Abstract
Albuminuria is a risk factor for progression of kidney disease. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers slow the progression to ESRD, an effect that is correlated with reduction in albuminuria. This has led to the hypothesis that albuminuria should be a target for therapy. This work argues that there are issues with this hypothesis. The previously reported studies were not designed to test the hypothesis that achieving a specific albuminuria target would be beneficial in and of itself irrespective the mechanism used to achieve that goal. One cannot assume that the beneficial effect observed was causally related to the effect on albuminuria or that it would extend to other interventions. Most importantly, it is not known if the approach of maximizing therapy to reduce proteinuria is safe. Recent studies have shown that combining renin-angiotensin system therapies decreases albuminuria without significant clinical benefit but with increased risk of adverse events. More studies are needed, but at this time, albuminuria has not jumped the hurdle needed to be accepted as a surrogate end point or target for treatment. Primum non nocere, first do no harm.
Keywords: albuminuria, CKD, surrogate outcome, renin angiotensin, system blockade
Introduction
The question posed to us is whether albuminuria is an appropriate target for treatment. In other words, after the use of maximally tolerated recommended doses of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), if we decrease patients’ albuminuria by additional treatment, do we decrease the risk of ESRD? Should we be actively trying to decrease albuminuria? Our answer to this question is that we do not yet have sufficient data to ensure that we would be helping, not harming, our patients with this approach.
The hypothesis that albuminuria should be a primary treatment target came from trials of renin angiotensin blockade in patients with CKD who were proteinuric (1–3). Reductions of albuminuria were associated with lower risk of ESRD; additional reduction in albuminuria explained a large proportion of the benefit of renin-angiotensin system (RAS) blockade on the risk of progression (4,5). In the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) Study, the decline in proteinuria with losartan accounted for 100% of the benefit of ARB in reduced risk of doubling of serum creatinine and 50% of the reduction in risk of progression to ESRD (1). Residual albuminuria while on ARB was a risk factor for progression (5). Although these findings support albuminuria as a target/surrogate, they are not sufficient. The studies were not designed to test albuminuria as a target. In addition, the benefit associated with reduction in albuminuria with ACEI or ARB cannot be assumed to extend to other interventions. In an extreme illustration of this, drugs that markedly reduced GFR, such as nonsteroidal anti-inflammatory drugs, reduce proteinuria but would not prevent ESRD!
To illustrate these issues, we can look at two analogous issues in cholesterol studies: decreased levels of serum HDL cholesterol as a risk factor for adverse cardiovascular outcomes and the use of LDL cholesterol as a target for treatment.
Multiple cohort studies have found that low HDL cholesterol is associated with the risk of coronary artery disease (6,7). HDL is presumed to be protective through reverse cholesterol transport, and animal studies supported the role of HDL in atherosclerosis (8). Low HDL is one of the factors in the Framingham risk score (9) and the more recently developed pooled cohort equations (6), which are used to determine who should be treated with lipid-lowering medications. However, despite the associated risk and biologic plausibility of the importance of HDL, trials of interventions that raise HDL have not shown a cardiovascular benefit (10–13). In one of the studies, despite raising HDL by 72% and lowering LDL by 25%, torcetrapib was associated with increased mortality (10). This does not detract from HDL’s role as a predictor of cardiovascular disease but means that it may not be a target for treatment (8) or that current interventions that lower HDL have other unintended consequences that outweigh this potential benefit.
Similarly, until recently, LDL cholesterol was considered a target for therapy to prevent primary and secondary cardiovascular events. Statin medications lower LDL, and the LDL lowering correlates with the decrease in cardiovascular risk (14). This led to cholesterol treatment guidelines, including those by the Kidney Disease Outcomes Quality Initiative, with target LDL cholesterol levels (15,16). More recently, it was recognized that the studies that led to this recommendation were not designed to test LDL as a target, because the trials used fixed statin doses rather than doses targeted to a particular LDL level. Importantly, other treatments that lower LDL cholesterol, such as fenofibrate or niacin, do not decrease cardiovascular risk. This past year, the revised American Heart Association/American College of Cardiology and Kidney Disease Improving Global Outcomes lipid guidelines no longer target LDL but recommend moderate or high doses of statins depending on the underlying risk of cardiovascular disease (17,18). The recently presented Improved Reduction of Outcomes: Vytorin Efficacy International Trial compared simvastatin with simvastatin/ezetimibe in patients with acute coronary syndrome; the median LDL cholesterol was 69.9 versus 53.2, respectively, in the two groups. The use of simvastatin/ezetimibe led to a small but statistically significant reduction in cardiovascular events (19). This suggests that lowering of LDL further may be beneficial in high-risk individuals but in context of the negative niacin and fenofibrate studies, that each intervention needs to be specifically tested in a large outcome study to prove efficacy.
