Abstract
Background and objectives
Induction therapy with IL-2 receptor antagonist (IL2-RA) is recommended as a first line agent in living donor renal transplantation (LRT). However, use of IL2-RA remains controversial in LRT with tacrolimus (TAC)/mycophenolic acid (MPA) with or without steroids.
Design, setting, participants, & measurements
The Organ Procurement and Transplantation Network registry was studied for patients receiving LRT from 2000 to 2012 maintained on TAC/MPA at discharge (n=36,153) to compare effectiveness of IL2-RA to other induction options. The cohort was initially divided into two groups based on use of maintenance steroid at time of hospital discharge: steroid (n=25,996) versus no-steroid (n=10,157). Each group was further stratified into three categories according to commonly used antibody induction approach: IL2-RA, rabbit anti-thymocyte globulin (r-ATG), and no-induction in the steroid group versus IL2-RA, r-ATG and alemtuzumab in the no-steroid group. The main outcomes were the risk of acute rejection at 1 year and overall allograft failure (graft failure or death) post-transplantation through the end of follow-up. Propensity score-weighted regression analysis was used to minimize selection bias due to non-random assignment of induction therapies.
Results
Multivariable logistic and Cox analysis adjusted for propensity score showed that outcomes in the steroid group were similar between no-induction (odds ratio [OR], 0.96; 95% confidence interval [95% CI], 0.86 to 1.08 for acute rejection; and hazard ratio [HR], 0.99; 95% CI, 0.90 to 1.08 for overall allograft failure) and IL2-RA categories. In the no-steroid group, odds of acute rejection with r-ATG (OR, 0.73; 95% CI, 0.59 to 0.90) and alemtuzumab (OR, 0.53; 95% CI, 0.42 to 0.67) were lower; however, overall allograft failure risk was higher with alemtuzumab (HR, 1.27; 95% CI, 1.03 to 1.56) but not with r-ATG (HR, 1.19; 95% CI, 0.97 to 1.45), compared with IL2-RA induction.
Conclusions
Compared with no-induction therapy, IL2-RA induction was not associated with better outcomes when TAC/MPA/steroids were used in LRT recipients. r-ATG appears to be an acceptable and possibly the preferred induction alternative for IL2-RA in steroid-avoidance protocols.
Keywords: acute allograft rejection, kidney transplantation, immunosuppression
Introduction
The ultimate goal of immunosuppression in kidney transplantation is to prevent acute rejection and maintain allograft function without causing adverse effects. Immunosuppressive agents are categorized as (1) induction therapy that is administered in the perioperative period and (2) maintenance therapy that transplant recipients require for lifelong use (1). However, optimal combinations of therapies remain controversial and the decision is mostly made in the context of the risk and benefit for each individual donor/recipient pair (2).
The most commonly used maintenance immunosuppressive combination in renal transplantation consists of tacrolimus (TAC) and mycophenolic acid (MPA) with steroids and is based on two open-label randomized studies (3,4). These agents have been part of clinical practice since the late 1990s (the TAC/MPA combination represented >90% in 2011) (5). More recently, induction therapy followed by steroid-sparing maintenance regimens with TAC/MPA alone have gained favor across all donor-recipient profiles. Induction therapy options currently comprise lymphocyte-depleting antibodies, such as polyclonal rabbit anti-thymocyte globulin (r-ATG) and monoclonal humanized anti-CD52 antibody (alemtuzumab), and nondepleting mAbs, such as basiliximab and daclizumab (both abrogate T cell activation; Supplemental Material) (6). Lymphocyte-depleting antibodies appear to be increasingly favored over IL-2 receptor antagonists (IL2-RAs) in the United States (the depleting agents were used in 57% of recipients in 2011) (5). Lymphocyte-depleting agents are mostly used in high immunologic risk factors for acute rejection and steroid-sparing protocols. IL2-RA and no-induction therapies are primarily utilized in patients with low immunologic risk (living related donor renal transplant) (6).
Prospective randomized multicenter studies and retrospective registry analysis demonstrate that no-induction therapy can achieve acceptable acute rejection rates (10%–20% at 1 year after transplant), with allograft and patient survival similar to other induction modalities in living donor renal transplantation (LRT) (7–10). However, the current Kidney Disease Improving Global Outcomes (KDIGO) Transplant Work Group guidelines recommend IL2-RA as a first-line induction agent in all types of donor-recipient profiles to reduce risk of acute rejection and allograft loss. These recommendations are mainly based on a meta-analysis that predominantly used cyclosporine-based maintenance immunosuppression (1,11). Nevertheless, use of IL2-RA remains controversial in LRT, partially because of the low risk of rejection in this population and the utilization of more potent maintenance immunosuppression combinations such as TAC/MPA with or without steroids. To explore the added benefit of IL2-RA induction therapy in LRT recipients maintained on TAC/MPA, we conducted a retrospective cohort analysis of the Organ Procurement and Transplantation Network (OPTN) registry to compare outcomes between IL2-RA and other induction options.
