Abstract
The presence of elevated levels of albuminuria is associated with an increased risk of progressive renal function loss over time. This association is found in various pathophysiological conditions, including diabetic nephropathy, hypertensive nephropathy, and various primary renal diseases, but also, the general, otherwise healthy population. Emerging data report that elevated albuminuria causes tubulointerstitial damage through activation of proinflammatory mediators, which ultimately leads to a progressive decline in renal function. Nowadays, various drugs are available that decrease the rate of GFR loss in patients with kidney disease. Well known are renin-angiotensin-aldosterone system inhibitors, but there are also other drugs and interventions, like intensive glucose control, anti-inflammatory agents (pentoxifylline), or a low-protein diet. These interventions have an additional effect beyond their original target, namely lowering albuminuria. Analyses from clinical trials show that the reduction in albuminuria observed during the first months of treatment with these drugs correlates with the degree of long-term renal protection: the larger the initial reduction in albuminuria, the lower the risk of ESRD during treatment. In addition, in treated patients, residual albuminuria is again the strongest risk marker for renal disease progression. These observations combined provide a strong argument that albuminuria is an appropriate therapeutic target in patients with CKD.
Keywords: albuminuria, CKD, urinary protein
Introduction
Measurement of urinary proteins became practice in nephrology already more than two centuries ago. Hermann Senator, a German physician, conducted important studies, in which he described that proteins could be detected in urine of otherwise healthy individuals and that this was a sign of CKD; he even provided suggestions for treatment (1). Shortly thereafter, it was shown that proteinuria in such individuals was associated with adverse health outcomes (2).
The pioneering work from Hermann Senator has since been confirmed in many large-scale observational studies in various populations. It is nowadays well known that leakage of even small amounts of albumin in the urine is one of the earliest signs of asymptomatic kidney damage. These small amounts of albumin convey an important message of what will happen with the kidney in the future: in the case that the urinary albumin concentration is increased, the chance of progressive renal function loss is significantly increased. More important than only establishing the presence of renal risk is the question of whether we can also modify this risk. Whereas albuminuria was traditionally viewed as merely a reflection of renal damage, recent studies have shown that it is also implicated in the causal pathway of progression of kidney disease. This means that albuminuria can be considered a modifiable risk factor and that targeting and lowering of albuminuria will lead to renoprotection. This paradigm shift has caused and is still causing a lot of debate among nephrologists.
In this article, we will review the evidence that albuminuria is a valid target for renoprotective therapy. Certain criteria should be met before one can accept albuminuria as an appropriate therapeutic target for renoprotective treatment (3). First, a biologic plausible explanation should exist as to how albuminuria causes renal damage. Second, evidence of a strong and consistent association between the level of albuminuria and renal end points during follow-up must be available. Third, clinical trial data must show that the effects of interventions that change albuminuria are directly associated with the same effects on these clinical end points. In this review, we will discuss the evidence that shows that these three criteria are met, and we will close by performing an assessment of the validity of albuminuria as a therapeutic target according the updated Biomarker Surrogacy Evaluation Schema for evaluating the validity of biomarkers as surrogate end points (4). The use of albuminuria as a cardiovascular risk marker and target for cardioprotective treatment is discussed elsewhere and will not be reviewed in this article (5).
Albumin Leakage Causes Renal Damage
Albuminuria has traditionally been viewed as merely a marker of renal damage. Indeed, damage to the glomerular filtration barrier leading to impaired size and charge selectivity results in increased albuminuria leakage. However, regardless of the origin of albumin leakage, emerging data show that albuminuria also has a direct toxic effect on renal tissue, leading to progressive function loss (6,7).
The mechanisms by which increased albuminuria causes or accelerates kidney damage involve multiple pathways that ultimately culminate in tubulointerstitial damage (8). Several studies have indicated that changes in the tubulointerstitial tissue compartment are a prominent feature of the pathophysiologic processes that lead to progression of kidney disease. First, the clinical kidney outcome of ESRD, for which dialysis or a renal transplantation is needed, is predicted by the severity of glomerular lesions but more strongly predicted by the severity of tubulointerstitial damage (tubular atrophy, interstitial inflammation, and interstitial fibrosis) (9). Second, numerous in vitro and in vivo studies have reported that increased glomerular albumin leakage stimulates proinflammatory and profibrotic signals that directly contribute to tubulointerstitial damage. Under normal physiologic conditions, the small amount of albumin that is filtered by the glomeruli is efficiently reabsorbed in the tubuli (10,11). However, in conditions of increased glomerular albumin leakage, the tubuli are exposed to increased albumin concentrations. Exposure of the tubuli to an overload of albumin triggers a toxic effect and inflammatory response (10,11). A large part of these deleterious effects seems to be mediated by the tubular uptake of albumin. The proximal tubule brush border reabsorbs albumin through the megalin and cubulin receptors (12–16). After it is internalized in endosomal vesicles, albumin dissociates from the cubulin-megalin complex and is transported to dendritic cells for the generation of antigenic peptides that elicit an inflammatory response (17). In vitro studies, indeed, showed that uptake of high concentrations of albumin exerts cytotoxic effects on proximal and distal tubular cells by activating a wide array of intracellular signaling pathways (e.g., extracellular-regulated kinase, NF-κB, and protein kinase C) (18–21). Activation of these signaling pathways, in turn, induces the release of inflammatory (monocyte chemotactic protein-1) (22,23), vasoactive (reactive oxygen species and endothelin) (24–26), fibrotic (TGF-β and collagens) substances (27–29), causing interstitial damage, tubulointerstittial dysfunction, and fibrosis and ultimately, leading to irreversible kidney damage. Thus, albuminuria is not solely a marker of the extent of glomerular damage but has a direct pathogenic effect precipitating renal function loss. It should be noted that, other than albumin itself, substances bound to albumin (such as free fatty acids), other proteins (such as proteins that form the complement system), and glycated albumin can also act as profibrotic and proinflammatory stimuli and aggravate tubular damage (30).
