Abstract
Background and objectives
Glycated hemoglobin (HbA1c) is used to diagnose diabetes mellitus (DM) and guide its management. The association between higher HbA1c and progression to ESRD and mortality has been demonstrated in populations with DM. This study examined the association between HbA1c and these end points in a population with CKD and without DM.
Design, setting, participants, & measurements
In the hospital-based NephroTest cohort study, measured GFR (mGFR) was taken by 51Cr-EDTA renal clearance and HbA1c in 1165 adults with nondialysis CKD stages 1–5 and without DM between January 2000 and December 2010. The median follow-up was 3.48 years (interquartile range, 1.94–5.82) for the competing events of ESRD and pre-ESRD mortality. Time-fixed and time-dependent Cox models were used to estimate hazard ratios (HRs) for ESRD and mortality according to HbA1c, treated continuously or in tertiles.
Results
At inclusion, the mean mGFR was 42.2±19.9 ml/min per 1.73 m2, and the mean HbA1c value was 5.5%±0.5%. During follow-up, 109 patients died, and 162 patients reached ESRD. Pre-ESRD mortality was significantly associated with HbA1c treated continuously: for every 1% higher HbA1c, the crude HR was 2.16 (95% confidence interval [95% CI], 1.27 to 3.68), and it was 1.85 (95% CI, 1.05 to 3.24) after adjustment for mGFR and other risk factors of death. After excluding incident diabetes over time, the updated mean of HbA1c remained significantly associated with higher mortality risk: adjusted HR for the highest (5.7%–6.4%) versus the lowest tertile (<5.3%) was 2.62 (95% CI, 1.16 to 5.91). There was no association with ESRD risk after adjustment for risk factors of CKD progression.
Conclusions
In a CKD cohort, HbA1c values in the prediabetes range are associated with mortality. Such values should be therefore included among the risk factors for negative outcomes in CKD populations.
Keywords: chronic kidney disease, glycation, mortality risk
Introduction
Glycated hemoglobin (HbA1c) is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time (from the last 4 weeks to 3 months) (1,2). In patients with diabetes mellitus (DM), higher concentrations indicate poorer control of blood glucose levels. HbA1c has been associated with the risk of cardiovascular disease, diabetic nephropathy, and retinopathy (3–6). It has been used as a central tool in management of DM. It has been also used as a diagnostic test for DM since the American Diabetes Association issued its 2010 guidelines (5). A 6.5% threshold has been designated as diagnostic. This threshold is also reported to predict the future risk of kidney disease and retinopathy in patients with diabetes (7); however, the authors of this study were unable to demonstrate the presence of a so-called glycemic threshold for the development of such complications. They did find that elevated values of HbA1c were associated with a higher risk of CKD, even in the absence of a DM diagnosis. They also observed an association between high nondiabetic HbA1c values and a higher risk of coronary heart disease, stroke, and death from any cause (6) and described the association between mortality and HbA1c as a J-shaped curve, with a higher hazard ratio (HR) of death for HbA1c <5% and >5.5%. Another study observed more complex coronary artery lesions for the highest HbA1c values in the nondiabetic range (8).
On the other hand, it has recently been demonstrated that CKD, even without DM, is associated with worse cardiovascular and nonvascular mortality (9). The risk of death after myocardial infarction in people with CKD without DM was similar to or higher than the risk for those with DM without CKD. The authors concluded that CKD is a specific risk factor that identifies people at higher risk of future coronary events or death.
The objective of our study was to determine whether and how HbA1c values are associated with ESRD or death in patients with CKD without known DM.
Materials and Methods
Study Population
The NephroTest study is an ongoing prospective hospital-based cohort, enrolling adults who are >18 years with any diagnosis of CKD stages 1–5, not pregnant, and neither on dialysis nor living with a kidney transplant (10). Three nephrology departments recruited these patients, who undergo extensive annual work-ups, including measured GFR (mGFR), in the hospitals’ physiology departments. Between January 2000 and December 2010, 1835 patients were enrolled. After exclusion of 121 patients lost to follow-up, 43 patients with missing baseline measurements of HbA1c, and 506 patients with diabetes defined by a diagnosis of DM in their medical history, use of an antidiabetic treatment, fasting plasma glucose ≥126 mg/dl (7 mmol/l), or HbA1c≥6.5% at the enrollment visit, this analysis covered 1165 patients (Figure 1). All patients signed written informed consents before inclusion in the cohort. The NephroTest study followed the Declaration of Helsinki and was approved by an ethics committee (Direction générale pour la recherche et l’innovation Comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé).
Figure 1.
Study flowchart. *Diabetes defined by a diagnosis of diabetes mellitus in their medical history, use of an antidiabetic treatment, fasting plasma glucose ≥1.26 mg/dl, or glycated hemoglobin (HbA1c) ≥6.5%. mGFR, measured GFR.