Materials and Methods
Design and Study Cohort
This study was a retrospective cohort analysis of the OPTN registry as of September 30, 2013, (the end of follow-up) that included all adults who received a LRT between January 1, 2000, and September 30, 2012, in the United States (n=76,266). Exclusion criteria were as follows: (1) pediatric patients aged <18 years; (2) multiorgan transplantations; (3) two or more previous kidney transplantations; (4) recipients of induction agents other than no-induction therapy, alemtuzumab, r-ATG, and IL2-RA; (5) patients with a positive cross-match; and (6) recipients of HLA zero mismatch (identical) kidneys. Data were further restricted to recipients whose maintenance immunosuppression at the time of hospital discharge was TAC and MPA. A total of 36,153 patients were included in the final analysis. The study population was initially divided into two groups based on the use of maintenance steroids at the time of hospital discharge (on-steroid [n=25,996] versus no-steroid [n=10,157] groups). Each group was further stratified into three categories according to commonly used antibody induction options: IL2-RA, r-ATG, and no-induction therapy in the steroid group versus IL2-RA, r-ATG, and alemtuzumab in the no-steroid group. The alemtuzumab category in the steroid cohort and the no-induction category in the no-steroid cohort were eliminated because of the small sample size.
Main Outcomes
The primary outcomes were incidence of acute rejection at 1 year (defined as biopsy-proven or clinically treated acute rejection) and overall allograft failure risk (graft failure or death) after transplantation (defined as return to dialysis, retransplant, or death with functioning allograft). Acute rejections were ascertained only up to 1 year after transplantation (available for all recipients), whereas overall allograft failures were included through the end of the follow-up period (September 30, 2013).
Statistical Analyses
Donor and recipient characteristics were described using frequencies or means±SDs. Comparisons between groups were made using the t test, Kruskal–Wallis test, or chi-squared test. Survival rates were estimated using the Kaplan–Meier product limit method. The log-rank test was used for comparison of the unadjusted survival curves. Logistic regression models were used to estimate the odds ratios of acute rejection. Cox regression models were used to estimate the hazard ratios associated with overall and death-censored allograft failure risk and patient mortality risk. P values < 0.05 were considered statistically significant. Statistical analyses were performed with SAS software (version 9.3; SAS Inc., Cary, NC).
Propensity Score Analyses
We controlled for potential selection bias due to nonrandom assignment of induction treatments using the propensity score (PS) method. PS is the probability that a patient would have been treated based on that patient’s observed pretreatment variables. We utilized multinomial logistic regression to estimate the PS as the conditional probability of a patient receiving a certain induction treatment given pretreatment covariates including donor (age, sex, and race), recipient (age, sex, race, diabetes status, cardiovascular comorbidities, retransplant status, dialysis before transplant, and panel reactive antibodies [PRAs]), and transplant factors (donor/recipient weight ratio, HLA mismatch, and transplant year) (12). Several adjustment methods integrating the estimated PS have been suggested, including matching (13), regression adjustment (14), and weighting (12,15). In this analysis, we utilized the inverse probability of treatment weight (IPTW), in which the weights were calculated as the inverse of the PS (15). Finally, PS-weighted regression models were fitted to compare the treatment effects, controlling for selection bias.
Covariates were balanced after IPTW adjustment, that is, after performing weighted regression (with one of the covariates as outcome, induction categories as a predictor, and PS as weights), the effect of induction therapy was no longer significant. For instance, before IPTW adjustment, the variable “recipient diabetes” was significantly different among induction groups in both steroid categories (P<0.001). After adjustment, the P values for recipient diabetes were 0.77 and >0.99 in the steroid and no-steroid groups, respectively.
Results
Characteristics of the Study Cohort
Recipient, donor, and transplant characteristics for each induction category stratified by use of steroid at discharge are summarized in Tables 1 and 2, indicating clinically equitable risk factor stratification among induction categories. P values before IPTW adjustment are mostly statistically significant in Tables 1 and 2. However, all P values became statistically insignificant after IPTW adjustment, suggesting that the PS-weighting method successfully controlled for the imbalance among covariates. In the context of steroids, compared with the no-induction and IL2-RA categories, the recipients of r-ATG were more likely to be black, were more likely to be sensitized (PRA>20%), and were more likely to have received higher HLA-mismatch (>3) kidneys. In the no-steroid group, IL2-RA induction was more likely to be used in recipients with a PRA< 20% and these patients were more likely to receive lower HLA-mismatch (<4) kidneys compared with the other two induction categories.
Table 1.
