CKD is a prevalent disorder found in over 13% of the population (1), making it more common than even diabetes mellitus. Similar to diabetes, the presence of CKD is strongly associated with cardiovascular disease (2). Because hypertension is a common complication of CKD, and, also, a major modifiable risk factor for both cardiovascular events and death, a focus on control of hypertension is a natural goal in the CKD population. Unfortunately, there is a paucity of evidence to support specific BP goals in CKD.
In this issue of CJASN, Palit et al. (3) describe the association of components of BP measured using the auscultated technique on one occasion at baseline with subsequent risk of all-cause mortality, ESRD, or cardiovascular events from a secondary analysis of the Veterans Administration–sponsored cooperative study program Homocysteinemia in Kidney and ESRD (HOST) Study. The HOST Study was a landmark, double-blind, multicenter, randomized, controlled trial, in which 2056 subjects with advanced CKD (estimated creatinine clearance of ≤30 ml/min, including those on peritoneal dialysis or hemodialysis) and elevated homendocysteine were randomized to placebo or an intervention to lower homocysteine. The intervention was a single pill containing a combination of vitamins: vitamin B6, cyanocobalamin, and folic acid. The primary outcome was all-cause mortality (4). Secondary objectives of this study were to test whether the vitamins decrease the following end points: (1) myocardial infarctions, (2) stroke, (3) amputation of the lower extremity, and (4) combinations of the preceding end points and all-cause mortality. In addition, the following outcomes were reported: (1) thrombosis of the vascular access in patients on hemodialysis and (2) time to initiation of dialysis. Although the vitamins were effective in significantly lowering an elevated homocysteine concentration by approximately 25% at 3 months, there was no significant effect on mortality, secondary outcomes, or adverse events.
Palit et al. (3) excluded subjects with ESRD or those who started dialysis within 3 months of enrollment, resulting in 1099 subjects included in their analysis. Participants were over 98% men, and mean eGFR calculated using the Modification of Diet in Renal Disease (MDRD) formula was 18 ml/min per 1.73 m2. Palit et al. (3) found that, in this cohort, over a median follow-up time of 2.9 years, pulse pressure was independently associated with cardiovascular events defined as symptomatic myocardial infarction, ischemic stroke, or limb amputation. The relationship was not a graded association; however, those in the highest quartile of pulse pressure had the greatest risk of cardiovascular events. Furthermore, cardiovascular events were not independently associated with either systolic or diastolic BP. In the case of ESRD, there was no graded association of systolic BP with this outcome; however, those in the highest quartile of systolic BP showed an elevated risk of ESRD. In contrast, diastolic BP exhibited a graded independent relationship with ESRD. However, pulse pressure had no significant association. In the case of all-cause mortality, Palit et al. (3) found no independent relationship for systolic BP, diastolic BP, or pulse pressure. In summary, pulse pressure predicted cardiovascular events, and systolic and diastolic BP predicted ESRD; however, none of the BP components predicted all-cause mortality.
The interpretation of the complex results of this report requires two considerations: (1) examine the limitations imposed by the design of the original trial and (2) review the current state of evidence for BP control in CKD. In the HOST Study, BP was measured at one visit; thus, misclassification of hypertension because of measurements made at a single visit is likely, because the diagnosis of hypertension requires an elevated BP at two different visits. As noted by Palit et al. (3), longitudinal BP measures would have been preferable, and recent evidence from the Chronic Renal Insufficiency Cohort shows that longitudinal BP values more strongly predict ESRD versus a single baseline value (5). Additionally, many such patients may have had white coat hypertension or masked hypertension. In fact, masked hypertension (elevated BP out of office and normal BP in office) has been associated with a 2-fold risk of ESRD (6), whereas white coat hypertension has a relatively benign prognosis in those with CKD. Such misclassifications inevitably weaken the associations between BP and outcomes. Properly classifying masked and white coat hypertension likely explains, in part, why home BP has had a much stronger association with death, ESRD, and cardiovascular compared with office BP in CKD (6,7). It should be noted that, in the past, a strong relationship has been reported between the onset of ESRD and both systolic and diastolic BP in veterans with CKD. In fact, the site that reported this association was also a participating site in the HOST Study. In addition, the number of patients in this earlier study (6) was much smaller than that in this analysis by Palit et al. (3), and therefore, BP measurement issues may be, as least in part, responsible for this discrepancy.