Albuminuria Predicts Renal Outcome
It is nowadays well established that higher levels of albuminuria precede and predict a faster rate of renal function decline and increased risk of ESRD (as well as cardiovascular disease) in various pathophysiologic conditions, such as diabetes, hypertension, and primary glomerular diseases but also, the general, otherwise healthy population (31,32). Importantly, large meta-analyses have shown that there is no lower threshold below which the association between albuminuria and renal outcomes plateaus (33). This indicates that even subtle increases in albuminuria within the normalbuminuria range still confer increased renal risk. A number of interesting observations have been made in the last few years.
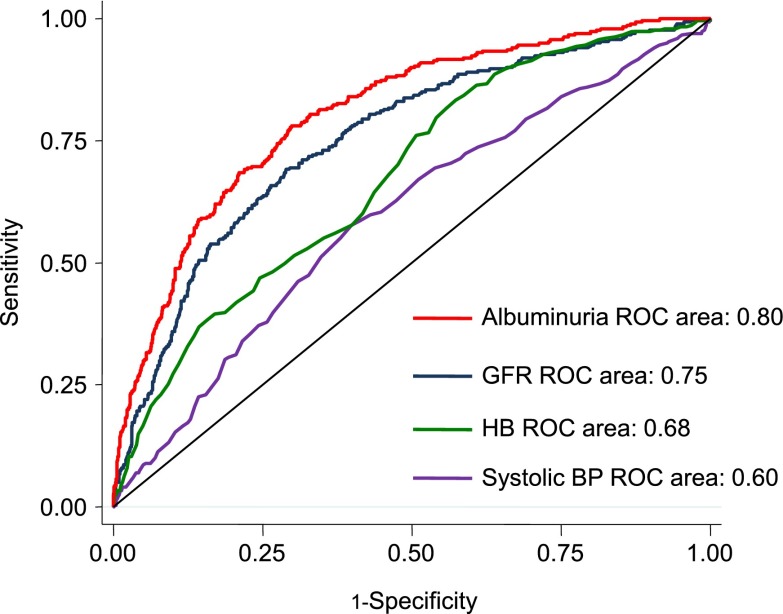
Among patients with diabetes and nephropathy, albuminuria consistently seems to be the strongest risk marker of ESRD. In patients with diabetic nephropathy in the Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) Trial (mean eGFR of 40 ml/min per 1.73 m2 and median albuminuria of 1246 mg/g), the area under the receiver operator characteristic curve for ESRD was significantly higher for albuminuria than for eGFR or any other clinical characteristic (Figure 1). Not only is albuminuria a very strong (if not strongest) renal risk marker among all clinical characteristics, it also stands out against novel renal risk markers. A recent prospective observational study in patients with type 2 diabetes and CKD reported that multiple novel renal risk markers predicted the progression of renal disease (34). However, when Agarwal et al. (34) adjusted their analyses for albuminuria, only fibroblast growth factor-23 remained statistically significantly associated with renal outcome. This indicates that a large part of the renal risk predicted by these novel renal risk markers can be explained by their association with albuminuria. Finally, in the Nord-Trøndelag Health Study, a large general population cohort study, albuminuria alone predicted ESRD risk significantly better than a clinical risk prediction score consisting of multiple risk factors, including age, sex, physical activity, diabetes, systolic BP, antihypertensive medication, and HDL cholesterol (35). Thus, albuminuria is a strong risk predictor for renal outcome and performs significantly better than any other clinically available renal risk marker.
Figure 1.
Area under the receiver operating characteristic (ROC) curve for prediction of ESRD of different renal risk markers in patients with diabetic nephropathy. HB, hemoglobin.
Some studies have shown that renal function can also decline in patients in the absence of micro- or macroalbuminuria, thereby challenging the paradigm of albuminuria-associated renal disease progression. The fact that some patients develop impaired renal function in the absence of micro- or macroalbuminuria is not surprising. Like hypertension, which is not the sole determinant of atherosclerotic disease progression, albuminuria is not the sole determinant of renal disease progression. This, however, does not disqualify BP and albuminuria as valid treatment targets. Indeed, many other risk factors have been implicated in the progression of renal disease, including high BP, serum uric acid, and blood glucose. Studies that report that a fraction of patients have progressive renal function loss without developing micro- or macroalbuminuria should, therefore, not be interpreted as evidence against the key role of albuminuria in initiating and accelerating renal damage. What matters is that, when high albuminuria is present, renal risk is significantly increased. Indeed, comparing patients with diabetes and CKD who are normo-, micro-, or macroalbuminuric, those with normoalbuminuria had a markedly stable GFR trajectory during prolonged follow-up, and none progressed to dialysis or died. In contrast, 26% of the patients with macroalbuminuria started dialysis, and 18% of patients died (36).
A Drug-Induced Reduction in Albuminuria Predicts a Reduction in Renal Outcome
A biologic plausible relation between a biomarker and renal disease progression and a strong association between a biomarker and renal outcome do not necessarily imply that the biomarker is a valid target for treatment. In the past, multiple seemingly promising biomarkers failed to be valid targets for treatments, because clinical trials showed a beneficial effect on the biomarker but no effect on clinical outcomes (37,38). The requirement that a drug-induced change in albuminuria predicts a change in renal outcome in a similar direction is, therefore, an important (if not the most important) criterion for validation of albuminuria as a treatment target.
