Abstract
Background and objective
The optimal BP target to reduce adverse clinical outcomes in patients with CKD is unclear. This study examined the relationship between BP and death, cardiovascular events (CVEs), and kidney disease progression in patients with advanced kidney disease.
Design, setting, participants, & measurements
The relationship of systolic BP (SBP), diastolic BP (DBP), and pulse pressure (PP) with death, CVE, and progression to long-term dialysis was examined in 1099 patients with advanced CKD (eGFR≤30 ml/min per 1.7 3m2; not receiving dialysis) who participated in the Homocysteine in Kidney and ESRD study. That study enrolled participants from 2001 to 2003. Cox proportional hazard models were used to examine the association between BP and adverse outcomes.
Results
The mean±SD baseline eGFR was 18±7 ml/min per 1.73 m2. During a median follow-up of 2.9 years, 453 patients died, 215 had a CVE, and 615 initiated long-term dialysis. After adjustment for demographic characteristics and confounders, SBP, DBP, and PP were not associated with a higher risk of death. SBP and DBP were also not associated with CVE. The highest quartile of PP was associated with a substantial higher risk of CVE compared with the lowest quartile (hazard ratio [HR], 1.67; 95% confidence interval [95% CI], 1.10 to 2.52). The highest quartiles of SBP (HR, 1.28; 95% CI, 1.01 to 1.61) and DBP (HR, 1.36; 95% CI, 1.07 to 1.73), but not PP, were associated with a higher risk of progression to long-term dialysis compared with the lowest quartile.
Conclusions
In patients with advanced kidney disease not undergoing dialysis, higher PP was strongly associated with CVE whereas higher SBP and DBP were associated with progression to long-term dialysis. These results suggest that SBP and DBP should not be the only factors considered in determining antihypertensive therapy; elevated PP should also be considered.
Keywords: blood pressure, cardiovascular disease, chronic kidney disease
Introduction
In patients with CKD, the optimal BP target remains controversial. Three large randomized controlled trials (African-American Study of Kidney Disease, Modification of Diet in Renal Disease, and Ramipril Efficacy in Nephropathy 2) did not find that treatment to a lower BP goal (mean arterial pressure <92 mmHg or BP<130/80 mmHg) reduced kidney-related end points compared with a higher goal (<140/90 mmHg or diastolic BP [DBP]<90 mmHg) (1,2). Furthermore, recent studies have found that lower BP goals may be associated with adverse outcomes (3,4). A recent cohort study of >600,000 patients with CKD found that participants with low systolic BP (SBP) and DBP (<120/80 mmHg) had the highest mortality rates (3). Furthermore, a low DBP (<70 mmHg) was associated with higher mortality even when combined with ideal SBP (3). Studies have also found a J-shaped relationship between SBP and cardiovascular outcomes in patients with CKD not undergoing dialysis (4). However, no randomized controlled trials have assessed BP targets and nonkidney outcomes in patients with advanced CKD.
Patients with CKD often have high pulse pressures (PPs) because of atherosclerosis or nonatherosclerotic arterial medial calcification (5,6). Increased PP is an independent predictor of cardiovascular disease (CVD), heart failure, and death in the general population (7,8). In dialysis patients, increased PP is associated with death (9,10). Small studies have shown that higher PP is associated with decline in kidney function in patients with mild to moderate CKD (11,12). However, the significance of PP on death, cardiovascular events (CVEs), and kidney disease progression in patients with advanced CKD not on dialysis has not been adequately studied.
This study sought to examine the relationship between SBP, DBP, and PP with death, CVE, and progression to long-term dialysis in patients with advanced kidney disease not requiring dialysis who participated in the Homocysteine in Kidney and ESRD (HOST) study (13). We tested the hypothesis that higher SBP, DBP, and PP would be associated with a higher risk of death, CVE, and progression to long-term dialysis.