Additionally, since the 1990s, when the HOST Study was designed, the definition of end points has evolved. For example, many myocardial infarctions are known to occur during a hospitalization in the absence of symptoms or electrocardiographic changes. These type 2 myocardial infarctions form the bulk of the myocardial infarctions among patients with advanced CKD, and they were likely missed during the HOST Study because of the diagnostic criteria used at the time. Those with hemorrhagic stroke (which may occur because of uncontrolled hypertension) were excluded. Perhaps most important, congestive heart failure is sensitive to hypertension control and was not defined as an end point in the original trial. These more updated end point definitions should be considered in any modern trial design investigating BP in the CKD population.
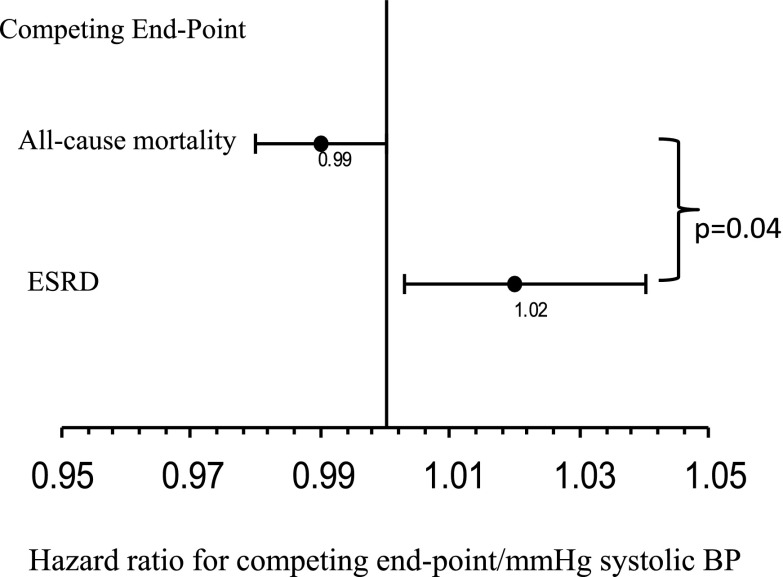
One intriguing possibility that emerges from the report by Palit et al. (3) is whether BP components have different relationships with the competing end points of death and ESRD. Although this report did not include a competing risks analysis, an earlier study did include such an analysis on a prospective cohort of veterans with CKD (8). Although the overall risk of ESRD or all-cause mortality was similar in a traditional Cox model, Agarwal et al. (8) also found that systolic BP may, indeed, have a different relationship with the end points of ESRD and death in a model that accounted for competing risks. For example, those with lower systolic BP have an increased hazard of death, but those with a higher systolic BP have an increased hazard of ESRD (Figure 1). Although the reasons why this may be so remain elusive, we speculate that low systolic BP is a marker of a more severe risk profile, such as the presence of inflammation, cancer, or advanced cardiac disease, that promotes the risk of death, whereas a higher BP indicates absence of these risk factors and the progression to ESRD. In the case of advanced CKD, it still remains unclear if lowering BP with lifestyle modifications or medications will protect from ESRD or come at the expense of reduced survival. Modeling of competing risks is possible with modern statistical methods and would be useful in interpreting the results of BP components in observational studies and randomized trials.
Figure 1.
Relationship between increasing systolic BP and competing outcomes in CKD. A higher systolic BP is a risk factor for ESRD, but a lower systolic BP is a marker for all-cause mortality in a competing risk model (data from ref. 8).
Most fundamentally, the results of this study come from an observational cohort derived from a trial that was designed to test the hypothesis that lowering of homocysteine reduces death, cardiovascular events, or ESRD. Specifically, the intervention in the original trial was not on BP, and therefore, as Palit et al. (3) appropriately note, the findings should not be interpreted as causal. In other words, no cause-and-effect relationship can be deduced from these observations.