Before we review the evidence that albuminuria reduction is associated with renoprotection in patients with CKD, we note that recent Kidney Disease Improving Global Outcomes guidelines already recommend targeting proteinuria. For instance, it is advised that patients with immunoglobin A nephropathy who have persistent proteinuria of >1 g/d despite 3–6 months of optimized supportive care receive a 6-month course of corticoidsteroid therapy to control proteinuria and improve renal prognosis (39). In addition, the same guideline states that patients with idiopathic membranous nephropathy should receive treatment with immunosuppressive agents when urinary protein excretion during supportive care persistently exceeds 4 g/d and remains at >50% of the baseline value. In light of these guidelines, it is remarkable that we continue to debate the validity of albuminuria as a therapeutic target in patients with CKD.
The renoprotective effects of drugs intervening in the renin-angiotensin-aldosterone system (RAAS) are well established. In addition, multiple studies in a variety of diseases and populations have shown that the initial reduction in albuminuria with these drugs correlates with the reduction in risk for renal disease progression (Table 1). These consistent findings suggest that targeting albuminuria confers renoprotection.
Table 1.
Short-term changes in albuminuria and subsequent renoprotection in different clinical trials
| Study | Population | eGFR (ml/min per 1.73 m2) | Proteinuriaa/ Albuminuria | Intervention | Renal End Point | Renal Risk Reduction in Albuminuria |
|---|---|---|---|---|---|---|
| RENAAL (54) | Type 2 diabetes and nephropathy | 40 | 1246 mg/g | Losartan versus placebo | Doubling serum creatinine, ESRD, or death | For each halving of albuminuria during the first 6 months, the risk of ESRD was statistically significantly reduced by one half |
| IDNT (64) | Type 2 diabetes and nephropathy | 47 | 1500 mg/g | Irbesartan versus Amlodipine versus placebo | Doubling serum creatinine or ESRD | For each halving of albuminuria during the first 12 months, the risk of kidney failure was statistically significantly reduced by more than one half (56%) |
| AASK (65) | Hypertensive nephrosclerosis | 46 | 80 mg/ga | Ramipril versus Metoprolol | ESRD | For each halving of albuminuria during the first 6 months, the risk of ESRD was statistically significantly reduced by more than one half (53%) |
| ROAD (55) | IgA nephropathy | 31 | 1800 mg/24 ha | Losartan or Benazepril | Doubling serum creatinine, ESRD, or death | Renal risk was 80% lower among subjects with a >50% reduction in proteinuria compared with those with a <25% reduction in proteinuria |
| REIN (66) | Nondiabetic nephropathy | 43 | 3500 mg/24 ha | Ramipril versus placebo | GFR decline | GFR decline was significantly slower in patients with a month 3 reduction in proteinuria (−0.28 ml/min per 1.73 m2 per month) versus patients without reduction in proteinuria at month 3 (−0.54 ml/min per 1.73 m2 per month) |
| IRMA-2 (67) | Type 2 diabetes and microalbuminuria | 72 | 54 µg/min | Irbesartan versus placebo | eGFR decline | eGFR decline was 1.1 ml/min per 1.73 m2 among subjects with a >50% decrease in albuminuria; eGFR decline decreased by 2.6 ml/min per 1.73 m2 among subjects with a >34% increase in albuminuria |
| ONTARGET (68) | High cardiovascular risk | 69 | 7 mg/g | Ramipril versus Telmisartan versus Ramipril and Telmisartan | Doubling serum creatinine or ESRD | A 2-fold decrease in albuminuria associated with a 27% relative renal risk reduction compared with no change in albuminuria |
| MDRD (51) study A | Nondiabetic nephropathy | 39 | 200 mg/24 ha | Low- versus usual protein diet | An initial reduction in proteinuria of 1.0 g/d was associated with a statistically significant 0.9-ml/min per year slower GFR decline during subsequent follow-up | |
| MDRD (51) study B | Nondiabetic nephropathy | 19 | 700 mg/24 ha |
RENAAL, Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan; IDNT, Irbesartan Diabetic Nephropathy Trial; AASK, African-American Study of Kidney Disease and Hypertension; ROAD, Renoprotection of Optimal Antiproteinuric Doses; IgA, immunoglobin A; REIN, Ramipril Efficacy in Nephropathy; IRMA-2, Irbesartan Microalbuminuria Type 2 Diabetes in Hypertensive Patients 2; ONTARGET, Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial; MDRD, Modification of Diet in Renal Disease.
Trials with proteinuria measurements.
Because the majority of the studies tested the effect of RAAS inhibition, one could raise the question of whether the reduction in albuminuria secondary to RAAS inhibition is driving renoprotection or whether inhibition of the RAAS per se is renoprotective. Experimental and human studies have provided important insights into this issue. Kramer et al. (40) treated proteinuric rats with the angiotensin converting enzyme inhibitor lisinopril and high- or low-salt diets. At the start of treatment, renal structural damage as assessed by focal glomerular sclerosis was negligible in the high- and low-salt lisinopril treatment groups. Six weeks of treatment with lisinopril in the low-salt group resulted in a significant fall in albuminuria, whereas albuminuria progressively increased in the high-salt lisinopril group. Importantly, focal glomerular sclerosis remained negligible in the low-salt lisinopril group but significantly worsened over time in the high-salt lisinopril group. Thus, inhibition of the RAAS does not improve renal structural damage if albuminuria is not reduced.