What Is Needed for Surrogate End Points?
In the Food and Drug Administration (FDA) guidance for exposure-response relationships, study design, data analysis, and regulatory applications, a number of terms are defined (Table 1): biomarkers, surrogate end points, and clinical benefit end points (20). A biomarker is defined as a “physiologic, pathologic or anatomic measure that is thought to relate to some aspect of normal or pathological biologic process” (20). A surrogate end point is a biomarker that is a “substitute for a clinically meaningful endpoint that is expected to predict the effect of the therapy” (20). Not all biomarkers are valid surrogate end points for clinical benefit, although they may have a role in drug development by assisting in understanding the drug’s mechanism of action, patient selection for studies, and evaluating potential risks and benefits. There are few biomarkers that the FDA currently accepts as valid surrogates for regulatory drug approval (e.g., BP reduction).
Table 1.
Definition of end points
| Term | Definition |
|---|---|
| Biomarker | Physiologic, pathologic, or anatomic measure that is thought to relate to some aspect of normal or pathologic biologic process |
| Surrogate end point | Biomarker that is a laboratory measurement or physical sign used in therapeutic trials as a substitute for a clinically meaningful end point that is expected to predict the effect of the therapy |
| Clinical benefit end point | Variable that reflects how a patient feels, functions, or survives |
Reprinted from reference 20, with permission.
What is needed to validate a surrogate end point is controversial, and there have been a number of different proposals (21). As discussed by Fleming and Powers (22), to validate a surrogate biomarker, one should have a comprehensive understanding of (1) the principal pathways through which the disease process affects the clinical end point, (2) the extent to which effects on the biomarker capture the meaningful on-target effects of the intervention on these causal pathways, and (3) any off-target effects of the intervention that are not captured by the biomarker (for both efficacy and safety).
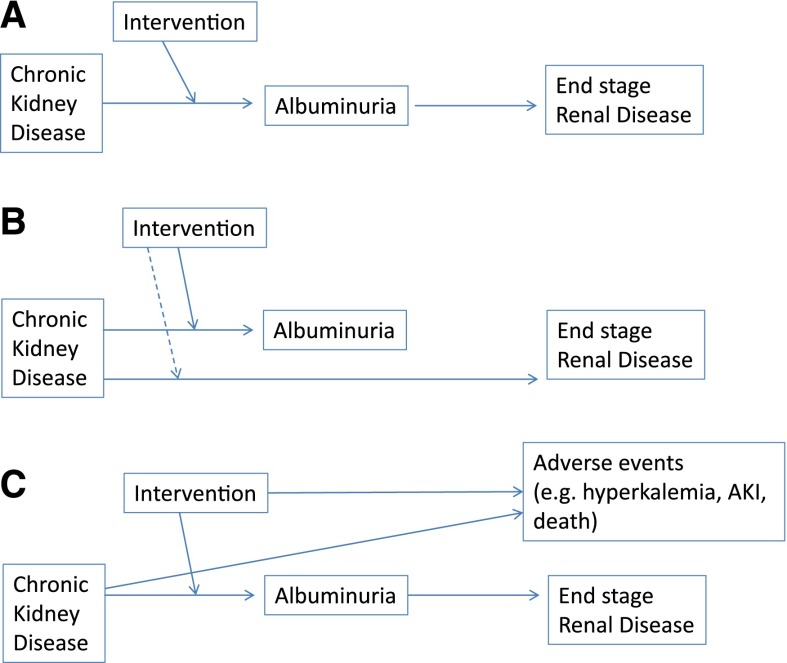
A valid surrogate outcome should be in the causal pathway, where the disease leads to a clinical outcome, and an intervention should be mediated through its effect on the surrogate (23). The best surrogate marker is on the only causal pathway to the disease (Figure 1). Furthermore, it is pointed out that a biomarker as a surrogate is context specific and cannot be assumed to be a general biomarker (22). That is, if one were to validate a biomarker in one setting (e.g., diabetic kidney disease) or intervention (RAS blockade), it cannot be assumed to be valid as a biomarker for efficacy or safety for other interventions or diseases (22). In addition, you cannot assume that, if the biomarker is validated in individuals with diabetes and an albumin/creatinine ratio >300 mg/g, you can extrapolate to lower levels of albuminuria.