Measurement
We measured HbA1c at each participant’s enrollment and follow-up visit, chromatographically, with the Variant II Hemoglobin Testing System (BioRad). GFR was measured by 51Cr-EDTA renal clearance at enrollment and each visit. Briefly, 1.8–3.5 MBq of 51Cr-EDTA (GE Healthcare, Velizy, France) was injected intravenously as a single bolus. An hour was allowed for distribution of the tracer in the extracellular fluid, and then the average renal 51Cr-EDTA clearance was determined for five to six consecutive 30-minute clearance periods (11,12).
Definitions and Outcome
We used the established definition of CKD with mGFR, according to the Kidney Disease Outcomes Quality Initiative. CKD stage 1 is defined by kidney damage, including proteinuria and/or hematuria, and GFR>90 ml/min per 1.73 m2, CKD stage 2 is defined by kidney damage and GFR between 60 and 89 ml/min per 1.73 m2, CKD stage 3 is defined by GFR from 30 to 59 ml/min per 1.73 m2, CKD stage 4 is defined by GFR from 15 to 29 ml/min per 1.73 m2, and CKD stage 5 is defined by GFR<15 ml/min per 1.73 m2 or need for dialysis (13).
Patients were passively followed-up through December 31, 2010. ESRD defined by dialysis or preemptive kidney transplantation was identified either from medical records or through linkage with the national Ramipril Efficacy In Nephropathy registry of dialysis and transplantation. Vital status was ascertained by linkage with the national death registry. All survival data were right-censored on December 31, 2010, or to the date of last visit for patients not identified in registries.
Statistical Analyses
Baseline clinical and laboratory data were expressed as percentages, means±SDs, or medians (interquartile range [IQR]), as appropriate. They were also described by tertiles of HbA1c: continuous variables were compared with the Wilcoxon test, and categorical variables were compared with the chi-squared or Fisher exact test.
We performed Cox regression models to estimate crude and adjusted cause-specific HRs and 95% confidence intervals for both pre-ESRD mortality and ESRD associated with HbA1c levels, with the lowest tertiles as the reference category. In each of these models, the competing events were treated as censored observations. The cause-specific approach has indeed been shown to be the most suitable to account for competing risks of concurrent events for etiologic studies (14). For the analysis of ESRD outcome, 63 patients with mGFR at <15 ml/min per 1.73 m2 at inclusion were excluded. For mortality, crude and adjusted HRs were also estimated with HbA1c treated continuously. Finally, association between HbA1c levels and risk of overall mortality was also studied using Cox regression models. Adjustment covariates were similar in all analyses: demographic characteristics (age, sex, ethnicity), center, and baseline mGFR. Traditional risk factors for CKD progression or death were also similar in all analyses: body mass index (BMI), high BP (>140/90 mmHg), history of cardiovascular disease, smoking, angiotensin-converting enzyme inhibitor (ACEi) or antireceptor blocker (ARB) treatment, log proteinuria, and low albuminemia (<3.5 g/dl). We tested the proportional-hazard assumption with Schoenfeld residuals against time for each covariate. Because it was not satisfied for mGFR in the cause-specific Cox model for ESRD, we stratified rather than adjusted for the baseline mGFR level, using six classes of mGFR (15–20, 20–30, 30–40, 40–50, 50–60, >60 ml/min per 1.73m2).
Finally, mortality analyses were repeated after exclusion of patients who developed diabetes during follow-up. To account for changes in HbA1c over time, we also estimated pre-ESRD and overall mortality associated with cumulative moving mean of HbA1c (further referred to as updated mean) (3) using time-dependent Cox models. The following adjustment variables were updated at each visit: mGFR, high BP, ACEi or ARB treatment, log proteinuria, and low albuminemia. When data were missing, we used the last available observation. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC) and R 3.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics of the Study Cohort
The mean age of participants at entry was 56.5±16.0 years, and the mean mGFR was 42.4±19.9 ml/min per 1.73 m2 (Table 1). Most patients were in CKD stages 3A (21.5%), 3B (28.2%), or 4 (25.8%), with 19.1% in stages 1–2 and only 5.4% in stage 5. In our study population, HbA1c values ranged from 3.4% to 6.4%, with a mean of 5.5%±0.5% and a median of 5.5 (IQR, 5.2–5.8). Patients with higher values of HbA1c were older and had a higher prevalence of cardiovascular disease history, BMI, systolic BP, fasting glycemia and uricemia, and prevalence of vascular nephropathy. Inversely, they had lower proteinuria (Table 1). Of note, there was no significant association with ethnicity, sex, LDL cholesterol level, hemoglobin, renal function, or serum albumin.
Table 1.
