Abstract
Background and objectives
This study compared the risk of subdural hematoma (SDH) and subsequent mortality in hemodialysis (HD) and peritoneal dialysis (PD) patients with ESRD.
Design, setting, participants, & measurements
Claims data were obtained from the National Health Insurance Administration Research Database in Taiwan. This retrospective cohort study comprised 10,136 PD patients and 10,136 HD patients with newly diagnosed ESRD from 1998 to 2010. Patients were matched by propensity score and year of dialysis initiation. Incidence rates and hazard ratios of SDH as well as odds ratios of subsequent 30-day deaths from SDH were evaluated from the date of the first dialysis session to the date when SDH was diagnosed, or the date of renal transplantation, death, withdraw from insurance, or the end of the follow-up period (December 31, 2011).
Results
Median (25th percentile, 75th percentile) follow-up times for SDH events were 3.61 years (1.91, 6.33) and 3.33 years (1.83, 5.66) in the HD and PD cohorts, respectively. The overall SDH incidence rate (95% confidence interval [95% CI]) was 61.4% higher in the HD cohort than in the PD cohort (34.7 [95% CI, 31.4 to 35.4] versus 21.5 [95% CI, 20.2 to 22.9] per 10,000 person-years, with an adjusted hazard ratio of 1.62 [95% CI, 1.17 to 2.33]). Approximately 152 of 253 (60%) of SDH events were associated with trauma. Subsequent 30-day SDH-related mortality was not statistically higher in HD patients than in PD patients (29.1% versus 25.3%; adjusted odds ratio, 1.30; 95% CI, 0.70 to 2.41).
Conclusions
HD patients have a higher risk of developing SDH than PD patients. Both patient groups have a high risk of mortality. Routine education on fall prevention is needed for dialysis patients.
Keywords: ESRD, hemodialysis, peritoneal dialysis, subdural hematoma, mortality
Introduction
Subdural hematoma (SDH) is a disorder in which traumatic head injury causes blood collection between the dura and the arachnoid membranes, resulting in high mortality (1,2). SDH usually occurs due to tearing of bridging veins between the cerebral cortex and the dural sinus. Arterial rupture causes approximately 20%–30% of SDH cases (3). The delicate brain tissue is damaged in patients with SDH, as a result of increased intracranial pressure with compression of brain structures. SDH has varied clinical manifestations, including acute, subacute, and chronic onset after bleeding; thus, early diagnosis requires a high degree of suspicion (4). Acute SDH more frequently occurs as a result of motor vehicle collisions in younger patients and falls in elderly patients, and acute SDH has worse outcomes (5). By contrast, chronic SDH often occurs in elderly individuals who sustain a minor injury or fall without a concomitant traumatic head injury (6).
In addition to head injury (direct trauma), studies have associated SDH risk with falls, advanced age, reduced cognitive function, use of anticoagulants or antiplatelet drugs, alcoholism, epilepsy, bleeding diathesis, and low intracranial pressure (6,7). Neurologic complications are important causes of morbidity and mortality in patients with ESRD (8). Studies also show that patients with ESRD are at higher risk of developing SDH (9–12). An earlier Japanese study found that acute SDH accounts for 8.6% of sudden deaths in patients receiving dialysis (11). Sood et al. reported that the risk of nontraumatic SDH in long-term hemodialysis (HD) patients is 10-fold higher than that of the general United States population (10). We recently found that HD-treated patients with ESRD were at nearly 4 times higher risk of SDH than study participants without kidney disease (12). However, the risk for patients on peritoneal dialysis (PD) was not comprehensively evaluated.
Dialysis modality may influence outcomes of patients with ESRD (13,14). Patients undergoing HD experience more hemodynamic changes as well as fluid, electrolyte, and acid-base status fluctuations than patients receiving PD (15). In addition, the use of anticoagulants such as heparin may exaggerate bleeding risk in HD patients. It is not clear whether the SDH risk for PD patients is similar to that for HD patients. In this study, we used Taiwan National Health Insurance Administration claims data to conduct retrospective cohort analyses to evaluate risks of SDH for HD patients and PD patients as well as mortality from SDH, according to dialysis modality. We used propensity score matching to reduce potential biases inherent to a retrospective cohort study.
