Abstract
Background
Mosquitoes become infected with Plasmodium when they ingest gametocyte-stage parasites from an infected person's blood. Plasmodium falciparum gametocytes are sensitive to the drug primaquine (PQ) and other 8-aminoquinolines (8AQ); these drugs could prevent parasite transmission from infected people to mosquitoes, and consequently reduce the incidence of malaria. However, PQ will not directly benefit the individual, and could be harmful to those with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
In 2010, The World Health Organization (WHO) recommended a single dose of PQ at 0.75 mg/kg, alongside treatment for P. falciparum malaria to reduce transmission in areas approaching malaria elimination. In 2013 the WHO revised this to 0.25 mg/kg due to concerns about safety.
Objectives
To assess whether giving PQ or an alternative 8AQ alongside treatment for P. falciparum malaria reduces malaria transmission, and to estimate the frequency of severe or haematological adverse events when PQ is given for this purpose.
Search methods
We searched the following databases up to 10 Feb 2014 for trials: the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; LILACS; metaRegister of Controlled Trials (mRCT); and the WHO trials search portal using 'malaria*', 'falciparum', and 'primaquine' as search terms. In addition, we searched conference proceedings and reference lists of included studies, and contacted researchers and organizations.
Selection criteria
Randomized controlled trials (RCTs) or quasi-RCTs comparing PQ (or alternative 8AQ) given as a single dose or short course alongside treatment for P. falciparum malaria with malaria treatment given without PQ/8AQ in adults or children.
Data collection and analysis
Two authors independently screened all abstracts, applied inclusion criteria, and extracted data. We sought evidence of an impact on transmission (community incidence), infectiousness (mosquitoes infected from humans) and potential infectiousness (gametocyte measures). We calculated the area under the curve (AUC) for gametocyte density over time for comparisons for which data were available. We sought data on haematological and other adverse effects, as well as secondary outcomes of asexual clearance time and recrudescence. We stratified by whether the malaria treatment regimen included an artemisinin derivative or not; by PQ dose category (low < 0.4 mg/kg; medium ≥ 0.4 to < 0.6 mg/kg; high ≥ 0.6 mg/kg); and by PQ schedules. We used the GRADE approach to assess evidence quality.
Main results
We included 17 RCTs and one quasi-RCT. Eight studies tested for G6PD status: six then excluded participants with G6PD deficiency, one included only those with G6PD deficiency, and one included all irrespective of status. The remaining ten trials either did not report on whether they tested (8), or reported that they did not test (2). Nine trials included study arms with artemisinin-based malaria treatment regimens, and eleven included study arms with non-artemisinin-based treatments.
Only two trials evaluated PQ given at low doses (0.25 mg/kg in one and 0.1 mg/kg in the other).
PQ with artemisinin-based treatments: No trials evaluated effects on malaria transmission directly (incidence, prevalence, or entomological inoculation rate), and none evaluated infectiousness to mosquitoes. For potential infectiousness, the proportion of people with detectable gametocytaemia on day eight was reduced by around two thirds with high dose PQ category (RR 0.29, 95% CI 0.22 to 0.37, seven trials, 1380 participants, high quality evidence), and with medium dose PQ category (RR 0.34, 95% CI 0.19 to 0.59, two trials, 269 participants, high quality evidence), but the trial evaluating low dose PQ category (0.1 mg/kg) did not demonstrate an effect (RR 0.67, 95% CI 0.44 to 1.02, one trial, 223 participants, low quality evidence). Reductions in log(10)AUC estimates for gametocytaemia on days 1 to 43 with medium and high doses ranged from 24.3% to 87.5%. For haemolysis, one trial reported percent change in mean haemoglobin against baseline, and did not detect a difference between the two arms (very low quality evidence).
PQ with non-artemisinin treatments: No trials assessed effects on malaria transmission directly. Two small trials from the same laboratory evaluated infectiousness to mosquitoes, and report that infectivity was eliminated on day 8 in 15/15 patients receiving high dose PQ compared to 1/15 in the control group (low quality evidence). For potential infectiousness, the proportion of people with detectable gametocytaemia on day 8 was reduced by around half with high dose PQ category (RR 0.44, 95% CI 0.27 to 0.70, three trials, 206 participants, high quality evidence), and by around a third with medium dose category (RR 0.62, 0.50 to 0.76, two trials, 283 participants, high quality evidence), but the single trial using low dose PQ category did not demonstrate a difference between groups (one trial, 59 participants, very low quality evidence). Reduction in log(10)AUC for gametocytaemia days 1 to 43 were 24.3% and 27.1% for two arms in one trial giving medium dose PQ. No trials systematically sought evidence of haemolysis.
Two trials evaluated the 8AQ bulaquine, and suggest the effects may be greater than PQ, but the small number of participants (n = 112) preclude a definite conclusion.
Authors' conclusions
In individual patients, PQ added to malaria treatments reduces gametocyte prevalence when given in doses greater than 0.4 mg/kg. Whether this translates into preventing people transmitting malaria to mosquitoes has rarely been tested in controlled trials, but there appeared to be a strong reduction in infectiousness in the two small studies that evaluated this. No included trials evaluated whether this policy has an impact on community malaria transmission either in low-endemic settings approaching elimination, or in highly-endemic settings where many people are infected but have no symptoms and are unlikely to be treated.
For the currently recommended low dose regimen, there is little direct evidence to be confident that the effect of reduction in gametocyte prevalence is preserved.
Most trials excluded people with G6PD deficiency, and thus there is little reliable evidence from controlled trials of the safety of PQ in single dose or short course.
PLAIN LANGUAGE SUMMARY
A single dose of primaquine added to malaria treatment to prevent malaria transmission
We conducted a review of the effects of adding a single dose (or short course) of primaquine to malaria treatment with the aim of reducing the transmission of malaria. We included 17 randomized controlled trials and one quasi-randomized controlled trial.
What is primaquine and how might it reduce transmission
Primaquine is an antimalarial drug which does not cure malaria illness, but is known to kill the gametocyte stage of the malaria parasite which infects mosquitoes when they bite humans. Primaquine is also known to have potentially serious side effects in people with an enzyme deficiency common in many malaria endemic settings (glucose-6-phosphate dehydrogenase (G6PD) deficiency). In these people, high doses of primaquine given over several days sometimes destroys red blood cells, causing anaemia and, in some cases, possibly life-threatening effects.
The World Health Organization (WHO) recommends adding a single dose of primaquine to malaria treatment with the intention of reducing malaria transmission and to contribute to malaria elimination. This recommendation was made in 2010, but in 2013 the WHO amended its recommendation from a dose of 0.75 mg/kg to 0.25 mg/kg due to concerns about safety, and indirect evidence suggesting this was as effective as the higher dose.This review examines the evidence of benefits and harms of using primaquine in this way, and looks for evidence that primaquine will reduce malaria transmission in communities.
What the research says
We did not find any studies that tested whether primaquine added to malaria treatment reduces the community transmission of malaria.
When added to current treatments for malaria (artemisinin-based combination therapy), we found no studies evaluating the effects of primaquine on the number of mosquitoes infected. However, primaquine does reduce the duration of infectiousness (the period that gametocytes are detected circulating in the blood) when given at doses of 0.4 mg/kg or above (high quality evidence). We only found one study using 0.1 mg/kg but this study did not conclusively show that primaquine was still effective at this dose (low quality evidence).
When added to older treatments for malaria, two studies showed that primaquine at doses of 0.75 mg/kg reduced the number of mosquitoes infected after biting humans (low quality evidence). Doses above 0.4 mg/kg reduced the duration of detectable gametocytes (high quality evidence), but in a single study of the currently recommended 0.25 mg/kg no effect was demonstrated (very low quality evidence).
Some studies excluded patients with G6PD deficiency, some included them, and some did not comment. Overall the safety of PQ given as a single dose was poorly evaluated across all studies, so these data do not demonstrate whether the drug is safe or potentially harmful at this dosing level.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| PQ for reducing P. falciparum transmission with artemisinin-based treatments | |||||
| Patient or population: People with symptomatic malaria | |||||
| Settings: Malaria-endemic areas | |||||
| Intervention: Single dose or short course PQ plus malaria treatment including an artemisinin derivative | |||||
| Control: Malaria treatment including an artemisinin derivative, without PQ | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | Number of participants(trials) | Quality of the evidence(GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | PQ | ||||
| Malaria incidence, prevalence or EIR | - | - | - | 0 trials | - |
| People infectious to mosquitoes | - | - | - | 0 trials | - |
| Participants with gametocytes on microscopy or PCR 1 (day 8) | Dose < 0.4 mg/kg | RR 0.67 (0.44 to 1.02) | 223 (1 trial) | ⊕⊕○○ 2,3,4 low | |
| 34 per 100 | 23 per 100 (15 to 35) | ||||
| Dose 0.4 to 0.6 mg/kg | RR 0.30 (0.16 to 0.56) | 219 (1 trial) | ⊕⊕⊕○ 2,4,5,6 moderate | ||
| 35 per 100 | 11 per 100 (6 to 20) | ||||
| Dose = 0.6 mg/kg | RR 0.29 (0.22 to 0.37) | 1380(7 trials) | ⊕⊕⊕○ high 7,8,9 | ||
| 30 per 100 | 9 per 100 (7 to 11) | ||||
| Mean percent change in haemoglobin 10 | The mean percent drop in Hb from baseline in the control group was15% | The mean percent drop in Hb from baseline in the intervention groups was 3% lower(from 10% lower to 4% higher) | - | 101(1 trial) | ⊕○○○ very low 10,11 |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| PQ: Primaquine; CI: Confidence interval; RR: Risk ratio; AUC: area under curve; G6PD: glucose-6-phosphate dehydrogenase; AS: artesunate; AQ: amodiaquine;DHAP: dihydroxyartemisinin-piperaquine; MQ: mefloquine; AL: artemether-lumefantrine. | |||||
| GRADE Working Group grades of evidence | |||||
| High quality: Further research is very unlikely to change our confidence in the estimate of effect. | |||||
| Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||
| Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | |||||
| Very low quality: We are very uncertain about the estimate. | |||||
1AUC estimates (log(10)AUC for day 1 to 43) are included as footnotes for each dosing strata.
2No serious risk of bias: includes one trial with no risk of bias detected.
3Downgraded by 2 for very serious imprecision: one small trial with CIs that include 50% reduction and no effect.
4There was no log(10)AUC day 1 to 43 % reduction data for this dose.
5Downgraded by 1 for serious imprecision. A single trial with few events.
6Not downgraded for serious indirectness: however this is a single trial from a single setting.
7Includes seven trials, with 11 comparisons: one trial included five separate comparisons with AS-AQ, DHAP, AS-MQ, and AL (Smithuis 2010).
8No serious inconsistency: whilst there is marked quantitative heterogeneity, the studies with no demonstrable effect had few events. Not downgraded.
9Log(10)AUC day 1 to 43 % reduction: range from 21.1% to 87.5%. We included four trials with 12 comparisons. We excluded one trial as high risk of bias (Vasquez 2009) due to small sample size and large difference in baseline gametocyte count in the two groups.
10Shekalaghe 2007 reported relative decrease in haemoglobin against baseline in both groups at day 8, 15, 29 and 43 in all participants irrespective of G6PD status. The comparison between those receiving PQ and those not did not demonstrate a difference at any time point. We presented day 43 in this table.
11Downgraded by 2 for very serious indirectness: the percentage of people with large drops in haemoglobin, not the mean change in the population, is the important safety outcome; and the estimates are averages in a small population (N = 99) that includes people with normal G6PD function so unlikely to detect effects in a small subgroup with a relatively uncommon adverse event.
Background
Malaria is a febrile illness due to infection with the plasmodium parasite, and is transmitted between humans via mosquitoes. Of the five plasmodium species known to cause illness in humans, P. falciparum is the most common, especially in sub-Saharan Africa, and causes the majority of severe illness and deaths. The clinical illness develops due to the presence of asexual stage parasites (sporozoites) in the persons bloodstream, but transmission to mosquitoes is via the sexual stage parasites (gametocytes), which develop from sporozoites at some point after infection.
Artemisinin-based combination therapies (ACTs) are currently recommended worldwide as the primary treatment for symptomatic P. falciparum malaria (WHO 2010). The artemisinin-derivatives treat the clinical illness by rapidly reducing the number of circulating sporozoites, which also reduces the potential for sporozoites to develop into gametocytes for onward transmission. The artemisinin-derivatives have been shown to kill early developing gametocytes, but they have no direct effects on mature gametocytes (Price 1996; Chotivanich 2006; Okell 2008a; Okell 2008b). The partner drugs in ACTs (mefloquine, amodiaquine, piperaquine, lumefantrine and sulfadoxine-pyrimethamine) are schizonticides with variable effects on gametocytes, and none adequately targets mature gametocytes (Drakeley 2006; Barnes 2008). In untreated infection, gametocytes can remain present for months as successive new generations are produced, and even following treatment they may persist for several weeks (Smalley 1977; Eichner 2001; Bousema 2010).
The mean circulation time of a mature P. falciparum gametocyte in humans has been estimated by microscopy or polymerase chain reaction (PCR) to be between 3.4 to 6.5 days (Smalley 1977; Eichner 2001; Bousema 2010). The minimum number of gametocytes required for transmission from an infected person to a mosquito has been estimated to be in the range of 100 to 300 per μL blood (Carter 1988), and the percentage of bites on humans that result in mosquito infection ranges between 0.3% and 46%, although most estimates are in the range of 1% to 10% (Graves 1988; Killeen 2006; Churcher 2013).
After uptake of a P. falciparum-infected blood-meal by the mosquito, gametocytes mature into male and female gametes. When fertilized, diploid oocysts develop on the mosquito's stomach wall and subsequently mature into sporozoites that migrate to the salivary glands, ready to be released when biting the next human. The median number of oocysts formed in wild caught infected mosquitoes is two to three (Rosenberg 2008). Each oocyst develops thousands of sporozoites, but only about 20% are thought to reach the mosquito salivary glands, and fewer than 25 sporozoites on average are ejected during mosquito blood-feeding (Rosenberg 1990; Rosenberg 2008).
Description of the intervention
Primaquine (PQ) is the only drug in common use which is known to kill mature P. falciparum gametocytes (Burgess 1961; Pukrittayakamee 2004; Chotivanich 2006), and with the recent emphasis on malaria elimination, there has been a renewed interest and emerging literature on PQ's potential value in reducing malaria transmission (White 2012; WHO 2012b; White 2013). PQ is an 8-aminoquinolone whose pharmacokinetic mode of action is not well understood, but it is known to be rapidly metabolized, with a half-life of six hours (White 1992). PQ does not directly affect the asexual stages of P. falciparum which cause the clinical illness (Arnold 1955; Pukrittayakamee 2004), and does not appear to affect the early or maturing gametocytes (Bhasin 1984; White 2008). Consequently, a combination of PQ and an artemisinin-derivative (as part of ACT) would target all stages of the gametocyte and have the greatest potential for reducing onward transmission to mosquitoes (White 2013; WHO 2012b).
One of the constraints to widespread use of PQ is that the drug is known to cause haemolysis in people with glucose-6-phosphate dehydrogenase (G6PD) deficiency. The deficiency is X-linked and expression highly variable with a wide variety of variants and levels of G6PD deficit (Howes 2013). PQ is a haemolytic trigger, and can cause a haemolytic anaemia that occasionally is serious with haemoglobinaemia and renal failure. The effect depends on the degree of enzyme deficiency, the dose of PQ, and the pattern of the exposure. These occasional, but clearly serious, adverse effects have led to a reputation of being "unsafe" although little is known about haemolysis at low doses of PQ.
The WHO 2010 Guidelines for the Treatment of Malaria recommended adding a single dose of PQ at 0.75 mg/kg to treatment for uncomplicated P. falciparum malaria in people who are not G6PD deficient with the goal of reducing transmission at the community level (WHO 2010). However, since testing for G6PD deficiency was rarely done, and due to the concerns about the safety of this single dose, the WHO convened a special expert review group in 2012 to reconsider this recommendation (WHO 2012a). The expert group concluded that 1) G6PD testing should be done more widely; 2) Countries already implementing single dose PQ should reduce the dose to 0.25 mg/kg in G6PD deficient patients; and 3) Countries not currently implementing single dose PQ but which are targeting malaria elimination, or are threatened by artemisinin resistance, should add 0.25 mg/kg PQ to treatment for uncomplicated P. falciparum malaria (White 2012; WHO 2012b).
How the intervention might work
A single dose of PQ could contribute to reducing malaria transmission through its effects on mature gametocytes, and it is reasonable to assume that reducing the density and duration of gametocytes in the blood of infected patients will reduce the duration of potential infectiousness to mosquitoes at the level of the individual (see Figure 1). However, any subsequent effects on the number of mosquitoes infected (infectiousness), or the number of new malaria infections in the community (transmission) are impossible to predict without measuring these effects using reliable methods.
Figure 1.
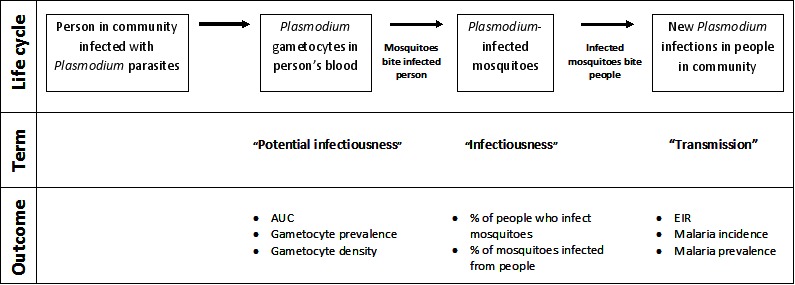
Review logic framework: The potential points in the Plasmodium parasite life cycle that could be impacted by PQ and the outcomes used to measure impact.
Infectiousness to mosquitoes can be measured directly by allowing mosquitoes to feed on infected individuals who have been treated with and without PQ (Killeen 2006; Bousema 2012), or estimated indirectly by measuring the infection rates of wild-caught mosquitoes (Graves 1990; Lines 1991).
Community level transmission can be measured through large cluster-randomized trials, or less reliably through controlled before and after studies. Within any community there are people who are carriers of P. falciparum gametocytes but who do not seek treatment (Bousema 2011). This is most apparent in areas of high endemicity, where much of the adult population has acquired immunity, and low level parasitemias do not produce symptoms. This reservoir of gametocytes in untreated adults will continue to facilitate community level transmission and may dilute any possible effect of PQ. Indeed, these dilutional effects may even be important in low transmission settings.
Recently, with the move toward a target of elimination, some policy makers are considering mass treatment strategies (von Seidlein 2003; Sturrock 2013) to reduce transmission or contain outbreaks once transmission is reduced to low levels. In this instance, it seems more likely that a higher proportion of the population with gametocytes will be detected or treated, or both, and that this could be effective in reducing or interrupting transmission. This policy is being considered in countries with lower intensity transmission, on islands or at the northern and southern fringes of malaria distribution, or both (GMAP 2008; Mendis 2009). Effective antimalarial drugs are likely to play a large role in this new strategy. One question in this effort is whether there is a role for PQ given in addition to curative antimalarial drugs, including artemisinin combination therapies (ACTs), to further reduce the infection transmissibility (White 2008).