Clinical and biologic characteristics of patients at baseline by tertiles of glycated hemoglobin
| Characteristic | HbA1c | ||||
|---|---|---|---|---|---|
| All Patients | First Tertile (<5.3%) | Second Tertile (5.3%–5.6%) | Third Tertile (5.7%–6.4%) | P Value | |
| Total no. of patients | 1165 | 335 | 390 | 440 | |
| Age (yr) | 56.5±16.0 | 49.8±16.7 | 56.5±15.9 | 61.6±13.5 | <0.001 |
| Male sex | 764 (65.6) | 216 (64.5) | 257 (65.9) | 291 (66.1) | 0.88 |
| Black ethnicity | 140 (12.5) | 40 (12.4) | 40 (10.7) | 60 (14.2) | 0.34 |
| Smoking status | 0.02 | ||||
| Past smoker | 348 (29.9) | 77 (23.0) | 133 (34.1) | 138 (31.4) | |
| Current smoker | 174 (14.9) | 58 (17.3) | 54 (13.8) | 62 (14.1) | |
| History of CV disease | 158 (13.9) | 31 (9.5) | 52 (13.6) | 75 (17.4) | 0.01 |
| BMI (kg/m2) | 25.4±4.6 | 24.1±4.6 | 25.3±4.2 | 26.5±4.6 | <0.001 |
| Systolic BP (mmHg) | 134±20 | 132±19 | 133±19 | 136±20 | 0.003 |
| Diastolic BP (mmHg) | 75±12 | 75±12 | 75±12 | 75±12 | 0.67 |
| ACEi or ARB treatment | 813 (74.7) | 230 (74.4) | 278 (76.6) | 305 (73.1) | 0.54 |
| Statin use | 444 (40.8) | 90 (29.1) | 146 (40.2) | 208 (49.9) | <0.001 |
| ESA use | 64 (5.9) | 31 (10.0) | 14 (3.9) | 19 (4.6) | 0.001 |
| mGFR (ml/min per 1.73 m2) | 39.6 (27.3–54.8) | 39.9 (26.9–59.8) | 41.9 (27.7–54.8) | 37.4 (26.9–52.0) | 0.10 |
| PCR (mg/mg) | 0.20 (0.09–0.75) | 0.30 (0.10–0.88) | 0.19 (0.17–0.62) | 0.18 (0.09–0.62) | 0.03 |
| Fasting plasma glucose (mg/dl) | 91.9±10.8 | 88.2±10.8 | 91.9±9.0 | 97.3±10.8 | <0.001 |
| Hemoglobin (g/dl) | 12.7±1.6 | 12.7±1.9 | 12.8±1.5 | 12.7±1.6 | 0.42 |
| Albumin (g/dl) | 3.98±0.45 | 4.01±0.52 | 3.98±0.43 | 3.96±0.41 | 0.11 |
| LDL cholesterol (mg/dl) | 116.2±38.2 | 116.2±40.9 | 115.4±37.1 | 116.2±37.1 | 0.94 |
| Triglycerides (mg/dl) | 129.2±84.1 | 124.8±79.6 | 134.5±85.0 | 127.4±86.7 | 0.12 |
| Uric acid (mg/dl) | 6.99±1.80 | 6.84±1.70 | 6.88±1.77 | 7.21±1.88 | 0.004 |
| Nephropathy type | <0.001 | ||||
| Glomerular | 219 (18.8) | 76 (22.7) | 84 (21.5) | 59 (13.4) | |
| Vascular | 315 (27.0) | 73 (21.8) | 106 (27.2) | 136 (30.9) | |
| Polycystic kidney disease | 88 (7.6) | 30 (9.0) | 27 (6.9) | 31 (7.0) | |
| Intersitial | 127 (10.9) | 50 (14.9) | 35 (9.0) | 42 (9.5) | |
| Other | 416 (35.7) | 106 (31.6) | 138 (35.4) | 172 (39.1) | |
Data are expressed as mean±SD, median (interquartile range), n (%), or as otherwise indicated. CV, cardiovascular; BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitor; ARB, antireceptor blocker; ESA, erythropoiesis-stimulating agents; mGFR, measured GFR; PCR, proteinuria/creatininuria ratio; HbA1c, glycated hemoglobin.
Follow-Up
The median follow-up was 3.48 years (IQR, 1.94–5.82) for the competing events of ESRD and pre-ESRD mortality and 4.17 years (IQR, 2.27–6.97) for overall mortality. Out of 1165 patients at baseline, 675 underwent at least a second visit, and 490 were only passively followed-up on (Figure 1). In the former, the median time between the first and last visits was 2.98 years (IQR, 1.93–4.95), and the mean number of visits was 3.7±2.0. Of the patients, 72 of them developed DM over time.