The current evidence base for BP goals in CKD stems from three large randomized trials. These three trials examined different BP goals and the important outcomes of all-cause mortality, ESRD, and cardiovascular events: the MDRD Trial (9), the African American Study of Kidney Disease and Hypertension Trial (10,11), and the Ramipril Efficacy in Nephropathy 2 Trial (12). All three trials were designed for a primary renal end point of decline in renal function or ESRD, and compared with usual lowering of BP, none of the three trials reported that more aggressive lowering of BP was more protective for the preservation of kidney function (13). Similar to the systematic review, a meta-analysis of large, randomized, controlled trials of BP lowering that included subjects with eGFR<60 ml/min per 1.73 m2 found no clear evidence of benefit for lower BP targets in the subgroups with mild CKD (mean eGFR =52 ml/min per 1.73 m2) to improve cardiovascular events or all-cause mortality (14).
Notably, the available evidence above on BP goals is derived from trials with primary renal end points or trials that recruited mostly subjects with normal renal function. The ongoing Systolic BP Intervention (SPRINT) Trial will address both shortcomings, because it has recruited over 9000 subjects age ≥50 years old with at least one additional cardiovascular risk factor, including CKD defined as eGFR=20–59 ml/min per 1.73 m2. Participants are randomized to intensive BP lowering with goal office systolic BP <120 mmHg versus control treatment of goal office systolic BP <140 mmHg, with a primary end point of cardiovascular events. Thus, the SPRINT Trial results should provide direct evidence of whether a low office BP goal may reduce cardiovascular events in the CKD population.
Treatment of hypertension in CKD is a major public health concern, and the report from Palit et al. (3) adds to the complexity of the interpretation of the BP components. Specifically, the use of pulse pressure for clinical decision-making is difficult. Currently, no antihypertensive agent specifically reduces pulse pressure. Most antihypertensive agents reduce systolic BP somewhat to a greater degree than diastolic BP, thereby reducing pulse pressure. However, the association of high pulse pressure and subsequent cardiovascular events does suggest that future investigations should include direct measures correlated with pulse pressure, including pulse wave velocity measurement, in the CKD population. Similarly, future studies should include out of office BP measures (whether home BP as recommended by the American Heart Association for the management of all hypertension or the gold standard of ambulatory BP monitoring as recommended by National Institute for Health and Care Excellent in the United Kingdom). Clinical trials, such as the SPRINT Trial, will now begin to provide direct evidence to rationally inform routine treatment of hypertension for the benefit of patients with CKD.
Disclosures
A.D.S. has nothing to disclose. R.A. has consulted for several pharmaceutical companies that make antihypertensive drugs, including Merck, Takeda, Novartis, Daiichi Sankyo, Abbvie, Bayer, and Johnson and Johnson.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Association of BP with Death, Cardiovascular Events, and Progression to Chronic Dialysis in Patients with Advanced Kidney Disease,” on pages 934–940.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Palit S, Chonchol M, Cheung AK, Kaufman J, Smits G, Kendrick J: Association of BP with death, cardiovascular events, and progression to chronic dialysis in patients with advanced kidney disease. Clin J Am Soc Nephrol 10: 934–940, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM; Veterans Affairs Site Investigators: Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: A randomized controlled trial. JAMA 298: 1163–1170, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER, 3rd, Rahman M, Steigerwalt S, Weir M, Wright JT, Jr., Feldman HI; Chronic Renal Insufficiency Cohort Study Investigators: Time-updated systolic blood pressure and the progression of chronic kidney disease: A cohort study. Ann Intern Med 162: 258–265, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal R, Andersen MJ: Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int 69: 406–411, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Andersen MJ: Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol 26: 503–510, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Bunaye Z, Bekele DM, Light RP: Competing risk factor analysis of end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 28: 569–575, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Wright JT, Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, Randall O, Rostand S, Sherer S, Toto RD, Wright JT, Jr., Wang X, Greene T, Appel LJ, Lewis J; AASK Study Group: Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 48: 739–751, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G; REIN-2 Study Group: Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): Multicentre, randomised controlled trial. Lancet 365: 939–946, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Upadhyay A, Earley A, Haynes SM, Uhlig K: Systematic review: Blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 154: 541–548, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, Cass A, Baigent C, Chalmers J, Li N, Woodward M, MacMahon S; Blood Pressure Lowering Treatment Trialists’ Collaboration: Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: Meta-analysis of randomised controlled trials. BMJ 347: f5680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]