Similar data are available in humans. In a post hoc analysis of the RENAAL Trial and the Irbesartan Diabetic Nephropathy Trial, the renoprotective effects of angiotensin receptor blockers (ARBs) were assessed with the study population stratified according to tertiles of sodium intake (assessed by 24-hour urinary sodium excretion). A statistically significant interaction was observed between ARB treatment and sodium intake. In the placebo arm, no differences in renal outcomes across tertiles of sodium intake were noted (41). In contrast, patients treated with ARBs in the lower tertile of sodium intake showed the largest reduction in albuminuria (−44% in the lowest tertile versus −21% in the highest tertile of sodium intake) and the lowest percentage of renal events defined as the occurrence of ESRD or doubling of serum creatinine (23% in the lowest tertile versus 37% in the highest tertile of sodium intake) (Figure 2). These observations indicate that low-salt intake by itself does not improve renal outcome and also, that an ARB by itself does not improve renal outcome per se. Only in the case that an ARB is combined with a moderate salt intake is albuminuria reduced and renal prognosis improved. Similar results were observed in an analysis of patients with nondiabetic nephropathy who were treated with the ACE inhibitor ramipril. In that study, proteinuria remained high in the upper tertile of sodium intake, despite treatment with ramipril. After 4 years of follow-up, 60% of the patients in the highest tertile and 20% of the patients in the lowest tertile had reached the renal end point of dialysis or doubling of serum creatinine (42). This effect was independent of BP and related to persistence of proteinuria in subjects with high sodium intake. Thus, in line with experimental data, intervention in the RAAS in patients with diabetic as well as nondiabetic nephropathy does not confer renoprotection in the absence of a reduction in albuminuria.
Figure 2.
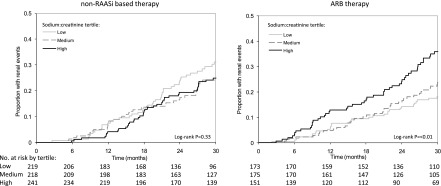
Risk of renal events depends on dietary sodium intake during ARB therapy. Kaplan–Meier curves according to tertiles of salt intake in patients who did not receive treatment intervening in the renin-angiotensin-aldosterone system (RAAS) (left panel) or received ARBs (right panel). RAASi, renin-angiotensin-aldosterone system inhibition; ARB, angiotensin-receptor blocker.
A limitation of the studies listed in Table 1 is that they are post hoc analyses of individual clinical trials. Another approach is to analyze multiple trials in a meta-analysis and correlate the placebo-adjusted treatment effect on albuminuria with the placebo-adjusted treatment effect on ESRD. Recently, two such meta-analyses have been published. The first meta-analysis included 32 small and large clinical trials published before 2007 and found that, for different drug classes, the direction of the treatment effect on initial change in albuminuria agreed with the direction of the treatment effect on long-term renal outcome (43). However, for many of the included studies, statistical power was insufficient to determine whether treatment effects on proteinuria associated with treatment effects on clinical outcome, and therefore, no definitive overall conclusion could be drawn. A more recent meta-analysis tried to avoid this methodologic shortcoming by including only randomized clinical trials (up to 2014) with >1000 patient-years of follow-up or 50 ESRD events and determined the effect of different interventions on albuminuria and ESRD. This meta-analysis included 21 clinical trials involving 78,342 patients (44). A strong correlation was observed between the early treatment effect on albuminuria and the long-term treatment effect on ESRD: for each 30% short-term reduction in albuminuria, the risk of ESRD during subsequent follow-up decreased by 23.7% (P=0.001). The strength of the association between reductions in albuminuria and ESRD was comparable for trials that tested the effect of interventions in the RAAS versus other interventions, such as lipid-lowering therapies, low-protein diet, or sulodexide.
Apart from meta-analyses of clinical trials, direct evidence from a prospective randomized, controlled clinical trial is available showing that targeting albuminuria confers renoprotection. In a trial that included patients with nondiabetic nephropathy and high albuminuria, Hou et al. (45) randomized patients either to a BP-lowering dose of an ACE inhibitor (benazepril) or ARB (losartan) or a dose of these agents uptitrated to achieve a maximal albuminuria-lowering effect. The maximal antialbuminuric dose of either of these agents resulted in additional albuminuria reduction without additional BP lowering and led to a marked reduction in the risk of ESRD of 50% during 3.7 years of follow-up. Targeting of albuminuria, thus, confers renoprotection independent of BP. These prospective findings are of great clinical importance, because they are the ultimate evidence that targeting albuminuria indeed optimizes renoprotection. To further strengthen the case, these findings should be replicated, especially in populations with lower-grade albuminuria.
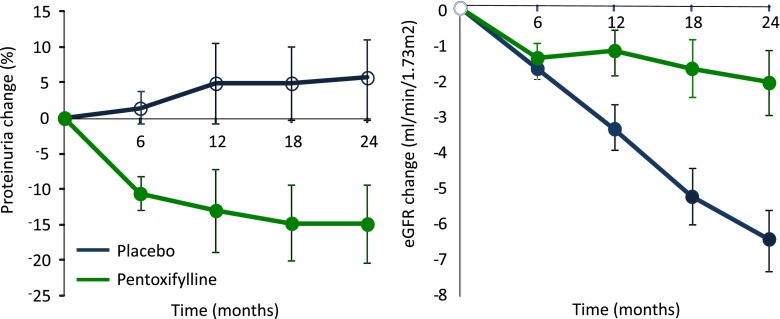
Are RAAS inhibitors the only drugs that decrease albuminuria and slow the progression of renal disease? In the last couple of years, several studies have shown that other drugs also decrease albuminuria and are renoprotective. Pentoxifylline, a xanthine derivative that has been in clinical use since 1971 to treat patients with peripheral arterial insufficiency, seems to possess anti-inflammatory properties by inhibiting TNF-α production. A recent randomized, controlled trial in patients with type 2 diabetes and nephropathy showed that pentoxifylline decreased albuminuria in the first months of treatment, which was sustained over time, and led to a significantly slower rate of eGFR decline during 2 years of follow-up compared with placebo (Figure 3) (46).