The transmission blocking potential of PQ has also been suggested as a strategy to reduce the spread of artemisinin resistant parasites in Southeast Asia (Breman 2012).
Why it is important to do this review
PQ could play a role in the next phase of P. falciparum malaria control, particularly malaria elimination and possibly eradication. Whether elimination or eradication can be accomplished, or at the very least, the efficiency with which PQ is deployed, depends on getting the details right on dose, timing and the situation in which it is used. Best use must be made of existing data and opportunities for filling in missing information (and not duplicating what already is known) should be created and moved on quickly. This review is intended to clarify what is and is not known, and to identify which missing pieces are critical to defining effective uses of PQ.
Objectives
1. To assess whether giving PQ or other 8AQ in addition to treatment for P. falciparum infection reduces:
malaria transmission intensity;
infectiousness of infected people to mosquitoes;
potential infectiousness (gametocyte prevalence and density over time).
2. To compare the effects of different 8AQs.
3. To determine whether the effects of PQ or other 8AQ differ if the primary treatment drug is artemisinin based or another antimalarial.
4. To estimate the frequency of severe or haematological adverse events associated with single dose or short course PQ when it has been used for this purpose.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or quasi-RCTs including individual- or cluster-RCTs. Cluster-RCTs must have had at least two clusters per arm.
Types of participants
Adults or children with P. falciparum infection or a mixed infection of P. falciparum and other Plasmodium species. For individual RCTs, eligible studies must have diagnosed patients by blood slide, rapid diagnostic test, or other valid molecular method; for cluster-RCTs, diagnosis could have been by clinical judgment if that was standard in the trial area at the time of the trial.
Types of interventions
Intervention
A single dose or short course (up to seven days) of PQ or other 8-aminoquinoline (8AQ) added to malaria treatment(s).
Control
Identical treatment for malaria not including PQ/8AQ (or substituting placebo for PQ/8AQ); or using a different 8AQ with same malaria treatment, or using different dose of PQ/8AQ with same malaria treatment(s).
Types of outcome measures
Primary outcomes
Figure 1 provides an outline of transmission of malaria that helps clarify these terms.
a) Transmission
Entomological inoculation rate
Malaria incidence
Malaria prevalence
b) Infectiousness
People who infect mosquitoes
Mosquitoes infected by direct feeding
c) Potential infectiousness
AUC of gametocyte density (y-axis) over time (x-axis)
Gametocyte prevalence (estimated by microscopy or PCR)
Gametocyte density (estimated by microscopy or PCR)
Gametocyte clearance time (duration of gametocyte carriage)
Adverse events
Serious adverse events leading to hospital admission or death
-
Haematologic effects
Haemolysis (higher prevalence)
Haemoglobin concentration (decline)
Packed cell volume (decline)
Secondary outcomes
Presence of asexual stage parasites (may be reported as treatment failure rate)
Asexual parasite clearance time (duration of asexual carriage)
Search methods for identification of studies
We attempted to identify all relevant trials, regardless of language or publication status (published, unpublished, in press, and in progress).
The search strategy is in Appendix 1.
Electronic searches
Databases
We searched the following databases up to 10 February 2014 using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 2 2014); MEDLINE (1966 to 10 Feb 2014); EMBASE (1980 to 10 Feb 2014) ; and LILACS (1982 to 10 Feb 2014). Also, we checked the metaRegister of Controlled Trials (mRCT) and the WHO trials search portal (both accessed 10 Feb 2014) using 'malaria*', 'falciparum', and 'Primaquine' as search terms.
Conference proceedings
We searched the following conference proceedings for relevant abstracts: the MIM Pan-African Malaria Conferences and the American Society of Tropical Medicine and Hygiene (ASTMH) to December 2009.
Searching other resources
Researchers and organizations
We contacted researchers at the London School of Hygiene and Tropical Medicine who were authors of some of the included and in-progress trials, and other experts in the field of malaria chemotherapy, including those based at WHO.
Reference lists
We checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Two authors (PMG and HG) independently screened all citations and abstracts identified by the search strategy, including ongoing studies, for potentially eligible studies. We independently assessed full reports of potentially eligible studies for inclusion in the review. Notably, we did not contact any trial authors for clarification regarding inclusion (although we later contacted several about trial details) because it was clear whether trials were or were not eligible for inclusion. We used translations of eight papers published in Chinese to assess eligibility. We resolved differences of opinion by discussion with PG. There was one instance of duplicate reports of the same trial in different languages.
Data extraction and management
Two authors (PMG and HG) independently extracted the following information for each trial using a data extraction form.
Characteristics of trial
Design (RCT or quasi-RCT, type of randomization)
Dates and duration of trial
Characteristics of participants
Number of participants
Age and sex of participants
Proportion with G6PD deficiency
Proportion with gametocytes at onset of trial
Inclusion criteria
Exclusion criteria
Characteristics of interventions
Type of drug, dose, and schedule
Presented outcomes
Description of outcomes presented in the papers
Other
Location of trial, setting, and source of funding
Local endemicity of malaria
Outcomes data
For each trial, PMG and HG extracted data on the trial outcomes eligible for inclusion in this review for the PQ and non-PQ groups. We extracted the number of participants randomized and the numbers analysed in each treatment group for each outcome. For dichotomous data outcomes (proportion of participants with gametocytes or asexual stages, proportion of participants infectious to mosquitoes, and proportion of mosquitoes infected), we extracted the number of participants experiencing the event of interest and the total number of patients or mosquitoes in each treatment arm of each trial. For continuous outcomes (AUC for gametocyte numbers over time), we extracted arithmetic or geometric means and standard deviations for each treatment group by day of assessment, together with the number of patients in each group. We noted details on the method of determining parasite presence and density, for example light microscopy (if so, the method of staining and number of fields examined), PCR or other methods.
For G6PD deficiency, we noted the sex of the carrier (if stated) and the method used to determine G6PD deficiency, either phenotypically (by enzyme function) or PCR (by genotype). We adopted the definition of 'deficient' used in the trials that assessed this outcome. We extracted adverse event data for each individual type of event wherever possible. Where adverse events were reported separately for more than one dose (for short-course regimens), we attempted to record the average number of people reporting each adverse event for each dose. If trials reported the occurrence of adverse events at more than one time point following a single dose, but did not record the total number of people reporting each event, we attempted to record the events occurring in the first time period.
In cases of disagreement, we double checked the data and we reached consensus through discussion between all three authors.
Assessment of risk of bias in included studies
PMG and HG independently assessed the risk of bias of the included trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For each included trial, we assigned a score of low, unclear or high risk of bias for the following components: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other biases.
For sequence generation and allocation concealment, we described the methods used, if given. For blinding, we described who was blinded and the blinding method. For incomplete outcome data, we reported the percentage and proportion of loss to follow-up (the number of patients for whom outcomes are not measured of the number randomized), if given. For selective outcome reporting, we stated any discrepancies between the methods and the results in terms of the outcomes measured and the outcomes reported; we also stated if we knew that an outcome was measured but was not reported in the publication. For other biases we described any other trial features that could have affected the trial's results (for example, whether a trial was stopped early or if no sample size calculation was included). We resolved any disagreements through discussion.
We reported the results of the risk of bias assessment in a 'Risk of bias' table and displayed them in a 'Risk of bias' summary and 'Risk of bias' graph (Figure 2; Figure 3).
Figure 2.
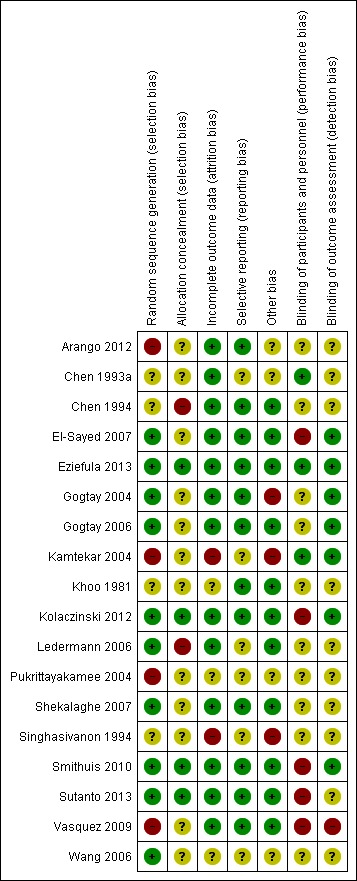
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Figure 3.
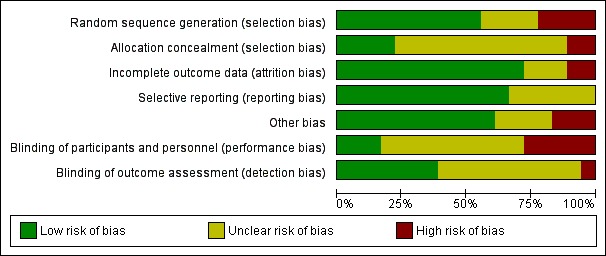
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
Measures of treatment effect
We analysed the data using Review Manager (RevMan). For dichotomous data, we estimated the Risk Ratio (RR) and used the Mantel-Haenszel method with fixed-effect, or with random-effects if there was heterogeneity. For continuous data, we estimated the mean difference (MD). All results are presented with 95% confidence intervals (CIs). We reported results only for days after the first day of PQ treatment, which, in some trials, was later than the beginning of primary treatment.
If trials reported gametocyte outcomes for days 1, 8, 15, 29, and 43, we estimated AUC using either the summary gametocyte measures reported by group in the paper, or by calculation from individual patient data supplied by the authors. Since few patients had gametocytes up to day 43, we also estimated AUC only up to day 15 and day 29 for the same trials. The AUC is a weighted sum of gametocyte densities, with weights proportional to the difference in time between adjacent sampling points as described by Dunyo 2006 and Mendez 2006 in trials assessing gametocytaemia after sulfadoxine-pyrimethamine (SP) treatment. However, Mendez 2006 used follow-up days 4 to 22 (reported as days 3 to 21 in trial), which do not encompass the early days of highest gametocytaemia nor the participants who still had gametocytes after day 21.
We used the following formulas:
AUC (days 1 to 15) = ((8-1)*(G1+G8)/2)+((15-8)*(G15+G8)/2)/14 for days 1 through 15
AUC (days 1 to 29) = ((8-1)*(G1+G8)/2)+((15-8)*(G15+G8)/2)+((29-15)*(G29+G15)/2)/28 for days 1 through 29
AUC (days 1 to 43) = ((8-1)*(G1+G8)/2)+((15-8)*(G15+G8)/2)+((29-15)*(G29+G15)/2)+((43-29)*(G43+G29)/2)/42 for days 1 through 43
where Gx = mean gametocyte density on day X (estimated using data from all participants still enrolled on day X). We estimated log(10)AUC values using geometric mean gametocyte density.
When one trial contained more than one comparison with the same placebo group and there was an analysis total or subtotal, we divided the placebo group participants between the comparisons to avoid underestimating the CI.
Unit of analysis issues
All the included trials were individually randomized and analysed. No cluster-RCTs met the inclusion criteria for the review.
Dealing with missing data
Where data were missing from the trials or details were unclear, we attempted to contact the authors. We used complete case analysis for trials with missing data.
Assessment of heterogeneity
We assessed heterogeneity between the trials by examining the forest plots to check for overlapping CIs, using the Chi2 test for heterogeneity with a 10% level of significance, and the I2 statistic using a value of 50% to represent moderate levels of heterogeneity.
Assessment of reporting biases
There were insufficient trials within each comparison to assess the likelihood of small trial effects, such as publication bias, by examining a funnel plot for asymmetry.
Data synthesis
We stratified trials by non-artemisinin or artemisinin based malaria treatment regimens and described which antimalarial drug was used for each comparison in the footnote. Also we stratified by PQ dose category: low (< 0.4 mg/kg) medium (≥ 0.4 to < 0.6 mg/kg); and high (≥ 0.6 mg/kg dose); by schedule (single dose day 1 or 2, single dose day 3 or 4, and multiple dose days 1 to 7) and grouped the 8AQ drugs as PQ and other. Throughout this review, we designated the first day of treatment as day 1 rather than day 0 as reported in some trials.
Where not stated as mg/kg, we reported the PQ dose as the adult dose with the equivalent dose reported as mg/kg; most trials stated that the dose was adjusted for children and we made this assumption if not.
When there was no statistically significant heterogeneity between trials, we applied the fixed-effect meta-analysis model. When we observed statistically significant heterogeneity within groups that could not be explained by subgroup or sensitivity analyses, we used a random-effects meta-analysis model. When we determined substantial heterogeneity from the assessments of heterogeneity (such as high I2 value, low Chi2 statistic P value, or when a pooled meta-analysis result was considered meaningless because of clinical heterogeneity) we did not undertake meta-analysis but instead presented a forest plot with the pooled effect suppressed.
Subgroup analysis and investigation of heterogeneity
In our protocol, we stated we would investigate heterogeneity in relation to drug resistance pattern, the parasite density before treatment and the local endemicity of malaria. However, we identified too few trials for this analysis. We stratified outcomes under comparisons 1 and 2 (non-artemisinin-based and artemisinin-based partner respectively) by time point after treatment, by dose and by schedule of PQ where possible.
We stratified Comparison 3 by artemisinin-based and non-artemisinin-based partners. In this case, we assessed the outcome of percentage of people with gametocytes on day 8 only and combined all trials in each subgroup that started PQ any time up to day 7.
When we did not detect statistically significant heterogeneity between trials, we applied the fixed-effect meta-analysis model. When there was statistically significant heterogeneity within groups that could not be explained by subgroup or sensitivity analyses, we used a random-effects meta-analysis model.
When substantial heterogeneity was determined from the assessments of heterogeneity (such as high I2 value, low Chi2 statistic P value, or when we considered a pooled meta-analysis result meaningless because of clinical heterogeneity), we did not perform meta-analysis but instead presented a forest plot with the pooled effect suppressed.
Sensitivity analysis
There were insufficient trials to conduct a sensitivity analysis to investigate the robustness of the results to the quality (risk of bias) components.
Results
Description of studies
Results of the search
In the first version of this review (Graves 2012), we identified 45 potentially relevant publications from literature searches. Two publications (in different languages) described the same trial (Chen 1994), leaving 44 distinct trials. We excluded 13 at abstract stage, excluded 20 after reading the full text article, and included 11 trials in the review.
For this update, we repeated the searches since we had expanded the scope of the review to include other 8AQ and comparisons of different doses of PQ and other 8AQ. We identified 65 more potential studies in addition to the 45 previously identified, which we rescreened due to revised inclusion criteria. We identified an additional 41 papers from reference lists, personal knowledge of new papers, or people consulted. Of the 151 abstracts we screened, we selected 73 for full text review. Five papers were duplicates, we could not locate three articles, and we included 18 trials (Figure 4). These 18 trials included a total of 30 distinct comparisons of different malaria treatment drugs, doses or schedules.
Figure 4.
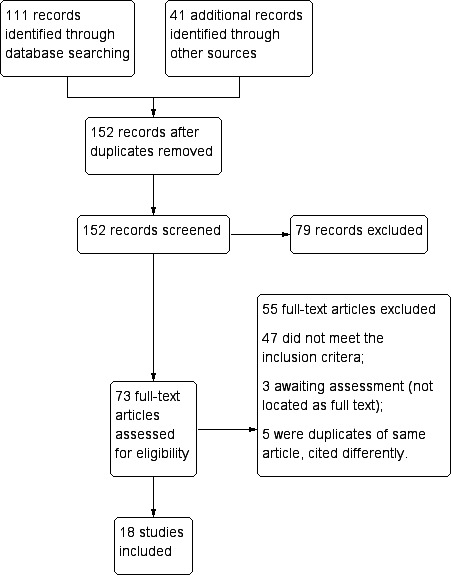
Study flow diagram.
Included studies
All 18 included trials were RCTs or quasi-RCTs. Two trials compared PQ and bulaquine, while 16 trials compared PQ versus no PQ. One trial of PQ (Khoo 1981) did not distinguish between short or long course of PQ and therefore no outcomes are included in this review. One trial did not include any gametocyte outcomes (Wang 2006).
Sixteen trials examined the impact of PQ or 8AQ on various measures of potential infectiousness, such as gametocyte prevalence over time or density in participants after treatment, gametocyte clearance time or gametocyte circulation time. Most trials assessed gametocyte prevalence by microscopy but two trials reported both microscopy and PCR (Shekalaghe 2007; Eziefula 2013) and one reported only PCR detection (El-Sayed 2007).
Two trials (Shekalaghe 2007; Eziefula 2013) reported log(10)AUC as a summary combined measure of gametocyte prevalence and density over time, using PCR estimates of density. For gametocytes detected by microscopy, we calculated the outcomes of AUC and log(10)AUC for four additional studies that provided appropriate information, either in the publications or from the authors (Vasquez 2009; Smithuis 2010; Kolaczinski 2012; Sutanto 2013).
For direct measures of infectiousness, two small trials in China (Chen 1993a; Chen 1994) evaluated the infectiousness to mosquitoes of people treated with mefloquine (MQ) compared to MQ+PQ.
Only five trials reported adverse effects quantitatively: three for anaemia outcomes (El-Sayed 2007; Shekalaghe 2007; Eziefula 2013) and two for other outcomes (Wang 2006; Sutanto 2013).
No community trials examining malaria transmission intensity (measuring incidence of malaria, prevalence or EIR) met the inclusion criteria.
Participants
Participants were people attending health clinics for treatment. Four trials did not state the participants' ages (Chen 1993a; Chen 1994; El-Sayed 2007; Khoo 1981), and three trials included children only: Singhasivanon 1994 (5 to 12 years); Shekalaghe 2007 (3 to 15 years); and Eziefula 2013 (1 to 10 years). Six trials used a wide age range of children and adults: Wang 2006 (6 to 60 years); Vasquez 2009 (≥ 1 year); Smithuis 2010 (= six months); Arango 2012 (1 to 75 years); Kolaczinski 2012 (3 to 70 years); and Sutanto 2013 (≥ 5 years). The remaining five studies included teenagers and adults only: Gogtay 2004 (= 18 years); Kamtekar 2004 (= 16 years); Pukrittayakamee 2004 (15 to 62 years); Gogtay 2006 (= 16 years); and Ledermann 2006 (≥ 15 years). See the Characteristics of included studies section.
For G6PD deficiency, two studies did not screen participants (Kamtekar 2004; Smithuis 2010), one trial screened and included all participants (Shekalaghe 2007), one trial included only G6PD-deficient participants (Khoo 1981), six studies included only non-deficient participants (Gogtay 2004; Pukrittayakamee 2004; Gogtay 2006; Ledermann 2006; Eziefula 2013; Sutanto 2013), and the remaining eight studies made no comment (Chen 1993a; Chen 1994; Singhasivanon 1994; Wang 2006; El-Sayed 2007; Vasquez 2009; Arango 2012; Kolaczinski 2012); see Table 1.