Figure 1.
Albuminuria as a surrogate. (A) If this is the true scenario, this would provide the best support for albuminuria as a target. Albuminuria would be in the causal pathway from CKD to ESRD, and the intervention would be mediated through its effect on albuminuria. (B) This illustrates other situations where albuminuria could fail as a surrogate. There are other pathways from CKD to ESRD, which may or may not be affected by an intervention, or albuminuria is not in the causal pathway to ESRD. (C) This illustrates a third issue: that there are significant safety effects of the intervention that lead to an adverse risk to benefit ratio, even if the intervention affects the surrogate and the clinical end point. Adapted from reference 23, with permission from the American College of Physicians.
The reason for this vigor in validation is that there are a number of cases where proposed surrogates have failed for either efficacy or safety when tested in larger studies. The prior cases also highlight the inability to extend a biomarker from one intervention to another and the need to understand the biology of how an intervention acts on a surrogate and the clinical outcome. As an illustration, we can look at bone mineral density (BMD). Low BMD predicts the risk of fracture (24). Bisphosphonates increased BMD and decreased the risk of vertebral and nonvertebral fractures (25), leading to the conclusion that BMD is an appropriate surrogate outcome for trials to prevent fracture (26). However, in subsequent studies using fluoride, which increased BMD, there was an increase in nonvertebral fractures (27). This is because there was a decrease in bone strength, despite the increase in BMD. BMD is used in the diagnosis of osteoporosis, predicts risk of future events, and may be useful as a marker of who should receive treatment, but it may not be a good surrogate for interventions. This is analogous to the situation with albuminuria, where persistent albuminuria is in the definition of CKD, albuminuria predicts risk of ESRD, and albuminuria indicates a population that benefits from RAS blockade but has not met the hurdle of being a good surrogate/target for interventions.
Why Is the Evidence Not Sufficient for Albuminuria?
Higher levels of albuminuria predict a greater risk of ESRD. Individuals with an eGFR≥60 ml/min per 1.73 m2 and an albumin-to-creatinine ratio ≥300 mg/g have an 18- to 67-fold higher risk of ESRD than individuals without albuminuria (28). A prediction model of the 2-year risk of ESRD in individuals with CKD includes log urine albumin in addition to age, sex, eGFR, serum albumin, and chemistries (29). Indeed, albuminuria is a more powerful predictor of renal progression than the creatinine itself. However, although albuminuria increases the risk of developing ESRD, many individuals reach ESRD without ever developing albuminuria. Thus, it is not a necessary step in the causal pathway from CKD to ESRD. This is in contrast to the currently accepted surrogate of doubling of serum creatinine. One cannot reach ESRD without a severe decline in GFR, which is evidenced by an increase in serum creatinine.
At this time, ACEI and ARB are the most effective treatments to reduce progression of kidney disease in individuals with albuminuria. The use of an ACEI or an ARB decreases progression of kidney disease by 25%–50% (1,2,30,31). The reduction in albuminuria correlates with decreased risk (3–5). Does this mean that the decline in albuminuria is the reason that ACEIs or ARBs are effective? Not necessarily. The medications could be affecting albuminuria and risk of ESRD through different paths (Figure 1). In the RENAAL Study, losartan decreased albuminuria on average in the group as a whole by 28%, but the albuminuric response to losartan was highly variable across individual participants, with some individuals decreasing by >60% and other individuals having increasing albuminuria levels (5). It may be that reduction in albuminuria is a sign of responsiveness to RAS blockade, but the actual mediator and mechanism of benefit are not related to change in albuminuria. If that is the case, other interventions that reduce albuminuria would not necessarily decrease risk. This would be analogous to the statin and LDL example above, where other interventions that decrease LDL do not decrease cardiovascular events. Simplistically, severity of cough correlates with the severity of pneumonia, and cough improves with antibiotics; however, codeine would suppress the cough and not treat the pneumonia.