Figure 3.
Effect of pentoxifylline versus placebo on albuminuria and eGFR in patients with diabetic nephropathy.
Intensive glucose control decreases the transition from normo- to microalbuminuira and from micro- to macroalbuminuria in patients with type 1 or type 2 diabetes. Long-term follow-up of these trials showed that intensive glucose control decreases the risk of new-onset CKD and ESRD (47–49). Importantly, in the Diabetes Control and Complications Trial, the beneficial effect of intensive glucose control was completely annihilated after statistical adjustment for change in albuminuria. This effect suggests that the biologic mechanism through which intensive glucose control exerts its beneficial effect on eGFR decline is mediated by its albuminuria-reducing effect.
Dietary protein restriction is another strategy to decrease proteinuria and slow the progression of renal disease. Patients assigned to the low-protein diet arm in the Modification of Diet in Renal Disease Trial had a greater reduction in proteinuria and a slower rate of long-term GFR decline after excluding the initial acute effect of the intervention on GFR (50,51).
Finally, some statins can also decrease albuminuria. In the Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease Trials, the renoprotective effects of atorvastatin and rosuvastatin were compared head to head in patients with proteinuria. During 12 months of follow-up, proteinuria was significantly reduced from baseline with atorvastatin but not rosuvastatin. In addition, eGFR decline was significantly smaller in the atorvastatin arm compared with rosuvastatin arm (52).
Although each of these individual strategies helps in slowing the progression of CKD, a multifactorial approach targeting proteinuria with different interventions will likely be most beneficial. Such an approach has been developed and implemented at the Mario Negri Institute in Italy. To test the efficacy of this approach, a study was performed in which the renal outcomes of patients who participated in the multifactorial program and had targeted proteinuria were compared with renal outcomes in matched historical reference subjects who had received conventional therapy titrated to a target BP. This study showed that multidrug treatment titrated to urinary protein level can be safely and effectively applied to normalize proteinuria and slow the loss of renal function (53).
Finally, during renoprotective treatments, the residual albuminuria level remains the strongest risk marker of renal disease progression in both diabetic and nondiabetic renal disease, like the albuminuria level in untreated patients (54,55). These observations combined provide a compelling argument that albuminuria is an appropriate therapeutic target.
Exceptions to a Rule Do Not Deny the Rule
Not all trials unequivocally reported that a short-term reduction in albuminuria translates into long-term renoprotection. Exceptions to the general rule were predominantly obtained in trials that tested the effect of dual RAAS blockade (i.e., combination of an ACE inhibitor and an ARB) versus single RAAS blockade. These trials reported a reduction in albuminuria with dual blockade but in general, a neutral effect on renal outcome (56–58). Several explanations exist for these seemingly discrepant findings. First, the reduction in albuminuria in these trials may have been too small to translate into clinically relevant reductions in risk of ESRD. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) Trial and the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints, dual RAAS blockade, indeed, decreased albuminuria by only 7% and 14%, respectively. Second, inappropriate populations may have been studied. The ONTARGET Trial enrolled a population at high cardiovascular risk, and the majority of subjects had normoalbuminuria and preserved eGFR, a population at very low renal risk. In such a population, it is difficult (if not impossible) to show a renoprotective effect of treatment. One could argue that the renoprotective effects of dual RAAS blockade only become apparent in a specific (renal) population. Indeed, renoprotective effects of single ACE inhibitors or ARB therapy trials have mainly been reported in populations with impaired renal function and high albuminuria (59). However, a subgroup analysis of the ONTARGET Trial showed that, even in patients with low eGFR and elevated albuminuria, dual RAAS blockade did not lead to renoprotection (60). Important in this context is the finding that, in this specific subgroup of the ONTARGET Trial, dual RAAS blockade also did not cause further reduction in albuminuria and BP compared with the control group that received single RAAS blockade (61). Another trial, Veterans Affairs Nephropathy in Diabetes, specifically enrolled patients at high renal risk and randomly assigned them to dual or single RAAS blockade. Dual RAAS blockade decreased albuminuria after 12 months treatment and decreased risk of ESRD during follow-up by 34%, although this treatment effect did not reach statistical significance, because the trial was stopped early due to side effects of dual RAAS blockade (58). Third, dual RAAS blockade may not always have resulted in additional renoprotection, because it exerted other effects, such as hypotension and consequently, AKI, that confound the association between initial albuminuria reduction and long-term renal outcome.
Given the aforementioned considerations, exceptions to the general rule that short-term reduction in albuminuria translates into long-term renoprotection can often be explained. However, even when no rationale is readily available, it does not imply that the general rule is invalid. For comparison, some trials that tested the cardiovascular protective effect of BP and cholesterol-lowering agents were negative, but this did not refute the paradigm that, in general, BP and cholesterol lowering is beneficial.