ESRD and Mortality
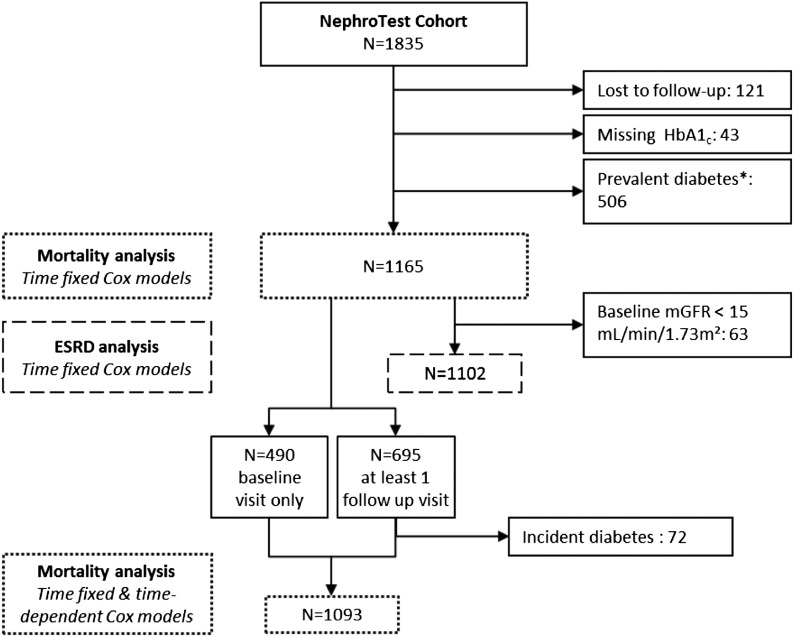
Among the 1165 cohort patients included in this analysis, 109 (9.4%) had died by the end of the follow-up period, 72 (6.1%) of them before reaching ESRD. HbA1c was significantly associated with both overall mortality and pre-ESRD mortality (log-rank test, P=0.002 and P=0.01, respectively) (Figure 2), with survival lowest in the highest tertile (HbA1c between 5.7% and 6.4%). The HRs for overall mortality and pre-ESRD mortality by baseline HbA1c are shown in Table 2. Adjustment for mGFR did not modify the crude association. After adjustment for confounders (see Supplemental Table 1 for HR), higher HbA1c was still associated with a significantly higher risk of overall mortality and pre-ESRD mortality.
Figure 2.
Kaplan–Meier curves for mortality end points (overall and pre-ESRD) stratified by glycated hemoglobin (HbA1c) tertiles. Log-rank test: P=0.002 (left plot) and P=0.01 (right plot).
Table 2.
Crude and adjusted hazard ratios (95% confidence intervals) for mortality (overall and pre-ESRD mortality) according to glycated hemoglobin values
| Overall Mortality | ||||
|---|---|---|---|---|
| HbA1c (%) | No. of Events/n | Crude HR | mGFR-Adjusted HR | Fully Adjusted HRa |
| Tertiles | ||||
| 1 (<5.3) | 27/335 | 1 (ref) | 1 (ref) | 1 (ref) |
| 2 (5.3–5.7) | 30/390 | 1.13 (0.67 to 1.91) | 1.20 (0.71 to 2.03) | 1.07 (0.60 to 1.88) |
| 3 (5.7–6.5) | 52/440 | 2.05 (1.28 to 3.29) | 2.18 (1.36 to 3.51) | 1.79 (1.06 to 3.00) |
| P value for trend | 0.002 | <0.001 | 0.02 | |
| Continuous | 109/1165 | 1.88 (1.24 to 2.86) | 1.98 (1.31 to 2.98) | 1.66 to (1.07 to 2.58) |
| P value | 0.003 | 0.001 | 0.02 | |
| Pre-ESRD Mortality | ||||
| HbA1c (%) | No. of Events/n | Crude HR | mGFR-Adjusted HR | Fully Adjusted HRa |
| Tertiles | ||||
| 1 (<5.3) | 14/335 | 1 (ref) | 1 (ref) | 1 (ref) |
| 2 (5.3–5.7) | 22/390 | 1.39 (0.71 to 2.72) | 1.36 (0.69 to 2.65) | 1.23 (0.60 to 2.54) |
| 3 (5.7–6.5) | 36/440 | 2.38 (1.28 to 4.42) | 2.28 (1.23 to 4.22) | 2.00 (1.02 to 3.92) |
| P value for trend | 0.004 | 0.01 | 0.03 | |
| Continuous | 72/1165 | 2.16 (1.27 to 3.68) | 2.07 (1.22 to 3.51) | 1.85 (1.05 to 3.24) |
| P value | 0.01 | 0.01 | 0.03 | |
HbA1c, glycated hemoglobin; ref, reference; HR, hazard ratio; mGFR, measured GFR.