Materials and Methods
Data Sources
The Taiwanese government has integrated several insurance programs into a single program (National Health Insurance) since 1995. The National Health Insurance Administration Research Database contains deidentified medical claims data for nearly all (99%) of the 23.7 million residents in Taiwan (16). Diagnoses in the claims data were coded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. This retrospective observational study complied with the Declaration of Helsinki guidelines and was approved by the China Medical University Research Ethics Committee (CMU-REC-101-012). Because this study involved a retrospective review of existing data, the China Medical University Institutional Review Board specifically waived the need for consent. All data were deidentified and analyzed anonymously.
Patient Selection and Definition of Dialysis Modality
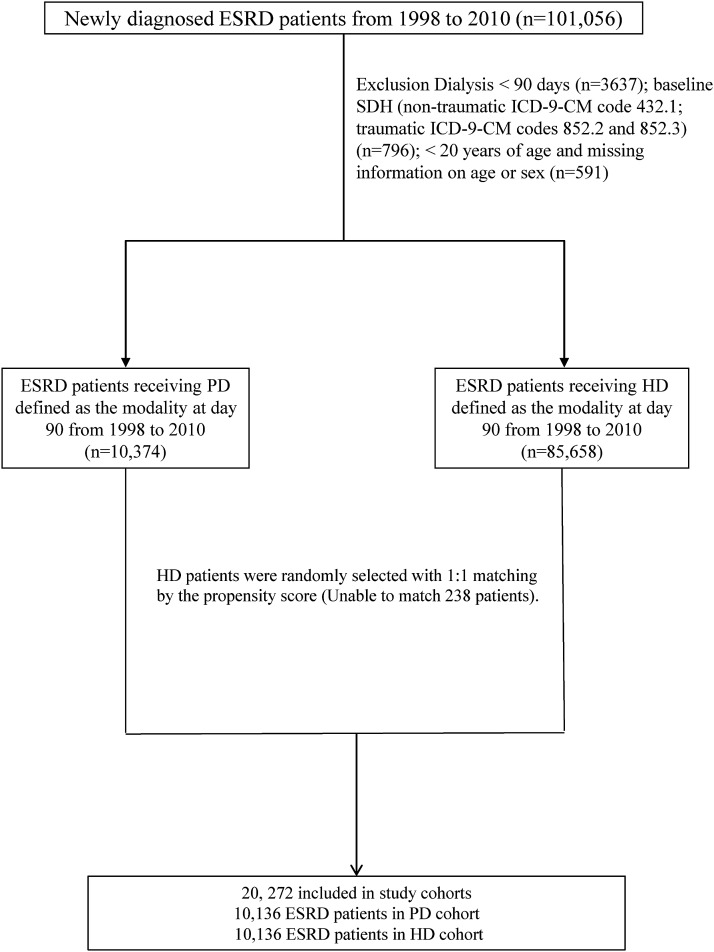
Figure 1 shows the process of selecting participants for the study cohorts. Patients who were newly diagnosed with ESRD from 1998 to 2010 and undergoing dialysis for ≥3 months were identified from the claims data (n=101,056). The index day was defined as day 90 after the first dialysis session. Patients with ESRD who died within 90 days after the first dialysis session were excluded (n=3637). Patients with a history of SDH (nontraumatic ICD-9-CM code 432.1; traumatic ICD-9-CM codes 852.2 and 852.3) (n=796) before the index date, as well as patients aged <20 years or those with incomplete age or sex information were also excluded (n=591).
Figure 1.
Flow chart of study cohort selection. HD, hemodialysis; ICD-9-CM, International Classification of Diseases Ninth Revision, Clinical Modification; PD, peritoneal dialysis; SDH, subdural hematoma.
Some PD patients may temporarily receive HD initially. Thus, dialysis modality was defined as the modality at day 90 after the first dialysis session. Patients were not censored if the dialysis modality changed and SDH was attributed to the modality at day 90. Because approximately 10% of patients with ESRD received PD, we selected the HD cohort with a similar sample size, matched by the propensity score and year of dialysis initiation. We used logistic regression to calculate the propensity score for each patient by estimating the probability of assignment based on baseline variables including age, sex, year of dialysis, comorbidity (coronary artery disease, congestive heart failure, stroke, hyperlipidemia, hypertension, diabetes, dementia, and atrial fibrillation), and medication (warfarin, clopidogrel, and aspirin). The C-statistic of the logistic regression model was 0.61. The level of precision in propensity score matching was 0.0001. We also considered a matching caliper of 0.12. The analyses took the matched nature of the cohorts into account. All participants were followed from the date of the first dialysis session to the date when SDH was diagnosed, or the date of renal transplantation, death, withdrawal from insurance, or the end of the follow-up period (December 31, 2011). Follow-up time was calculated in person-years for each participant.