Materials and Methods
Study Cohort
The HOST study was a multicenter, prospective, randomized, double-blind, placebo-controlled trial examining the effects of high doses of folic acid, pyridoxine hydrochloride (vitamin B6), and cyanocobalamin (vitamin B12) on death and CVE in patients with advanced kidney disease and elevated plasma homocysteine concentrations (13). The trial enrolled 2056 adult participants from 36 Veterans Affairs medical centers between September 2001 and October 2003. Patients were included in the study if they were 21 years of age or older with ESRD receiving hemodialysis or peritoneal dialysis (n=751), or with an estimated creatinine clearance (calculated by the Cockroft–Gault formula) of <30 ml/min but not yet receiving long-term dialysis (n=1305), in addition to an elevated plasma homocysteine concentration of ≥15 µmol/L. The primary results of the HOST study were negative. Each center’s institutional review board approved the study, and all participants provided informed consent. All participants of the HOST study with advanced CKD not requiring maintenance dialysis at the 3-month follow-up visit were included in the present analysis, resulting in a final cohort of 1099 participants.
Demographic, Laboratory, and Clinical Data
Information collected at the time of randomization included a complete history and physical examination, demographic characteristics, smoking status, cause of kidney disease, history of hypertension, diabetes and CVD identified by self-report and chart review, and use of medications (including antihypertensive agents, β-blockers, and lipid-lowering drugs). SBP and DBP were measured manually using a calibrated aneroid sphygmomanometer with the patient in a seated position after 5 minutes of rest, with the arm at heart level, using an appropriate-size cuff. Three BP measurements were taken; the average was recorded and used for the analysis in this study. PP was determined by subtracting the DBP from SBP. Serum albumin and hemoglobin were measured at local laboratories. Although the Cockcroft–Gault formula was used to assess eligibility for the HOST study, the four-variable Modification of Diet in Renal Disease prediction equation was used to determine the eGFR in this analysis.
Outcomes
The outcomes for this analysis were (1) time to death from any cause, (2) time to any CVE (defined as a composite of myocardial infarction, stroke, and amputation of all or part of a lower extremity), and (3) time to initiation of maintenance dialysis that occurred at least 3 months after randomization. These outcomes were prespecified endpoints of the original HOST study and were adjudicated by an independent end points committee. All fatal events were reviewed and classified using information obtained from the hospital discharge summary, autopsy report, Medicare ESRD Death Notification, and/or death certificate. Deaths were also tracked with the Beneficiary Identification and Records Locator Subsystem, a Veterans Affairs file used to record death and dates. Nonfatal CVE and initiation of maintenance dialysis were determined through self-reporting by participants in response to specific queries during quarterly follow-up contacts and by review of the patients’ medical record. Myocardial infarction was diagnosed when two of the following three criteria were present: (1) typical cardiac symptoms, (2) elevated serum cardiac biomarker concentrations, and (3) diagnostic electrocardiographic changes. Stroke was defined as an acute onset of persistent neurologic deficits from an obstruction in the arterial system in the brain. Trauma, cerebral hemorrhage, and infection had to be ruled out before the participant was diagnosed with a thrombotic stroke. Amputation of all or part of the lower extremity was determined through self-reporting. All diagnoses were verified using information provided by discharge summaries, neurologic examinations, imaging results, serum cardiac biomarkers, and electrocardiograms.
Statistical Analyses
We excluded participants who had initiated long-term hemodialysis within 3 months after randomization (n=34; 2.6%) and those for whom other data were missing (n=172; 13.2%), resulting in a final sample of 1099 participants for the present study. Compared with excluded patients, demographic characteristics and event rates were similar among participants who were retained in this analysis (data not shown). We used Cox proportional hazard models to examine the association between BP and time to death, CVE, and initiation of long-term dialysis. Quartiles of SBP, DBP, and PP were chosen as the primary predictor variables, with the lowest quartile serving as the reference group. BP was also examined as a continuous variable. The quartiles for SBP, DBP, and PP are as follows:
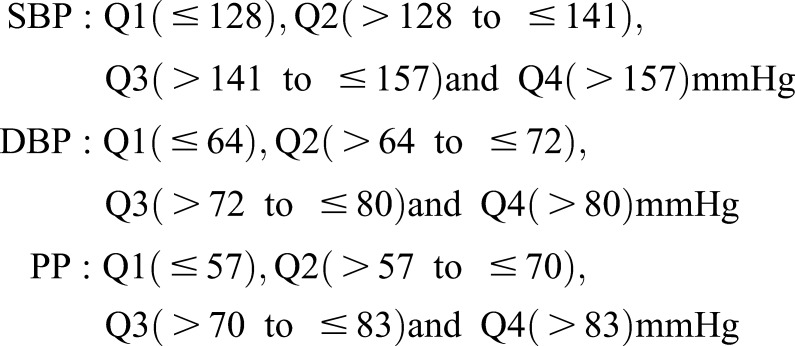 |
Covariates used for adjustment in the statistical models were identified a priori. Model 1 included age, sex, and race; model 2 included covariates in model 1 plus smoking status, body mass index (BMI), history of hypertension, diabetes and CVD, eGFR, serum hemoglobin, serum albumin, and HOST randomized assignment. Because SBP, DBP, and PP are all highly collinear and we were interested in the effect of each variable on the outcome, we did not adjust the analyses for the respective other BP measures because doing so might have confounded the statistical analysis. Two-tailed P values <0.05 were considered to represent statistically significant differences. All statistical analyses were performed with SAS software, version 9.13 (SAS Institute, Cary, NC).