Additional Tables
Table 1.
G6PD status, partner drugs, gametocyte status at onset, and PQ dose and treatment schedule
| Comparator | Trial | Comparison | Place | G6PD status | Parasite species | Partner or alternative drug | Proportion with gametocytes at onset (by microscopy unless noted) | PQ day(s)* | Target PQ dose per day |
|---|---|---|---|---|---|---|---|---|---|
| Non-artemisinin partner | |||||||||
| CQ or (CQ+SP) | Kamtekar 2004 | a | India (Mumbai) | Not screened | Pf only | CQ days 1 to 3 or CQ days 1 to 3 + SP day 1 | 100% (within 3 days) (N = 46) | day 4 | 45 mg (˜0.75 mg/kg) |
| Khoo 1981 | Malaysia (Sabah) | Only deficient (method: Brewer's methaemoglobin reduction test) | Pf, Pv or mixed | CQ days 1 to 3 | Not reported | days 1 to 3 | 25 mg (˜0.42 mg/kg) | ||
| Kolaczinski 2012 | a | Pakistan (3 Afghan refugee camps) | Not reported | Pf only | CQ days 1 to 3 | 17.8% (N = 152) | day 3 | 0.5 mg/kg | |
| Ledermann 2006 | a | Indonesia (Central Java) | Only non-deficient (method: semiquantitative glucose-6-phosphate dehydrogenase (G6PD) assay) | Pf only | CQ days 1 to 3 + SP day 1 | Not reported (N = 60) | day 1 | 45 mg (˜0.75 mg/kg) | |
| Ledermann 2006 | b | Indonesia (Central Java) | Only non-deficient (method: semiquantitative glucose-6-phosphate dehydrogenase (G6PD) assay) | Pf only | CQ days 1 to 3 + SP day 1 | Not reported (N = 60) | day 3 | 45 mg (˜0.75 mg/kg) | |
| SP | Kolaczinski 2012 | b | Pakistan (2 Afghan refugee camps) | Not reported | Pf only | SP day 1 | 27.1% (N = 85) | day 1 | 0.5 mg/kg |
| AQ+SP | Arango 2012 | a | Colombia | Not reported | Pf only | AQ days 1 to 3 + SP day 1 | 22.5% (N = 40) | day 2 | 0.75 mg/kg |
| MQ or (MQ+SP) | Chen 1993a | China | Not reported | Pf only | MQ day 1 | 100% (N = 12) | day 1 | 45 mg (˜0.75 mg/kg) | |
| Chen 1994 | China (Hainan province) | Not reported | Pf only | MQ day 1 | 100% (N = 18) | day 1 | 45 mg (˜0.75 mg/kg) | ||
| Singhasivanon 1994 | Thailand (Bangkok) | Not reported | Pf only | MQ+SP fixed day 1 | Not reported (N = 18) | day 1 | 0.75 mg/kg | ||
| QN | Kamtekar 2004 | b | India (Mumbai) | Not screened | Pf only | QN i.v. days 1 to 2 and orally days 1 to 7 | 100% (within 3 days) (N = 43) | day 8 | 45 mg (˜0.75 mg/kg) |
| Pukrittayakamee 2004 | a | Thailand | Patients with G6PD deficiency were excluded from getting PQ (method not reported) | Pf only | QN days 1 to 7 | 18.6% (N = 59) | days 1 to 7 | 0.25 mg base/kg | |
| Pukrittayakamee 2004 | b | Thailand | Patients with G6PD deficiency were excluded from getting PQ (method not reported) | Pf only | QN days 1 to 7 | 22.4% (N = 67) | days 1 to 7 | 0.5 mg base/kg | |
| Artemisinin-based partner | |||||||||
| AS or ACT | Arango 2012 | b | Colombia | Not reported | Pf only | AS+MQ days 1 to 3 | 17.1% (N = 42) | day 2 | 0.75 mg/kg |
| El-Sayed 2007 | Sudan (east) | Not reported | Pf only | AS+SP days 1 to 3 | 3.8% (N = 104) 11.6% PCR (N = 95) | day 4 | 0.75 mg/kg | ||
| Eziefula 2013 | a | Uganda | Patients without normal G6PD were excluded (method fluoresence spot test) | Pf only | AL days 1 to 3 | 24.3% (N = 115) 86.7% PCR (N = 113) | day 3 | 0.1 mg/kg | |
| Eziefula 2013 | b | Uganda | Patients without normal G6PD were excluded (method fluoresence spot test) | Pf only | AL days 1 to 3 | 20.4% (N = 113) 78.7% PCR (N = 108) | day 3 | 0.4 mg/kg | |
| Eziefula 2013 | c | Uganda | Patients without normal G6PD were excluded (method fluoresence spot test) | Pf only | AL days 1 to 3 | 22.4% (N = 116) 82.0% PCR (N = 111) | day 3 | 0.75 mg/kg | |
| Pukrittayakamee 2004 | c | Thailand | Patients with G6PD deficiency were excluded from getting PQ (method not reported) | Pf only | AS days 1 to 7 | 26.0% (N = 50) | days 1 to 7 | 0.5 mg base/kg | |
| Shekalaghe 2007 | Tanzania (North east) | Screened and all included (method: detection of single nucleotide polymorphisms in the human G6PD gene (G202A,A376G) by a simple high throughput PCR using sequence specific oligo-nucleotide probes (SSOPs) and ELISA testing) | Pf only | AS+SP days 1 to 3 | 22.6% (N = 106) 87.7% PCR (N = 106) | day 4 | 0.75 mg/kg | ||
| Smithuis 2010 | a | Myanmar (3 states) | Not screened | Pf or mixed | AS+AQ days 1 to 3 | 34% (N = 155) | day 1 | 0.75 mg/kg | |
| Smithuis 2010 | b | Myanmar (3 states) | Not screened | Pf or mixed | AL days 1 to 3 | 33% (N = 152) | day 1 | 0.75 mg/kg | |
| Smithuis 2010 | c | Myanmar (3 states) | Not screened | Pf or mixed | AS+MQ fixed dose days 1 to 3 | 30% (N = 169) | day 1 | 0.75 mg/kg | |
| Smithuis 2010 | d | Myanmar (3 states) | Not screened | Pf or mixed | AS days 1 to 3 + MQ day 1 loose | 29% (N = 161) | day 1 | 0.75 mg/kg | |
| Smithuis 2010 | e | Myanmar (3 states) | Not screened | Pf or mixed | DHAP days 1 to 3 | 38% (N = 161) | day 1 | 0.75 mg/kg | |
| Sutanto 2013 | Indonesia (south Sumatra) | Screened and only normals included (method: qualitative test) | Pf only | DHAP days 1 to 3 | 20.6% (on day 3) (N = 349) | day 3 | 0.75 mg/kg | ||
| Vasquez 2009 | Colombia (Antioquia) | Not reported | Pf only | AS+MQ days 1 to 3 (MQ only on day 2 for children < 6) | 20.0% (N = 50) | day 3 | 45 mg (˜0.75 mg/kg) | ||
| Wang 2006 | Gabon | Not reported | Pf | AS i.m. days 1 to 5 | Not reported (N = 204) | days 1 to 5 | 22.5 mg (˜0.38 mg/kg) | ||
| Comparison of different 8AQ | |||||||||
| PQ versus Bulaquine | Gogtay 2004 | India (Mumbai) | Only non-deficient (method: not stated) | Pf | QN + doxycycline days 1 to 7 + BQ day 4 | 100% (N = 22) | day 4 | 45 mg (˜0.75 mg/kg) | |
| Gogtay 2006 | India | Only non-deficient (method: not stated) | Pf | QN + doxycycline days 1 to 7 + BQ day 4 | 100% (N = 93) | day 4 | 45 mg (˜0.75 mg/kg) | ||
* first day of any treatment = day 1
Abbreviations: G6PD = glucose-6-phosphate dehydrogenase; PQ = primaquine; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; MQ = mefloquine; QN = quinine; AS = artesunate; ACT = artemisinin-based combination therapy; 8AQ: 8-aminoquinoline; AQ = amodiaquine; AL = artemether-lumefantrine; DHAP = dihydroxyartemisinin-piperaquine; BQ = bulaquine; i.v. = intravenous injection; i.m. = intramuscular injection; Pf = P. falciparum; Pv = P. vivax.
Interventions
Non-artemisinin-based regimens
Twelve studies (15 treatment arms) evaluated PQ given alongside non-artemisinin-based treatments: chloroquine alone (CQ) (two trials), CQ+sulfadoxine-pyrimethamine (SP) (one trial), CQ alone or CQ+SP (one trial), SP (one trial), mefloquine (MQ) (two trials), MQ+SP (two trials), quinine (QN) (two trials), and QN plus doxycycline (two trials).
Artemisinin-based regimens
Eight studies (15 treatment arms) evaluated PQ given alongside artemisinin-based treatments: artesunate (AS) (two trials), AS+SP (two trials), AS+MQ (four trials), AS+amodiaquine (AQ) (one trial), artemether-lumefantrine (AL) (four trials) and dihydroxyartemisinin-piperaquine (DHAP) (two trials).
Dose
Most trials used a target dose equivalent to 0.75 mg/kg PQ per day (adult dose 45 mg/day), see Table 1. The exceptions were:
Khoo 1981: adult dose of 25 mg or approximately 0.42 mg/kg/day;
Kolaczinski 2012: (two comparisons) 0.5 mg/kg or adult dose 30 mg/day;
Pukrittayakamee 2004: the trial with QN had two arms, one with 0.25 mg/kg and the other 0.5 mg/kg per day (adult dose 15 mg or 30 mg per day respectively); the comparison with AS used 0.5 mg/kg per day (adult dose 30 mg per day).
Wang 2006: adult dose of 22.5 mg or approximately 0.38 mg/kg per day.
Eziefula 2013: evaluated 0.1, 0.4 and 0.75 mg/kg and placebo.
Schedule
Most trials used a single dose of PQ given on the following days, and we regarded the first day of any treatment as day 1:
Day 1: Chen 1993a; Chen 1994; Singhasivanon 1994; Ledermann 2006 (one of two comparisons); Smithuis 2010 (five comparisons); and Kolaczinski 2012 (one of two comparisons);
Day 2: Arango 2012 (two comparisons);
Day 3: Ledermann 2006 (one of two comparisons); Vasquez 2009; Kolaczinski 2012 (one of two comparisons); Eziefula 2013; and Sutanto 2013;
Day 4: Gogtay 2004; Kamtekar 2004 (one of two comparisons); Gogtay 2006; El-Sayed 2007; and Shekalaghe 2007;
Day 8: Kamtekar 2004 (one of two comparisons).
Three trials used a longer course of PQ:
Prevalence of gametocytes at start of trial
Five trials only included people with gametocytes at onset (as detected by microscopy) (Chen 1993a; Chen 1994; Gogtay 2004; Kamtekar 2004 (both comparisons); Gogtay 2006). However Kamtekar 2004 reported this variable as "within 3 days" rather than on day 1. Four trials did not report this statistic (Khoo 1981; Singhasivanon 1994; Ledermann 2006 (both comparisons); Wang 2006).
In the remaining trials, one had low gametocyte prevalence at onset (El-Sayed 2007, prevalence by microscopy 3.8%, by PCR 11.8%). Trials with initial prevalence between 17.1% and 27.1% were: Pukrittayakamee 2004 (all three comparisons); Vasquez 2009; Arango 2012; Kolaczinski 2012 (both comparisons); Eziefula 2013; and Sutanto 2013. Shekalaghe 2007 reported gametocyte prevalence by microscopy of 22.6% but by PCR of 87.7%. Eziefula 2013 observed a similar ratio between microscopy and PCR prevalence, with microscopy prevalence by arm of 20.4% to 24.3% and PCR prevalence of 78.4% to 86.7%. Excluding trials that included only gametocyte carriers, the five arms of the Smithuis 2010 trial showed the highest prevalences, with gametocyte prevalence (microscopy) between 29% and 38%.
The details of the trial locations, malaria treatments, gametocyte prevalence, PQ doses and schedules are in Table 1.
Excluded studies
We have listed the reasons for exclusion of 47 trials in the Characteristics of excluded studies section. Some additional details are given here.
Six community-based trials did not meet criteria for inclusion. Both Hii 1987 (MDA with SP+PQ (30 mg adult dose, 0.5 mg/kg) + insecticide treated net (ITN) versus ITN only in Sabah, Malaysia) and Shekalaghe 2011 (MDA with SP+AS+PQ (0.75 mg/kg) versus placebo) did not have appropriate comparison groups. Doi 1989 was a community-based observational trial of mass test and treat with SP+PQ (0.7 to 1 mg/kg) in one intervention village, two schools in two other intervention villages, and one control village (SP only) on the coast of north Sumatra, Indonesia. There was no 'before' data from these villages, and in the control site it appears that some children received treatment with PQ. Kaneko 1989, also in north Sumatra, Indonesia, tested mass fever test and treat and/or mass test and treat in school children. The drugs used were SP+PQ in one intervention village and SP in one control village. Apart from there being only one cluster (village) per arm and non-randomized, the main reason for exclusion was the intensity of effort on case detection appeared much greater in the intervention village, resulting in 75% of people in the intervention village being treated over a 29-day period versus 18% in the control village over a 14-day period. The Barber 1932 trial in Liberia was a trial of MDA that administered the 8AQ plasmoquine approximately twice weekly to ˜133 people for periods ranging from nine to 28 days with follow up for several weeks. Plasmoquine had a large (although short-lived) impact on transmission in this trial. However the main reasons for its exclusion were the lack of malaria treatment given together with plasmoquine, non-comparable control site and lack of parasite outcomes in the control group. In the MDA trial of Clyde 1962 in Tanzania, AQ+PQ was given every 1, 2 or 4 weeks to over 93% of the populations residing in three sites near Morogoro, Tanzania for periods ranging by site between 26 and 39 weeks. The dose of PQ was 30 mg (˜0.5 mg/kg for an adult) given to everyone over six years of age, with half dose given to those aged between 0 and five years. Transmission was greatly reduced, especially in the sites receiving MDA every one or two weeks (although transmission was not interrupted). We excluded this trial because everyone received malaria treatment as well as PQ, so the additional impact of PQ cannot be assessed.
Several controlled or uncontrolled before-and-after studies, and non-randomized comparative case series or trials, were excluded. They were generally studies of small numbers of individuals on whom mosquitoes were fed before and after they ingested PQ, with or without other malaria treatment. These studies, which include Barber 1929, Barber 1932, Jerace 1933, Mackerras 1949, Jeffery 1956, Young 1959, Gunders 1961, Jeffery 1963, Rieckmann 1968, Rieckmann 1969, Clyde 1970, and Clyde 1971, have been reviewed by White 2012 and White 2013 but did not meet our inclusion criteria. Abay 2013 also reviewed two of these before-and-after studies which had four patients in total (Rieckmann 1968; Clyde 1971). Two studies used varying doses of PQ (Jeffery 1956, Rieckmann 1969), as did Burgess 1961 and Bunnag 1980. However, Burgess 1961 gave doses according to participants' age rather than testing different doses in comparable patients, and there was no other malaria treatment drug given. In Bunnag 1980 all received malaria treatment (SP) in addition to PQ.
We sought publications for Chinese trials cited in White 2012, White 2013 and by personal communication from Professor Li Guo Qiao. We were unable to locate two (Chen 1993b; Li 2006); the others were translated where required. We excluded the following studies on the basis of no appropriate comparison (either all groups got PQ or there was no comparator group with same dose of malaria treatment drug but no PQ) (Yang 1989; Che 1990; Che 1987; Huang 1996; Lin 2004; and Sun 2011) or lack of randomization (Cai 1985 and Huang 1993). Three other trials of artemether with and without PQ in Africa (Huang 2001, Li 2007, and Li 2010) were stated to be randomized, but were excluded due to the late administration of PQ (after five to seven days of artemether) and lack of gametocyte outcomes.
Risk of bias in included studies
Of the 18 included studies, the risk of bias assessment for concealment of allocation was adequate in 4 studies; methods of random allocation were adequate in 10; and blinding of outcome assessment adequate in 7; see Figure 2 and Figure 3.
Pukrittayakamee 2004 excluded G6PD-deficient people from the PQ group post-randomization. We had no reason to suppose it biased the primary outcomes but it could have affected assessment of adverse effects.
Effects of interventions
See: Summary of findings for the main comparison Summary of findings table 1; Summary of findings 2 Summary of findings table 2
For malaria transmission intensity (prevalence, incidence or EIR) we found no community cluster-RCTs measuring these outcomes. Regarding infectiousness, two trials (Chen 1993a; Chen 1994) measured this in 12 and 18 patients respectively for non-artemisinin drugs, (in both cases MQ) with and without PQ.
All other trials reported potential infectiousness: the effects of PQ on gametocyte prevalence, density or clearance time, or all three outcomes. Only Shekalaghe 2007 and Eziefula 2013 reported a summary measure of potential infectiousness using AUC of gametocyte density over time; we calculated this for four other trials with available data. We estimated the AUC for microscopy-determined densities for trials of both non-artemisinin and artemisinin based malaria treatments. In the former category we had only Kolaczinski 2012 (two comparisons). There were four trials with this information for artemisinin-based partners: Shekalaghe 2007, Smithuis 2010 (five comparisons), Sutanto 2013, and Vasquez 2009. The estimate used the mean (or geometric mean) gametocyte density by group at a sequence of reported days of measurement. Since trials were not consistent in the days on which they estimated gametocyte density, we used the days on which measurements were available for all trials (days 1, 8, 15, 29 and 43; see Methods section). We estimated AUC up to day 15 (Table 2), day 29 (Table 3) and day 43 (Table 4). Results are presented separately by non-artemisinin-based and artemisinin-based malaria treatments below and given for log(10)AUC in the summary of findings tables for days 1 to 43.
Table 2.