Independent Variables
For each participant, baseline information including demographic characteristics, comorbidities, and medications was obtained from the claims data. The following covariates were included: sex, age, and comorbidity including coronary artery disease (ICD-9-CM codes 410–413, 414.01–414.05, 414.8, and 414.9), congestive heart failure (ICD-9-CM codes 428, 398.91, 402.x1), stroke (ICD-9-CM codes 430–438), hyperlipidemia (ICD-9-CM code 272), atrial fibrillation (ICD-9-CM code 427.31), hypertension (ICD-9-CM codes 401–405), diabetes (ICD-9-CM code 250), and dementia (ICD-9-CM codes 290.0, 290.1, 290.2, 290.3, 290.4, 294.1 and 331.0), as well as selected medications (warfarin, clopidogrel, and aspirin).
Statistical Analyses
Values are expressed as means±SD, medians (25th percentile, 75th percentile), or numbers and proportions. We first calculated and plotted the chronological yearly age-standardized incidence rates of SDH for HD and PD patients separately from 1998 to 2011, using 1998 as the reference. Baseline distributions of demographic and clinical characteristics of the study groups are expressed as means±SD for continuous variables and as percentages for categorical variables. Distributions between the PD and HD cohorts were examined using the t test or chi-squared test as appropriate. Incident SDH was computed for each cohort by separately measuring traumatic and nontraumatic events. The proportion of patients who were free of SDH in the PD and HD cohorts was plotted using the Kaplan–Meier method, and the difference was assessed with the log-rank test. Cox proportional hazards regression analysis was used to estimate hazard ratios (HR) and 95% confidence intervals (95% CIs) of developing SDH in both cohorts. We further used multivariable Cox models to measure HRs for SDH by demographic status, comorbidity, and medication among PD and HD patients. Thirty-day mortality rates for patients with SDH were estimated for both cohorts. Logistic regression analysis was used to measure odds ratios (ORs) of 30-day mortality from SDH between the two modalities. All analyses were carried out with SAS statistical software (version 9.2 for Windows; SAS Institute Inc., Cary, NC). All statistical tests were performed at the two-tailed significance level of 0.05.
Results
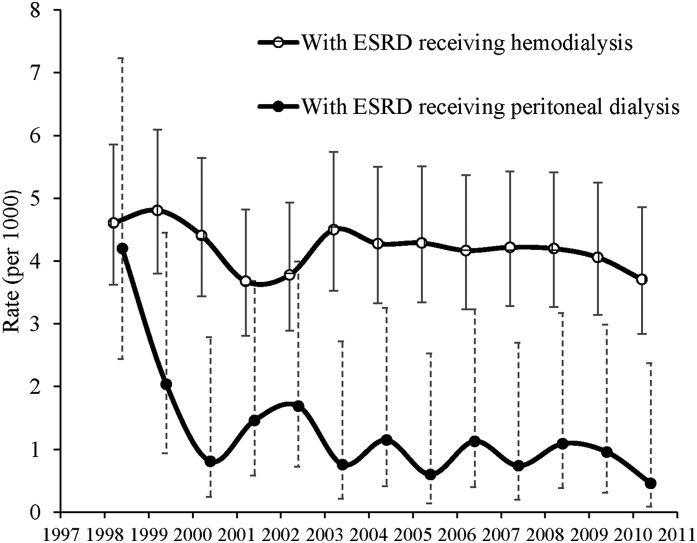
The annual age-standardized incidence of SDH was much higher in HD patients than in PD patients (P<0.001), with a significant decreasing trend in PD patients from 1998 to 2010 (P for trend, P=0.01 and 0.09 in PD and HD patients, respectively) (Figure 2).
Figure 2.
The annual age-standardized incidence rates and 95% CIs of SDH in patients with ESRD receiving HD or PD from 1998 to 2010. 95% CI, 95% confidence interval; SDH, subdural hematoma.
Table 1 presents the baseline characteristics of the HD cohort compared with the propensity score–matched PD cohort. There were no significant differences in baseline characteristics between the PD and HD cohorts.