Characteristics of donor, recipient and transplant factors in steroid group (n=25,996)
| Characteristic | Steroid Induction Categories | P Value | |||
|---|---|---|---|---|---|
| IL2-RA | r-ATG | No Induction | Before IPTW | After IPTW | |
| No. (%) | 9741 (37.5) | 8552 (32.9) | 7703 (29.6) | ||
| Donors | |||||
| Age (yr) | 41.4±11.4 | 41.1±11.4 | 40.6±11.2 | <0.001 | 0.56 |
| Women | 61.0 | 60.3 | 59.8 | 0.26 | 0.74 |
| Race | <0.001 | 0.84 | |||
| White | 68.2 | 71.1 | 66.8 | ||
| Black | 12.2 | 15.7 | 13.8 | ||
| Hispanic | 14.4 | 9.3 | 14.0 | ||
| Other | 5.2 | 3.9 | 5.5 | ||
| Recipients | |||||
| Age (yr) | 47.4±14.3 | 46.8±13.7 | 46.6±14.1 | <0.001 | 0.53 |
| Women | 37.0 | 41.3 | 39.2 | <0.001 | 0.91 |
| Race | <0.001 | 0.60 | |||
| White | 65.8 | 67.3 | 64.6 | ||
| Black | 13.8 | 18.4 | 15.6 | ||
| Hispanic | 14.5 | 9.3 | 14.1 | ||
| Other | 5.9 | 5.0 | 5.7 | ||
| Diabetes mellitus (yes) | 28.4 | 27.8 | 31.6 | <0.001 | 0.77 |
| Cardiovascular disease (yes) | 5.8 | 5.8 | 5.3 | 0.33 | 0.97 |
| Retransplant | 2.8 | 2.5 | 4.2 | <0.001 | 0.96 |
| Dialysis before transplant | <0.001 | 0.92 | |||
| Preemptive | 30.7 | 32.1 | 32.2 | ||
| <1 yr | 29.4 | 28.5 | 28.0 | ||
| 1–3 yr | 28.6 | 27.2 | 29.3 | ||
| >3 yr | 11.3 | 12.2 | 10.4 | ||
| Panel reactive antibody | <0.001 | 1.00 | |||
| <20 | 43.9 | 34.5 | 51.0 | ||
| 20–80 | 3.8 | 7.1 | 4.8 | ||
| >80 | 0.7 | 2.1 | 1.0 | ||
| Missing | 51.5 | 56.3 | 43.2 | ||
| Transplant | |||||
| Weight ratio (donor/recipient) | 1.02±0.30 | 1.02±0.31 | 1.02±0.31 | 0.39 | 0.87 |
| HLA mismatch | <0.001 | 1.00 | |||
| 1 | 5.9 | 5.3 | 6.1 | ||
| 2 | 18.3 | 16.7 | 20.2 | ||
| 3 | 29.9 | 28.2 | 31.5 | ||
| 4 | 16.2 | 17.5 | 15.3 | ||
| 5 | 19.3 | 20.9 | 17.4 | ||
| 6 | 10.6 | 11.5 | 9.6 | ||
| Transplant year | <0.001 | 1.00 | |||
| 2000–2001 | 11.2 | 3.5 | 20.1 | ||
| 2002–2003 | 16.1 | 12.0 | 20.2 | ||
| 2004–2005 | 12.5 | 15.7 | 20.0 | ||
| 2006–2007 | 15.7 | 16.7 | 13.9 | ||
| 2008–2009 | 18.2 | 20.0 | 12.9 | ||
| 2010–2012 | 26.3 | 32.2 | 12.9 | ||
Data are presented as means±SD or percentages unless otherwise indicated. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit anti-thymocyte globulin; IPTW, inverse probability of treatment weight.
Table 2.
Characteristics of donor, recipient, and transplant factors in the no-steroid group (n=10,157)
| Characteristic | No-Steroid Induction Categories | P Value | |||
|---|---|---|---|---|---|
| IL2-RA | r-ATG | Alemtuzumab | Before IPTW | After IPTW | |
| No. (%) | 1,483 (14.6) | 4,905 (48.3) | 3,769 (37.1) | ||
| Donors | |||||
| Age (yr) | 41.7±11.4 | 41.6±11.5 | 40.6±11.5 | <0.001 | 0.80 |
| Women | 60.9 | 59.7 | 60.8 | 0.51 | 0.93 |
| Race | <0.001 | 1.00 | |||
| White | 71.3 | 71.7 | 65.2 | ||
| Black | 8.6 | 11.0 | 12.2 | ||
| Hispanic | 13.8 | 12.2 | 19.5 | ||
| Other | 6.3 | 5.0 | 3.0 | ||
| Recipients | |||||
| Age (yr) | 50.7±15.1 | 48.5±13.7 | 48.4±13.4 | <0.001 | 0.36 |
| Women | 35.8 | 36.5 | 36.6 | 0.86 | 0.97 |
| Race | <0.001 | 1.00 | |||
| White | 70.1 | 69.5 | 61.6 | ||
| Black | 9.3 | 12.5 | 13.9 | ||
| Hispanic | 13.8 | 12.3 | 20.2 | ||
| Other | 6.8 | 5.7 | 4.5 | ||
| Diabetes mellitus (yes) | 33.0 | 31.3 | 36.7 | <0.001 | 0.97 |
| Cardiovascular disease (yes) | 5.4 | 7.1 | 4.1 | <0.001 | 0.81 |
| Retransplant | 2.0 | 2.5 | 1.9 | <0.001 | 0.97 |
| Dialysis before transplant | <0.001 | 1.00 | |||
| Preemptive | 35.4 | 37.5 | 32.9 | ||
| <1 yr | 26.6 | 28.7 | 29.6 | ||
| 1–3 yr | 29.1 | 24.3 | 27.2 | ||
| >3 yr | 8.9 | 9.4 | 10.3 | ||
| Panel reactive antibody | <0.001 | 0.62 | |||
| <20 | 45.6 | 39.1 | 36.2 | ||
| 20–80 | 3.8 | 2.9 | 3.6 | ||
| >80 | 0.2 | 0.7 | 0.64 | ||
| Missing | 50.4 | 57.3 | 59.5 | ||
| Transplant | |||||
| Weight ratio (donor/recipient) | 1.01±0.33 | 1.00±0.31 | 0.99±0.30 | 0.02 | 0.89 |
| HLA mismatch | <0.001 | 1.00 | |||
| 1 | 6.9 | 4.9 | 5.3 | ||
| 2 | 21.2 | 16.3 | 17.3 | ||
| 3 | 29.9 | 28.7 | 28.2 | ||
| 4 | 14.0 | 17.2 | 17.3 | ||
| 5 | 18.0 | 21.5 | 20.3 | ||
| 6 | 9.9 | 11.4 | 11.6 | ||
| Transplant year | <0.001 | 0.73 | |||
| 2000–2001 | 6.5 | 0.1 | 0.0 | ||
| 2002–2003 | 5.5 | 3.7 | 2.1 | ||
| 2004–2005 | 17.7 | 15.0 | 10.7 | ||
| 2006–2007 | 20.6 | 22.6 | 17.8 | ||
| 2008–2009 | 20.8 | 26.2 | 25.2 | ||
| 2010–2012 | 28.9 | 32.3 | 44.2 | ||
Data are presented as means±SD or percentages unless otherwise indicated. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit anti-thymocyte globulin; IPTW, inverse probability of treatment weight.