Results
Table 1 shows the baseline characteristics of the 1099 participants. The mean±SD age and eGFR of the participants were 69±11 years and 18±6 ml/min per 1.73 m2, respectively. Most participants (n=718; 65%) had an eGFR of 15–29 ml/min per 1.73 m2. Nearly all participants had a history of hypertension (n=1060; 96.5%). The mean SBP and DBP were 143±23 and 73±13 mmHg, respectively. Most patients were male, and >50% were white. There was also a high prevalence of diabetes and history of CVD in this cohort. In most patients the primary cause of kidney disease was diabetes (41.3%) and hypertension (31.9%).
Table 1.
Baseline characteristics of study participants
| Characteristic | Value |
|---|---|
| Age (yr) | 68.8±10.7 |
| Sex, n (%) | |
| Male | 1081 (98.4) |
| Female | 18 (1.6) |
| Race, n (%) | |
| White | 622 (56.6) |
| Black | 290 (26.4) |
| Other | 187 (17.0) |
| Cause of kidney disease, n (%) | |
| Diabetes | 454 (41.3) |
| Hypertension | 351 (31.9) |
| GN | 52 (4.7) |
| Obstructive nephropathy | 23 (2.1) |
| Polycystic kidney disease | 22 (2.0) |
| Tubulointerstitial disease | 6 (0.5) |
| Vascular disease | 27 (2.5) |
| Other | 81 (7.4) |
| Unknown | 83 (7.6) |
| History of diabetes, n (%) | 604 (55.0) |
| History of hypertension, n (%) | 1060 (96.5) |
| History of cardiovascular disease, n (%) | 625 (56.9) |
| Current smoker, n (%) | 201 (18.3) |
| Body mass index (kg/m2) | 28.0±4.8 |
| Systolic BP (mmHg) | 143.4±23.1 |
| Diastolic BP (mmHg) | 73.0±12.6 |
| eGFR (ml/min per 1.73 m2) | 18.2±6.4 |
| Serum albumin (g/dl) | 4.1±0.5 |
| Hemoglobin (g/dl) | 11.9±1.7 |
| LDL cholesterol (mg/dl) | 94.8±32.8 |
| HDL cholesterol (mg/dl) | 42.6±14.8 |
| Serum calcium (mg/dl) | 8.9±0.7 |
| Serum phosphorus (mg/dl) | 4.4±1.3 |
Values expressed with a plus/minus sign are mean±SD.
BP and Risk of Death from All Causes
Over a median (25th–75th percentile) follow-up of 1.9 (0.99–2.5) years, 453 (41%) deaths from all causes occurred. Of the patients who died, 128 (28.3%) also had a CVE during the study. In unadjusted analyses, higher quartiles of DBP (quartiles 2–4) were associated with a lower risk of death compared with the lowest quartile (Table 2). In unadjusted analyses, the highest quartile of PP was associated with a higher risk of death. However, there was no association between the quartiles of SBP, DBP, or PP and all-cause mortality in adjusted analyses (Table 2). There was also no association between BP and death when SBP, DBP, and PP were examined as continuous variables.
Table 2.