Studies, including the RENAAL, the Irbesartan Diabetes Nephropathy Trial, and the Benazapril for Advanced Renal Disease Study, used fixed doses of an ACEI or an ARB (1,2,32). They were not designed to test whether proteinuria should be a target. There is one study that did aim to test proteinuria as a target. The Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study randomized 360 individuals with proteinuria (>1 g/d) and nondiabetic kidney disease to conventional doses (50 mg losartan or 10 mg benzapril) or doses titrated to maximal proteinuria lowering (10–40 mg benazepril or 50–100 mg losartan) (33). Individuals who did not respond in the titration group (reduction in albuminuria <10%) were maintained on 50 mg losartan (7%) or 10 mg benazepril (6%). The mean dose of benazepril was 20.8 mg, with 24% requiring >20 mg; the mean dose of losartan was 117.7 mg, with 29% requiring >100 mg. The risk of doubling of serum creatinine, ESRD, or death was approximately one half in the titrated RAS blockade group. This would seem to suggest that titrating to albuminuria would be beneficial. However, it is important to note that the doses chosen for the control conventional group were one half of the doses used in the RENAAL Study (100 mg losartan) (1) and the Benazapril for Advanced Renal Disease Study (20 mg benazepril) (32). The final optimal dose for the majority of participants in the ROAD Study was close to the doses used in these studies. Therefore, an alternative interpretation of the ROAD Study is that it compared suboptimal dosing with the previously shown effective doses. Indeed, the findings are confounded by the dosing of the RAS blockers.
If albuminuria was a surrogate end point that could be used for all interventions, interventions that decrease albuminuria should decrease risk of ESRD, whereas interventions that increase albuminuria should increase the risk. The former is difficult to evaluate, because with the exception of ACEIs and ARBs, we do not have interventions that have been shown to decrease the risk of ESRD. However, both loop and thiazide diuretics decrease albuminuria when used with RAS agents (34,35) but have not been shown to decrease progression. The Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension Study randomized 11,506 individuals with hypertension and increased cardiovascular risk to benazepril plus hydrochlorthiazide or benazepril plus amlodipine (36). The risk of doubling of serum creatinine, ESRD, or death was lower in the benazepril plus amlodipine group (0.73; 95% confidence interval, 0.64 to 0.84), despite greater proteinuria lowering in the benazepril and hydrochlorthiazide group. In contrast, in the Bardoxolone Methyl Evaluation in Patients with CKD and Type 2 Diabetes Mellitus: The Occurrence of Renal Events Study, bardoxolone increased albuminuria and GFR; although there was an increase in the risk of cardiovascular death and heart failure with bardoxolone, there was not an increased risk of ESRD (hazard ratio, 0.82; 95% confidence interval, 0.55 to 1.24) (37). Avosentan, an endothelin inhibitor, decreased proteinuria but led to an increased risk of heart failure (38). Therefore, there is a separation between change in proteinuria with interventions and risk of cardiovascular events.
An advantage to surrogate end points is that they require smaller numbers of individuals and shorter studies to determine efficacy. However, smaller and shorter-term studies may not give adequate information regarding safety (39). In the Aliskiren in the Evaluation of Proteinuria in Diabetes Study, 599 individuals with diabetes and proteinuria were treated with 100 mg losartan and randomized to aliskiren or placebo for 6 months. Proteinuria was reduced in the aliskiren group by 20%, and twice as many individuals halved their proteinuria with combination therapy compared with monotherapy (24.7% versus 12.5%) (40). There was no difference in the overall event rate in the two arms. This led to the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints Study, which tested the addition of aliskiren to underlying ACEI or ARB in 21,157 individuals with diabetes and kidney disease (41). The study was stopped early, because there was greater risk of adverse events that could not be offset by a reduction in cardiovascular events or renal progression (safety and futility). This is despite the greater reduction in albuminuria with aliskiren. Similarly, in both the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial and the Veterans Affairs Nephropathy in Diabetes Study, there was an increased risk of adverse events, including hyperkalemia, hospitalization, and AKI, without a reduction in progression or cardiovascular events; in both studies, albuminuria was decreased by combination therapy (42,43). From a clinical point of view, how could you target albuminuria after treating with appropriate doses of an ACEI or an ARB? Combination therapy of ACEI plus ARB is associated with a higher risk of adverse events. Loop or thiazide diuretics have not been shown to be beneficial. Mineralocorticoid receptor blockers decrease risk, but in the work by Mehdi et al. (44), the addition of spironolactone to 80 mg lisinopril in patients with diabetes and proteinuria significantly decreased proteinuria but caused hyperkalemia >6 mEq/L in 50% of individuals (44,45). Lower BP may be beneficial in individuals with proteinuria (46). Lowering systemic BP to below currently recommended goals is being studied and could result in further albuminuria reductions, but the safety of this is in question and was not demonstrably beneficial in patients with type 2 diabetes in the Action to Control Cardiovascular Risk Study (47). Thus, the potential available additional therapies to reduce albuminuria are of unproven benefit and may decrease safety.