Table 1.
Distributions of demographic and clinical characteristics between propensity score–matched HD and PD cohorts
| Characteristic | HD (n=10,136) | PD (n=10,136) | P Value |
|---|---|---|---|
| Sex | 0.64 | ||
| Female | 5475 (54.0) | 5508 (54.3) | |
| Male | 4661 (46.0) | 4628 (45.7) | |
| Age, yr | 0.16 | ||
| <50 | 4378 (43.2) | 4229 (41.7) | |
| 50–59 | 2535 (25.0) | 2577 (25.4) | |
| 60–69 | 1743 (17.2) | 1830 (18.1) | |
| ≥70 | 1480 (14.6) | 1500 (14.8) | |
| Mean | 53.5±14.6 | 53.3 (14.9) | 0.34 |
| Comorbidity | |||
| Coronary artery disease | 2368 (23.4) | 2297 (22.7) | 0.24 |
| Congestive heart failure | 1793 (17.7) | 1756 (17.3) | 0.49 |
| Stroke | 950 (9.4) | 921 (9.1) | 0.48 |
| Hyperlipidemia | 4371 (43.1) | 4388 (43.3) | 0.81 |
| Hypertension | 9056 (89.3) | 8998 (88.8) | 0.19 |
| Diabetes | 3587 (35.4) | 3627 (35.8) | 0.56 |
| Dementia | 125 (1.2) | 121 (1.2) | 0.80 |
| Atrial fibrillation | 201 (2.0) | 216 (2.1) | 0.46 |
| Medication | |||
| Warfarin | 302 (3.0) | 308 (3.0) | 0.81 |
| Clopidogrel | 940 (9.3) | 933 (9.2) | 0.87 |
| Aspirin | 5888 (58.1) | 5870 (57.9) | 0.80 |
| Year of dialysis initiation | 0.97 | ||
| 1998 | 265 (2.6) | 282 (2.8) | |
| 1999 | 363 (3.6) | 362 (3.6) | |
| 2000 | 474 (4.7) | 481 (4.8) | |
| 2001 | 604 (6.0) | 581 (5.7) | |
| 2002 | 645 (6.4) | 603 (6.0) | |
| 2003 | 646 (6.4) | 665 (6.6) | |
| 2004 | 675 (6.7) | 669 (6.6) | |
| 2005 | 768 (7.6) | 770 (7.6) | |
| 2006 | 854 (8.4) | 865 (8.5) | |
| 2007 | 1087 (10.7) | 1124 (11.1) | |
| 2008 | 1212 (12.0) | 1238 (12.2) | |
| 2009 | 1244 (12.3) | 1239 (12.2) | |
| 2010 | 1299 (12.8) | 1257 (12.4) |
Data are presented as n (%) or mean±SD. Distributions were compared using the t test for mean age and the chi-squared test for sex, age, comorbidity, and medication. HD, hemodialysis; PD, peritoneal dialysis.
Median follow-up time for SDH events was longer in the HD cohort than in the PD cohort (3.61 [1.91, 6.33] versus 3.33 [1.83, 5.66] years, respectively; Table 2). The overall incidence density of SDH was higher in the HD cohort than in the PD cohort (34.7 [95% CI, 31.4 to 35.4] versus 21.5 [95% CI, 20.2 to 22.9] per 10,000 person-years). The adjusted HR of SDH was 1.62 (95% CI, 1.17 to 2.33) for the HD cohort compared with the PD cohort. The HD cohort had a higher incidence for both traumatic SDH (19.8 [95% CI, 18.6 to 21.1] versus 13.1 [95% CI, 12.3 to 14.0] per 10,000 person-years) and nontraumatic SDH (13.5 [95% CI, 12.6 to 14.4] versus 8.38 [95% CI, 7.83 to 8.97] per 10,000 person-years) than the PD cohort. The adjusted HR of traumatic SDH was 1.74 (95% CI, 1.15 to 2.64) for the HD cohort compared with the PD cohort. Of 158 SDH events in HD patients, 131 (82.9%) occurred on the day of HD treatment (data not shown).
Table 2.