Association of BP with all-cause mortality
| BP (mm Hg) | Unadjusted Hazard Ratio (95% CI) | Model 1 Hazard Ratio (95% CI) | Model 2 Hazard Ratio (95% CI) |
|---|---|---|---|
| Systolic BP | |||
| Q1 (≤128) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>128–≤141) | 0.94 (0.72 to 1.23) | 0.90 (0.69 to 1.18) | 0.94 (0.72 to 1.24) |
| Q3 (>141–≤157) | 0.96 (0.74 to 1.26) | 0.92 (0.70 to 1.20) | 0.99 (0.76 to 1.29) |
| Q4 (>157) | 1.61 (0.90 to 1.50) | 1.15 (0.90 to 1.49) | 1.17 (0.89 to 1.52) |
| Diastolic BP | |||
| Q1 (≤64) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>64–≤72) | 0.76 (0.59 to 0.97) | 0.81 (0.63 to 1.05) | 0.91 (0.71 to 1.18) |
| Q3 (>72–≤80) | 0.70 (0.54 to 0.91) | 0.84 (0.65 to 1.09) | 0.97 (0.74 to 1.27) |
| Q4 (>80) | 0.74 (0.58 to 0.95) | 1.01 (0.78 to 1.32) | 1.16 (0.89 to 1.52) |
| Pulse pressure | |||
| Q1 (≤57) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>57–≤70) | 0.98 (0.75 to 1.28) | 0.86 (0.66 to 1.12) | 0.78 (0.59 to 1.02) |
| Q3 (>70–≤83) | 1.14 (0.87 to 1.50) | 0.97 (0.74 to 1.28) | 0.94 (0.71 to 1.24) |
| Q4 (>83) | 1.39 (1.08 to 1.80) | 0.97 (0.74 to 1.28) | 1.01 (0.77 to 1.33) |
Model 1: adjusted for age, sex, and race. Model 2: adjusted for covariates in model 1 plus smoking status, body mass index, history of hypertension, diabetes and cardiovascular disease, eGFR, serum hemoglobin, serum albumin, and Homocysteine in Kidney and ESRD randomized assignment. Q, quartile; 95% CI, 95% confidence interval.
BP and Risk of Cardiovascular Events
A total of 215 (19.5%) CVEs occurred over a median (25th–75th percentile) follow-up of 1.4 (0.5–2.3) years. The association between SBP, DBP, and PP with CVEs is shown in Table 3. When examined as a continuous variable, every 1 mmHg higher SBP was associated with a 0.8% higher risk of CVEs after adjustment for age, sex, and race (P=0.003). After further adjustment for BMI, smoking status, history of diabetes, hypertension and CVD, eGFR, serum hemoglobin, serum albumin, and treatment group, however, SBP was no longer significantly associated with CVEs. In model 1, the highest quartile of SBP (quartile 4) was associated with a 51% higher risk of CVEs compared with the lowest quartile (hazard ratio [HR], 1.51; 95% confidence interval [95% CI], 1.04 to 2.18). However, in the fully adjusted model (model 2), the highest quartile of SBP was no longer significantly associated with a higher risk of CVEs (HR, 1.34; 95% CI, 0.91 to 1.96). Quartiles 2 and 3 were not associated with a higher risk of CVEs compared with the lowest quartile in either model.
Table 3.
Association of BP with cardiovascular events
| BP (mmHg) | Unadjusted Hazard Ratio (95% CI) | Model 1 Hazard Ratio (95% CI) | Model 2 Hazard Ratio (95% CI) |
|---|---|---|---|
| Systolic BP | |||
| Q1 (≤128) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>128–≤141) | 1.08 (0.73 to 1.60) | 1.08 (0.72 to 1.60) | 1.03 (0.69 to 1.54) |
| Q3 (>141–≤157) | 0.86 (0.57 to 1.30) | 0.84 (0.55 to 1.27) | 0.82 (0.54 to 1.24) |
| Q4 (>157) | 1.57 (1.09 to 2.27) | 1.51 (1.01 to 2.18) | 1.35 (0.92 to 1.97) |
| Diastolic BP | |||
| Q1 (≤64) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>64–≤72) | 0.71 (0.48 to 1.03) | 0.69 (0.47 to 1.01) | 0.73 (0.50 to 1.07) |
| Q3 (>72–≤80) | 0.73 (0.50 to 1.07) | 0.73 (0.49 to 1.07) | 0.79 (0.53 to 1.18) |
| Q4 (>80) | 0.91 (0.64 to 1.30) | 0.93 (0.64 to 1.36) | 1.03 (0.70 to 1.52) |
| Pulse pressure | |||
| Q1 (≤57) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>57–≤70) | 1.49 (0.99 to 2.23) | 1.44 (0.95 to 2.16) | 1.28 (0.85 to 1.93) |
| Q3 (>70–≤83) | 1.28 (0.82 to 2.00) | 1.26 (0.81 to 1.98) | 1.16 (0.74 to 1.81) |
| Q4 (>83) | 2.16 (1.45 to 3.21) | 2.03 (1.35 to 3.04) | 1.67 (1.10 to 2.52) |
Model 1: adjusted for age, sex, and race. Model 2: adjusted for covariates in model 1 plus smoking status, body mass index, history of hypertension, diabetes and cardiovascular disease, eGFR, serum hemoglobin, serum albumin, and Homocysteine in Kidney and ESRD randomized assignment. Q, quartile; 95% CI, 95% confidence interval.
