of low T3 syndrome with sudden death in this study is particularly meaningful because this result was consistent after adjustment for demographic risk factors, previous cardiovascular disease history, and inflammation and nutritional factors. The finding was also robust even when accounting for competing risks from other CV or infectious cause of death. Our findings extend from those described by Meuwese et al., which demonstrated that low T3 syndrome predicts survival and CV deaths in prevalent HD patients when assessed in a time-varying manner, and also when assessed at baseline (13). However, in that study, sudden death was included in CV death; thus, the association of low T3 syndrome with sudden death could not be specified.
Interestingly, the significant association of low T3 syndrome with other CV death disappeared when accounting for competing risks from sudden death or infectious death. In the competing risk analysis, the competing risk is not handled as a censoring event such as in the conventional Cox models, and cumulative survival probability is lowered by competing events. Therefore, the competing risk method could be more appropriate to yield unbiased estimates of the cumulative probabilities for cause-specific mortality. Considering the different results between the two models, we cautiously supposed that low T3 might be more related to heart disease, rather than other CV disease including cerebrovascular disease or peripheral vascular disease.
T3 is particularly important for control of myocardial contractility, vascular tone, and myocardial cell growth because myocytes cannot convert T4 into T3 (21). A significant decrease in serum T3 was observed in patients with CHF, and T3 replacement therapy produces favorable effects in these patients (22). Animal studies have shown that treatment with T3 significantly improves cardiac function, with normalization of some, but not all, of the changes in gene expression in the acute MI model (23). Therefore, low T3 might not only be a prognostic marker, but may also be directly implicated in the development and aggravation of cardiac disease in dialysis patients.
Despite the high risk for sudden death in the dialysis population, there have been very few prospective studies about risk factors associated with sudden death. Unlike the general population, coronary artery disease and CHF are not the major risk factors in dialysis patients (16). In prevalent HD patients, LVH was associated with sudden death (17,18); in another study of PD patients, systolic dysfunction was the most significant predictor followed by a high systolic BP (24). These results might explain the association of low T3 syndrome with sudden death considering that low T3 was associated with left ventricular dysfunction and LVH in a previous study (7). Low T3 syndrome was also associated with an increase in the occurrence of ventricular late potentials and in the risk of ventricular tachyarrhythmia, which contribute to sudden death (25). In prevalent PD patients, RRF was associated with sudden death (26). Szeto et al. showed that anuric PD patients had a higher rate of CV death compared with those with RRF, and the difference was largely caused by higher prevalence of sudden death in anuric patients. The difference in the distribution of cause of death was particularly prominent in patients without preexisting cardiovascular disease and could not be explained by the longer duration of dialysis or dialysis adequacy or nutritional status in anuric patients (26). In PD patients, RRF is the most important predictor of outcome and is significantly associated with inflammation, anemia, malnutrition, LVH, volume overload, hypertension, and CV disease, and interacts with these factors to increase CV mortality (27,28). Similar evidence is now emerging in HD patients (29). Considering the vital association of RRF with outcomes in PD patients, it is meaningful that the T3 level had an independent negative association with RRF in our study. These findings should be considered in light of certain limitations. First, this is an observational study and we cannot infer a causal relationship between low T3 and outcomes. Second, the inclusion of only incident PD patients may have led to a selection bias, which could limit the generalizability of our results and render our results not applicable to prevalent PD and HD patients. Third, we could not examine cardiac function directly, which makes it difficult to elucidate the pathogenesis of low T3 syndrome on CV outcome in our study. This study also has several strengths. The data come from a relatively large cohort of incident PD patients with several years of follow-up, providing a data set with which to examine the long-term association of baseline low T3 with specific causes of mortality and the association of T3 level with RRF. The more rapid decrease of RRF in HD compared with PD patients is likely responsible for the poor data regarding the effect of RRF on low T3 syndrome in HD patients. In addition, the data were analyzed by the careful scrutiny of all deaths for accurate determination of cause.
In summary, the T3 level at the initiation of PD was a strong independent predictor of long-term CV mortality, particularly sudden death, even after adjusting for well known risk factors. Given the findings from this report relating low T3 syndrome to CV outcomes, nephrologists need to be attuned to thyroid dysfunction as a prognostic marker of CV outcome including sudden death at the initiation of dialysis in patients with CKD. In addition, the observed independent associations between T3 and RRF, as well as data showing the association of both factors with CV outcome, indicate the need for studies that examine the effects of preserving RRF on T3 changes and outcomes in dialysis patients.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Low-T3 Syndrome in Peritoneal Dialysis: Metabolic Adaptation, Marker of Illness, or Mortality Mediator?,” on pages 917–919.