Incidence (per 10,000 person-years) and HRs of SDH in the propensity score–matched cohorts
| Characteristic | HD | PD |
|---|---|---|
| Follow-up, person-years | 47,442 | 44,133 |
| Median | 3.61 (1.91–6.33) | 3.33 (1.83–5.66) |
| SDH | ||
| Overall | ||
| Event, n | 158 | 95 |
| Incidence rate | 34.7 (31.4 to 35.4) | 21.5 (20.2 to 22.9) |
| HR | 1.62 (1.17 to 2.33) | 1 (reference) |
| Traumatic | ||
| Events, n | 94 | 58 |
| Incidence rate | 19.8 (18.6 to 21.1) | 13.1 (12.3 to 14.0) |
| HR | 1.74 (1.15, 2.64) | 1 (reference) |
| Nontraumatic | ||
| Event, n | 64 | 37 |
| Incidence rate | 13.5 (12.6 to 14.4) | 8.38 (7.83 to 8.97) |
| HR | 1.44 (0.86 to 2.40) | 1 (reference) |
Data are presented as the median (interquartile range), incidence rate (95% CI), or HR (95% CI) unless otherwise indicated. HR, hazard ratio; SDH, subdural hematoma; HD, hemodialysis; PD, peritoneal dialysis; 95% CI, 95% confidence interval.
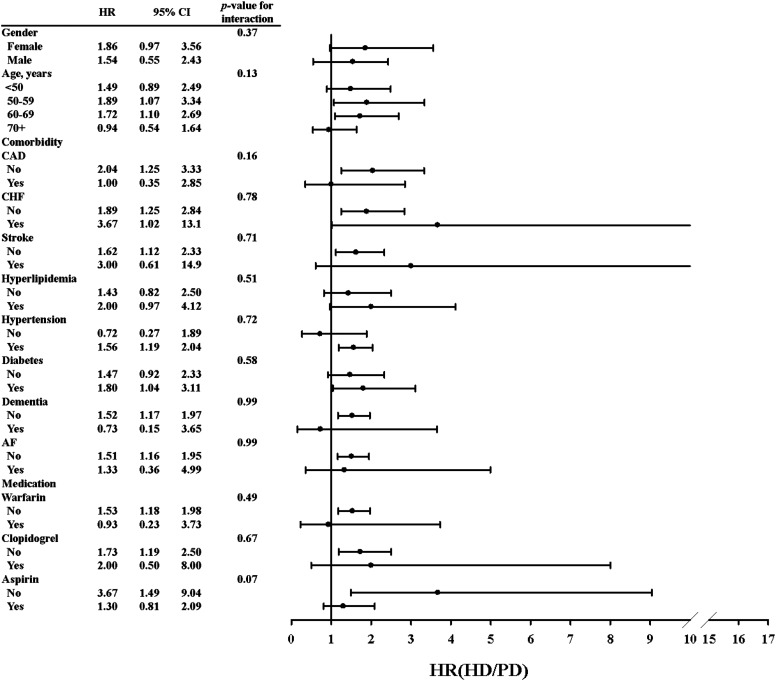
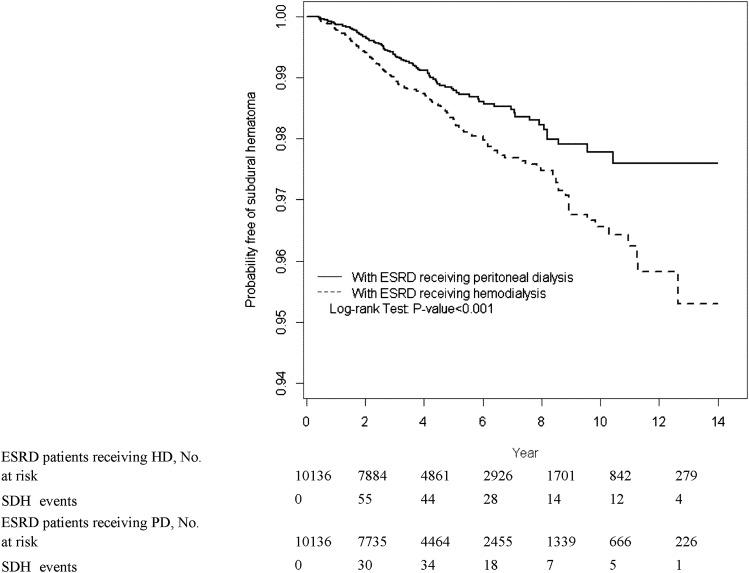
Figure 3 and Supplemental Table 1 show HRs of SDH for both cohorts, stratified by sex, age, comorbidity, and medication. HRs of SDH were higher for patients aged 50–59 and 60–69 years in the HD cohort compared with the PD cohort (Supplemental Table 1). In general, the presence of comorbidity or medication use was associated with a higher incidence of SDH in both cohorts; patients in both cohorts who used warfarin, clopidogrel, or aspirin had a higher incidence of SDH than those who did not. Figure 4 shows the proportion of study participants who were free of SDH after 13 years of follow-up. Compared with the PD cohort, the proportion of study participants who developed SDH was 2.38% higher in the HD cohort (log-rank test P<0.001).