PP was strongly and significantly associated with CVEs (Table 3). After adjustment for age, race, and sex (model 1), every 1 mmHg higher PP was associated with a 1.1% higher risk of CVEs (P<0.001). In the fully adjusted model (model 2), every 1 mmHg higher PP was associated with a 0.8% higher risk of CVE (P=0.03). When examined in quartiles, in model 1, the highest quartile of PP was associated with a 2-fold higher risk of CVEs compared with the first quartile (HR, 2.03; 95% CI, 1.35 to 3.04). The highest quartile remained significantly associated with a higher risk of CVEs in the fully adjusted model (HR, 1.67; 95% CI, 1.10 to 2.52). Quartiles 2 and 3 were not associated with CVEs. When DBP was examined in quartiles or as a continuous variable, there was no association between DBP and CVE (Table 3).
PP was the only measure associated with CVE in the fully adjusted model. Given this finding, we further investigated the relationships between PP and clinical measures. PP correlated with age (r= 0.19; P<0.001), serum albumin level (r=−0.11; P<0.001), serum hemoglobin level (r=−0.11; P=0.02), serum HDL cholesterol level (r=0.06; P=0.05), and smoking (r=−0.10; P=0.001). PP did not correlate with eGFR, sex, history of hypertension, CVD, BMI, serum calcium, phosphorus, or LDL cholesterol. In multiple linear regression analysis, PP was independently associated with the following: age (0.47 mmHg higher PP per year of age), serum LDL cholesterol (0.06 mmHg higher PP per 1 mg/dl higher LDL cholesterol), serum HDL cholesterol (0.09 mmHg higher PP per 1 mg/dl higher HDL), and serum albumin (4.67 mmHg lower PP per 1 mg/dl higher albumin).
BP and Risk of Progression to Long-Term Dialysis
A total of 615 patients (56%) initiated long-term dialysis after a median (25th–75th percentile) follow-up of 1.1 (0.6–1.9) years. The relationship between BP and long-term dialysis initiation is shown in Table 4. Higher SBP was associated with a higher risk of dialysis initiation. In model 1, the third and fourth quartiles of SBP were associated with a 43% and 57% higher risk of progression to long-term dialysis, respectively (HR, 1.43 [95% CI, 1.13 to–1.79] and 1.57 [95% CI, 1.23 to 1.98], respectively). In the fully adjusted model, the highest quartile of SBP remained significantly associated with a 28% higher risk of progression to dialysis (HR, 1.28; 95% CI, 1.01 to 1.61). When examined as a continuous variable, every 1 mmHg higher SBP was associated with a 0.7% higher risk of progression to dialysis after adjustment for age, sex, and race (P<0.001). In the fully adjusted model, SBP was no longer significantly associated with a higher risk of progression to long-term dialysis (P=0.07). In model 1, DBP was not associated with progression to long-term dialysis. However, in the fully adjusted model (model 2), the highest quartile of DBP was associated with a 36% higher risk of progression to dialysis (HR, 1.36; 95% CI, 1.07 to 1.73). Similar results were found when DBP was examined as a continuous variable. In the fully adjusted model, every 1 mmHg higher DBP was associated with a 0.9% higher risk of progression to long-term dialysis (P=0.01). After adjustment for age, sex, and race, increasing quartiles of PP were associated with a higher risk of progression to long-term dialysis compared with quartile 1 (Table 4). However, after adjustment for all covariates, PP was no longer significantly associated with progression to dialysis. Similar results were found when PP was examined as a continuous variable.