AUC of gametocyte density over time, days 1 to 15 after treatment
| Other malaria treatment type | Trial | Malaria treatment | Dose PQ mg/kg | AUC with PQ days 1 to 15 | AUC without PQ days 1 to 15 | % reduction AUC days 1 to 15 | log(10)AUC with PQ days 1 to 15 | log(10)AUC without PQ days1 to 15 | % reduction log(10)AUC days 1 to 15 |
|---|---|---|---|---|---|---|---|---|---|
| Non-artemisinin- based | Kolaczinski 2012 | a: CQ | 0.5 | 485.26 | 4266.61 | 88.6 | 2.69 | 3.63 | 26.0 |
| Kolaczinski 2012 | b: SP | 0.5 | 924.87 | 5964.77 | 84.5 | 2.97 | 3.78 | 21.4 | |
| Artemisinin- based | Shekalaghe 2007 | AS+SP | 0.75 | 40.15 | 65.06 | 38.3 | 1.60 | 1.81 | 11.6 |
| Smithuis 2010 | a: AS+AQ | 0.75 | 140.35 | 428.51 | 67.3 | 2.15 | 2.63 | 18.4 | |
| Smithuis 2010 | b: AL | 0.75 | 196.24 | 242.86 | 19.2 | 2.29 | 2.39 | 3.9 | |
| Smithuis 2010 | c: AS+MQ fixed | 0.75 | 237.78 | 510.03 | 53.4 | 2.38 | 2.71 | 12.2 | |
| Smithuis 2010 | d: AS+MQ loose | 0.75 | 183.51 | 293.28 | 37.4 | 2.26 | 2.47 | 8.3 | |
| Smithuis 2010 | e: DHAP | 0.75 | 295.24 | 709.60 | 58.4 | 2.47 | 2.85 | 13.4 | |
| Sutanto 2013 | DHAP | 0.75 | 1252.48 | 2355.84 | 46.8 | 3.10 | 3.37 | 8.1 | |
| Vasquez 2009 | AS+MQ | 0.75 | 500.40 | 305.67 | -63.7 | 2.70 | 2.49 | -8.6 | |
Abbreviations: AUC = area under curve; PQ = primaquine; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AS = artesunate; AQ = amodiaquine; AL = artemether-lumefantrine; MQ = mefloquine; DHAP = dihydroxyartemisinin-piperaquine.
Table 3.
AUC of gametocyte density over time, days 1 to 29 after treatment
| Other malaria treatment type | Trial | Malaria treatment | Dose PQ mg/kg | AUC with PQ days 1 to 29 | AUC without PQ days 1 to 29 | % reduction AUC days 1 to 29 | log(10)AUC with PQ days 1 to 29 | log(10)AUC without PQ days 1 to 29 | % reduction log(10)AUC days 1 to 29 |
|---|---|---|---|---|---|---|---|---|---|
| Non-artemisinin- based | Kolaczinski 2012 | a: CQ | 0.5 | 732.98 | 8777.24 | 91.7 | 2.87 | 3.94 | 27.3 |
| Kolaczinski 2012 | b: SP | 0.5 | 2111.55 | 12847.37 | 83.6 | 3.32 | 4.11 | 19.1 | |
| Artemisinin- based | Shekalaghe 2007 | AS+SP | 0.75 | 40.30 | 87.69 | 54.0 | 1.61 | 1.94 | 17.4 |
| Smithuis 2010 | a: AS+AQ | 0.75 | 141.08 | 649.46 | 78.3 | 2.15 | 2.81 | 23.6 | |
| Smithuis 2010 | b: AL | 0.75 | 197.57 | 318.23 | 37.9 | 2.3 | 2.5 | 8.3 | |
| Smithuis 2010 | c: AS+MQ fixed | 0.75 | 240.79 | 535.40 | 55.0 | 2.38 | 2.73 | 12.7 | |
| Smithuis 2010 | d: AS+MQ loose | 0.75 | 183.51 | 321.96 | 43.0 | 2.26 | 2.51 | 9.7 | |
| Smithuis 2010 | e: DHAP | 0.75 | 307.14 | 952.93 | 67.8 | 2.49 | 2.98 | 16.5 | |
| Sutanto 2013 | DHAP | 0.75 | 1363.10 | 3108.05 | 56.1 | 3.13 | 3.49 | 10.3 | |
| Vasquez 2009 | AS+MQ | 0.75 | 526.40 | 349.04 | -50.8 | 2.72 | 2.54 | -7.0 | |
Abbreviations: AUC = area under curve; PQ = primaquine; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AS = artesunate; AQ = amodiaquine; AL = artemether-lumefantrine; MQ = mefloquine; DHAP = dihydroxyartemisinin-piperaquine.
Table 4.
AUC of gametocyte density over time, days 1 to 43 after treatment
| Other malaria treatment type | Trial | Malaria treatment | Dose PQ | AUC with PQ days 1 to 43 | AUC without PQ days 1 to 43 | % reduction AUC days 1 to 43 | log(10)AUC with PQ days 1 to 43 | log(10)AUC without PQ days 1 to 43 | % reduction log(10)AUC days 1 to 43 |
|---|---|---|---|---|---|---|---|---|---|
| Non-artemisinin- based | Kolaczinski 2012 | CQ | 0.5 | 71.02 | 279.13 | 74.6 | 1.85 | 2.45 | 24.3 |
| SP | 0.5 | 85.47 | 445.65 | 80.8 | 1.93 | 2.65 | 27.1 | ||
| Artemisinin- based | Shekalaghe 2007 | AS+SP | 0.75 | 1.16 | 3.22 | 64.1 | 0.06 | 0.51 | 87.5 |
| Smithuis 2010 | AS+AQ | 0.75 | 3.36 | 19.29 | 82.6 | 0.53 | 1.29 | 59.1 | |
| AL | 0.75 | 4.70 | 8.12 | 42.1 | 0.67 | 0.91 | 26.1 | ||
| AS+MQ fixed | 0.75 | 5.73 | 12.75 | 55.0 | 0.76 | 1.11 | 31.4 | ||
| AS+MQ loose | 0.75 | 4.37 | 7.74 | 43.5 | 0.64 | 0.89 | 27.9 | ||
| DHAP | 0.75 | 7.38 | 25.49 | 71.1 | 0.87 | 1.41 | 38.3 | ||
| Sutanto 2013 | DHAP | 0.75 | 32.73 | 83.15 | 60.6 | 1.51 | 1.92 | 21.1 | |
| Vasquez 2009 | AS+MQ | 0.75 | 12.53 | 8.87 | -41.3 | 1.10 | 0.95 | -15.8 | |
Abbreviations: AUC = area under curve; PQ = primaquine; CQ = chloroquine; SP = sulfadoxine-pyrimethamine; AS = artesunate; AQ = amodiaquine; AL = artemether-lumefantrine; MQ = mefloquine; DHAP = dihydroxyartemisinin-piperaquine.
Primaquine plus non-artemisinin-based treatment regimens (Comparison 1)
Eleven trials contributed comparisons to this analysis, of which one trial tested a low dose PQ regimen (Pukrittayakamee 2004). One trial (Khoo 1981) did not report results in a usable manner.
Gametocyte prevalence
There were fewer people with gametocytes (detected by microscopy) in the PQ group at days 8, 15, 22, 29 and 36 (Analysis 1.1). The largest number of trials and comparisons was included at day 8 (RR 0.60, 95% CI 0.50 to 0.73, six trials, 498 participants, nine comparisons) and the effect appeared larger at day 15 (RR 0.31, 95% CI 0.22 to 0.43, four trials, 366 participants, seven comparisons).
The trials included three with CQ or CQ combination partner treatment (Kamtekar 2004; Ledermann 2006 (two comparisons); Kolaczinski 2012); one with SP (Kolaczinski 2012); one with AQ+SP (Arango 2012 (one comparison)); one with MQ (Chen 1993a) and two with quinine (Kamtekar 2004 (one comparison); Pukrittayakamee 2004 (two comparisons)).
Gametocyte clearance time or duration of gametocyte carriage (the average number of days each person has gametocytes)
Gametocyte clearance time (in days) was significantly reduced in the PQ group in Singhasivanon 1994 (which had MQ+SP partner) with a mean difference of -14.90 days (95% CI -18.18 to -11.62). (Analysis 1.2). The median gametocyte clearance time was also reduced in Pukrittayakamee 2004 (two comparisons; partner QN) from 216 hours to 48 hours with 0.5 mg/kg PQ, or 87 hours with 0.25 mg/kg PQ, although results were not presented in a form that could be shown graphically.
AUC of gametocyte density over time
Gametocyte density over time up to day 43 was assessed by microscopy in the trial of Kolaczinski 2012 (two comparisons). We analysed the data further using the AUC and log(10)AUC measures for days 1 to 15, 1 to 29 and 1 to 43, estimated from data provided by the authors (Table 2; Table 3; Table 4)
Reductions in AUC for non-artemisinin malaria treatment regimens were 84.5% and 88.6% up to day 15, 83.6% and 91.7% up to day 29, and 74.6% and 80.8% up to day 43 (one trial, two comparisons). Using the log(10)AUC, for non-artemisinin malaria treatment regimens the estimates were 21.4% and 26.0% to day 15, 19.1% and 27.3% to day 29, and 24.3% and 27.1% to day 43 (one trial, two comparisons).
Infectiousness to mosquitoes
Two small trials in China (Chen 1993a; Chen 1994), with only six and nine participants per group respectively, directly tested the impact of PQ added to MQ on infectiousness to mosquitoes. On day 1 all patients in the trial were infectious to Anopheles dirus mosquitoes, but after a dose of PQ on day 1 the proportion of people infectious was reduced to 0 when measured on days 2, 5 and 8 (Analysis 1.3). By day 15 and day 22 the difference was attenuated as infectiousness in the control group declined.
Chen 1994 also reported the number of mosquitoes infected after feeding on trial participants (Analysis 1.4; note the CIs are not corrected for repeated observations in the same patients). None of the mosquitoes feeding on people receiving PQ were infected, with over 64% infected at day 5 after feeding on the group not receiving PQ, with the effect still evident up to day 22, although the proportion infected in the control group declined over time.
Asexual parasites at day 29 (recrudescence or reinfection)
In Kolaczinski 2012 there was no effect of PQ (added to either CQ or SP) on prevalence of asexual parasites at day 29 (parasitological treatment failure), whether or not the results were adjusted for new infections detected by PCR (Analysis 1.5).
Asexual parasite clearance
There was no effect of PQ on asexual clearance time in Singhasivanon 1994 (added to MQ+SP) or Pukrittayakamee 2004 (two comparisons, added to QN) (Analysis 1.6).
Adverse effects
Patients with G6PD deficiency were excluded (two trials), the only patients included (one trial), not screened for (two trials), or not reported or commented on (six trials).
The trials with non-artemisinin regimens did not report adverse effects well or consistently. None of these trials reported on haemolysis, other haematological measures or severe adverse events.
Singhasivanon 1994 found no difference in frequency of reported adverse effects (nausea, vomiting or dizziness) over 28 days follow-up (Analysis 1.7).
We could not use data from one trial with non-artemisinin partner, CQ, because it did not distinguish between patients with P. falciparum and P. vivax and their respective treatments (Khoo 1981). There was a much higher risk of adverse haemolytic events in those who received PQ in the Khoo 1981 trial (OR of 22.27 for both haemolysis and need for blood transfusion), but we could not include the results because the groups combined participants receiving a short course (three days) of PQ with those receiving a 14-day regimen. The most unusual aspect of the trial, however, is that it only included individuals with G6PD deficiency.
Dose and schedule
Dose of PQ: We stratified trials into low, medium and high dose category PQ. Only Pukrittayakamee 2004 used the low dose category (0.25 mg/kg per day, given for seven days in conjunction with QN). It used medium dose category (0.5 mg/kg) at the same schedule also with QN, while Kolaczinski 2012 used the 0.5 mg/kg medium dose in two comparisons (with CQ and SP). Three trials used the high dose category 0.75 mg/kg of PQ: Arango 2012 in one comparison with AQ + SP); Ledermann 2006 in two comparisons, both with CQ+SP, and Kamtekar 2004 in one comparison with CQ or (CQ+SP).
With the low dose, there was no difference detected between the groups with and without PQ (Analysis 1.8, one trial), even though this dose of PQ was given for seven days (RR 1.76, 95% CI 0.97 to 3.18, 59 participants, one trial).
Both the medium and high dose reduced the prevalence of gametocytes at day 8: RR = 0.62 (95% CI 0.50 to 0.76) for medium dose, three comparisons; and RR 0.39 (95% CI 0.25 to 0.62 for high dose, five comparisons) (Analysis 1.8).
Schedule of PQ: We stratified this comparison into three groups: single dose on day 1 or 2, single dose day 3 or 4, and multiple doses day 1 to 7. The comparison is indirect, although the schedule on day 3 or 4 seemed to have a greater effect. One arm of Ledermann 2006 received PQ on day 1 and the other on day 3. There was no apparent difference in the outcome between these two arms (Analysis 1.9).
Primaquine plus artemisinin-based treatment regimens (Comparison 2)
Nine trials contributed comparisons to this analysis, including ACTs (seven trials) and artemisinin monotherapy (two trials) for malaria treatment. Only one trial tested a low dose PQ regimen, 0.1 mg/kg total dose.
Gametocyte prevalence
Microscopy analysis revealed that PQ clearly reduced the number of people with gametocytes on day 8 (RR 0.24, 95% CI 0.10 to 0.55, six trials, 1121 participants, 10 comparisons), day 15 (RR 0.09, 95% CI 0.04 to 0.19, four trials, 995 participants, eight comparisons), day 22 (RR 0.10, 95% CI 0.03 to 0.32, three trials, 858 participants, seven comparisons, four with estimable results, 507 participants) and day 29 (RR 0.17, 95% CI 0.04 to 0.72, four trials, eight comparisons, four with estimable results) (Analysis 2.1). We used the random-effects model due to heterogeneity.
In Smithuis 2010, new gametocytaemia (by microscopy) on day 8 was also reduced by PQ (one of 272 versus 10 of 268; RR 0.1, 95% CI 0.01 to 0.76, P = 0.006).
Three trials examined gametocytes by PCR rather than (or in addition to) microscopy. In Shekalaghe 2007 and Eziefula 2013, a reduction in gametocyte prevalence was observed on day 8 and day 15 (Analysis 2.2). However, in El-Sayed 2007, giving PQ did not lead to a detectable difference between the two groups on these two follow-up days, although there were very few participants with gametocytes in the control group. Given the clear statistical and clinical heterogeneity between the two estimates (related to different numbers of participants with gametocytes in the comparator arm in these two studies) we used the random-effects model to combine the trials in meta-analysis (Analysis 2.2). In Shekalaghe 2007 which had additional follow-up on day 29, reduction in gametocyte prevalence was significant (RR 0.23, 95% CI 0.08 to 0.62, one trial, 90 participants), and on day 43, it was not (one trial, 79 participants, Analysis 2.2).
Note that the trial of Eziefula 2013 was reported as a non-inferiority analysis comparing lower dose groups with the previously recommended 0.75 mg/kg. In this review, in order to combine trials for analysis, results in each arm have been compared with the single placebo group, and where meta-analysis is done, numbers in the placebo group have been divided into three to avoid biasing the CIs.
Gametocyte clearance time or duration of gametocyte carriage (the length of time each person has gametocytes)
Several authors presented gametocyte clearance time, sometimes described as "duration of gametocyte carriage". This was presented in Shekalaghe 2007 and was significantly lower (by PCR) in the PQ group (6.3 days, 95% CI 4.7 to 8.5) than in the non-PQ group (28.6 days, 95% CI 17.0 to 48.0, P < 0.001). In Eziefula 2013, also by PCR, the gametocyte clearance time was not significantly longer in the 0.1 mg/kg group (8.0 days, 95% CI 6.6 to 9.4) or the 0.4 mg/kg group (6.3 days, 95% CI 5.1 to 7.5) than the 0.75 mg/kg group (6.6 days, 95% CI 5.3 to 7.8); however this group had significantly shorter gametocyte clearance time than the placebo group (12.4 days, 95% CI 9.9 to 15.0). Smithuis 2010, using microscopy, also reported significantly lower gametocyte clearance time in the PQ groups, reported as person-gametocytaemia-weeks standardized per 1000 person-weeks of follow-up. This was 5.5 weeks in the ACT+PQ groups versus 65.5 weeks in the non-PQ groups (RR 11.9, 95% CI 7.4 to 20.5, P < 0.001) and the difference was very large for each individual malaria treatment regimen. Although the duration of gametocyte carriage (without PQ) was significantly longer for AS+AQ, AL and DHAP than for AS+MQ, there was no significant difference in length of gametocyte carriage between the ACT groups when PQ was added (Smithuis 2010).
Gametocyte circulation time
Another outcome related to gametocytes estimated by PCR in Eziefula 2013 and Shekalaghe 2007 was the mean life (circulation time) of gametocytes. In Eziefula 2013 the circulation time per gametocyte was significantly longer in the 0.1 mg/kg group (1.47 days, 95% CI 1.22 to 1.73) than in the other two groups (0.95 and 0.98 days in the 0.4 and 0.75 mg/kg groups respectively), and was similar to the placebo group (1.97 days, 95% CI 1.64 to 2.31). In Shekalaghe 2007, the mean gametocyte circulation time was reduced from 4.6 days (95% CI 2.9 to 7.3) after AS+SP alone to 0.5 days (95% CI 0.2 to 1.2) after AS+SP plus PQ (P < 0.001).
AUC of gametocyte density over time
Gametocyte density over time was assessed by microscopy in the artemisinin-based regimen trials of Shekalaghe 2007, Smithuis 2010 (five comparisons), Sutanto 2013 and Vasquez 2009, and we analysed this further using data provided by the authors (Table 2; Table 3; Table 4). All trials except Vasquez 2009 demonstrated reduction in the AUC after PQ. The reduction ranged from -63.7% to 67.3% up to day 15, -50.8% to 91.7% up to day 29, and from -41.3% to 82.6% up to day 43. Using the log(10)AUC, the reduction ranged from -8.6% to 18.4% up to day 15, from -7.0% to 27.3% up to day 29 and -15.8% to 87.5% up to day 43.
Vasquez 2009 was an exception suggesting an increase in AUC after PQ, possibly due to the small sample size and differing mean gametocyte counts by group at baseline in this trial. Excluding Vasquez 2009, reductions in AUC varied from 19.2% to 67.3% for days 1 to 15, from 37.9% to 91.7% for days 1 to 29, and 42.1% to 82.6% for days 1 to 43, using the mean gametocyte density. Using the log(10)AUC, the reduction ranged from 3.9% to 18.4% for days 1 to 15, 8.3% to 27.3% for days 1 to 29 and 24.3% to 87.5% for days 1 to 43.
Eziefula 2013 used a duration of 14 days to estimate AUC using PCR, but the results were not reported separately by group and day so cannot be shown in Table 2. However the log(10)AUC in the intervention groups were not significantly different from placebo. It was 3.8 (95% CI 1.7 to 8.2) gametocytes per μL per day in the placebo group, 3.8 (1.8 to 7.8) in the 0.1 mg/kg group, 2.1 (1.0 to 4.5) in the 0.4 mg/kg group, and 2.0 (0.9 to 4.3) in the 0.75 mg/kg group.
Using PCR-detected gametocyte density estimates, Shekalaghe 2007 provided geometric mean and interquartile range (IQR) values on days 1, 4, 8, 15, 29 and 43. Mean density was consistently lower in the PQ than the non-PQ group, for days when gametocytes were detected (with PQ: 5.8, IQR 0.8 to 55.1; without PQ: 15.8, IQR 4.1 to 85.8).