In summary, if we are to look critically at the albuminuria data, we should conclude that (1) albuminuria is a predictor of progression of kidney disease, (2) ACEIs or ARBs, which lower albuminuria, decrease risk, but studies were not designed to test whether albuminuria itself was an appropriate target, and (3) we cannot assume that all treatments that lower albuminuria will decrease the risk of progression of kidney disease. At this time, we should conclude that albuminuria is, therefore, a biomarker but not a surrogate end point or target for treatment. We may be doing our patients more harm than good by targeting albuminuria. However, we await additional studies to prove or disprove this hypothesis.
Disclosures
None.
Acknowledgments
The opinions expressed in this article are those of the authors and do not necessarily represent those of the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Perna A, Remuzzi G; GISEN Group Investigators: Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ: Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 45: 281–287, 2005 [DOI] [PubMed] [Google Scholar]
- 5.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr., Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129[Suppl 2]: S49–S73, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Sacks FM: Why cholesterol as a central theme in coronary artery disease? Am J Cardiol 82[10B]: 14T–17T, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Rader DJ, Hovingh GK: HDL and cardiovascular disease. Lancet 384: 618–625, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation 97: 1837–1847, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators: Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357: 2109–2122, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W; AIM-HIGH Investigators: Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365: 2255–2267, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr., Cushman WC, Simons-Morton DG, Byington RP; ACCORD Study Group: Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362: 1563–1574, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM; ILLUSTRATE Investigators: Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 356: 1304–1316, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists’ (CTT) Collaborators: Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366: 1267–1278, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease Outcomes Quality Initiative (K/DOQI) Group: K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease. Am J Kidney Dis 41[4 Suppl 3]: I–IV, S1–S91, 2003 [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr., Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129[Suppl 2]: S1–S45, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members: KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int 85: 1303–1309, 2014 [DOI] [PubMed] [Google Scholar]
- 19.IMPROVE IT-LBCT: IMPROVE IT-LBCT Final. Available at: clinicaltrialresults.org/Slides/AHA2014/Cannon_IMPROVEIT.ppt. Accessed December 17, 2014.
- 20.Food and Drug Administration: Guidance for Industry, Exposure-Response Relationships—Study. Design, Data Analysis, and Regulatory Applications. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072109.pdf. Accessed October 6, 2014.
- 21.Lassere MN: The Biomarker-Surrogacy Evaluation Schema: A review of the biomarker-surrogate literature and a proposal for a criterion-based, quantitative, multidimensional hierarchical levels of evidence schema for evaluating the status of biomarkers as surrogate endpoints. Stat Methods Med Res 17: 303–340, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Fleming TR, Powers JH: Biomarkers and surrogate endpoints in clinical trials. Stat Med 31: 2973–2984, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming TR, DeMets DL: Surrogate end points in clinical trials: Are we being misled? Ann Intern Med 125: 605–613, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Johnell O, Kanis J: Epidemiology of osteoporotic fractures. Osteoporos Int 16[Suppl 2]: S3–S7, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr., Dequeker J, Favus M; The Alendronate Phase III Osteoporosis Treatment Study Group: Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333: 1437–1443, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Wasnich RD, Miller PD: Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 85: 231–236, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Riggs BL, Hodgson SF, O’Fallon WM, Chao EY, Wahner HW, Muhs JM, Cedel SL, Melton LJ, 3rd: Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 322: 802–809, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P; The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW: Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, Guo ZJ, Jiang JP: Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: A randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 18: 1889–1898, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM: Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 16: 474–481, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V, Chiang YT, Weber MA; ACCOMPLISH Trial investigators: Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): A prespecified secondary analysis of a randomised controlled trial. Lancet 375: 1173–1181, 2010 [DOI] [PubMed] [Google Scholar]
- 37.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM; BEACON Trial Investigators: Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G; ASCEND Study Group: Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeffer MA, Sacks FM: Leapfrogging data: No shortcuts for safety or efficacy information. Circulation 118: 2491–2494, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK; AVOID Study Investigators: Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA; ALTITUDE Investigators: Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators: Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET investigators: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD: Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 20: 2641–2650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Buren PN, Adams-Huet B, Nguyen M, Molina C, Toto RD: Potassium handling with dual renin-angiotensin system inhibition in diabetic nephropathy. Clin J Am Soc Nephrol 9: 295–301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appel LJ, Wright JT, Jr., Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group: Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cushman WC, Evans GW, Byington RP, Goff DC, Jr., Grimm RH, Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group: Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]