Table 4.
Association of BP with progression to long-term dialysis
| BP (mmHg) | Unadjusted Hazard Ratio (95% CI) | Model 1 Hazard Ratio (95% CI) | Model 2 Hazard Ratio (95% CI) |
|---|---|---|---|
| Systolic BP | |||
| Q1 (≤128) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>128–≤141) | 1.13 (0.89 to 1.42) | 1.14 (0.90 to 1.44) | 1.07 (0.84 to 1.35) |
| Q3 (>141–≤157) | 1.36 (1.08 to 1.71) | 1.43 (1.13 to 1.79) | 1.22 (0.97 to 1.53) |
| Q4 (>157) | 1.50 (1.20 to 1.89) | 1.57 (1.25 to 1.98) | 1.28 (1.01 to 1.61) |
| Diastolic BP | |||
| Q1 (≤64) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>64–≤72) | 1.07 (0.85 to 1.35) | 1.04 (0.83 to 1.32) | 1.07 (0.84 to 1.35) |
| Q3 (>72–≤80) | 1.31 (1.04 to 1.64) | 1.17 (0.92 to 1.47) | 1.22 (0.97 to 1.55) |
| Q4 (>80) | 1.44 (1.15 to 1.80) | 1.17 (0.92 to 1.49) | 1.36 (1.07 to 1.73) |
| Pulse pressure | |||
| Q1 (≤57) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>57–≤70) | 1.16 (0.94 to 1.45) | 1.38 (1.10 to 1.72) | 1.10 (0.88 to 1.37) |
| Q3 (>70–≤83) | 1.14 (0.90 to 1.44) | 1.33 (1.04 to 1.69) | 1.05 (0.83 to 1.34) |
| Q4 (>83) | 1.23 (0.98 to 1.55) | 1.54 (1.21 to 1.95) | 1.09 (0.86 to 1.40) |
Model 1: adjusted for age, sex, and race. Model 2: adjusted for covariates in model 1 plus smoking status, body mass index, history of hypertension, diabetes and cardiovascular disease, eGFR, serum hemoglobin, serum albumin, and Homocysteine in Kidney and ESRD randomized assignment. Q, quartile; 95% CI, 95% confidence interval.
Because of the potential risk of informative censoring of incident dialysis by the competing risk of death, the association of SBP, DBP, and PP with the composite outcome of progression to long-term dialysis or death was examined. Similar results were observed for the association between DBP and PP when the composite outcome of progression to long-term dialysis or death was examined (Table 5). However, SBP was not significantly associated with the composite outcome of progression to long-term dialysis or death.
Table 5.
Association of BP with composite outcome of progression to long-term dialysis or death
| BP (mm Hg) | Model 1 Hazard Ratio (95% CI) | Model 2 Hazard Ratio (95% CI) |
|---|---|---|
| Systolic BP | ||
| Q1 (≤128) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>128–≤141) | 1.04 (0.86 to 1.27) | 0.98 (0.80 to 1.20) |
| Q3 (>141–≤157) | 1.26 (1.04 to 1.53) | 1.15 (0.95 to 1.40) |
| Q4 (>157) | 1.40 (1.15 to 1.69) | 1.19 (0.97 to 1.44) |
| Diastolic BP | ||
| Q1 (≤64) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>64–≤72) | 0.95 (0.79 to 1.16) | 1.00 (0.82 to 1.21) |
| Q3 (>72–≤80) | 1.08 (0.89 to 1.31) | 1.16 (0.95 to 1.41) |
| Q4 (>80) | 1.10 (0.90 to 1.34) | 1.24 (1.01 to 1.52) |
| Pulse pressure | ||
| Q1 (≤57) | 1.00 (reference) | 1.00 (reference) |
| Q2 (>57–≤70) | 1.16 (0.95 to 1.40) | 0.98 (0.80 to 1.18) |
| Q3 (>70–≤83) | 1.29 (1.05 to 1.58) | 1.09 (0.89 to 1.33) |
| Q4 (>83) | 1.36 (1.11 to 1.66) | 1.03 (0.84 to 1.27) |
Model 1: adjusted for age, sex, and race. Model 2: adjusted for covariates in model 1 plus smoking status, body mass index, history of hypertension, diabetes and cardiovascular disease, eGFR, serum hemoglobin, serum albumin, and Homocysteine in Kidney and ESRD randomized assignment. Q, quartile; 95% CI, 95% confidence interval.