Shekalaghe 2007 also presented a statistical comparison of AUC of gametocyte density (by PCR) over a 43-day period, with a 95% CI derived from generalized estimation equations. There was a significant reduction in AUC in the PQ groups over 43 days after treatment, reported as mean of 1.5 (IQR 0.3 to 8.8) in the PQ group versus 11.1 (IQR 2.2 to 53.8) in the non-PQ group (P < 0.001).
Asexual parasite prevalence
Analysis 2.3 shows the participants who had asexual parasites at several time points after treatment. This analysis suggests a lower proportion of asexual parasites at day 29 in the PQ group reflecting possible recrudescence, late treatment failure or reinfection. However, Wang 2006 did not adjust for reinfections by PCR. In the other trials and time periods, there was no difference between PQ and non-PQ groups in the low proportion of people with asexual parasites.
Asexual parasite clearance
Pukrittayakamee 2004 noted no difference in parasite clearance time (Analysis 2.4).
Adverse effects
For haematologic adverse events, Smithuis 2010 stated that there were no cases of severe anaemia (< 5 g/dL) or blackwater fever in any group. El-Sayed 2007 showed that there was no difference in packed cell volume between groups at day 7 (34.2% (15% to 44%) versus 36.2% (26% to 42%)) or day 14 (35.2% versus 35.4%). The difference was not significant at either day 7 (0.78, (-0.75 to 0.23), P = 0.32) or day 14 (0.86, (-0.31 to 2.0), P = 0.15). Sutanto 2013 observed no significant difference in mean haemoglobin between groups at days 1, 8 and 43.
In Shekalaghe 2007, although there was also no reduction in mean haemoglobin by group (Analysis 2.5), there was a significantly greater change (decrease) in haemoglobin status in the PQ group on day 8; haemoglobin decreased by 5% in the PQ group compared to 1% in the non-PQ group (Analysis 2.6). These findings suggest that rather than looking at population mean of haemoglobin, it would be more meaningful to examine the proportion of individuals who had serious adverse events: Shekalaghe 2007 stated that 8 of 52 children in the PQ group had a 20% reduction in haemoglobin by day 8, compared to 0 of 53 children in the control group. However, Shekalaghe 2007 also stated that no child developed clinical symptoms related to anaemia or a haemoglobin below 5 g/dL. The effect on haemoglobin in the PQ group was transient and was no longer significant by day 15.
Eziefula 2013 used the outcome 'mean max decrease in haemoglobin' from enrolment to day 29 and this was not significantly different between the intervention groups or with placebo. Other haematological outcomes were 'day of haemoglobin nadir' - day 3 in all groups; maximum % decrease in haemoglobin (significantly lower in the 0.75 mg/kg group than placebo, P = 0.023) and % of patients with haemoglobin < 50 g/L (no differences between groups). No children needed blood transfusion or had black urine or any other severe adverse events.
El-Sayed 2007 assessed the minor adverse effects of vomiting, insomnia and itching and found no difference between groups. Smithuis 2010 found a higher percentage of patients in the PQ groups had abdominal pain (16%; N = 397 versus 11%; N = 411, P = 0.05); frequencies of dizziness, nausea, anorexia, diarrhoea, palpitations, sleeplessness, headache and vomiting were not increased in the PQ groups. Sutanto 2013 and Wang 2006 observed no differences between groups regarding other adverse effects (Analysis 2.7).
Dose and schedule
Dose of PQ: We stratified trials into low, medium and high dose PQ category and assessed the prevalence of gametocytes at day 8 by either microscopy or PCR (Analysis 2.8).
Only Eziefula 2013 used a low dose category - in this case 0.1 mg/kg. There were fewer patients with gametocytes in the PQ group, but the analysis was underpowered (Analysis 2.8). Two trials used a medium dose category, with no detectable effect in one trial with few patients with PQ in the control group (Pukrittayakamee 2004); but with clear effect in Eziefula 2013 with 0.4 mg/kg single dose (Analysis 2.8), and an overall effect estimate in this dose category of RR 0.34 (95% CI 0.19 to 0.59; two trials, 269 participants). The high dose category of ≥ 0.6 mg/kg reduced the prevalence of gametocytes at day 8: RR = 0.29 (0.22 to 0.37), seven trials, 1380 participants, 11 comparisons; Analysis 2.8.
Schedule of PQ: We stratified this comparison into three groups: single dose on day 1 or 2, single dose day 3 or 4, and multiple doses day 1 to 7 (Analysis 2.9). This indirect comparison suggested a greater effect of single dose on days 1 to 2 than on days 3 to 4. There was only one trial of multiple doses on days 1 to 7.
Summary analysis of gametocytes on day 8 (Comparison 3)
We have shown a single outcome, percent of participants with microscopy or PCR-detected gametocytes on day 8, for this comparison as a representative outcome across the trials. We subgrouped the data by whether or not the malaria treatment was artemisinin-based. We included only PCR data from the one trial that reported both microscopy and PCR outcomes, to avoid duplicate reporting of the same patients (Shekalaghe 2007).
We excluded the QN comparison of Kamtekar 2004 because PQ was not given until day 8, but included all other trials with gametocyte outcomes (all trials except Khoo 1981 and Wang 2006).
The day on which PQ was given and the gametocyte detection method varied and are presented for each trial in the footnotes in Analysis 3.1. This comparison shows that, overall, PQ reduced the prevalence of gametocytaemia on day 8 following treatment (RR 0.43, 95% CI 0.32 to 0.58, 12 trials, 2141 participants). The individual trials nearly all trended in the direction of a reduction, but an indirect comparison suggested a smaller effect for non-artemisinin-based (RR 0.63, 95% CI 0.44 to 0.88, six trials, 499 participants) than for artemisinin-based malaria treatments (RR 0.33, 95% CI 0.21 to 0.52, eight trials, 1642 participants, Analysis 3.1).
Comparison of different 8AQ (Comparison 4)
Two small trials compared the effect of bulaquine and PQ on gametocyte prevalence at day 8 (Gogtay 2004; Gogtay 2006). Both trials suggested a greater reduction of gametocytes by bulaquine (OR 0.41 95% CI 0.26 to 0.66, two trials, 112 participants, Analysis 4.1). Neither trial concealed allocation.
ADDITIONAL SUMMARY OF FINDINGS [Explanation]
| PQ for reducing P. falciparum transmission with non-artemisinin-based treatments | |||||
| Patient or population: People with symptomatic malaria | |||||
| Settings: Malaria-endemic areas | |||||
| Intervention: Single dose or short course PQ plus malaria treatment which does not include an artemisinin derivative | |||||
| Control: Malaria treatment not including an artemisinin derivative, without PQ | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | Number of participants(trials) | Quality of the evidence(GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | PQ | ||||
| Malaria incidence, prevalence or EIR | - | - | - | - (0 trials) | - |
| People infectious to mosquitoes (day 8) | 93 per 100 | 7 per 100 (1 to 39) | RR 0.07 1 (0.01 to 0.45) | 30(2 trials) | ⊕⊕○○ low2,3 |
| Participants with gametocytes on microscopy (day 8) | Dose < 0.4 mg/kg | - | - (0 trials) | - | |
| Dose 0.4 to 0.6 mg/kg | RR 0.60 (0.49 to 0.75) | 216 (1 trial) | ⊕⊕⊕⊕ high 4,5 | ||
| 70 per 100 | 43 per 100 (35 to 53) | ||||
| Dose = 0.6 mg/kg | RR 0.39 (0.25 to 0.62) | 186 (4 trials) | ⊕⊕⊕⊕ high 6,7 | ||
| 35 per 100 | 14 per 100 (9 to 22) | ||||
| Evidence of haemolysis | - | - | - 0 trials6,7 | - | |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| PQ: Primaquine; CI: Confidence interval; RR: Risk ratio; EIR: entomological inoculation rate; G6PD: glucose-6-phosphate dehydrogenase. | |||||
| GRADE Working Group grades of evidence | |||||
| High quality: Further research is very unlikely to change our confidence in the estimate of effect. | |||||
| Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||
| Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | |||||
| Very low quality: We are very uncertain about the estimate. | |||||
1High category dose PQ used in both studies = 0.6 mg/kg.
2Downgraded by 1 for serious risk of bias: allocation was not concealed; both experiments were performed by the same author team and the methods were unclear.
3Downgraded by 1 for serious imprecision: the sample size is small (N = 30; downgraded by 2) but the effect is large (upgraded by 1). In the control group, infectivity wanes by day 15.
4No serious imprecision: although the sample size is limited, the effect is large.
5Log(10)AUC day 1 to 43 % reduction relative decrease: 2 estimates from one trial of 24.3% to 27.1% (N = 219), assessed as moderate quality evidence.
6We could not use data from Khoo 1981 because it did not distinguish between patients with P. falciparum and P. vivax and their respective treatments. There was a much higher risk of adverse haemolytic events in those who received PQ (OR of 22.27 for both haemolysis and need for blood transfusion), but the participants included those receiving a short course (three days) of PQ with those receiving a 14-day regimen.
7For the 10 included trials, the G6PD status of participants varied: two trials excluded patients with G6PD deficiency trials), one trial included only those with G6PD deficiency, two trials did not screen for G6PD deficiency, and five trials did not report or comment on screening for G6PD status. No trials systematically reported on adverse effects.
Discussion
Summary of main results
PQ with artemisinin-based regimens
See Summary of findings for the main comparison.
When added to artemisinin-based therapy, a single dose of PQ above 0.4 mg/kg reduces the proportion of people with detectable gametocytaemia on day eight by around two thirds (high quality evidence), and can reduce the log(10) AUC estimates for gametocytaemia by up to 87.5%. However, only a single trial evaluated lower doses (0.1 mg/kg) and did not demonstrate an effect (low quality evidence). No trials evaluated effects on infectiousness to mosquitoes, or on malaria transmission directly (incidence, prevalence, or entomological inoculation rate).
PQ with non-artemisinin-based regimens
See Summary of findings 2.
When added to non-artemisinin-based treatment regimens, a single dose of PQ above 0.4 mg/kg reduces the proportion of people with detectable gametocytaemia on day 8 by around a third (high quality evidence), and may reduce infectiousness to mosquitoes (low quality evidence).The single trial of lower doses (0.25 mg/kg) did not demonstrate an effect on gametocytaemia (very low quality evidence). No trials assessed effects on malaria transmission directly.
Overall completeness and applicability of evidence
What is known
Adding PQ to treatment doses of artemisinin-based combination therapies appears to be an effective strategy to reduce the duration of potential infectiousness to mosquitoes (gametocytaemia). Large effects have been seen across trials from different epidemiological settings (Tanzania, Uganda, Myanmar, and Sumatra), and with different artemisinin-based treatments (AS+SP, AL, AS+AQ, AS+MQ, AS, and DHAP). Further studies evaluating this at single doses above 0.4 mg/kg are therefore probably unnecessary.
What is unknown
While reducing the duration of gametocytaemia can be assumed to reduce infectiousness at the level of the individual, it remains unclear whether it will impact on community level transmission as reliable trials have never evaluated this. The older trials, which are often cited as proof of an effect on community transmission were excluded from this review because either: i) they lacked an adequate control group (Clyde 1962), ii) did not apply the interventions equally in intervention and control groups or had no 'before' data (Doi 1989; Kaneko 1989), iii) administered PQ alongside vector control co-interventions which may equally be responsible for any effect seen (Hii 1987; Kaneko 2000), or iv) administered PQ alone and not alongside treatment regimens (which is the policy currently being considered) (Barber 1932).
In moderate and high transmission settings, a policy of adding PQ to malaria treatment regimens is highly unlikely to impact community transmission as most adults have a level of acquired immunity that reduces the probability that parasitaemia will cause clinical symptoms, and consequently they never seek care. These asymptomatic people are thought to represent the major source of gametocytes, and so will continue to facilitate transmission unless they are also treated with gametocytocidal drugs. Low transmission settings, especially those approaching elimination, are therefore more suited to the current policy as most people will develop symptoms following infection. However, Johnston and colleagues recently modelled the effect of PQ in low-transmission areas, and concluded that the most important factor predicting success is the percentage of infected individuals treated with an ACT, and that adding PQ is of negligible benefit (Johnston 2014). They estimated that "it would require switching 180 people from ACTs to ACTs plus PQ to achieve the same transmission reduction as switching a single individual from untreated to treated with ACTs".
Mass treatment programmes (where everyone in the community is treated at specified intervals until transmission is stopped) are a potential strategy to overcome the problem of asymptomatic gametocyte carriers (von Seidlein 2003; GMAP 2008; Mendis 2009; Sturrock 2013). However, in theory, mass treatment consisting only of primary treatment (an ACT alone), would interrupt transmission, and the effects of additional PQ (interrupting transmission more quickly, or at a lower coverage levels) are unknown (Poirot 2013).
Perhaps even more importantly, it also remains unknown whether the current WHO-recommended dose of 0.25 mg/kg PQ has the effects seen on gametocytes with higher doses. Whilst there is some indirect evidence in narrative summaries and analyses that have proposed widespread programmatic implementation of PQ (for example, Bunnag 1980, with no difference in gametocyte outcomes between 15 mg PQ for five days and single doses of 30 mg or 45 mg PQ in adults), the only two controlled studies which have evaluated doses below 0.4 mg/kg failed to demonstrate reliable effects. The WHO revised recommendation therefore appears to be based more on concerns about safety than on proven efficacy.
In terms of safety, it is highly likely that adverse effects in people with G6PD deficiency will be less common with lower doses of PQ. However, the evidence to reliably demonstrate this with doses 0.25 mg/kg does not currently exist. Most of the trials included in this review excluded individuals with G6PD deficiency, but in the few trials that included them no severe haematological effects have been reported. However, there are hundreds of variants of G6PD deficiency (albeit only a few that are common) and an adequate picture of how PQ affects even the common ones is lacking. Without this evidence, national malaria control programmes may remain reluctant to introduce a drug for use in population-based programmes which has the potential to harm individuals. Health professionals and programme managers around the world are aware of the association between haemolysis, G6PD deficiency and PQ and will be wary of expanded use without evidence of relative safety.
The trials included in this review do not mention PQ resistance as a potential threat. PQ clearly should be used where it is of clinical importance, especially for P. vivax and P. ovale. Its effectiveness against those parasites could be compromised by resistance if used at low doses in populations where P. falciparum, P. vivax and P. ovale coexist. As for all antimalarial drugs, there is a global responsibility to maintain the effectiveness of PQ for as long as possible without withholding it when needed. In this case, that translates into using it to reduce transmission only if there is reasonable evidence that it actually has that effect. Otherwise, it will be used to little or no effect but its value for radical cure may be diminished by the development of resistant Plasmodium parasites.
Potential biases in the review process
None known.
Agreements and disagreements with other studies or reviews
In a Cochrane Review of mass drug administration (MDA) (Poirot 2013), the authors found no studies directly comparing MDA regimens that included 8AQs with regimens that did not. In a secondary analysis, the authors then subgrouped included non-randomized controlled trials by regimens with and without 8AQs. In high endemicity areas, two studies included PQ, and one study did not. During multiple MDA rounds, there were substantial drops in parasitaemia regardless of whether PQ was included. At 1 to 3 months, the studies without PQ showed larger impact than the one study that did, but this single observation from a non-randomized comparison cannot be relied upon. In a second similar subgroup analysis of four before and after studies, the stratified analysis was uninformative.
In an opinion piece in the Lancet, the possibility that doses lower than 0.5 to 0.75 mg/kg might still be very effective in blocking transmission is raised (White 2013). Based on the current review, however, there is insufficient information to know whether the effect is preserved with doses of less than 0.4 mg/kg.
A review in the Malaria Journal provides an analysis of published and unpublished data on 158 subjects with different drug exposures spanning 80 years (White 2012). The methods and sources of data are not clearly specified, but the authors argue this provides evidence that PQ decreases infectivity much faster than the effect on gametocytes would suggest. Our review identified very limited data from controlled studies testing the effect of PQ on infectiousness to mosquitoes (2 trials, with 30 participants, in people given non-artemisinin treatments), so was not able to confirm or refute this early effect on gametocyte viability.
A paper whose title implies it was a systematic review of the impact of artemisinin derivatives and PQ on infectiousness (Abay 2013) included only two before-and-after trials using PQ with small numbers of patients. Neither of these studies met our inclusion criteria (Rieckmann 1968; Clyde 1971). A historical review of patients treated at clinics in India with either AS+SP+PQ (single dose 0.75 mg/kg on the third treatment day, nine sites) or AS+SP alone (12 sites) observed that PQ reduced the gametocyte clearance time by 45% and the AUC of gametocyte density over time (up to 28 days) by about the same proportion (Shah 2013). They expressed the reduction as a hazard ratio with PQ increasing the rate of gametocyte clearance by 1.9 (95% CI 1.1 to 1.3). These results are consistent with our findings.
In relation to current WHO guidelines, no direct evidence was available to include in this systematic review to support the currently recommended 0.25 mg/kg dose as effective in reducing transmission either from individuals or in communities (WHO 2012b). Ongoing studies may help inform this current gap in our knowledge (Eziefula 2012).
Authors' conclusions
Implications for practice
Current policy recommendations that 0.25 mg/kg PQ should be added as a single dose to primary treatment for P. falciparum malaria in areas that are targeting elimination or are facing artemisinin resistance are based on judgements and inferences rather than reliable evidence of an effect at this dose.
Decisions must be specific to the country or area where the introduction is being considered. In a high endemicity area, the dilutional effects are likely to be important. If PQ is given as part of clinical treatment in a low endemicity area, what might be important is whether most people with parasitaemia are likely to be seen at clinic. Thus the evidence presented in this review is likely to be used to lead to different decisions depending on a variety of epidemiological, malarial and logistic factors.
Whilst harm at the currently recommended dose of 0.25 mg/kg is likely to be much less than at higher doses, these trials are insufficient to evaluate whether the drug is safe (for widespread use) at any dose.
Implications for research
Future RCTs of low dose PQ should include measures of infectiousness to mosquitoes over an extended period including the days immediately following treatment, and stratify the analysis by participants with and without gametocytes at baseline.
The impact on individuals with G6PD deficiency (an agreed upon set of the most widespread genotypes and those with the greatest likelihood for harm) should be assessed.
Given the high frequency of asymptomatic infections, information on individual patients should be combined with modelling or community-based trials of treatment regimens with and without PQ to determine the malaria and population characteristics under which PQ is likely to be an important additional contributor to malaria elimination programmes (including mass treatment and 'test and treat').
Acknowledgments
We thank authors of Shekalaghe 2007, Vasquez 2009, Smithuis 2010, Kolaczinski 2012 and Sutanto 2013 for providing unpublished data for this review. Dr Isabela Ribeiro assisted with assessing trials in Portuguese for inclusion. Dr Adam Ye and Dr Yang Wu helped with translation of Chinese trials and Dr Nick White provided links to Chinese trials. Dr David Sinclair was the Academic Editor for this review, and assisted with the Summary of Findings tables and advised on structuring the discussion in this revision. We are grateful to our affiliated institutions and organizations, and thank the referees and editors for their comments and encouragement. The editorial base for the Cochrane Infectious Disease Group is funded by the Department for International Development (DFID), UK, for the benefit of developing countries.