Figure 3.
The HR of SDH (HD versus PD) by sex, age, comorbidity, and medication. HR, hazard ratio; 95% CI, 95% confidence interval; CAD, coronary artery disease; CHF, congestive heart failure; AF, atrial fibrillation; HR, hazard ratio; SDH, subdural hematoma; HD, hemodialysis; PD, peritoneal dialysis.
Figure 4.
The proportion of patients with ESRD receiving HD or PD who were free of SDH. HD, hazard ratio; SDH, subdural hematoma; PD, peritoneal dialysis.
Table 3 shows that 30-day mortality for patients who developed SDH was higher in HD patients than in PD patients (29.1% versus 25.3%). The difference in SDH mortality between HD and PD patients was not statistically significant.
Table 3.
ORs of 30-day mortality from SDH in the propensity score–matched cohorts
| Mortality | HD | PD |
|---|---|---|
| Death/SDH | 46/158 | 24/95 |
| Rate | 29.1 | 25.3 |
| Crude OR | 1.22 (0.68, 2.16) | 1 (reference) |
| Adjusted ORa | 1.30 (0.70, 2.41) | 1 (reference) |
Data are presented as the number of death events/total SDH events, percentage, or OR (95% CI). OR, odds ratio; SDH, subdural hematoma; HD, hemodialysis; PD, peritoneal dialysis.
Multivariate analysis controlling for age, sex, coronary artery disease, congestive heart failure, stroke, hyperlipidemia, hypertension, diabetes, dementia, atrial fibrillation, warfarin, clopidogrel, aspirin, and type of SDH.
Discussion
This large population-based study used propensity score matching to compare SDH risk between HD and PD patients. This matching method was used to reduce the selection bias inherent to retrospective cohort studies. Our findings demonstrate that HD patients were more likely to develop SDH (adjusted HR, 1.62) compared with PD patients. Among HD patients, 82.9% of SDH events occurred on the day of a HD session. The difference in SDH risks between HD and PD patients was consistent with the chronologic trends between patients on HD and PD from 1998 to 2010. These findings suggest that there was a possible causal relationship between dialysis treatment and SDH occurrence.
Mehrotra et al. found that outcomes in PD patients improved and there was no significant difference in outcomes between PD and HD patients (17). Improved treatment in PD patients may have resulted in the decreasing SDH incidence from 1998 to 2010 in Taiwan; however, this explanation may not be adequate. Our further data analysis failed to show that the decrease in the incidence of SDH was associated with the use of warfarin among PD patients (data not shown). Our findings also show no significant difference in 30-day mortality between HD patients and PD patients. Therefore, the risks of death could be similar if a dialysis patient develops SDH regardless of the dialysis modality. To our knowledge, these findings have not previously been reported.
Several factors may explain the higher incidence of SDH in HD patients. The routine use of anticoagulants with heparin during HD sessions may have exaggerated bleeding diathesis. For example, the risk of peptic ulcer bleeding is near 3-fold greater in HD patients than PD patients (18). HD patients are also at higher risk of hemorrhagic stroke than PD patients (19). Bleeding complications are more likely associated with higher SDH risk, which is greater in HD patients than in PD patients.
Falls are common in dialysis patients and are associated with a higher risk of traumatic SDH. Studies have shown that the incidence of falls in HD patients is about 1.2–1.6 per patient-year, or 0.2–0.37 per patient-year for patients with severe conditions requiring emergency care (20–22). A recent prospective cohort study showed that the incidence of falls may increase to 1.7 per patient-year for older PD patients (23). To our knowledge, no study has directly compared the risk of falls between PD and HD patients. Another recent study in Taiwan revealed a 2.2-fold greater incidence of hip fracture in HD patients than in PD patients (13.6 versus 6.25 per 1000 person-years) (24). It is likely that the HD procedure may increase the risk of falls and subsequent head injury as a result of needing frequent transportation for dialysis, greater electrolyte and fluid shifts, and subsequent dizziness, hypotension, and arrhythmia.