Discussion
Among patients with advanced kidney disease not undergoing dialysis, higher PP was independently associated with a higher risk of CVE. SBP and DBP were not associated with CVE but were independently associated with a higher risk of progression to long-term dialysis. Unlike other studies in patients with CKD, this study did not find an association between SBP, DBP, or PP and death. Furthermore, lower SBP or DBP was not associated with adverse outcomes in our patient population.
To date, no adequately powered randomized controlled clinical trials have evaluated BP treatment targets and hard cardiovascular outcomes in patients with stage 3 or more advanced kidney disease. Previous observational studies in the dialysis population and in patients with less severe kidney disease have found that both higher and lower SBP and DBP were associated with a higher risk of death and CVD (9,14–19). A recent study by Kovesdy et al. examined >600,000 veterans with CKD and found that patients in whom both SBP and DBP were very high or very low had the highest mortality rates (3). Furthermore, those with a DBP <70 mmHg had the highest risk of death. We did not find an association between BP and death in our cohort. Our patients had more advanced kidney disease compared with the patients in the study reported by Kovesdy and colleagues (mean±SD eGFR, 18±6 ml/min per 1.73 m2 versus 50±14 ml/min per 1.73 m2); unlike Kovesdy et al., we did not examine specific combinations of SBP and DBP, which may partly explain the different findings.
In patients with CKD, the leading cause of death is CVD. Compared with the general population, patients with CKD have increased arterial stiffness, which may be due to accelerated medial calcification or atherosclerosis. This likely explains the high PP seen in CKD (5,6). Controversy exists as to whether PP or SBP is the most important predictor of CVD. In our cohort, PP was the only measure significantly associated with CVE. To our knowledge, our study is the first to show a relationship between PP and CVE in patients with advanced CKD not undergoing dialysis. Current hypertension treatment strategies primarily target SBP instead of DBP. However, the consequences of tight control of SBP needs to be considered. As shown in our study, elevated PP may be detrimental by resulting in a higher risk of adverse CVEs. Elevated PP can damage the vascular wall and increase the stress on the left ventricle, leading to ventricular hypertrophy and failure (20,21).
High BP is a known independent risk factor for faster decline of GFR (22–24). Consistent with these findings, we found that higher SBP (>157 mmHg) and DBP (>80 mmHg) were associated with progression to long-term dialysis. We did not find a U-shaped relationship with SBP or DBP because lower levels were not associated with a higher risk. Our results suggest that SBP and DBP may be more important for predicting progression to long-term dialysis than PP is because PP was not associated with progression. However, other studies have found an association between PP and kidney disease progression. A study of 329 patients with CKD stages 2–4 found that baseline PP was the only predictor of kidney disease progression (11). Differences in the findings may be related to differences in the two cohorts as our patients had much more advanced kidney disease than the previous study (mean±SD eGFR, 18±6 ml/min per 1.73 m2 versus 39±18 ml/min per 1.73 m2). In addition, studies have found an association between PP and rapid decline in kidney function (25,26). Unfortunately, we did not have multiple measurements of kidney function to determine whether this relationship was present in our study. More studies are needed to determine the effect of PP on kidney disease progression.
This study has several limitations. First, as an observational study, it cannot establish a causal relationship between BP and clinical outcomes. Second, >50% of our patients were diabetic and likely had some degree of proteinuria, but we were unable to control for proteinuria because these data were not available in the HOST study. Third, we had data on BP from only one time point. Using multiple BP measurements would have strengthened our findings because we would have been able to account for temporal changes in BP. Fourth, the definition of kidney disease was based on eGFR rather than more precise measures of kidney function, such as iothalamate clearance. In addition, we had data on kidney function from only one time point. Finally, this study consisted mainly of male veterans with high homocysteine levels, so caution should be used in extrapolating these results to other populations.
Despite these limitations, our study has several important strengths. First, to our knowledge this is the first study examining the association of PP with death, CVEs, and kidney disease progression in patients with advanced CKD not requiring dialysis. Second, the HOST study from which these data were obtained had a large patient population and a comprehensive dataset that allowed for adjustment for important cardiac and CKD risk factors. Third, BP and laboratory values were measured in a standard fashion.