APPENDICES
Appendix 1. Search strategy
| Search set | CIDG SR1 | CENTRAL | MEDLINE2 | EMBASE2 | LILACS2 |
|---|---|---|---|---|---|
| 1 | malaria | MALARIA, FALCIPARUM/ DRUG THERAPY | MALARIA, FALCIPARUM/ DRUG THERAPY | MALARIA FALCIPARUM/DRUG THERAPY | Malaria AND falciparum |
| 2 | primaquine OR pamaquine OR plasmoquine OR plasmochin OR plasmocide OR rhodoquine OR plasmocid OR quinocine OR pentaquine OR isopentaquine OR bulaquine OR tafenoquine OR 8-aminoquinoline* | Malaria AND falciparum ti, ab | Malaria AND falciparum ti, ab | Malaria AND falciparum ti, ab | primaquine OR pamaquine OR plasmoquine OR plasmochin OR plasmocide OR rhodoquine OR plasmocid OR quinocine OR pentaquine OR isopentaquine OR bulaquine OR tafenoquine OR 8-aminoquinoline$ |
| 3 | 1 and 2 | 1 or 2 | 1 or 2 | 1 or 2 | 1 and 2 |
| 4 | - | PRIMAQUINE/ ADMINISTRATION AND DOSAGE/ THERAPEUTIC USE | PRIMAQUINE/ ADMINISTRATION AND DOSAGE/ THERAPEUTIC USE | PRIMAQUINE | - |
| 5 | - | primaquine OR pamaquine OR plasmoquine OR plasmochin OR plasmocide OR rhodoquine OR plasmocid OR quinocine OR pentaquine OR isopentaquine OR bulaquine OR tafenoquine OR 8-aminoquinoline* ti, ab | primaquine OR pamaquine OR plasmoquine OR plasmochin OR plasmocide OR rhodoquine OR plasmocid OR quinocine OR pentaquine OR isopentaquine OR bulaquine OR tafenoquine OR 8-aminoquinoline* ti, ab | primaquine OR pamaquine OR plasmoquine OR plasmochin OR plasmocide OR rhodoquine OR plasmocid OR quinocine OR pentaquine OR isopentaquine OR bulaquine OR tafenoquine OR 8-aminoquinoline* ti, ab | - |
| 6 | - | 4 or 5 | 4 or 5 | 4 or 5 | - |
| 7 | - | 3 and 6 | 3 and 6 | 3 and 6 | - |
| 8 | - | - | Limit 7 to Humans | Limit 7 to Human | - |
1Cochrane Infectious Diseases Group Specialized Register.
2Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2011); Upper case: MeSH or EMTREE heading; Lower case: free text term.
WHAT'S NEW
Last assessed as up-to-date: 5 January 2015.
| Date | Event | Description |
|---|---|---|
| 4 February 2015 | New citation required but conclusions have not changed | Search updated and minor errors corrected. |
| 4 February 2015 | New search has been performed | Search updated to 5 January 2015. We simplified the text. We corrected data extraction errors in one study and corrected the results and 'Summary of findings' table. We adjusted the wording around "evidence of no effect". We took into account comments and criticisms received. These criticisms were also published in the Malaria Journal in 2014, so this revision corrects the minor data extraction errors pointed out in this article. The conclusions are not changed. |
HISTORY
Protocol first published: Issue 4, 2009
Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 24 June 2014 | New search has been performed | New studies added. |
| 24 June 2014 | New citation required and conclusions have changed | We stratified the analysis by dose or primaquine and added new studies. We clarified the excluded studies and adjusted the conclusions. |
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
Arango 2012
| Methods | Quasi-RCT: alternate allocation to AQ+SP and AQ+SP+PQ, then MQ+AS and MQ+AS+PQ | |
| Participants | Inclusion criteria: 1. Uncomplicated malaria 2. Pf only 3. Not pregnant 4. Voluntary consent Colombia 82 patients, aged one to 75 years (mean age ranged from 24 to 35 years in four groups) Gametocytes in 23/82 (28%) | |
| Interventions | All loose combinations: 1. AQ+SP 2. AQ+SP+PQ 3. MQ+AS 4. MQ+AS+PQ AQ: 25 mg/kg total dose divided into 10 mg/kg on day 1 and 7.5 mg/kg on days 2 and 3 SP: 25 mg/kg/1.25 mg/kg single dose on day 1 MQ: 25 mg/kg total dose, divided into 8.3 mg/kg per day for 3 days AS: 4 mg/kg per day for 3 days PQ: 0.75 mg/kg, total single dose on day 2 | |
| Outcomes | Day 1 (pretreatment with schizonticide), 4 and 8 Asexual and gametocyte counts in thick smears Gametocyte prevalence Gametocyte density | |
| Notes | No mention of G6PD status | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Nothing obvious. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
Chen 1993a
| Methods | RCT | |
| Participants | 18 participants with P. falciparum | |
| Interventions | A: MQ 750 mg B: MQ 750 mg + SP (1500 mg/75 mg) C: MQ 750 mg + PQ 45 mg All single doses Follow-up: 28 days for gametocytes and 21 days for infectiousness | |
| Outcomes | Gametocyte prevalence at days 3, 8, 14 and 21 Infectiousness to An. dirus Sporozoite infections in mosquitoes Infectivity of sporozoite-infected mosquitoes to subsequent patients | |
| Notes | Only abstract available. Mosquitoes fed on the patients were allowed to develop sporozoites which were then fed on uninfected people. One of the MQ + PQ group passed the infection to a new person. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors stated it was randomized in abstract. |
| Allocation concealment (selection bias) | Unclear risk | No information in abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up. |
| Selective reporting (reporting bias) | Unclear risk | No information in abstract. |
| Other bias | Unclear risk | None known. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated to be double blind in title. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information in abstract. |
Chen 1994
| Methods | Possibly individually RCT (stated to be randomized but no information given). Dates of trial not reported. | |
| Participants | 27 patients with slide positive P. falciparum including both asexual stages and gametocytes. No information given on age or sex. All dosages appear to be adult dosages. Site: malaria-endemic Hainan Island, China. Exclusion criteria: history of antimalarial treatment for present attack. | |
| Interventions | 1. Artemisinin: 1200 mg per day for 5 days (not included in review) 2. MQ 750 mg single dose day 1 (reported as day 0) 3. MQ 750 mg single dose + PQ 45 mg single dose day 1 (reported as day 0) | |
| Outcomes | 1. Gametocyte density: days 5, 8, 15, 22 and 29 (reported in paper as days 4, 7, 14, 21 and 28 since first day was day 0). Given as % of initial density on chart only 2. Percentage of participants infectious to An. dirus: days 5, 8, 15 and 22 (reported as days 4, 7, 14 and 21) 3. Percentage of mosquitoes infected: days 5, 8, 15 and 22 (reported as days 4, 7, 14 and 21) | |
| Notes | For gametocyte density, graph only of percentages; no raw numbers given except range of asexuals and gametocyte numbers reported for each group on day 1 (reported as day 0). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation. Trial authors described process as participants "divided into groups A, B, and C". Equal number in each group and lack of detail suggests randomization not done adequately. |
| Allocation concealment (selection bias) | High risk | No data to suggest any measures to conceal allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing participants in intervention groups 2 and 3. |
| Selective reporting (reporting bias) | Low risk | No obvious selective reporting. |
| Other bias | Low risk | No indication of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
El-Sayed 2007
| Methods | Individually RCT Dates of trial: Randomization June 2004; trial done 17 August 2004 to 3 September 2004. | |
| Participants | 104 people with asymptomatic P. falciparum positive by slide and positive for gametocytes by PCR. No information given on age and sex. Site: Two villages in East Sudan where there is seasonal malaria, mainly P. falciparum, during October to December. Exclusion criteria: Pregnancy, history of sulfa allergy, fever or other symptoms, Plasmodium spp other than P. falciparum present. | |
| Interventions | 1. AS: Children < 50 kg: 4 mg/kg; All = 50 kg: 200 mg (two 100 mg tabs) days 1, 2, and 3 (reported as days 0, 1, and 2). SP: Children < 50 kg: 25 mg/kg S + 1.25 mg/kg P; All = 50 kg: 3 tablets of 500 mg S + 25 mg P. 2. As for 1. above plus PQ 0.75 mg/kg day 4 (reported as day 3). | |
| Outcomes | 1. Proportion of people with P. falciparum parasites by PCR days 4, 8 and 15 (reported as days 3, 7 and 14) 2. Proportion of people with gametocytes by RT-PCR days 8 and 15 (reported as days 7 and 14) 3. Adverse events days 2, 3, 4, 8 and 15 (reported as days 1, 2, 3, 7 and 14) 4. Packed cell volume days 1, 8 and 15 (reported as days 0, 7 and 14) | |
| Notes | The trial was conducted about two months after the initial screening for positives (asymptomatic carriers). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The list of carriers was sorted according to village and age to ensure that the treatment groups were balanced with respect to these two variables. The random allocation of this ordered list into the treatment arms was then created using restricted randomization with a block size of 12 in STATA v7" |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only three of 104 participants did not complete follow-up. |
| Selective reporting (reporting bias) | Low risk | No obvious selective reporting. |
| Other bias | Low risk | No indication of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and health staff were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Lab staff doing PCR were blinded. |
Eziefula 2013
| Methods | Individually randomized placebo-controlled, double blind trial conducted December 2011 to March 2013 | |
| Participants | 468 randomized, aged one to 10 years old, male and female Inclusion criteria: 1. P. falciparum mono infection with parasite density lower than 500,000 parasites/μL 2. Normal G6PD enzyme function 3. Fever or history of fever in past 24 hours | |
| Exclusion criteria: 1. Signs of severity 2. Haemoglobin concentration < 80 g/L 3. Known allergy to the trial drugs 4. Antimalarials taken within the past two days 5. PQ taken within the past four weeks 6. Blood transfusion within the past 90 days | ||
| Interventions | 1. AL standard three day (twice per day) course + placebo (given with 5th AL dose, ie, with 1st dose on 3rd day of treatment) 2. AL + 0.1 mg/kg PQ 3. AL + 0.4 mg/kg PQ 4. AL + 0.75 mg/kg PQ (reference) | |
| Outcomes | Primary efficacy: Mean duration of gametocyte carriage Secondary efficacy: Point prevalence of gametocytes on days 7, 10 and 14; gametocyte circulation time (days), AUC of gametocyte density Primary safety: Arithmetic mean maximum decrease in haemoglobin concentration from enrolment to day 28 Secondary safety: Day of haemoglobin nadir, maximum percentage decrease in haemoglobin, percentage of participants with haemoglobin concentration lower than 50 g/L, requirement for blood transfusion, evidence of black urine, and frequency of severe adverse events. | |
| Notes | G6PD enzyme function based on a fluorescence spot test (R&D Diagnostics, Aghia Paraskevi, Greece) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated four-digit treatment assignment codes and allocated these to random dose groups in block sizes of 16. |
| Allocation concealment (selection bias) | Low risk | Only the pharmacist was aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 8% of patients were lost to follow-up. No group significantly different from others. |
| Selective reporting (reporting bias) | Low risk | None detected or suspected. |
| Other bias | Low risk | None detected or suspected. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Masking syrup" added to all treatments to mask taste of drug. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded. |
Gogtay 2004
| Methods | Allocated by "simple, computer-generated randomization code" | |
| Participants | Twenty-two patients in Mumbai, India Inclusion criteria: 1. = 18 years 2. Normal G6PD 3. = 55 P. falciparum gametocytes/μL on admission | |
| Exclusion criteria: 1. Complicated malaria | ||
| Interventions | 1. Quinine days 1 to 7: 30 mg/kg/day + PQ (45 mg) 2. Quinine days 1 to 7: 30 mg/kg/day + bulaquine (approximately 75 mg base) | |
| Outcomes | Asexual and gametocyte counts on days 1, 4, 8, 15, 22 and 29 Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Unbalanced allocation (9 versus 13) and small number of participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory technician reading blood smears was blinded. |
Gogtay 2006
| Methods | Randomized (computer-generated) to PQ or bulaquine (1:2 ratio) after primary treatment with quinine + doxycycline | |
| Participants | 93 participants in India Inclusion criteria: 1. = 16 years 2. Male 3. Uncomplicated Pf only 4. = 55 P. falciparum gametocytes/μL on admission | |
| Exclusion criteria: 1. Antimalarial treatment in previous two weeks 2. Allergy to trial drug 3. G6PD deficient | ||
| Interventions | All patients: Quinine days 1 to 7: 30 mg/kg/day (10 mg/kg/day three times per day) + 100 mg doxycycline Randomization and treatment on day 4 1. PQ 2. Bulaquine | |
| Outcomes | Gametocyte prevalence, density and viability on days 1, 4, 15, 22 and 29 Adverse events | |
| Notes | Gametocyte viability assessed by Shute's technique (exflagellation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete data. Three participants (two bulaquine, one PQ) did not return for follow-up. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None noted. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Slide readers were blinded. |
Kamtekar 2004
| Methods | Individually RCT, comprising two distinct comparisons a: (CQ or [CQ+SP]) with and without PQ and b: QN with and without PQ. Dates of trial: Not given. | |
| Participants | 57 people aged ≥ 16 years with symptomatic uncomplicated and 46 with severe (WHO criteria) P. falciparum malaria, diagnosed by thick and thin blood slides. Gametocytaemic within first 72 hrs with = 55 P. falciparum gametocytes/μL Site: Urban areas of Mumbai, India. Exclusion criteria: Pregnant or lactating, treatment for malaria within last two weeks, co-infection with P.vivax, history of PQ allergy. | |
| Interventions | Comparison a: for uncomplicated malaria All received CQ (some also got SP) Day 4: Randomized to PQ or placebo (45 mg) Comparison b: For severe malaria All received quinine Day 8: Randomized to PQ or placebo (dose 45 mg) Doses background drugs: CQ 10 mg/kg on days 1 and 2; 5 mg/kg on day 3; SP 1500 mg; quinine dose 10 mg/kg every 8 hrs for 24 to 48 hrs and orally for total of 7 days | |
| Outcomes | 1. Proportion of people with gametocytes, days 1, 4, 8, 15, 22 and 29 2. Proportion of people with viable gametocytes (exflagellation), days 1, 4, 8, 15, 22 and 29 3. Gametocyte density (given as range) days 1, 4, 8, 15, 22 and 29 | |
| Notes | No screening for G6PD deficiency. It is not stated how many got SP in addition to CQ or why. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "simple computer generated randomization code". Not all patients had gametocytes on day 1. Inclusion criteria were that the person had to have gametocytes in the first 72 hours (from day 1?). This suggests some post randomization inclusions or exclusions. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Originally there were 57 people included in uncomplicated comparison (a), of whom 2 were lost to follow-up and 9 were not evaluated as they showed CQ resistance. There were 46 in severe comparison (b), of whom 3 were lost to follow-up. The final numbers evaluated in each group were (a) 22 and 24 (b) 22 and 21. |
| Selective reporting (reporting bias) | Unclear risk | No obvious selective reporting. |
| Other bias | High risk | It was not clear why some patients got SP and others did not, and the numbers in each group are not given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial used a placebo for PQ. Patients and health workers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Slide readers were blinded. |
Khoo 1981
| Methods | Individually RCT Dates of trial: between June 1976 and March 1978. | |
| Participants | 69 people (adults and children of both sexes, no ages specified) with G6PD deficiency (full or partial by Brewer's methaemoglobin reduction test) who were slide positive for malaria (P. falciparum, P. vivax or mixed). Site: Sabah, Malaysia. Exclusion criteria: Other associated clinical conditions. | |
| Interventions | 1. CQ: 1.5 g CQ over 3 days for P. falciparum, P. vivax or mixed, less for children 2. CQ + PQ: CQ as above plus 75 mg PQ over 3 days for P. falciparum; 210 mg PQ over 14 days for P. vivax and mixed infections; less for children 3. SP (not included in this review): 1.5 g S and 75 mg P, single dose | |
| Outcomes | 1. Haemolysis 2. Proportion cleared parasites by 72 hours 3. Need for blood transfusion 4. Renal failure | |
| Notes | The participants are not divided by P. falciparum, P. vivax or mixed, so it is not possible to use the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "those found G6PD deficient were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given. |
| Selective reporting (reporting bias) | Low risk | No apparent selective reporting. |
| Other bias | Low risk | No indication of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Kolaczinski 2012
| Methods | Individually RCT Dates of trial: between July and January, from 2000 to 2003. | |
| Participants | 237 individuals aged from 3 to 70 years, in 5 villages for Afghan refugees in Pakistan. Inclusion: = 2 years of age, P. falciparum mono-infection, confirmed by slide, will be resident during entire follow-up period. Exclusions: Pregnancy, signs of severe malaria, report of antimalarial drug in past 21 days, other serious disease | |
| Interventions | 1. CQ: 3 days 25 mg/kg. 2. CQ+PQ: CQ as in 1; PQ on day 3 (0.5 mg/kg). 3. SP: 25(S)/1.25(P) mg/kg in single dose. 4. SP+PQ: SP as in 3; PQ on same day (0.5 mg/kg). | |
| Outcomes | 1. Clinical treatment failure (PCR non-adjusted and adjusted). 2. Gametocytes on day 8. 3. Gametocyte density on days 1 to 8 of follow-up. 4. Genotyping of resistant strains for CQ and SP-specific mutations. | |
| Notes | Also included CQ + AS and SP + AS arms, compared with CQ +/- PQ and SP +/- PQ arms, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients numbered sequentially at enrolment. Random numbers with treatment assignment from Excel-generated lists, then paired with patient numbers. |
| Allocation concealment (selection bias) | Low risk | Patient number concealed until after enrolment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 209 of 237 randomized completed treatment and at least one follow-up test. 47 (13%) of those randomized did not contribute data. Variable numbers tested during follow-up (see analyses). |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None noted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Identified in report as 'single-blind'. Manager (gave Rx) not blinded; patients, microscopists and health workers 'partially blinded' due to different drug appearance and times of follow-up. No placebos used, but vitamin given to those in non-PQ arms. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Implied only. |
Ledermann 2006
| Methods | Individually RCT Date of trial: July to Oct 2001. | |
| Participants | 117 malaria cases with P. falciparum ≥ 400 asexual stages/μL (thick film) recruited by mass blood survey and passive case detection. Symptoms not required. Age: ≥ 15 years Site: Central Java, Indonesia, an area with high CQ resistance and resurgent malaria approximately equal P. falciparum and P. vivax. Exclusion criteria: Pregnancy, breast feeding, body weight < 40kg, G6PD deficiency, history of antimalarial or antibiotic in last seven days, severe or complicated malaria, history or allergy or adverse reaction to trial medications, Pv or mixed infection. | |