Brain atrophy is common in patients with ESRD, affecting as many as 77.5% of those receiving HD (25), and may increase the length of the bridging veins, which are vulnerable to tearing. Cortical atrophy in HD patients may be related to repeated hypotensive episodes (26). Cognitive impairment, including dementia, is also prevalent in patients with ESRD and affects 16%–38% of patients (27). Compared with PD patients, HD patients are at higher risk of impaired cognitive function because of more severe anemia and less efficiency in clearing middle molecules (28,29). HD patients are also at higher risk of hospitalization for psychiatric illness (30). By contrast, PD patients have greater stability in intracranial pressure and cerebral blood flow due to smaller changes in urea clearance, acid-base status, serum osmolality, and fluid status (31,32). Fewer fluid shifts in PD also contribute to fewer changes in brain volume. Furthermore, pressure within the subdural cavity is reduced during HD sessions (33). Thus, HD patients are at a higher risk of SDH than PD patients.
This study has some limitations. First, data on lifestyle, Glasgow coma scale score, frailty, and laboratory measurements were unavailable in the National Health Insurance Administration Research Database. Second, we were unable to differentiate acute and chronic SDH because neuroimaging records were not included in the claims reports. Third, the validity of diagnoses of SDH and comorbidity in the claims data could not be verified because diseases were identified by ICD-9-CM codes. However, dialysis patients made frequent visits to their healthcare professionals, and the claims data are reliable and audited by the National Health Insurance Administration. Finally, although we established the study cohorts matched by propensity score to reduce selection bias, there was a possibility of residual confounding.
In conclusion, this study demonstrates that SDH risk is higher in HD patients than in PD patients. Because of the high mortality from SDH for dialysis patients, preventive measures designed to reduce SDH risk in patients with ESRD, especially for those on HD, are urgently needed.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Sciences Council, Executive Yuan (NSC 100-2621-M-039-001), China Medical University Hospital (1MS1, DMR-100-101, DMR-101-016, DMR-103-013, and DMR-104-015), Research Laboratory of Pediatrics, Children’s Hospital of China Medical University, Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM103010096), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006), Tseng-Lien Lin Foundation, Taiwan Brain Disease Foundation, and Katsuzo and Kiyo Aoshima Memorial Funds.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08140814/-/DCSupplemental.
References
- 1.Harvey LA, Close JC: Traumatic brain injury in older adults: Characteristics, causes and consequences. Injury 43: 1821–1826, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Jacobsson LJ, Westerberg M, Lexell J: Demographics, injury characteristics and outcome of traumatic brain injuries in northern Sweden. Acta Neurol Scand 116: 300–306, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Maxeiner H, Wolff M: Pure subdural hematomas: A postmortem analysis of their form and bleeding points. Neurosurgery 50: 503–508, discussion 508–509, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Burn DJ, Bates D: Neurology and the kidney. J Neurol Neurosurg Psychiatry 65: 810–821, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman RF, Komotar R, Hillis AE: Neurologic aspects of traumatic brain injury. Int Rev Psychiatry 15: 302–309, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK: Chronic subdural haematoma in the elderly. Postgrad Med J 78: 71–75, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordström A, Nordström P: Cognitive performance in late adolescence and the subsequent risk of subdural hematoma: An observational study of a prospective nationwide cohort. PLoS Med 8: e1001151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouns R, De Deyn PP: Neurological complications in renal failure: A review. Clin Neurol Neurosurg 107: 1–16, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Power A, Hamady M, Singh S, Ashby D, Taube D, Duncan N: High but stable incidence of subdural haematoma in haemodialysis—a single-centre study. Nephrol Dial Transplant 25: 2272–2275, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Sood P, Sinson GP, Cohen EP: Subdural hematomas in chronic dialysis patients: Significant and increasing. Clin J Am Soc Nephrol 2: 956–959, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Harada A, Okuda S, Fujimi S, Oh Y, Hattori F, Motomura K, Hirakata H, Fujishima M: Sudden death in chronic