In conclusion, we have demonstrated that higher PP was strongly and independently associated with a higher risk of CVEs. In contrast, SBP and DBP were not associated with CVEs but were associated with a higher risk of progression to long-term dialysis. Our results suggest that SBP and DBP should not be the only factors considered in determining antihypertensive therapy; elevated PP should also be considered.
Disclosures
A.K.C. is a consultant for Amgen and Baxter.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants K23-DK087859 and 1R01-DK081473-01 and by an American Heart Association Award 13BGIA16850035.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “BP Components in Advanced CKD and the Competing Risks of Death, ESRD, and Cardiovascular Events,” on pages 911–913.
References
- 1.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G, REIN-2 Study Group : Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 365: 939–946, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, Quarles LD, Kalantar-Zadeh K: Blood pressure and mortality in U.S. veterans with chronic kidney disease: A cohort study. Ann Intern Med 159: 233–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Levey AS, Elsayed E, Griffith JL, Salem DN, Sarnak MJ: Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol 18: 960–966, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG: Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23: 586–593, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Peralta CA, Shlipak MG, Wassel-Fyr C, Bosworth H, Hoffman B, Martins S, Oddone E, Goldstein MK: Association of antihypertensive therapy and diastolic hypotension in chronic kidney disease. Hypertension 50: 474–480, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME: Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 160: 1085–1089, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Millar JA, Lever AF, Burke V: Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens 17: 1065–1072, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF, Jr: Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA 287: 1548–1555, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Banerjee D, Brincat S, Gregson H, Contreras G, Streather C, Oliveira D, Nelson S: Pulse pressure and inhibition of renin-angiotensin system in chronic kidney disease. Nephrol Dial Transplant 21: 975–978, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Arulkumaran N, Diwakar R, Tahir Z, Mohamed M, Kaski JC, Banerjee D: Pulse pressure and progression of chronic kidney disease. J Nephrol 23: 189–193, 2010 [PubMed] [Google Scholar]
- 12.Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM, RENAAL Study Group : Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAAL study. Arch Intern Med 163: 1555–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM, Veterans Affairs Site Investigators : Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: A randomized controlled trial. JAMA 298: 1163–1170, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Iseki K, Miyasato F, Tokuyama K, Nishime K, Uehara H, Shiohira Y, Sunagawa H, Yoshihara K, Yoshi S, Toma S, Kowatari T, Wake T, Oura T, Fukiyama K: Low diastolic blood pressure, hypoalbuminemia, and risk of death in a cohort of chronic hemodialysis patients. Kidney Int 51: 1212–1217, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Weiss JW, Johnson ES, Petrik A, Smith DH, Yang X, Thorp ML: Systolic blood pressure and mortality among older community-dwelling adults with CKD. Am J Kidney Dis 56: 1062–1071, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duranti E, Imperiali P, Sasdelli M: Is hypertension a mortality risk factor in dialysis? Kidney Int Suppl 55: S173–S174, 1996 [PubMed] [Google Scholar]
- 17.Fleischmann EH, Bower JD, Salahudeen AK: Risk factor paradox in hemodialysis: Better nutrition as a partial explanation. ASAIO J 47: 74–81, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW: Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33: 507–517, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesal P: . “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Winston GJ, Palmas W, Lima J, Polak JF, Bertoni AG, Burke G, Eng J, Gottesman R, Shea S: Pulse pressure and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Hypertens 26: 636–642, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Rourke M, Frohlich ED: Pulse pressure: Is this a clinically useful risk factor? Hypertension 34: 372–374, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J: Blood pressure and end-stage renal disease in men. N Engl J Med 334: 13–18, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Locatelli F, Marcelli D, Comelli M, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A, Northern Italian Cooperative Study Group : Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Nephrol Dial Transplant 11: 461–467, 1996 [DOI] [PubMed] [Google Scholar]
- 25.McIntyre NJ, Fluck RJ, McIntyre CW, Fakis A, Taal MW: Determinants of arterial stiffness in chronic kidney disease stage 3. PLoS ONE 8: e55444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CS, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kim SW: Association of pulse wave velocity and pulse pressure with decline in kidney function. J Clin Hypertens (Greenwich) 16: 372–377, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