Adjusted for measured GFR, age, sex, body mass index (<19, 20–24, 25–29, ≥30), race (black/other), urinary protein/creatinine ratio (log), elevated BP (>140/90 mmHg), history of cardiovascular disease (yes or no), smoking status (current smoker, former smoker, or never smoker), angiotensin-converting enzyme inhibitor or antireceptor blocker treatment, and serum albumin (>3.5 g/dl or <3.5 g/dl).
After exclusion of the 72 patients who developed DM during follow-up, HbA1c remained significantly associated with both overall mortality and pre-ESRD mortality whether treated continuously or in tertiles (Table 3). Finally, in time-dependent Cox models adjusted for updated mGFR and other covariates, including log proteinuria, associations with the updated mean of HbA1c were attenuated, but remained statistically significant for pre-ESRD mortality in the highest versus the lowest tertile. Additional adjustment for erythropoiesis-stimulating agents (ESAs) did not significantly modify our results, with the HRs of pre-ESRD mortality in HbA1c tertiles 3 and 2 versus tertile 1 as 2.18 (IQR, 1.10–4.33) and 1.39 (IQR, 0.66–2.92), respectively (P for trend, 0.02).
Table 3.
Crude and adjusted hazard ratios (95% confidence intervals) for mortality (overall and pre-ESRD mortality) according to glycated hemoglobin values, after exclusion of 72 participants who developed diabetes mellitus over time (n=1093)
| Overall Mortality | ||||
|---|---|---|---|---|
| Cox Model Type | Time-Fixed Covariates | Time-Dependent Covariatesb | ||
| HbA1c (%) | Crude HR | Fully Adjusted HRa | Crude HR | Fully Adjusted HRa |
| Tertiles | ||||
| 1 (<5.3) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 2 (5.3–5.7) | 1.16 (0.68 to 2.00) | 1.34 (0.74 to 2.41) | 1.46 (0.86 to 2.49) | 1.44 (0.81 to 2.56) |
| 3 (5.7–6.4) | 2.40 (1.47 to 3.92) | 2.08 (1.21 to 3.59) | 2.30 (1.36 to 3.90) | 1.75 (0.97 to 3.16) |
| P value for trend | <0.001 | 0.01 | 0.002 | 0.07 |
| Continuous | 2.10 (1.35 to 3.27) | 1.80 (1.14 to 2.86) | 2.00 (1.21 to 3.30) | 1.49 (0.87 to 2.56) |
| P value | 0.001 | 0.01 | 0.01 | 0.14 |
| Pre-ESRD Mortality | ||||
| Cox Model Type | Time-Fixed Covariates | Time-Dependent Covariatesb | ||
| HbA1c (%) | Crude HR | Fully Adjusted HRa | Crude HR | Fully Adjusted HRa |
| Tertiles | ||||
| 1 (<5.3) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 2 (5.3–5.7) | 1.68 (0.83 to 3.41) | 1.83 (0.85 to 3.94) | 2.22 (1.04 to 4.72) | 2.08 (0.94 to 4.63) |
| 3 (5.7–6.4) | 2.96 (1.52 to 5.78) | 2.52 (1.21 to 5.27) | 3.28 (1.55 to 6.92) | 2.62 (1.16 to 5.91) |
| P value for trend | <0.001 | 0.01 | 0.001 | 0.02 |
| Continuous | 2.44 (1.38 to 4.31) | 1.99 (1.09 to 3.62) | 2.53 (1.34 to 4.79) | 1.84 (0.93 to 3.63) |
| P value | 0.002 | 0.02 | 0.004 | 0.08 |
HbA1c, glycated hemoglobin; ref, reference; HR, hazard ratio.
Adjusted for measured GFR, age, sex, body mass index (<19, 20–24, 25–29, ≥30), race (black/other), urinary protein to creatinine ratio (log), elevated BP (>140/90 mmHg), history of cardiovascular disease (yes or no), smoking status (current smoker, former smoker, or never smoker), angiotensin-converting enzyme inhibitor or antireceptor blocker treatment, and serum albumin (>3.5 g/dl or <3.5 g/dl).
Updated mean of glycated hemoglobin is studied in time-dependent covariates Cox models. Values of other biologic parameters and treatments were updated at each visit for adjustment variables.