Outcomes
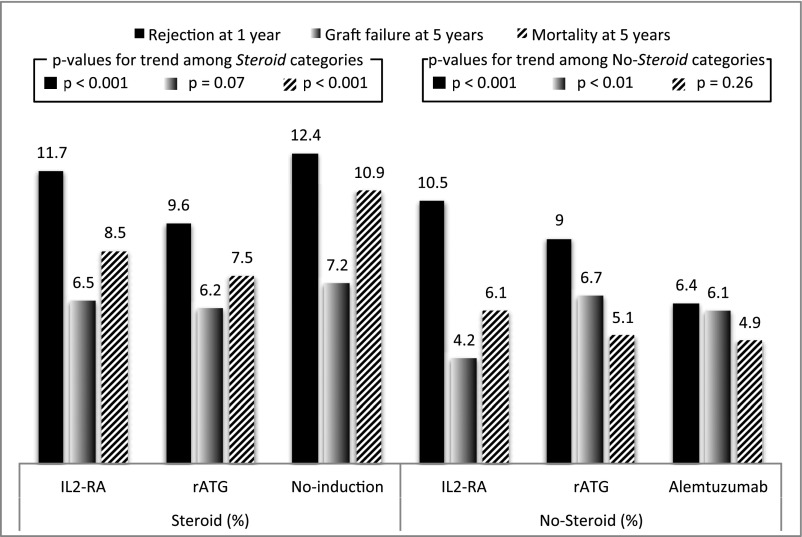
Median (25th, 75th percentiles) follow-up time was 4.3 (2.1, 7.1) and 3.8 (2.0, 5.8) years for the steroid and no-steroid groups, respectively. Observed frequencies of different components of the primary outcomes are shown in Figure 1. Acute rejection was the most common outcome across induction categories for both steroid groups. In the steroid group, the recipients who received no-induction and IL2-RA therapy had a slightly higher rate of acute rejection at 1 year after transplantation compared with r-ATG. In the no-steroid group, acute rejection rates at 1 year were lower with r-ATG and alemtuzumab than those observed with IL2-RA. During the study period, the risks for acute rejection and graft failure steadily declined (Supplemental Figures 1 and 2). Causes of death and allograft failures are summarized in Supplemental Tables 1 and 2. Acute and chronic rejections were the most common cause of allograft failure in the IL2-RA category for both steroid groups. The cause of death was not specified in one half of the recipients. Malignancy accounted for approximately 20% of mortalities in the r-ATG arm.
Figure 1.
Observed frequencies of outcomes by induction type, with or without steroids at discharge. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit anti-thymocyte globulin.
Acute Rejection Risk at 12 Months
Table 3 displays the results of PS-weighted and covariate-adjusted multivariable logistic regression models for acute rejection risks. Among the patients receiving steroids at discharge, the relative risk (RR) of acute rejection was significantly lower in the r-ATG category compared with IL2-RA, whereas there was no significant difference between the no-induction and IL2-RA categories. In the no-steroid group, the adjusted risk of acute rejection in patients induced with r-ATG and alemtuzumab was lower compared with those induced with IL2-RA.
Table 3.
Comparison of the estimated association of induction treatments on acute rejection at 1 year using multivariable logistic regression models
| Multivariable Model | Induction Type | OR (95% CI) | P Value |
|---|---|---|---|
| Steroid | |||
| Logistic regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 0.80 (0.71 to 0.89) | <0.001 | |
| No induction | 1.04 (0.92 to 1.16) | 0.55 | |
| PS-weighted logistic regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 0.78 (0.70 to 0.88) | <0.001 | |
| No induction | 0.96 (0.86 to 1.08) | 0.48 | |
| No Steroid | |||
| Logistic regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 0.72 (0.58 to 0.90) | 0.004 | |
| Alemtuzumab | 0.52 (0.40 to 0.66) | <0.001 | |
| PS-weighted logistic regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 0.73 (0.59 to 0.90) | 0.004 | |
| Alemtuzumab | 0.53 (0.42 to 0.67) | <0.001 |
Adjusted for donor factors (age, sex, and race), recipient factors (age, sex, race, cardiovascular morbidity, panel reactive antibody, retransplant status, and dialysis status) and transplant factors (donor/recipient weight ratio, HLA mismatch, and transplant year). PS, propensity score; IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit anti-thymocyte globulin; OR, odds ratio; 95% CI, 95% confidence interval.