| Interventions | 1. CQ only (not included in this review) 2. CQ+SP: CQ 150 mg base, 10, 10 and 5 mg/kg on days 1, 2, 3 (reported as days 0, 1, 2). SP 500 mg S 25 mg P on day 1 (reported as day 0) 3. CQ+SP as for group 2 above plus PQ 45 mg on day 1 (reported as day 0) 4. CQ+SP as for group 2 above plus PQ 45 mg on day 3 (reported as day 2) | |
| Outcomes | 1. Parasite clearance time assessed at days 1, 3, 8, 15, 22, 29 or day of recurrent parasitaemia (reported as days 0, 2, 7, 14, 21, 28) 2. Fever clearance time at days 2, 3, 4, 5, 8, 12, 15, 19, 22, 29 3. Proportion of people with gametocytes (from chart) days 1 to 29 4. Adverse events | |
| Notes | Some comparisons in the results reported include the CQ only group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial subject codes were assigned to treatment arms by a random process (not specified). |
| Allocation concealment (selection bias) | High risk | Eligibles were assigned a sequential participant number by the screening physician. Pre-packaged treatment but not stated whether allocation was concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% of participants withdrew before day 28. |
| Selective reporting (reporting bias) | Unclear risk | Abstract states that drugs were well tolerated and safe but no evidence is given in report. |
| Other bias | Low risk | No indication of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was implied only. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was implied only. |
Pukrittayakamee 2004
| Methods | Individually RCT Dates of trial not stated. | |
| Participants | 176 patients with acute uncomplicated P. falciparum. After exclusion of QN+tetracycline group: 146. Age 14 to 62. All male. Site: Hospital for Tropical Diseases, Bangkok, Thailand. Exclusion criteria: Severe malaria, mixed malaria infection, history of drug hypersensitivity, any antimalarial within last 48 hrs, urine positive for sulfonamide or 4AQ. People with G6PD deficient phenotype were excluded from receiving PQ. | |
| Interventions | 1. QN: QN sulfate (300 mg salt/tab) at 10 mg salt/kg, three times per day for 7 days 2. QN+tetracycline (excluded from this review) 3. QN+PQ low dose: QN as above in 1 plus PQ 15 mg base/tab, 0.25 mg/kg base (adult dose 15 mg base) daily for 7 days 4. QN+PQ high dose: QN as above in 1 plus PQ 0.50 mg/kg base (adult dose 30 mg base) daily for 7 days 5. AS: AS 50 mg salt/tab 3.3 mg/kg (adult dose 200 mg) on day 1 and 1.65 mg/kg (adult dose 100 mg) daily on days 2 to 7 6. AS+PQ (high dose): AS as above plus PQ 0.5 mg/kg base daily on days 1 to 7 | |
| Outcomes | 1. Parasite clearance time: measured at 12 hrs until clearance 2. Gametocyte clearance time: median, 12 hrs until clearance 3. Fever clearance time (measured every 4 hr at first and then every 6 to 12 hrs until resolution of fever) 4. Parasite reduction ratio at 48 hrs 5. Reappearance of infection P. falciparum/P. vivax up to 28 days 6. Prevalence of gametocytes on admission/after treatment/total 7. Gametocyte carriage: total number of hours for which gametocytes were detectable | |
| Notes | Patients with recrudescence of P. falciparum or relapse of P. vivax were re-treated with 7 day QN+tetracycline or 'standard doses' of CQ+PQ respectively; not clear if they were excluded from further trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method not stated. Patients with G6PD deficiency were excluded from getting PQ which suggests randomization was biased. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 122/142 of the original participants in the 5 groups studied here completed follow-up. Patients with recrudescences of P. falciparum or relapse of P. vivax were re-treated with QN+tetracycline or CQ+PQ respectively; not clear if they were excluded from further trial. |
| Selective reporting (reporting bias) | Unclear risk | Not detected. |
| Other bias | Unclear risk | Those who were unable to stay in hospital until clearance of both fever and parasites were excluded from trial of fever clearance time. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
Shekalaghe 2007
| Methods | Individually RCT Dates of trial: June to Sept 2006. | |
| Participants | 108 children with fever = 37.5°C or history of fever in last 48 hours and P. falciparum mono-infection 500 to 100,000/μL. Age three to 15 years. Both sexes. Site: Mynuzi health centre, North-Eastern Tanzania, a hyperendemic area with rainy seasons in March to June and October to December Exclusion criteria: Hb < 8, inability to take drugs orally, known hypersensitivity to meds, reported anti-malarial treatment in last two weeks, evidence of chronic disease or acute infection other than malaria, domicile outside trial area, signs of severe malaria, eligible for other malaria studies. | |
| Interventions | 1. AS+SP: AS: 4 mg/kg once daily for 3 days; SP: S 25 mg/kg and P: 1.125 mg/kg 2. AS+SP+PQ: As above for AS and SP plus PQ base 0.75 mg/kg on the third day | |
| Outcomes | 1. Proportion of people with gametocytes (by microscopy) days 1, 4, 8, 15, 29 and 43 (reported as 0, 3, 7, 14, 28 and 42) 2. Proportion with gametocytes (by PCR), same time points 3. Gametocyte density by PCR 4. AUC for gametocyte presence 5. Adverse events 6. Adequate clinical and parasitological response 7. Haemoglobin | |
| Notes | Hb outcome assessed with respect to G6PD variant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated in STATA 8.0 using restricted randomization with block size of 20. |
| Allocation concealment (selection bias) | Unclear risk | Pre-prepared envelopes (but person who opened envelope administered treatment). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 out of 108 failed to complete follow-up. |
| Selective reporting (reporting bias) | Low risk | No information given. |
| Other bias | Low risk | No indication of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial physician evaluated patients, opened envelopes, and administered treatment. Other staff were blinded. Not clear if participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
Singhasivanon 1994
| Methods | Individually RCT Dates of trial: not stated. | |
| Participants | 23 people with uncomplicated P. falciparum malaria, parasitaemia between 1 to 5 per 1000 rbc. Age 5 to 12 years, sex not stated. Exclusion criteria: Antimalarial drugs, urine with quinoline and sulfonamide drugs, other diseases, hematocrit ≤ 20%, inability to take oral medication. | |
| Interventions | 1. MSP: MQ 20 mg/kg; S 40 mg base/kg; P 2 mg/kg; single dose 2. MSP + PQ: As above plus PQ 0.75 mg/kg single dose. MSP+PQ crushed and mixed with 30 mL syrup (83% dextrose) | |
| Outcomes | 1. Gametocyte clearance time (days) (assessed twice daily until negative, then once daily, by blood slide) 2. Adverse drug reactions, assessed once daily in first week then once a week 3. Parasite clearance time (hrs) 4. Fever clearance time (hrs) 5. Cure rate | |
| Notes | Those who vomited within three hours of Rx were excluded - this is a post randomization exclusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes only reported for 18 of the 23 participants. |
| Selective reporting (reporting bias) | Unclear risk | No information given. |
| Other bias | High risk | Those who vomited within three hours of Rx were excluded- this is a post randomization exclusion. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
Smithuis 2010
| Methods | Individually RCT (5 comparisons - 10 arms). Follow-up: Patients were asked to return weekly for 9 weeks for assessment and at any other time they were unwell. Dates: December 2008 to March 2009. | |
| Participants | Number: 808 people attending clinics in Myanmar. Inclusion criteria: Age = 6 months, weight = 5 kg, P. falciparum mono-infection 500 to 200,000 parasites/µL or co-infection with P. vivax, informed consent. Exclusion criteria: Pregnancy, signs of severe malaria, severe malnutrition, history of hypersensitivity to any of the trial drugs, severe malnutrition, concomitant febrile illness, history of psychiatric disorder, a full course of MQ in the previous nine weeks or any other antimalarial in the previous 48 hrs. | |
| Interventions | Each of the five trial arms was divided into two where one half also received a one-off dose of 0.75 mg/kg PQ on day 1. Groups: 1+2. AS plus amodiaquine, fixed-dose combination: 25 mg/67.5 mg or 50 mg/135 mg or 100 mg/270 mg tablets. • AS 4 mg/kg once daily for 3 days 1. AQ 10.8 mg base/kg once daily for 3 days 3+4. AL, fixed-dose combination: 20 mg/120 mg tablets. • A 3.3 mg/kg in two divided doses each day for 3 days • L 19.8 mg/kg in two divided doses each day for 3 days • Advised to consume fatty food or breast feed before each dose 5+6. AS plus MQ, fixed-dose combination: 25 mg/55 mg or 100 mg/220 mg tablets (artesunate: Guilin, Lariam: Hoffman-La Roche) • AS 4 mg/kg once daily for 3 days • MQ 8.8 mg/kg once daily for 3 days 7+8. Artesunate plus MQ, loose combination (artesunate: Guilin, Lariam: Hoffman-La Roche) • AS 4 mg/kg once daily for 3 days • MQ 25 mg base/kg as a single dose on day 1 (reported as day 0) 9+10. DHAP, fixed-dose combination: 40 mg/320 mg or 20 mg/160 mg tablets (Artekin: Holleykin) • DHA 2.5 mg/kg once daily for 3 days • P 20 mg/kg once daily for 3 days First dose supervised, all others unsupervised. | |
| Outcomes | 1. Recurrent parasitaemia at day 15, 29, 43 and 64 (reported as days 14, 28, 42 and 63) 2. Treatment failure due to P. falciparum 3. Gametocytaemia prevalence 4. Person-gametocyte weeks 5. Haemoglobin on days 1 and 64 6. Adverse events (monitoring not described) | |
| Notes | Funding: Médecins sans Frontières (Holland). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After patients were screened and enrolled in the study, they were stratified prospectively into three age groups (1 to 4 years, 5 to 14 years and older than 14 years). Patients were randomly assigned in equal numbers to receive one of the five different treatments. They were then randomly assigned either a single dose of PQ …or not". |
| Allocation concealment (selection bias) | Low risk | "Treatment allocations were put in sealed envelopes in blocks of 50 for each age group, and random assignment was achieved by patients drawing an envelope from a box after enrolment. When the box was empty, another 50 envelopes were added". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition is low in absolute numbers and unlikely to have introduced significant bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No indication of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial for patients and medical staff. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Microscopists were blinded. |
Sutanto 2013
| Methods | Two-arm open-label RCT Follow-up: Days 1, 2, 3, 7, 14, 21, 28, 35 and 42, and any other day in between if they felt ill. Thin and thick blood smears and dried blood spot for genotyping Dates of randomization: December 2008 to March 2010 | |
| Participants | 188 (178 left on day 3) + 186 (171 day 3). Analysis based on those still present on day 3 Setting: Hanura Primary Health Center, Padang Cermin district, Lampung province located at the southern end of Sumatra. Endemicity: Low malaria endemicity with a malaria prevalence of 1.8% across all age groups. Seasonal transmission. | |
| Inclusion criteria: 1. Parasite density ≥ 1000 parasites/μL 2. Age ≥ 5 years 3. Normal glucose-6-phosphate dehydrogenase (G6PD) enzyme levels based on a qualitative test 4. Haemoglobin level ≥ 8 g/dL 5. Negative pregnancy test (assessed by human chorionic gonadotropin urine test) or not breastfeeding 6. No signs of severe malnutrition 7. No other chronic diseases 8. No history of allergy to the trial drugs 9. Ability to return for 42 days of follow-up | ||
| Interventions | 1. Standard 3-day DHAP (fixed-dose tablets of 40 mg dihydroartemisinin and 320 mg piperaquine; D-ARTEPP, Guilin Pharmaceutical Co, Ltd) 2. DHAP as in intervention 1; PQ: Day 3, single dose of 0.75 mg/kg, rounded to the nearest half tablet. Mean dose was 0.74 mg/kg (range, 0.5 to 0.94 mg/kg) | |
| Outcomes | 1. Gametocyte prevalence-days 7, 14, 21, 28, 35 and 42 2. Gametocyte clearance rates by day 42 in patients with gametocytes on day 3 3. Recurrence of asexual stages of P. falciparum, PCR adjusted and unadjusted for reinfections 4. Gametocyte development by day 42 in patients who were gametocyte free on day 3 5. Gametocyte densities between days 3 and 42 inclusive 6. Asexual infection recurrence by PCR 7. Hemoglobin on days 7, 42 8. Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated sequences in blocks of four. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used in order at the health centre. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of differential attrition. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None detected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, no PQ placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
Vasquez 2009
| Methods | Individually RCT Dates of trial: April 2007 to Feb 2008. | |
| Participants | 50 people with uncomplicated P. falciparum diagnosis by thick blood slide, 150 to 50,000 parasites/μL Age one year and over, both sexes. Exclusion criteria: Pregnant, mixed infection, danger signs and complications, allergy to antimalarials, serious illness at time or presentation, antimalarial treatment in last 72 hrs, MQ in last four weeks. | |
| Interventions | 1. AS+MQ Age 1 to 6: AS 50 mg on days 1, 2, 3 (reported as 0, 1, 2); MQ 250 mg on day 2 Age 7 to 13: AS 100 mg on days 1, 2, 3; MQ 250 mg on days 1, 2, 3 Age = 13: AS 200 mg on days 1, 2, 3; MQ 500 mg on days 1, 2, 3 2. AS+MQ+PQ As above plus PQ: Age 1 to 6: 0.3 to 0.6 mg/kg day 3 (reported as day 2). Age 7 to 13: 22.5 mg/kg day 3. Age = 13: 45 mg day 3. | |
| Outcomes | Assessed on days 2, 3, 4, 8, 15, 22, 29, 36, and 43. 1. Clinical recurrence 2. Parasitemia prevalence 3. Parasite density 4. Fever resolution 5. Prevalence of gametocytes 6. Density of gametocytes 7. Adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Seems to be alternate allocation following order of arrival ("segun el orden de llegada"). |
| Allocation concealment (selection bias) | Unclear risk | Not clear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts noted. |
| Selective reporting (reporting bias) | Low risk | No evidence of bias. |
| Other bias | Low risk | No suggestion of other bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Does not seem to be blinded ("con determination no ciega del efecto en grupos iguales"). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
Wang 2006
| Methods | Individually RCT | |
| Participants | Number of participants: 214 (no dropouts mentioned) Gabon International Tropical Medicine Institute Age range: 6 to 60 All have P. falciparum malaria clinical symptoms and blood smear positive. 1. Trial group: 108, male 50, female 58, age 16.4 ± 10.5 2. Control group: 106, male 52, female 54, age 18.2 ± 9.4 | |
| Exclusion criteria: N/A | ||
| Interventions | 1. Artesunate IM injection, daily for 5 days, 1.2 mg/kg each dose, first dose double. PQ 3 tablets (base 7.5 mg/tablet, children use half) once a day, for 5 days 2. Only artesunate IM injection daily for 5 days, 1.2 mg/kg each dose, the first dose double total 5 days | |
| Outcomes | 1. Fever clearance time: (hrs) below 37°C continuously measured four times 2. Clinical cure rate at day 7 3. Adverse events (not specified) 4. Recrudescence rate: Symptoms appeared again after clinical cure; parasite appeared in blood smear by 28 days Follow-up: 28 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated to be randomized, and the fact that numbers per group are not equal supports this contention. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Unknown. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated to be blinded. |
AL = artemether-lumefantrine; AQ = amodiaquine; AS = artesunate; CQ = chloroquine; DHAP = dihydroartemisinin-piperaquine; G6PD = glucose-6-phosphate dehydrogenase; IM = intramuscular; MQ = mefloquine; PCR = polymerase chain reaction; Pf = Plasmodium falciparum; PQ = primaquine; QN = quinine; RCT = randomized controlled trial; SP = sulfadoxine-pyrimethamine.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baird 2002 | Outcome is cure of asexual infection. No gametocyte outcomes. |
| Barber 1929 | Not a RCT or quasi-RCT. No controls. |
| Barber 1932 | MDA with PQ; no other drug. |
| Brueckner 1998 | Participants were not infected. Safety only trial. |
| Bunnag 1980 | Comparison of SP plus either five day PQ 15 mg, single dose PQ 30 mg or single dose PQ 45 mg in patients with and without gametocytes at presentation. No regimen without PQ. Not a RCT or quasi-RCT. Authors state they will do further studies, including transmission. No difference in gametocyte outcomes between regimens, and gametocytes persisted for up to 21 days. |
| Burgess 1961 | Comparison of 15 mg, 30 mg and 45 mg dose of PQ. Outcomes were gametocyte prevalence, density, percent of mosquitoes infected and mean oocysts per mosquito up to eight days. Not a RCT or quasi-RCT. No other drug. Although this trial used different doses by group (12 participants total), they were assigned to participants based on age or body size, and therefore it was not a valid comparison of different doses. |
| Cai 1985 | Not a RCT. |
| Carter 2011 | No 8AQ in trial. |
| Che 1987 | No mention of randomization. No valid comparison group (pyronaridine phosphate plus sulfadoxine plus PQ versus pyronaridine phosphate only). |
| Che 1990 | No appropriate control group. |
| Chevalley 2010 | In vitro studies only. Not a RCT. |
| Clyde 1962 | All patients got PQ. |
| Clyde 1970 | Individual before-and-after study, but small number of patients and not controlled. |
| Clyde 1971 | Individual before-and-after study, but small number of patients and not controlled. |
| da Silva 1984 | Trial of treatment regimens, some including PQ, for P. vivax and P. falciparum. |
| Degowin 1966 | No 8AQ in trial. |
| Doi 1989 | Community observational study. Except for a small pilot study, everyone in the intervention villages got PQ. There no 'before' data from these villages. In the control site, some children received treatment. |
| Giao 2004 | No appropriate control group (trial of CV8 (contains PQ) versus atovaquone-proguanil). |
| Gogtay 1999 | Compares QN+PQ against QN+bulaquine. Not a relevant comparison. |
| Gunders 1961 | Before-and-after studies of gametocytes and mosquito feeds on people with gametocytes given pyrimethamine and PQ in doses ranging from 10 mg to 40 mg base. No group without other drug. |
| Hii 1987 | Controlled before-and-after study comparing SP+PQ+ITN versus SP+PQ only. Only one cluster per arm and no group without PQ. |
| Huang 1993 | Not a RCT. Unbalanced groups. |
| Huang 1996 | PQ given to both intervention groups in same regimen. Malaria treatment regimen was varied (low and higher dose pyronardine/SP). |
| Huang 2001 | No gametocyte outcomes. |
| Jeffery 1956 | Non-randomized comparison of gametocytes and infectivity of artificially infected patients treated with CQ or CQ+PQ. |
| Jeffery 1963 | Observational study of gametocytes and infectivity of two patients given PQ. |