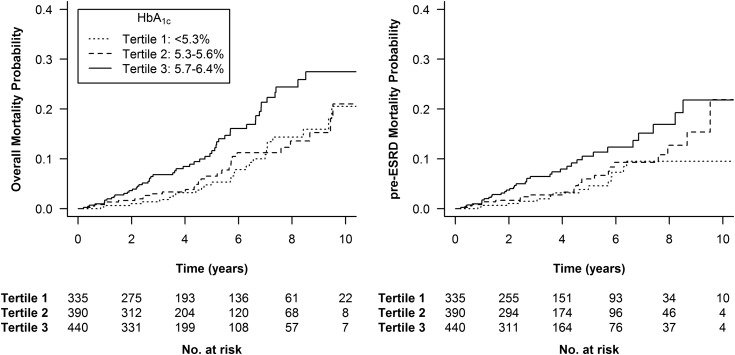
We analyzed renal survival in 1102 patients with CKD stages 1–4. At the end of follow-up, 162 patients (14.7%) had reached ESRD. We observed a significantly lower risk of ESRD in patients with intermediate baseline HbA1c values (between 5.3% and 5.6%) in the univariate analysis and after adjustment for mGFR (Figure 3, Table 4). However, the difference was no longer significant after adjustment for the following risk factors for CKD progression: age, sex, BMI, ethnicity, log proteinuria, BP, smoking status, previous cardiovascular event, or ACEi or ARB treatment.
Figure 3.
Kaplan–Meier curves for ESRD end points stratified by glycated hemoglobin (HbA1c) tertiles. Log-rank test: P=0.02. The analysis included 1102 patients with CKD stages 1–4 (exclusion of 63 patients with CKD stage 5).
Table 4.
Crude and adjusted hazard ratios for ESRD according to glycated hemoglobin tertiles
| HbA1c (%) | No. of Events/n | HR Model 0a | HR Model 1b | HR Model 2c |
|---|---|---|---|---|
| Tertiles | ||||
| 1 (<5.3) | 58/309 | 1 (ref) | 1 (ref) | 1 (ref) |
| 2 (5.3–5.7) | 41/371 | 0.61 (0.41 to 0.91) | 0.60 (0.40 to 0.90) | 0.67 (0.43 to 1.04) |
| 3 (5.7–6.5) | 63/422 | 0.97 (0.68 to 1.38) | 0.81 (0.57 to 1.17) | 0.96 (0.65 to 1.43) |
| P–Wald | 0.03 | 0.05 | 0.15 |
The analysis included 1102 patients with CKD stages 1–4 (exclusion of 63 patients with CKD stage 5). HbA1c, glycated hemoglobin; HR, hazard ratio; ref, reference.
Model 0: crude model.
Model 1: stratification for baseline measured GFR levels with 6 classes of measured GFR (15–20, 20–30, 30–40, 40–50, 50–60, >60 ml/min per 1.73 m2).
Model 2: model 1 plus adjustment for age, sex, body mass index (<19, 20–24, 25–29, ≥30), race (black/other), urinary protein/creatinine ratio (log), elevated BP (>140/90 mmHg), history of cardiovascular disease (yes or no), smoking status (current smoker, former smoker, or never smoker), angiotensin-converting enzyme inhibitor or antireceptor blocker treatment, and serum albumin (>3.5 g/dl or <3.5 g/dl).
Discussion
In this observational study, we have demonstrated that HbA1c in nondiabetic CKD patients is significantly and independently associated with patient survival. The HbA1c value was also associated with better renal survival. However, this latter finding was not confirmed after adjustment for the most common variables. To our knowledge, this is the first study of the predictive value of HbA1c in a cohort of nondiabetic CKD patients.
The HbA1c values in our population, previously reported, are interesting per se. It has been reported that in the United States, among those without a diagnosis of DM, >2.4 million have an HbA1c value >6.5% and 7 million have an HbA1c value >6.0% (15). For the purpose of the study, we applied a strict definition of nondiabetic patients, excluding patients with HbA1c values ≥6.5%. Our findings show that patients with CKD with an HbA1c value >5.7% have a higher risk of death than patients with lower values, even after adjustment for other risk factors and independently of baseline fasting glucose levels. This 5.7% threshold is consistent with the prediabetes status defined by the American Diabetes Association (16). These findings are consistent with those already reported in the general population of the United States (6), in a study in which the authors determined that these patients were also at higher risk for coronary heart disease and stroke. Moreover, they demonstrated that risk reclassification for coronary heart disease was improved by the inclusion of HbA1c in adjusted models. On the other hand, Chonchol and colleagues did not find any significant relation between HbA1c and survival in nondiabetic older adults, but mortality was higher with patients with an abnormal response to glycemic load, defining prediabetes (17).
Tonelli et al. have explored the relative roles of DM and CKD in cardiovascular and all-cause mortality (9). One major conclusion was that CKD could be added to the list of criteria defining people at highest risk of future coronary events, in view of the finding that the rates of hospital admission for myocardial infarction and the risk of death after this event in people with CKD without diabetes were similar to or higher than rates in those with diabetes without CKD.