Overall Allograft Failure
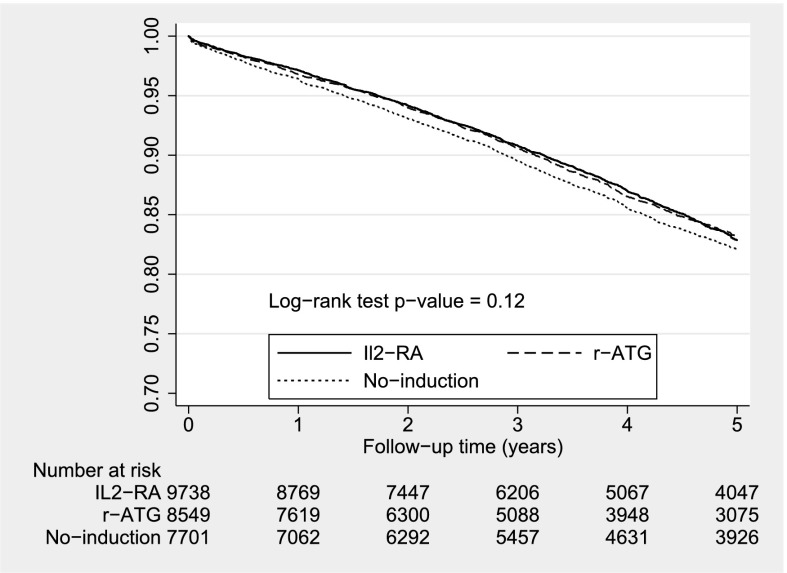
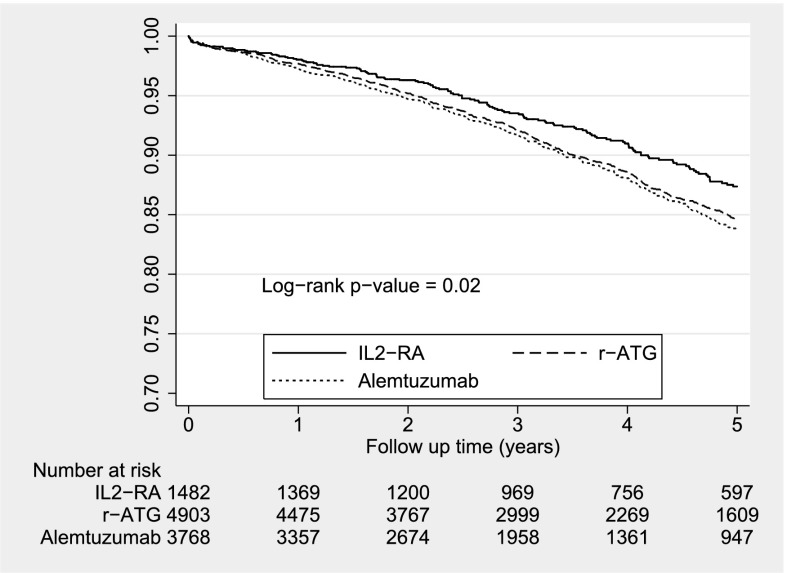
Unweighted Kaplan–Meier curves for overall graft survival are given in Figures 2 and 3. The survival curves did not differ across induction therapies in the steroid group, but they were significantly different in the no-steroid group. Table 4 shows the PS-weighted and covariate-adjusted multivariable Cox models for overall allograft failure. In the context of steroids, the hazard ratios for overall graft failure were not significantly different between the r-ATG and no-antibody induction categories compared with the IL2-RA category. When steroids were absent, overall allograft failure risk was statistically significantly higher with alemtuzumab but not with r-ATG compared with IL2-RA.
Figure 2.
Unweighted Kaplan–Meier survival estimates for induction types with steroid. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit anti-thymocyte globulin.
Figure 3.
Unweighted Kaplan–Meier survival estimates for induction types without steroid. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit anti-thymocyte globulin.
Table 4.
Comparison of the estimated association of induction treatments on overall allograft failure through the end of the follow-up using multivariable Cox regression models
| Multivariable Model | Induction Type | HR (95% CI) | P Value |
|---|---|---|---|
| Steroid | |||
| Cox regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 1.00 (0.91 to 1.09) | 0.93 | |
| No induction | 1.00 (0.91 to 1.09) | 0.92 | |
| PS-weighted Cox regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 0.99 (0.91 to 1.08) | 0.79 | |
| No induction | 0.99 (0.90 to 1.08) | 0.76 | |
| No Steroid | |||
| Cox regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 1.14 (0.93 to 1.39) | 0.21 | |
| Alemtuzumab | 1.21 (0.98 to 1.49) | 0.07 | |
| PS-weighted Cox regression | |||
| IL-2 RA | 1 | ||
| r-ATG | 1.19 (0.97 to 1.45) | 0.01 | |
| Alemtuzumab | 1.27 (1.03 to 1.56) | 0.02 |
Adjusted for donor factors (age, sex, and race), recipient factors (age, sex, race, cardiovascular morbidity, panel reactive antibody, retransplant status, and dialysis status) and transplant factors (donor/recipient weight ratio, HLA mismatch, and transplant year). PS, propensity score; IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit anti-thymocyte globulin; HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
In LRT, the incidence of acute rejection at 1 year decreased over the observed period of the study (2000–2012) and stayed below 15%. Short-term graft survival steadily improved at a comparable level among all induction categories including no-induction therapy over the past decade. These findings raise the following important issues: (1) clinical utility of induction therapy in LRT in the setting of TAC/MPA maintenance immunosuppression (9,16), and (2) improved outcomes that may be accounted for by other factors, such as sensitive HLA antibody detection (17–19), more sensitive cross-match techniques (20,21), implementation of virtual cross-match in 2006 (22,23), and utilization of a calculated PRA system in 2009 into a routine allocation system (19). A large sample size (a patient population ranging from 1600 to 7000) needed to detect small differences in observed outcomes among induction types is most likely prohibitive to prospective randomized trials (10). In the current literature, there are a limited number of randomized studies to compare the effectiveness of induction modalities in the setting of TAC/MPA maintenance immunosuppression, and LRT recipients are also significantly under-represented in those clinical trials. Moreover, comparison of sensitized patients using lymphocyte-depleting agents with nonsensitized patients more frequently using either no-induction or IL2-RA therapies is challenging unless advanced statistical adjustments for selection bias or stratification for immunologic risk groups are performed.