Quantification of the Strength of the Albuminuria-ESRD Relation
The recently developed Biomarker Surrogacy Evaluation Schema aims to provide an instrument to quantify the evidentiary strength of the association between a biomarker and clinical outcomes (62). This instrument is endorsed by various stakeholders and has been reported to have important face and content validity (63). It consists of four panels: study design, target outcome, strength of the biomarker for the target by statistical evaluation, and generalizability. The study design criterion ranks the quality of studies used to validate the surrogacy status, with the highest rank being at least three randomized, controlled trials in each of three known drug classes of an intervention evaluating the relationship between the surrogate and target. The target outcome criterion ranks the target (outcome) against which the surrogate has been tested and indicates if the target in patient centered and clinically meaningful. ESRD is used in clinical trials of CKD progression, and it is a patient-centered outcome of great clinical importance and would, therefore, be ranked highest. The statistical evaluation criterion considers the strength of the association between the change in the surrogate and change in the target. The correlation between the change in the marker and target can be determined by using individual patient data from a single trial or measured in a meta-analytic framework across multiple trials (so-called trial-level analysis). For individual trials, the early change in albuminuria calculated from baseline to month 6 can be correlated with subsequent renal risk. For multiple trials, a metaregression analysis can be performed, in which the treatment effect relative to placebo on albuminuria and ESRD is analyzed across all trials. The statistics criterion also considers the surrogate threshold effect (STE), which is defined as the minimal change in the surrogate below which no benefit on the target can be expected. The STE proportion is calculated by dividing the STE by the range of changes in the surrogate. We refer to Lassere (62) for a detailed review of biomarker surrogate end point literature.
As shown in Table 2, albuminuria scores the maximum number of points for each component of the instrument, providing comprehensive qualitative and quantitative support for the robust relationship between albuminuria and ESRD and albuminuria being a valid treatment target.
Table 2.
The Biomarker Surrogacy Evaluation Schema
| Criterion | Evaluation and Scores |
|---|---|
| Study design | 3 points |
| 0: Biologic plausibility and lower-quality clinical studies | Multiple clinical trials measured both proteinuria and recorded ESRD outcomes. These trials assessed the effects of different drugs, including RAAS inhibition, low-protein diet, and corticosteroids. |
| 1: At least two high-quality prospective observational studies | |
| 2: At least two high-quality adequately powered RCTs measuring S and T | |
| 3: At least three high-quality adequately powered RCTs measuring S and T | |
| Target outcome | 3 points |
| 0: Reversible disease-centered biomarker of harm | ESRD is a patient-centered clinical end point of irreversible organ morbidity. |
| 1: Irreversible disease-centered biomarker of harm | |
| 2: Patient-centered end point of reversible organ morbidity or burden of disease or clinical harm | |
| 3: Patient-centered clinical end point of irreversible organ morbidity or burden of disease or clinical harm or death | |
| Statistical strength of biomarker for target | 2.5 points |
| 0: Poor | In a meta-analysis of 21 randomized, controlled trials, the R2 from a weighted regression analysis was 0.46 (44). The same meta-analysis showed that the lower boundary of the 95% confidence interval intersects the horizontal axis at approximately 30% (44). This is the STEP. Under the assumption that the percentage change in albuminuria ranges between −70% and +20% (values derived from refs. 43 and 44), the STEP equals 0.33. Analyses of individual patient data of diabetic and nondiabetic CKD trials show in all trials that, within trials, the R2 exceeds 0.6 (45,54,64,65). The statistical strength, thus, ranges between good and excellent. |
| 1: Fair: R2trial≥0.2, STEP≥0.1, and R2ind≥0.2 | |
| 2: Good: R2trial≥0.4, STEP≥0.2, and R2ind≥0.4 | |
| 3: Excellent: R2trial≥0.6, STEP≥0.3, and R2ind≥0.6 | |
| Generalizability of biomarker-target relationship; clinical evidence across different risk populations and pharmacologic evidence across different drug class mechanisms | 3 points |
| 0: No clinical or pharmacologic evidence | Albuminuria predicts renal outcome across different populations and CKD disease etiologies. Therapy–induced short-term changes in albuminuria predict renal outcome across different populations, CKD disease etiologies, and interventions (RAAS intervention, intensive glucose control, anti-inflammatory drugs, or low-protein diet). |
| 1: Clinical or pharmacologic evidence | |
| 2: Clinical and pharmacologic evidence | |
| 3: Consistent clinical RCT and pharmacologic RCT evidence |
The Biomarker Surrogacy Evaluation Schema consists of four domains: (1) study design, (2) target outcome, (3) statistical strength, and (4) generalizability. The overall summed score ranges from 0 to 12. The score is converted to a surrogate/outcome level of evidence, with the most convincing (level 1) being a score of 12 and the least convincing (level 5) being a score of 0, 1, or 2. Intermediates are level 2 (scores 9–11), level 3 (scores 6–8), and level 4 (scores 3–5). RAAS, renin-angiotensin-aldosterone system; RCT, randomized controlled trial; S, surrogate (i.e., albuminuria); T, target outcome (i.e., ESRD); STEP, surrogate threshold effect proportion.
Conclusion
Experimental evidence unambiguously shows that albuminuria has a direct pathogenic effect on renal tissue. In line, a strong association exists between albuminuria and risk of renal outcomes not only in untreated patients but also in patients on renoprotective treatments. Finally, clinical trials show a strong association between short-term drug-induced changes in albuminuria and long-term treatment effects on ESRD. In light of these lines of evidence, albuminuria should be considered an appropriate therapeutic target for interventions to slow progression of renal disease.