We note that all previous studies have based their CKD classification on a single determination of serum creatinine value, which might have resulted in the incorrect classification of some participants. Because such misclassification would tend to underestimate the risk associated with CKD, the potential value of using kidney disease as a risk equivalent might be higher than suggested. For instance, the study reporting that HbA1c predicts a higher risk of future CKD was on the basis of a single creatinine serum assay several years after the HbA1c measurement (7). Moreover, an analysis on the basis of a single creatinine value could be biased by the confounding inherent in the fact that weight and age are both determinants of serum creatinine and established risk factors for cardiovascular disease. One major strength of our study is that we have used the direct measurement of GFR, which is the best available definition for the diagnosis and classification of CKD, and did it repeatedly.
Another end point we sought to explore was the risk of CKD progression according to HbA1c values. A crude analysis of the data using ESRD as the end point showed that a better prognosis was associated with intermediate HbA1c values. This result suggests a J-shaped relation between the HbA1c value and ESRD risk, like those already described for the HbA1c values for predicting the risk of death in the general population (6) and in diabetic populations with or without CKD (3,4). Although the cause for this J-shape is unclear, one may speculate that the patients in the lower HbA1c may have partial undernutrition, a recognized factor for progression to ESRD. At the other end of the curve, the patients in the highest tertile may experience nephrotoxicity related to that observed in diabetic nephropathy. In the latter, the exact cause of the development of renal lesions is unknown, but various mechanisms have been postulated, including hyperglycemia (causing hyperfiltration and renal injury), kidney hypoxia, advanced glycosylation products, activation of cytokines, protein kinase C, and oxidative stress. Few studies have examined the association of HbA1c with incident kidney disease in the absence of recognized DM. It has been previously demonstrated that there is an independent association between HbA1c and risk of CKD in individuals with DM even in the absence of albuminuria (18). However, the question addressed in our study is different because our patients already had CKD that was not related to DM. We acknowledge that this association was no longer statistically significant when the standard confounding factors for CKD progression were included in the analysis. This might be explained by the specificity of our population, who entered the study with CKD, mostly stages 3 or 4. The presence of another nephropathy may also explain the negative outcome in the long term. We might speculate that a kidney already impaired as a result of another cause could be more sensitive to the impaired glucose metabolism demonstrated by higher HbA1c values.
In our study, patients with prediabetes diagnosed on HbA1c value have a higher risk of death compared with patients with an HbA1c value <5.7%. This observation may lead to the proposition to follow and/or even to propose to treat preemptively CKD patients with high HbA1c values. Several studies demonstrated the benefit of an intervention in prediabetes to prevent DM. Lifestyle intervention, metformin, glitazone, acarbose, or bariatric surgery were all associated with the reduction of DM outcome (19–23).
However, in all of these studies, the mean BMI was >30 (and most were >34), whereas this value is 26 for the patients in the highest HbA1c tertile in this study. Moreover, most oral diabetes medications should not be used in case of impaired renal function. Of note, the use of valsartan in the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research trial (24) reduced incidence of DM, and ramipril increased regression to normoglycemia in patients with impaired glucose tolerance in the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication Trial (25). However, none of the studies were able to show a reduction of cardiovascular events. Therefore, if these observations suggest the potential usefulness of ACEi or ARB treatment in patients with HbA1c between 5.7 and 6.4, no studies in prediabetes patients have demonstrated an effect on mortality.
This study has some limitations. First, in patients with ESRD, HbA1c is known to underestimate blood glucose control, mainly because of the modification of hemoglobin turnover and its carbamylation as a result of uremia (26–30). Hence some authors recommend using glycated albumin or fructosamine to assess glycemic control in patients with diabetes with renal failure (31–33). Unfortunately, these biomarkers were not available in our cohort. However, most patients in our cohort have moderate CKD, and HbA1c could be considered as an accurate biomarker. Second, we included in the analysis patients receiving ESA; however, it has been suggested that its use might modify the value of HbA1c. We must emphasize that only 64 patients in the cohort received ESA at inclusion. Additional adjustment for ESA did not significantly modified our results. Furthermore, the modification of the HbA1c value in patients with ESA is still debated, especially in steady state. Indeed once the Hb levels are stabilized, it is unlikely that there is a significant variation of HbA1c according to ESA use. Third, the high percentage of patients with only baseline measurements may have limited our analyses using time-dependent covariates in Cox models. Finally, because this is an observational study, we cannot exclude the possibility of residual confounding by unmeasured risk factors such as physical activity.
Strengths of this study include the large sample, the availability of both fasting glucose and HbA1c measurements, the rigorous measurement of potentially confounding factors, and the prospective follow-up of participants with high retention (>90%).
In summary, in patients with CKD without diabetes, HbA1c is independently associated with patient survival and in some instance with CKD progression to ESRD. HbA1c, even <6.5%, should be therefore added to the risk factors for negative outcomes in CKD populations. Other studies are necessary to better understand the mechanisms of this outcome.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08540814/-/DCSupplemental.