Our study constitutes the largest analysis of the OPTN registry on main outcomes in LRT recipients maintained on TAC/MPA with or without steroids since 2000. It challenges the concept of routine use of IL2-RA induction agent in all kidney transplant recipients (deceased and living), which is suggested by the KDIGO guidelines. Below, we review current recommendations and compare our findings against moderate- to high-quality evidence in the literature.
Steroid Maintenance
The KDIGO guidelines recommend induction therapy (a biologic agent, either a lymphocyte-depleting agent or IL2-RA, at the time of transplant) in all kidney transplant recipients (1A equals high-quality evidence) and IL2-RA as the first-line agent (1B equals moderate-quality evidence). However, supportive evidence in the current literature since the guidelines were published is unclear, mostly because of heterogeneity in the recipient’s immunologic risks and difference in maintenance immunosuppressive agents (choice of calcineurin inhibitors and antiproliferative agents).
A large meta-analysis (32 studies, n=5854), mainly including cyclosporine-based maintenance immunosuppression (30 of 32), compared IL2-RA induction to placebo (no induction). The authors demonstrated that biopsy-proven acute rejection was reduced by 28% (RR, 0.72; 95% confidence interval [95% CI], 0.64 to 0.81) and overall allograft failure was reduced by 25% (RR, 0.75; 95% CI, 0.62 to 0.90) (11,24). The rate of acute rejection at 1 year after transplant was 38% in the placebo/no-induction group and 27% in the IL2-RA arm. In a subgroup analysis, the outcomes were not different when use of cyclosporine and TAC maintenance regimens were compared. However, two pivotal large prospective studies reported significantly lower rates of biopsy-proven rejection at 1 year among kidney transplant recipients receiving IL2-RA induction and TAC/MPA maintenance (15.4% and 7.5%, respectively) (3,4). A registry analysis (n=26,686) compared IL2-RA induction use to no-induction therapy in primary LRT recipients discharged on TAC/MPA/prednisone maintenance (9). The incidence of rejection at 1 year was significantly different between groups (11.6% for IL2-RA versus 13% for no-induction therapy), but this did not translate into a graft or patient survival benefit.
In our study, based on the multivariable logistic and Cox regression analyses adjusted for PS weighting and covariates, IL2-RA neither decreased the incidence of acute rejection rate nor did it improve overall graft survival after transplant compared with no-induction therapy. However, one does not truly know whether the IL2-RA cohort was treated the same as the no-induction cohort in terms of maintenance immunosuppression (especially target TAC levels and MPA dosing). It is possible that LRT recipients maintained on TAC/MPA with steroids are at low risk for acute rejection and graft loss, and the advantage of using IL2-RA induction may be too small. In this setting, especially considering adverse effects and cost, no-induction therapy is a reasonable option. By contrast, compared with IL2-RA, r-ATG decreased the RR of acute rejection by 22%, but it did not improve graft survival. It is a plausible strategy to limit the use of r-ATG to patients at increased risk for acute rejection to keep a favorable balance between benefits and serious adverse effects.
Steroid Avoidance
Early steroid avoidance has gained interest over the past decade in the United States, mainly to minimize metabolic side effects and negative effects on quality of life. Steroids have been a mainstay of immunosuppression for decades, and data evaluating minimization of steroids are sparse. In addition, many of the side effects attributed to steroids have been observed with higher doses. The association of low-dose steroid protocols (prednisone 5 mg daily) with major side effects is not well defined. Early steroid withdrawal studies were associated with high rejection rates and graft failures (25–27); however, incorporation of antibody induction into standard immunosuppression protocols produced acceptable results (28–31). The KDIGO guidelines also suggest using a lymphocyte-depleting agent, rather than an IL2-RA, for kidney transplant recipients at high immunologic risk for rejection prevention (2B equals a moderate evidence suggestion).