References
- 1.Lim VS: Thyroid function in patients with chronic renal failure. Am J Kidney Dis 38[Suppl 1]: S80–S84, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Warner MH, Beckett GJ: Mechanisms behind the non-thyroidal illness syndrome: An update. J Endocrinol 205: 1–13, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Meuwese CL, Dekkers OM, Stenvinkel P, Dekker FW, Carrero JJ: Nonthyroidal illness and the cardiorenal syndrome. Nat Rev Nephrol 9: 599–609, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Iglesias P, Díez JJ: Thyroid dysfunction and kidney disease. Eur J Endocrinol 160: 503–515, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Song SH, Kwak IS, Lee DW, Kang YH, Seong EY, Park JS: The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant 24: 1534–1538, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C, Tripepi G, Cutrupi S, Pizzini P, Mallamaci F: Low triiodothyronine: A new facet of inflammation in end-stage renal disease. J Am Soc Nephrol 16: 2789–2795, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Zoccali C, Benedetto F, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P, Malatino LS, Bonanno G, Seminara G: Low triiodothyronine and cardiomyopathy in patients with end-stage renal disease. J Hypertens 24: 2039–2046, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ozen KP, Asci G, Gungor O, Carrero JJ, Kircelli F, Tatar E, Sevinc Ok E, Ozkahya M, Toz H, Cirit M, Basci A, Ok E: Nutritional state alters the association between free triiodothyronine levels and mortality in hemodialysis patients. Am J Nephrol 33: 305–312, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Tatar E, Kircelli F, Asci G, Carrero JJ, Gungor O, Demirci MS, Ozbek SS, Ceylan N, Ozkahya M, Toz H, Ok E: Associations of triiodothyronine levels with carotid atherosclerosis and arterial stiffness in hemodialysis patients. Clin J Am Soc Nephrol 6: 2240–2246, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatar E, Sezis Demirci M, Kircelli F, Gungor O, Yaprak M, Asci G, Basci A, Ozkahya M, Ok E: The association between thyroid hormones and arterial stiffness in peritoneal dialysis patients. Int Urol Nephrol 44: 601–606, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Carrero JJ, Qureshi AR, Axelsson J, Yilmaz MI, Rehnmark S, Witt MR, Bárány P, Heimbürger O, Suliman ME, Alvestrand A, Lindholm B, Stenvinkel P: Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med 262: 690–701, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P: Low triiodothyronine and survival in end-stage renal disease. Kidney Int 70: 523–528, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Meuwese CL, Dekker FW, Lindholm B, Qureshi AR, Heimburger O, Barany P, Stenvinkel P, Carrero JJ: Baseline levels and trimestral variation of triiodothyronine and thyroxine and their association with mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol 7: 131–138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr DJ, Singh VK, Tsakiris D, McConnell KN, Junor BJ, Alexander WD: Serum and peritoneal dialysate thyroid hormone levels in patients on continuous ambulatory peritoneal dialysis. Nephron 43: 164–168, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Enia G, Panuccio V, Cutrupi S, Pizzini P, Tripepi G, Mallamaci F, Zoccali C: Subclinical hypothyroidism is linked to micro-inflammation and predicts death in continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 22: 538–544, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Green D, Roberts PR, New DI, Kalra PA: Sudden cardiac death in hemodialysis patients: An in-depth review. Am J Kidney Dis 57: 921–929, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Krane V, Heinrich F, Meesmann M, Olschewski M, Lilienthal J, Angermann C, Störk S, Bauersachs J, Wanner C, Frantz S, German Diabetes and Dialysis Study Investigators : Electrocardiography and outcome in patients with diabetes mellitus on maintenance hemodialysis. Clin J Am Soc Nephrol 4: 394–400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostovaya IM, Bots ML, van den Dorpel MA, Goldschmeding R, den Hoedt CH, Kamp O, Levesque R, Mazairac AH, Penne EL, Swinkels DW, van der Weerd NC, Ter Wee PM, Nubé MJ, Blankestijn PJ, Grooteman MP: Left ventricular mass in dialysis patients, determinants and relation with outcome. Results from the COnvective TRansport STudy (CONTRAST). PLoS ONE 9: e84587, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Geriatr Soc 58: 783–787, 2010. 20345862 [Google Scholar]
- 21.Klein I, Ojamaa K: Thyroid hormone and the cardiovascular system. N Engl J Med 344: 501–509, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, Chopra IJ, Moriguchi JD, Hage A: Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. Am J Cardiol 81: 443–447, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Ojamaa K, Kenessey A, Shenoy R, Klein I: Thyroid hormone metabolism and cardiac gene expression after acute myocardial infarction in the rat. Am J Physiol Endocrinol Metab 279: E1319–E1324, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE: Sudden cardiac death in end-stage renal disease patients: A 5-year prospective analysis. Hypertension 56: 210–216, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Jaroszyński AJ, Głowniak A, Chrapko B, Sodolski T, Małecka T, Widomska-Czekajska T, Ksiazek A: Low-T3 syndrome and signal-averaged ECG in haemodialysed patients. Physiol Res 54: 521–526, 2005 [PubMed] [Google Scholar]
- 26.Szeto CC, Wong TY, Chow KM, Leung CB, Li PK: Are peritoneal dialysis patients with and without residual renal function equivalent for survival study? Insight from a retrospective review of the cause of death. Nephrol Dial Transplant 18: 977–982, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Marrón B, Remón C, Pérez-Fontán M, Quirós P, Ortíz A: Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl 108: S42–S51, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, Sanderson JE: Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol 15: 2186–2194, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Vilar E, Farrington K: Emerging importance of residual renal function in end-stage renal failure. Semin Dial 24: 487–494, 2011 [DOI] [PubMed] [Google Scholar]