See related editorial, “Should Hemoglobin A1C Be Routinely Measured in Patients with CKD?,” on pages 914–916.
References
- 1.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE: Defining the relationship between plasma glucose and HbA(1c): Analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 25: 275–278, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A: Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med 295: 417–420, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD: Survival as a function of HbA(1c) in people with type 2 diabetes: A retrospective cohort study. Lancet 375: 481–489, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M, Alberta Kidney Disease Network : Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: A population-based cohort study. Arch Intern Med 171: 1920–1927, 2011 [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association : Diagnosis and classification of diabetes mellitus. Diabetes Care 33[Suppl 1]: S62–S69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL: Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362: 800–811, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvin E, Ning Y, Steffes MW, Bash LD, Klein R, Wong TY, Astor BC, Sharrett AR, Brancati FL, Coresh J: Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 60: 298–305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda N, Iijima R, Hara H, Moroi M, Nakamura M, Sugi K: Glycated hemoglobin is associated with the complexity of coronary artery disease, even in non-diabetic adults. J Atheroscler Thromb 19: 1066–1072, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR, Alberta Kidney Disease Network : Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 380: 807–814, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Moranne O, Froissart M, Rossert J, Gauci C, Boffa J-J, Haymann JP, M’rad MB, Jacquot C, Houillier P, Stengel B, Fouqueray B, NephroTest Study Group : Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20: 164–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantler C, Garnett ES, Parsons V, Veall N: Glomerular filtration rate measurement in man by the single injection methods using 51Cr-EDTA. Clin Sci 37: 169–180, 1969 [PubMed] [Google Scholar]
- 12.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 14.Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ: When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28: 2670–2677, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Selvin E, Zhu H, Brancati FL: Elevated A1C in adults without a history of diabetes in the U.S. Diabetes Care 32: 828–833, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association : Standards of medical care in diabetes--2013. Diabetes Care 36[Suppl 1]: S11–S66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chonchol M, Katz R, Fried LF, Sarnak MJ, Siscovick DS, Newman AB, Strotmeyer ES, Bertoni A, Shlipak MG: Glycosylated hemoglobin and the risk of death and cardiovascular mortality in the elderly. Nutr Metab Cardiovasc Dis 20: 15–21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokoyama H, Kanno S, Takahashi S, Yamada D, Itoh H, Saito K, Sone H, Haneda M: Determinants of decline in glomerular filtration rate in nonproteinuric subjects with or without diabetes and hypertension. Clin J Am Soc Nephrol 4: 1432–1440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group : Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR: Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 368: 1096–1105, 2006 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD, ACT NOW Study : Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 364: 1104–1115, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trail Research Group : Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 359: 2072–2077, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I: Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med 122: 248–256, e5, 2009 [DOI] [PubMed] [Google Scholar]
- 24.NAVIGATOR Study Group. McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM: Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 362: 1477–1490, 2010 [DOI] [PubMed] [Google Scholar]
- 25.DREAM Trial Investigators. Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR: Effect of ramipril on the incidence of diabetes. N Engl J Med 355: 1551–1562, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Light RP: Relationship between glycosylated hemoglobin and blood glucose during progression of chronic kidney disease. Am J Nephrol 34: 32–41, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Cordeiro AC, Carrero JJ, Bárány P, Qureshi AR, Heimbürger O, Lindholm B, Stenvinkel P: Influence of erythropoiesis-stimulating agents on glycated hemoglobin in nondiabetic kidney diseases at the start of dialysis. Am J Nephrol 33: 17–24, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Bloomgarden ZT: A1C: Recommendations, debates, and questions. Diabetes Care 32: e141–e147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Hörkkö S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL: Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 13: 1176–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Joy MS, Cefalu WT, Hogan SL, Nachman PH: Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 39: 297–307, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Meyer L, Chantrel F, Imhoff O, Sissoko A, Serb L, Dorey F, Fleury D, Smagala A, Kepenekian L, Krummel T, Le Floch JP, Kessler L: Glycated albumin and continuous glucose monitoring to replace glycated haemoglobin in patients with diabetes treated with haemodialysis. Diabet Med 30: 1388–1389, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Fukuoka K, Nakao K, Morimoto H, Nakao A, Takatori Y, Arimoto K, Taki M, Wada J, Makino H: Glycated albumin levels predict long-term survival in diabetic patients undergoing haemodialysis. Nephrology (Carlton) 13: 278–283, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Freedman BI, Andries L, Shihabi ZK, Rocco MV, Byers JR, Cardona CY, Pickard MA, Henderson DL, Sadler MV, Courchene LM, Jordan JR, Balderston SS, Graham AD, Mauck VL, Russell GB, Bleyer AJ: Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol 6: 1635–1643, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.