Woodle et al. compared outcomes (graft failure, death, acute rejection) of adult renal transplant recipients (LRT comprising 58% of the study cohort, n=386) who received antibody induction, TAC/MPA maintenance immunosuppression, and early steroid cessation (7 days) versus chronic low-dose steroid in a randomized double-blinded study (32). At 5 years, the authors did not observe any difference in primary end points. In the corticosteroid cessation group, biopsy-proven rejection was significantly higher in recipients inducted with IL2-RA (24.4%) compared with r-ATG (14.4%). A recent meta-analysis (n=1934) included eight randomized clinical trials of early steroid withdrawal in kidney transplant recipients treated with r-ATG or IL2-RA induction and calcineurin inhibitors (TAC or cyclosporine A)/MPA with or without steroid-maintenance immunosuppression (33). Compared with conventional steroid use, when TAC and MPA were used, the no-steroid arm was not associated with higher acute rejection (RR, 1.06; 95% CI, 0.79 to 1.42), death (RR, 1.09; 95% CI, 0.50 to 2.37), and graft loss (RR, 1.29; 95% CI, 0.71 to 2.34). Similar findings were observed in a prospective, randomized multicenter trial (Thymoglobulin in Renal Transplantation for Induction and Minimization of Steroids study, n=153), which evaluated early corticosteroid withdrawal in LRT recipients who received r-ATG induction and TAC/MPA maintenance (8). In another steroid withdrawal randomized controlled trial (TAC/MPA maintenance regimen), Hanaway et al. stratified recipients based on their immunologic risk; low-risk patients (n=335) were randomized to alemtuzumab or basiliximab, whereas high-risk patients (n=139) received alemtuzumab or r-ATG (34). The incidence of rejection at 1 year in the low-risk group was lower with alemtuzumab versus basiliximab (3% versus 20%, P<0.001) and similar among high-risk patients (10% for alemtuzumab versus 13% for r-ATG, P=0.53). Nevertheless, these differences in the lower rejection rates did not translate to better death-censored graft survival or function.
In our multivariable PS-weighted analysis of LRT recipients maintained on TAC/MPA without steroids at discharge, induction with r-ATG and alemtuzumab lowered the RR of acute rejection, compared with IL2-RA, by 27% and 47%, respectively. Only alemtuzumab significantly increased the RR of overall graft failure after transplant by 27%, as previously shown in another OPTN/United Network for Organ Sharing (UNOS) analysis (35).
We agree with the KDIGO suggestion that, in the setting of steroid withdrawal, lymphocyte-depleting agents are more effective for decreasing risk of rejection and r-ATG seems to be safer and preferable over alemtuzumab to minimize graft loss and death. Nevertheless, in terms of pharmacoeconomics, IL2-RA induction is initially less costly, compared with r-ATG, as a result of shorter initial hospitalization and lower serious infectious complications (36). However, this initial higher cost can easily be offset by reducing hospitalization rates for acute rejection episodes and preventing graft failures. Clinicians should base their induction choice on the risk/benefit ratio for each recipient.
Cost
Alemtuzumab offers a significant cost savings compared with r-ATG and IL2-RA based on the average wholesale price (Red Book Online 2014, http://www.redbook.com/redbook/online). The cost of a typical course of alemtuzumab induction (typically 30 mg intravenously ×1) was $2118 in 2010. Alemtuzumab is no longer commercially available but is distributed only under research protocols with an institutional review board approval by its manufacturer. Basiliximab (IL2-RA) is usually administered as two doses of 20 mg (postoperative days 0 and 4) and costs $6489.14 (20-mg unit price $3244.57). Thymoglobulin (r-ATG) is typically given as four doses of 1.5 mg/kg (1.5 mg/kg×70 kg×4 doses=420 mg for a 70-kg standard adult patient) and costs $13,554.95 (unit price $797.35 per 25-mg vial, 17 vials×$797.35=$13,554.95). One should also keep in mind that these values are the costs of the drugs but they do not reflect the cost of administration, inpatient hospital stay, incidence, and cost of induction therapy–related complications.
Strengths and Limitations of the Study
The large sample size powers our study to detect small differences in the outcomes. Minimization of selection bias in patients undergoing different induction treatments (approximation to randomization) was mostly achieved by using PS weighting. Despite these strengths, our study has some limitations that are inherent in observational studies using registry data. Definitions and reporting of acute rejection episodes are left to the discretion of individual transplant centers, which are likely to be underreported. The lack of maintenance immunosuppression doses and trough levels (TAC) in the OPTN/UNOS database can introduce bias in acute rejection rates as a result of the difference in TAC/MPA exposure among induction categories. Finally, rate of malignancy and infectious complications could not be accurately assessed.
In LRT, when TAC/MPA/steroids are used, IL2-RA induction does not improve the outcomes compared with no-induction therapy. r-ATG appears to be an acceptable and preferred induction alternative for IL2-RA in steroid-avoidance protocols.
Disclosures
None.
Supplementary Material
Acknowledgments
This research is partly supported by the University of Texas Southwestern O’Brien Kidney Research Core Center (Grant P30-DK079328). On the basis of OPTN data as of September 30, 2013, this work was supported in part by Health Resources and Services Administration Contract 234-2005-370011C.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08710814/-/DCSupplemental.
See related editorial, “Induction Therapy in Renal Transplantation: Why? What Agent? What Dose? We May Never Know,” on pages 923–925.