Disclosures
H.J.L.H. has consultancy agreements with AbbVie, Astellas, Johnson & Johnson, Reata, and Vitae. All honoraria are paid to his employer, the University Medical Center Groningen. R.T.G. has consultancy agreements with Amgen, AbbVie, Baxter, Bayer, Ipsen, Otsuka, and Sequela and received research grants from these companies. Honoraria and grants are paid to his employer, the University Medical Center Groningen.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gansevoort RT, Ritz E: Hermann Senator and albuminuria—forgotten pioneering work in the 19th century. Nephrol Dial Transplant 24: 1057–1062, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Williams PS, Fass G, Bone JM: Renal pathology and proteinuria determine progression in untreated mild/moderate chronic renal failure. Q J Med 67: 343–354, 1988 [PubMed] [Google Scholar]
- 3.Harmonised Tripartite Guideline ICH: ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med 18: 1905–1942, 1999 [PubMed] [Google Scholar]
- 4.Johnson KR: Strengths and weaknesses of renal markers as risk factors and surrogate markers. Kidney Int 79: 1272–1274, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Parving HH, Persson F, Rossing P: Microalbuminuria: A parameter that has changed diabetes care. Diabetes Res Clin Pract 107: 1–8, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Bertani T: Is glomerulosclerosis a consequence of altered glomerular permeability to macromolecules? Kidney Int 38: 384–394, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Wang Y, Tay YC, Harris DC: Proteinuria and tubulointerstitial injury. Kidney Int Suppl 61: S60–S62, 1997 [PubMed] [Google Scholar]
- 9.Okada T, Nagao T, Matsumoto H, Nagaoka Y, Wada T, Nakao T: Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology (Carlton) 17: 68–75, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Birn H, Christensen EI: Renal albumin absorption in physiology and pathology. Kidney Int 69: 440–449, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gekle M: Renal tubule albumin transport. Annu Rev Physiol 67: 573–594, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R: Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock CA, Poronnik P: Albumin transport and processing by the proximal tubule: Physiology and pathophysiology. Curr Opin Nephrol Hypertens 16: 359–364, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow TE, Moestrup SK, Christensen EI: Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 105: 1353–1361, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen EI, Birn H: Megalin and cubilin: Synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 280: F562–F573, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Storm T, Tranebjærg L, Frykholm C, Birn H, Verroust PJ, Nevéus T, Sundelin B, Hertz JM, Holmström G, Ericson K, Christensen EI, Nielsen R: Renal phenotypic investigations of megalin-deficient patients: Novel insights into tubular proteinuria and albumin filtration. Nephrol Dial Transplant 28: 585–591, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Macconi D, Chiabrando C, Schiarea S, Aiello S, Cassis L, Gagliardini E, Noris M, Buelli S, Zoja C, Corna D, Mele C, Fanelli R, Remuzzi G, Benigni A: Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol 20: 123–130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morigi M, Macconi D, Zoja C, Donadelli R, Buelli S, Zanchi C, Ghilardi M, Remuzzi G: Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J Am Soc Nephrol 13: 1179–1189, 2002 [PubMed] [Google Scholar]
- 19.Dixon R, Brunskill NJ: Activation of mitogenic pathways by albumin in kidney proximal tubule epithelial cells: Implications for the pathophysiology of proteinuric states. J Am Soc Nephrol 10: 1487–1497, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Drumm K, Bauer B, Freudinger R, Gekle M: Albumin induces NF-kappaB expression in human proximal tubule-derived cells (IHKE-1). Cell Physiol Biochem 12: 187–196, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Lee EM, Pollock CA, Drumm K, Barden JA, Poronnik P: Effects of pathophysiological concentrations of albumin on NHE3 activity and cell proliferation in primary cultures of human proximal tubule cells. Am J Physiol Renal Physiol 285: F748–F757, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Chen J, Chen L, Tay YC, Rangan GK, Harris DC: Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol 8: 1537–1545, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M, Remuzzi G: Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int 53: 1608–1615, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Vlachojannis JG, Tsakas S, Petropoulou C, Goumenos DS, Alexandri S: Endothelin-1 in the kidney and urine of patients with glomerular disease and proteinuria. Clin Nephrol 58: 337–343, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Zoja C, Morigi M, Figliuzzi M, Bruzzi I, Oldroyd S, Benigni A, Ronco P, Remuzzi G: Proximal tubular cell synthesis and secretion of endothelin-1 on challenge with albumin and other proteins. Am J Kidney Dis 26: 934–941, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Whaley-Connell AT, Morris EM, Rehmer N, Yaghoubian JC, Wei Y, Hayden MR, Habibi J, Stump CS, Sowers JR: Albumin activation of NAD(P)H oxidase activity is mediated via Rac1 in proximal tubule cells. Am J Nephrol 27: 15–23, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Diwakar R, Pearson AL, Colville-Nash P, Brunskill NJ, Dockrell ME: The role played by endocytosis in albumin-induced secretion of TGF-beta1 by proximal tubular epithelial cells. Am J Physiol Renal Physiol 292: F1464–F1470, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Goumenos DS, Tsakas S, El Nahas AM, Alexandri S, Oldroyd S, Kalliakmani P, Vlachojannis JG: Transforming growth factor-beta(1) in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant 17: 2145–2152, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Stephan JP, Mao W, Filvaroff E, Cai L, Rabkin R, Pan G: Albumin stimulates the accumulation of extracellular matrix in renal tubular epithelial cells. Am J Nephrol 24: 14–19, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Zoja C, Abbate M, Remuzzi G: Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration [published online ahead of print August 2, 2014]. Nephrol Dial Transplant gfu261 [DOI] [PubMed] [Google Scholar]
- 31.Roscioni SS, Lambers Heerspink HJ, de Zeeuw D: Microalbuminuria: Target for renoprotective therapy PRO. Kidney Int 86: 40–49, 2014 [DOI] [PubMed] [Google Scholar]