dialysis patients. Nephrol Dial Transplant 12: 952–955, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Wang IK, Lin CL, Wu YY, Kuo HL, Lin SY, Chang CT, Yen TH, Chuang FR, Cheng YK, Huang CC, Sung FC: Subdural hematoma in patients with end-stage renal disease receiving hemodialysis. Eur J Neurol 21: 894–900, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Vonesh EF, Snyder JJ, Foley RN, Collins AJ: The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int 66: 2389–2401, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Wang IK, Kung PT, Kuo WY, Tsai WC, Chang YC, Liang CC, Chang CT, Yeh HC, Wang SM, Chuang FR, Wang KY, Lin CY, Huang CC: Impact of dialysis modality on the survival of end-stage renal disease patients with or without cardiovascular disease. J Nephrol 26: 331–341, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Rodby RA, Vonesh EF, Korbet SM: Blood pressures in hemodialysis and peritoneal dialysis using ambulatory blood pressure monitoring. Am J Kidney Dis 23: 401–411, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Lu JF, Hsiao WC: Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood) 22: 77–88, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Huang KW, Leu HB, Luo JC, Chan WL, Hou MC, Lin HC, Lee FY, Kuan YC: Different peptic ulcer bleeding risk in chronic kidney disease and end-stage renal disease patients receiving different dialysis. Dig Dis Sci 59: 807–813, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Wang HH, Hung SY, Sung JM, Hung KY, Wang JD: Risk of stroke in long-term dialysis patients compared with the general population. Am J Kidney Dis 63: 604–611, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Cook WL, Tomlinson G, Donaldson M, Markowitz SN, Naglie G, Sobolev B, Jassal SV: Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol 1: 1197–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Desmet C, Beguin C, Swine C, Jadoul M, Université Catholique de Louvain Collaborative Group : Falls in hemodialysis patients: Prospective study of incidence, risk factors, and complications. Am J Kidney Dis 45: 148–153, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Rossier A, Pruijm M, Hannane D, Burnier M, Teta D: Incidence, complications and risk factors for severe falls in patients on maintenance haemodialysis. Nephrol Dial Transplant 27: 352–357, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Farragher J, Chiu E, Ulutas O, Tomlinson G, Cook WL, Jassal SV: Accidental falls and risk of mortality among older adults on chronic peritoneal dialysis. Clin J Am Soc Nephrol 9: 1248–1253, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YJ, Kung PT, Wang YH, Huang CC, Hsu SC, Tsai WC, Hsu HC: Greater risk of hip fracture in hemodialysis than in peritoneal dialysis. Osteoporos Int 25: 1513–1518, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Savazzi GM, Cusmano F, Musini S: Cerebral imaging changes in patients with chronic renal failure treated conservatively or in hemodialysis. Nephron 89: 31–36, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Mizumasa T, Hirakata H, Yoshimitsu T, Hirakata E, Kubo M, Kashiwagi M, Tanaka H, Kanai H, Fujimi S, Iida M: Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: A 3-year prospective study. Nephron Clin Pract 97: c23–c30, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kurella Tamura M, Yaffe K: Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int 79: 14–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buoncristiani U, Alberti A, Gubbiotti G, Mazzotta G, Gallai V, Quintaliani G, Gaburri M: Better preservation of cognitive faculty in continuous ambulatory peritoneal dialysis. Perit Dial Int 13[Suppl 2]: S202–S205, 1993 [PubMed] [Google Scholar]
- 29.Wolcott DL, Wellisch DK, Marsh JT, Schaeffer J, Landsverk J, Nissenson AR: Relationship of dialysis modality and other factors to cognitive function in chronic dialysis patients. Am J Kidney Dis 12: 275–284, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Kimmel PL, Thamer M, Richard CM, Ray NF: Psychiatric illness in patients with end-stage renal disease. Am J Med 105: 214–221, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E: Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 27: 1861–1869, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Stefanidis I, Bach R, Mertens PR, Liakopoulos V, Liapi G, Mann H, Heintz B: Influence of hemodialysis on the mean blood flow velocity in the middle cerebral artery. Clin Nephrol 64: 129–137, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kopitnik TA, Jr, de Andrade R, Jr, Gold MA, Nugent GR: Pressure changes within a chronic subdural hematoma during hemodialysis. Surg Neurol 32: 289–293, 1989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.