References
- 1.Kidney Disease Improving Global Outcomes Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S: KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 56: 189–218, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Silva HT, Jr, Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, Dhadda S, Holman J, Fitzsimmons W, First MR: One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 7: 595–608, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF, ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 Annual Data Report: Kidney. Am J Transplant 14[Suppl 1]: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Hardinger KL, Brennan DC, Klein CL: Selection of induction therapy in kidney transplantation. Transpl Int 26: 662–672, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Ahsan N, Holman MJ, Jarowenko MV, Razzaque MS, Yang HC: Limited dose monoclonal IL-2R antibody induction protocol after primary kidney transplantation. Am J Transplant 2: 568–573, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC, TRIMS Study Investigators : A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with thymoglobulin in living-donor kidney transplantation. Clin Transplant 24: 73–83, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Gralla J, Wiseman AC: The impact of IL2ra induction therapy in kidney transplantation using tacrolimus- and mycophenolate-based immunosuppression. Transplantation 90: 639–644, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Willoughby LM, Schnitzler MA, Brennan DC, Pinsky BW, Dzebisashvili N, Buchanan PM, Neri L, Rocca-Rey LA, Abbott KC, Lentine KL: Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: Application of statistical approaches to reduce bias in observational comparisons. Transplantation 87: 1520–1529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster AC, Playford EG, Higgins G, Chapman JR, Craig J: Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev (1): CD003897, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Spreeuwenberg MD, Bartak A, Croon MA, Hagenaars JA, Busschbach JJ, Andrea H, Twisk J, Stijnen T: The multiple propensity score as control for bias in the comparison of more than two treatment arms: An introduction from a case study in mental health. Med Care 48: 166–174, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Rubin DB, Thomas N: Matching using estimated propensity scores: Relating theory to practice. Biometrics 52: 249–264, 1996 [PubMed] [Google Scholar]
- 14.D’Agostino RB, Jr: Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17: 2265–2281, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, Robins JM: Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 163: 262–270, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Emami S, Huang E, Kuo HT, Kamgar M, Bunnapradist S: Multivariate analysis of antibody induction therapy and their associated outcomes in live donor kidney transplantation in the recent era. Clin Transplant 26: 351–358, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S: Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation 87: 1681–1688, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Vaidya S, Partlow D, Susskind B, Noor M, Barnes T, Gugliuzza K: Prediction of crossmatch outcome of highly sensitized patients by single and/or multiple antigen bead Luminex assay. Transplantation 82: 1524–1528, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Cecka JM, Kucheryavaya AY, Reinsmoen NL, Leffell MS: Calculated PRA: Initial results show benefits for sensitized patients and a reduction in positive crossmatches. Am J Transplant 11: 719–724, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Mulley WR, Kanellis J: Understanding crossmatch testing in organ transplantation: A case-based guide for the general nephrologist. Nephrology (Carlton) 16: 125–133, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Bryan CF, Baier KA, Nelson PW, Luger AM, Martinez J, Pierce GE, Ross G, Shield CF, 3rd, Warady BA, Aeder MI, Helling TS, Muruve N: Long-term graft survival is improved in cadaveric renal retransplantation by flow cytometric crossmatching. Transplantation 66: 1827–1832, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Bielmann D, Hönger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S: Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant 7: 626–632, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bingaman AW, Murphey CL, Palma-Vargas J, Wright F: A virtual crossmatch protocol significantly increases access of highly sensitized patients to deceased donor kidney transplantation. Transplantation 86: 1864–1868, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Webster AC, Playford EG, Higgins G, Chapman JR, Craig J: Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev (1): CD003897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, Wilkinson A, Ewell M, McIntosh M, Stablein D, Hodge E, Steroid Withdrawal Study Group : Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil—a prospective randomized study. Transplantation 68: 1865–1874, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Vanrenterghem Y, Lebranchu Y, Hené R, Oppenheimer F, Ekberg H: Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 70: 1352–1359, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kasiske BL, Chakkera HA, Louis TA, Ma JZ: A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol 11: 1910–1917, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J, FREEDOM Study Group : A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant 8: 307–316, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Woodle ES, Vincenti F, Lorber MI, Gritsch HA, Hricik D, Washburn K, Matas AJ, Gallichio M, Neylan J: A multicenter pilot study of early (4-day) steroid cessation in renal transplant recipients under simulect, tacrolimus and sirolimus. Am J Transplant 5: 157–166, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Heilman RL, Reddy KS, Mazur MJ, Moss AA, Post DJ, Petrides S, Mulligan DC: Acute rejection risk in kidney transplant recipients on steroid-avoidance immunosuppression receiving induction with either antithymocyte globulin or basiliximab. Transplant Proc 38: 1307–1313, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, Roth D, Kupin W, Tueros L, Flores S, Hanson L, Vianna R, Burke GW, 3rd: Randomized trial of three induction antibodies in kidney transplantation: Long-term results. Transplantation 97: 1128–1138, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P, Astellas Corticosteroid Withdrawal Study Group : A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564–577, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Pascual J, Royuela A, Galeano C, Crespo M, Zamora J: Very early steroid withdrawal or complete avoidance for kidney transplant recipients: A systematic review. Nephrol Dial Transplant 27: 825–832, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, Croy R, Holman J, INTAC Study Group : Alemtuzumab induction in renal transplantation. N Engl J Med 364: 1909–1919, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Sampaio MS, Kadiyala A, Gill J, Bunnapradist S: Alemtuzumab versus interleukin-2 receptor antibodies induction in living donor kidney transplantation. Transplantation 88: 904–910, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Lilliu H, Brun-Strang C, Le Pen C, Büchler M, Al Najjar A, Priol G, Reigneau O, Lebranchu Y: Cost-minimization study comparing Simulect vs. thymoglobulin in renal transplant induction. Clin Transplant 18: 247–253, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.