- 32.van der Velde M, Halbesma N, de Charro FT, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT: Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol 20: 852–862, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium: Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal R, Duffin KL, Laska DA, Voelker JR, Breyer MD, Mitchell PG: A prospective study of multiple protein biomarkers to predict progression in diabetic chronic kidney disease. Nephrol Dial Transplant 29: 2293–2302, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR: Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 20: 1069–1077, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigalleau V, Lasseur C, Raffaitin C, Beauvieux MC, Barthe N, Chauveau P, Combe C, Gin H: Normoalbuminuric renal-insufficient diabetic patients: A lower-risk group. Diabetes Care 30: 2034–2039, 2007 [DOI] [PubMed] [Google Scholar]
- 37.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators: Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 321: 406–412, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis I, Sollano JA, Shannon J, Tandon PK, DeMets DL: Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 325: 1468–1475, 1991 [DOI] [PubMed] [Google Scholar]
- 39.KDIGO Clincial Practice Guideline Working Group: KDIGO clinical practice guideline for glomerulonephritis. Kidney Int 2: 139–274, 2012 [Google Scholar]
- 40.Kramer AB, Bos H, van Goor H, Navis GJ: Sodium intake modifies the negative prognostic value of renal damage prior to treatment with ACE inhibitors on proteinuria induced by adriamycin. Nephron Physiol 103: 43–52, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, de Graeff PA, de Zeeuw D: Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 82: 330–337, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P: Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol 23: 165–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T; Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI): Early change in proteinuria as a surrogate end point for kidney disease progression: An individual patient meta-analysis. Am J Kidney Dis 64: 74–85, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambers Heerspink HJ, Kropelin TF, Hoekman J, De Zeeuw D: A drug induced reduction in albuminuria is associated with subsequent renoprotection; a meta-analysis [published online ahead of print November 24, 2014]. J Am Soc Nephrol ASN.2014070688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, Guo ZJ, Jiang JP: Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: A randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol 18: 1889–1898, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, Del Castillo N, Rivero A, Getino MA, García P, Jarque A, García J: Effect of Pentoxifylline on Renal Function and Urinary Albumin Excretion in Patients with Diabetic Kidney Disease: The PREDIAN Trial. J Am Soc Nephrol 26: 220–229, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Group DR; The Diabetes Control and Complications (DCCT) Research Group: Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 47: 1703–1720, 1995 [DOI] [PubMed] [Google Scholar]
- 48.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B; DCCT/EDIC Research Group: Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M, Mogensen CE, Poulter N, Mancia G, Cass A, Patel A, Zoungas S; ADVANCE Collaborative Group: Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 83: 517–523, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, Klahr S: Dietary protein restriction and the progression of chronic renal disease: What have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J Am Soc Nephrol 10: 2426–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 52.de Zeeuw D, Anzalone D, Cain VA, Cressman MD, Lambers Heerspink HJ, Molitoris BA, Monyak JT, Parving H-H, Remuzzi G, Sowers JR, Vidt DG: Different renal protective effects of atorvastatin and rosuvastatin in patients with proteinuric diabetic and non-diabetic renal disease; result from the PLANET Trials[published online ahead of print February, 4, 2015]. Lancet Diabetes Endocrinol 10.1016/S2213-8587(14)70260-8 [Google Scholar]
- 53.Ruggenenti P, Perticucci E, Cravedi P, Gambara V, Costantini M, Sharma SK, Perna A, Remuzzi G: Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 19: 1213–1224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Xie D, Hou FF, Fu BL, Zhang X, Liang M: High level of proteinuria during treatment with renin-angiotensin inhibitors is a strong predictor of renal outcome in nondiabetic kidney disease. J Clin Pharmacol 51: 1025–1034, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET investigators: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA; ALTITUDE Investigators: Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators: Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS; AIPRD Study Group. Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease: Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, Teo KK, Yusuf S, Mann JF; ONTARGET and TRANSCEND Investigators: Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: Results from the ONTARGET and TRANSCEND studies. Circulation 123: 1098–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Lambers Heerspink HJ, de Zeeuw D: ONTARGET still OFF-TARGET? Circulation 123: 1049–1051, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Lassere MN: The Biomarker-Surrogacy Evaluation Schema: A review of the biomarker-surrogate literature and a proposal for a criterion-based, quantitative, multidimensional hierarchical levels of evidence schema for evaluating the status of biomarkers as surrogate endpoints. Stat Methods Med Res 17: 303–340, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Moynihan R: Surrogates under scrutiny: Fallible correlations, fatal consequences. BMJ 343: d5160, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ: Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 45: 281–287, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Lea J, Greene T, Hebert L, Lipkowitz M, Massry S, Middleton J, Rostand SG, Miller E, Smith W, Bakris GL: The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: Results of the African American study of kidney disease and hypertension. Arch Intern Med 165: 947–953, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Ruggenenti P, Perna A, Remuzzi G; GISEN Group Investigators: Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Roscioni SS, de Zeeuw D, Hellemons ME, Mischak H, Zürbig P, Bakker SJ, Gansevoort RT, Reinhard H, Persson F, Lajer M, Rossing P, Lambers Heerspink HJ: A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia 56: 259–267, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G, Weber MA, McQueen M, Koon T, Yusuf S; ONTARGET Investigators: Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 22: 1353–1364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]