| Jerace 1933 | Case series studying gametocytes and infectivity of patients given PQ. |
| Kaneko 1989 | Non-randomized community trial comparing SP+PQ in one village with SP only in another. Only one cluster per arm. The trial was predominantly mass fever test and treat but 75% of people in the intervention village were treated versus 18% in the control village. |
| Karbwang 1991 | Not randomized, no gametocyte outcomes. |
| Karbwang 1992 | Pharmacokinetic study; no gametocyte outcomes or control group. |
| Kyaw 1994 | No control group. All patients got PQ. |
| Li 2007 | No gametocyte outcomes. |
| Li 2010 | No gametocyte outcomes. |
| Lin 2004 | All patients got PQ. |
| Mackerras 1949 | No other malaria treatment; one patient fed on before and after PQ. |
| Rieckmann 1968 | Two patients given 45 mg PQ only and fed on by mosquitoes before and after. No other malaria treatment or control group. |
| Rieckmann 1969 | 18 patients given CQ alone (N = 2), CQ plus 45 mg PQ (N = 3), or PQ alone in doses ranging from 15 to 45 mg, at either single dose or at one to two week intervals, and fed on before and after one of the doses of PQ. Non-randomized or quasi-randomized. |
| Santana 2007 | Study of 14 day regimen of 15 mg PQ. Some P. falciparum cases were included but study did not distinguish between the patients with P. falciparum and P. vivax. Study was a comparison of association between methaemoglobinaemia after 14 day PQ in people with and without G6PD deficiency. |
| Shah 2013 | Review of 21 trials from national drug resistance monitoring system of India. Compares 9 sites where AS+SP+PQ was used with 12 sites where it was not. |
| Shekalaghe 2010 | Randomized comparison of anaemia after SP+AS+PQ versus placebo. Children with haemoglobin < 8 g were excluded from receiving PQ. |
| Shekalaghe 2011 | Trial was a comparison of SP+AS+PQ versus placebo. No comparison of groups with and without PQ. |
| Sun 2011 | Artesunate + PQ versus Quinimax only. No appropriate control group. |
| Suputtamongkol 2003 | Comparison of MQ+AS versus MQ + PQ. No appropriate control group. |
| Tangpukdee 2008 | Comparison of Artequick (contains PQ) with MQ+AS. No appropriate control group and no gametocyte outcomes. |
| Yang 1989 | All patients got PQ, though different doses of PQ and other malaria treatments. |
| Yeramian 2005 | PQ given only to P. vivax patients for 14 days. |
| Young 1959 | No other malaria treatment; case series of PQ given either daily, twice a week or weekly to Pf patients. |
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 1993b
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study not yet located |
Ishii 2009
| Methods | Unclear |
| Participants | Residents of trial villages in Solomon Islands (number not given) |
| Interventions | Testing of clinical malaria patients for G6PD and addition of single dose PQ to other malaria treatment if appropriate |
| Outcomes | Village prevalence of malaria |
| Notes | |
Li 2006
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study not yet located |
Characteristics of ongoing studies [ordered by study ID]
D'Alessandro (started 2013)
| Trial name or title | Primaquine's Gametocytocidal Efficacy in Malaria Asymptomatic Carriers Treated With Dihydroartemisinin-Piperaquine in The Gambia | |
| Methods | Randomized, open label | |
| Participants | 1200 participants will be recruited Inclusion criteria: • Age ≥ 1 year • Weight = 10 kg • P. falciparum mono-infection, density of at least 20 parasites/μL • Axillary temperature < 37.5°C • Resident in the trial area and willingness to reside for the duration of the trial • Written informed consent (plus an assent in children = 12 years of age) | |
| Exclusion criteria: • G6PD deficiency haemoglobin < 8 g/dL • Known allergy to any of the trial medications • Known pregnancy or breastfeeding • Clear/documented history of anti-malarial treatment two weeks before contact with trial team • History of blood transfusion in the previous three months • Any chronic or acute conditions that might interfere with the trial as judged by the research clinician • History of sickle cell anaemia | ||
| Interventions | 1. Control: complete course of DHA-PPQ (Eurartesim) 2. Experimental: DHA-PPQ plus single dose PQ at 0.75 mg/kg body weight 3. Experimental: DHA-PPQ plus single dose PQ at 0.4 mg base/kg body weight 4. Experimental: DHA-PPQ plus single dose PQ at 0.2 mg base/kg body weight | |
| Outcomes | Primary: Prevalence of P. falciparum gametocyte carriers (QT-NASBA) (time frame: Day 7) Secondary: 1. Prevalence of P. falciparum gametocytes carriers on days 3, 10, 14, 28 and 42 as determined by QT-NASBA 2. Proportion of individuals infectious to mosquitoes (DMFA) on day 7, with direct membrane feeding assay 3. Haemoglobin change from day 0 on days 3, 7, 10, 14, 21, 28, 35 and 42, as mean (±SD) difference in haemoglobin measured between baseline (day 0) and each follow-up visit day by trial arm 4. Prevalence of infection (asexual stages) on day 3 5. Proportion of participants with recurrent infection (PCR adjusted and unadjusted) from day 7 to day 42 6. Occurrence of adverse events and serious adverse events | |
| Starting date | August 2013; December 2014 (final data collection date for primary outcome measure) | |
| Contact information | udalessandro@mrc.gm +220-4495442-6 ext 4001 (Umberto D'Alessandro) jokebe@mrc.gm +220-4495442-6 ext 4009 (Joseph Okebe) | |
| Notes | ClinicalTrials.gov identifier: NCT01838902 | |
Gosling (recruitment ended 2014)
| Trial name or title | Phase 2a Dose Escalation Study of the Efficacy, Safety, and Pharmacokinetics of Low Dose Primaquine for Gametocytocidal Activity Against P. falciparum in Sub-Saharan Africa and South East Asia |
| Methods | Randomized, single blind (outcomes assessor blinded) |
| Participants | 50 participants being recruited Inclusion criteria: • Male • Age ≥ 18 years and < 50 years • Malaria blood thick film positive • Presence of gametocytes on thick blood film • Agrees to admission to trial ward for 26 hours post diagnosis and available for follow-up visits • No allergies to trial drugs • Hemoglobin ≥ 8 g/dL • No evidence of severe or chronic disease • Written, informed consent |
| Interventions | Group 1: Active comparator: dihydroartemisinin-piperaquine (DP) only Group 2: Experimental: DP and 0.125 mg/kg PQ Group 3: Experimental: DP and 0.5 mg/kg PQ |
| Outcomes | Primary: mosquito infectivity assessed through membrane feeding at baseline, and 1, 2 and 7 days Secondary: 1. Gametocyte prevalence and density, at baseline, 2, 6, 12 and 24 hours, and at 2, 3, 7, 14 and 28 days 2. PQ pharmacokinetics - AUC of parent drug and metabolite at 1, 2, 3, 4, 6, 8, 12 and 24 hours 3. Asexual parasite prevalence and density baseline at baseline, 2, 6, 12, and 24 hours, and at 2, 3, 7, 14 and 28 days 4. Safety measurements including haemoglobin and signs of haemolysis baseline at baseline, 1, 2, 3, 7 14 and 28 days |
| Starting date | September 2014 (final data collection date for primary outcome measure) |
| Contact information | goslingr@globalhealth.ucsf.edu 415 597 8114 |
| Notes | ClinicalTrials.gov identifier: NCT01743820 Sites in Mali and Thailand |
Indonesia (started 2013)
| Trial name or title | Surveillance and Treatment With Dihydroartemisinin-piperaquine Plus Primaquine (MTC Belu) Sub-title: Impact of Mass Screening and Selective Treatment With Dihydroartemisinin-piperaquine Plus Primaquine on Malaria Transmission in High Endemic Area, Belu Regency, Nusa Tenggara Timur Province, Indonesia: a Randomized Cluster Trial | |
| Methods | Allocation: randomized; endpoint classification: efficacy study; intervention model: parallel assignment; masking: open label, primary purpose: treatment Cluster randomized. | |
| Participants | Target sample size: 1488 participants Inclusion criteria: • All villagers of all the selected clusters | |
| Exclusion criteria: • Pregnant women during their first trimester • Single dose PQ should not be given for infants under 1 year-old, pregnant women in all trimesters of pregnancy, breast-feeding mothers and patients with G6PD deficiency Age minimum: N/AAge maximum: N/A Gender: Both | ||
| Interventions | Drug: dihydroartemisinin-piperaquine Drug: PQ 1. Intervention arm of mass screening and treatment with interval of 6 weeks 2. Intervention arm of mass screening and treatment with interval of three months 3. Control arm without mass screening and treatment. The intervention arm with six weeks interval represents a new proposed method to detection malaria infections, while the intervention arm with three month interval represents the Ministry of Health current policy of active case detection in Indonesia, and the third arm will serve as the control for Ministry of Health's policy. No arm without PQ | |
| Outcomes | Malaria incidence (time frame: six months) Anaemia (time frame: six months) | |
| Starting date | June 2013 | |
| Contact information | Indonesia University/Walter and Eliza Hall Institute of Medical Research | |
| Notes | ClinicalTrials.gov identifier: NCT01878357 Stated to be completed | |
Mwaiswelo (started 2014)
| Trial name or title | Efficacy and Safety of a Single Low-dose Primaquine Added to Standard Artemether-lumefantrine Treatment for the Clearance of Plasmodium Falciparum Gametocytes | |
| Methods | Phase 4, randomized, safety/efficacy study, parallel assignment, single blind (subject). Primary purpose: Prevention | |
| Participants | Target sample size (N = 220) in Tanzania Inclusion criteria: 1. Gender: Both 2. Age of 1 year and above and neither pregnant nor breast feeding 3. Weight over 10 kg 4. Body temperature = 37.5°C or history of fever in the last 24 hours 5. P. falciparum mono-infection | |
| Exclusion criteria: 1. Evidence of severe illness malaria or danger signs 2. Known allergy to study medications 3. Hemoglobin < 8 g/dL 4. Antimalarials taken within last 2 weeks 5. Blood transfusion within last 90 days and evidence of recent use (within 14 days) of or will be taking other drugs known to cause hemolysis in G6PD-deficient subjects | ||
| Interventions | Control: AL + placebo. The first dose of AL will be administered concomitantly with a single-dose placebo. A volume of normal saline will be measured based on weight bands and then will be given to patients. Intervention: AL+PQ. All the recruited patients will be treated with a six doses, 3 days AL treatment regimen. However, patients randomized to the AL+PQ arm will be given 0.25 mg/kg single-dose PQ concomitantly with AL first dose. | |
| Outcomes | Primary outcome: Number of days per treatment arm for gametocytes to become undetectable using Quantitative nucleic acid sequence based assay (QT-NASBA) (time frame: 14 days) Secondary outcome: Mean maximal fall in haemoglobin (g/dL) from enrolment to day 28 of follow-up defined as mean greatest negative difference in haemoglobin per treatment arm (time frame: 28 days) | |
| Starting date | Date of registration 16 March 2014 Date of first enrolment July 2014 | |
| Contact information | richiemwai@yahoo.com +255 717 043 970 (Richard O Mwaiswelo, MSc. PE) Andreas Martensson, PhD, Karolinska Institutet | |
| Notes | ClinicalTrials.gov identifier: NCT02090036 Primary sponsor: Muhimbili University of Health and Allied Sciences Secondary sponsor: Karolinska Institutet | |
Sarr (started 2014)
| Trial name or title | A trial of the safety of low dose primaquine in addition to ACTs commonly used in Senegal | |
| Methods | Parallel, randomized | |
| Participants | 300 participants will be recruited in Senegal Inclusion criteria: 1. Age minimum: 20 years old, age maximum: 50 years old 2. Gender: Both 3. Adult patients with acute uncomplicated P. falciparum malaria 4. P. falciparum monospecific infection with parasite density ranged from 1000 to 100,000 trophozoite/µL 5. Willingness to participate in the planed study investigations and to remain in the study area for the duration of the study 6. Participants informed consent 7. Absence of known chronic illness such as hypertension, diabetes, renal and liver disease | |
| Exclusion criteria: 1. Known allergy to the study medications 2. Presence of severe anaemia (haemoglobin 8 g/dL) at enrolment 3. Pregnancy, or breastfeeding | ||
| Interventions | Control: ACT treatment, standard malaria treatment, 1 daily dose, 3 days duration (N = 150) Experimental: ACT plus PQ treatment; ACT treatment 1 daily dose for 3 days, PQ single low dose 0.25 mg/kg at day 1 (N = 150) | |
| Outcomes | Primary outcome 1. Mean haemoglobin difference from day 0 to day 7 after treatment with ACT and PQ | |
| Secondary outcomes 1. Mean haemoglobin level at day 14 of follow-up in each treatment arm 2. Mean haemoglobin level at day 28 of follow-up in each treatment arm | ||
| Starting date | Registered 10/11/2014; Date of first enrolment 15 November 2014 | |
| Contact information | roger.tine@ucad.edu.sn +00 221 77 637 05 31 (Prof Roger Tine) bathie65@yahoo.fr +00 221 77 647 09 99 (Dr Samba Cor Sarr) khadimesylla@yahoo.fr +00 221 77 521 76 44 (Dr Khadime Sylla) | |
| Notes | Pan African Clinical Trials identifier: PACTR201411000937373 Primary sponsor: University Cheikh Anta DIOP of Dakar | |
Saunders (started 2012)
| Trial name or title | Active Surveillance for P. falciparum Drug Resistance With Assessment of Transmission Blocking Activity of Single Dose Primaquine in Cambodia | |
| Methods | Randomized, open label | |
| Participants | 150 male and female participants Inclusion criteria: 1. Volunteer with uncomplicated P. falciparum malaria (volunteers with mixed P. falciparum and P. vivax infections may be enrolled), 18 to 65 years of age 2. Baseline asexual parasite density between 1000 to 200,000 parasites/µL 3. Able to provide informed consent 4. Available and agree to follow-up for anticipated trial duration including three day treatment course at the MTF and weekly follow-up for the 42-day period 5. Authorized by local commander to participate if active duty military | |
| Exclusion criteria: 1. Allergic reaction or contraindication to DHA, piperaquine or PQ 2. Significant acute comorbidity requiring urgent medical intervention 3. Signs or symptoms and parasitological confirmation of severe malaria 4. Use of any antimalarial within the past 14 days. 5. Class I or II G6PD deficiency (defined as severe) as determined at screening 6. Pregnant or lactating female, or female of childbearing age, up to 50 years of age, who does not agree to use an acceptable form of contraception during the trial 7. Clinically significant abnormal EKG, including a QTcF interval = 500 ms at enrolment. 8. Known or suspected concomitant use of QTc prolonging medications. 9. Judged by the investigator to be otherwise unsuitable for trial participation | ||
| Interventions | Group 1: DHA-piperaquine 3-day course plus 45 mg single dose PQ Group 2: DHA-piperaquine 3-day course of DHA-piperaquine | |
| Outcomes | Primary: Efficacy rates at 42 days for DP with and without single dose PQ for uncomplicated P. falciparum diagnosed by positive PCR-corrected malaria microscopy Secondary: Efficacy of PQ to treat sexual stage gametocyte infection and prevent transmission of P. falciparum gametocytes to mosquitoes. | |
| Starting date | December 2012; December 2014 (final data collection date for primary outcome measure) | |
| Contact information | David Saunders, MD, MPH 66-2-696-2798 david.saunders@afrims.org Chanthap Lon, MD, MCTM 855 23 881 845 chanthapl@afrims.org | |
| Notes | ClinicalTrials.gov identifier: NCT01849640 | |
Sawa DAPPI-1 (started 2014)
| Trial name or title | A Double Blind Randomized Controlled Trial of Dihydroartemisinin-Piperaquine Alone and in Combination With Single Dose Primaquine to Reduce Post-treatment Malaria Transmission | |
| Methods | Phase 3 randomized, safety/efficacy study, parallel assignment, double blind (subject, caregiver, investigator, outcomes assessor) | |
| Participants | 120 participants will be recruited in Kenya Inclusion criteria: 1. Microscopically detectableP. falciparum gametocyte carriage 2. Age 5 years to 15 years 3. Gender: both | |
| Exclusion criteria: 1. Age < 5 years or = 15 years 2. Non-falciparum malaria co-infection 3. Malaria parasite density = 200,000 parasites/µL 4. Clinical symptoms indicating severe malaria 5. Axillary temperature = 39°C 6. Body Mass Index (BMI) below 16 or above 32 kg/m2 7. Haemoglobin concentration below 9.5 g/dL 8. Antimalarials taken in last 2 days 9. For women: Pregnancy (assessed by clinical examination and urine pregnancy test) or lactation 10. Known hypersensitivity to DP or PQ 11. History or symptoms, or both indicating chronic illness 12. Current use of tuberculosis or anti-retroviral medication 13. Unable to give written informed consent 14. Unwillingness to participate in two membrane feeding assays 15. Travel history to Angola, Cameroon, Chad, Central African Republic, Congo, DR Congo, Equatorial Guinea, Ethiopia, Gabon, Nigeria and Sudan 16. Family history of congenital prolongation of the QTc interval or sudden death or with any other clinical condition known to prolong the QTc interval such as history of symptomatic cardiac arrhythmias, with clinically relevant bradycardia or with severe cardiac disease 17. Taking drugs that are known to influence cardiac function and to prolong QTc interval, such as class IA and III: neuroleptics, antidepressant agents, certain antibiotics including some agents of the following classes - macrolides, fluoroquinolones, imidazole, and triazole antifungal agents, certain non-sedating antihistaminics (terfenadine, astemizole) and cisapride 18. Known disturbances of electrolyte balance, eg hypokalaemia or hypomagnesaemia 19. Taking drugs which may be metabolized by cytochrome enzyme CYP2D6 (for example, flecainide, metoprolol, imipramine, amitriptyline, clomipramine) 20. Blood transfusion within last 90 days | ||
| Interventions | Control: DHAP (Artekin) combination alone Experimental: DHAP (Artekin) combination alone plus single-dose 0.25 mg/kg PQ | |
| Outcomes | Primary outcome 1. Gametocyte prevalence on day 7 after initiation of treatment (time frame: day 7 of follow-up) | |
| Secondary outcomes 1. Gametocyte carriage during follow-up (time frame: 14 days during follow-up) 2. Gametocyte sex-ratio (time frame: 14 days of follow-up) 3. Haematological recovery (time frame: 14 days during follow-up) 4. Transmission to An. gambiae mosquitoes (time frame: day 3 and 7 during follow-up) | ||
| Starting date | Registration date 29 September 2014; first enrolment October 2014 | |
| Contact information | psawa@icipe.org +254 59 22620 (Patrick Sawa MD, ICIPE) teun.bousema@lshtm.ac.uk +31243617574 (Teun J Bousema, PhD) | |
| Notes | ClinicalTrials.gov identifier: NCT02259426 Primary sponsor: London School of Hygiene and Tropical Medicine | |
Shekalaghe (started 2013)
| Trial name or title | The Optimal Timing of Primaquine to Prevent Malaria Transmission After Artemisinin-Combination Therapy | |
| Methods | Randomized, open label | |
| Participants | 250 male and female participants Inclusion criteria: • Age 3 years to 17 years • Residents of research area • Willingness to come for complete scheduled follow-up • Uncomplicated malaria with P. falciparum mono-infection • Axillary temperature = 37.5°C and < 39.5°C, or history of fever in previous 48 hours • No history of adverse reactions to trial medication • Understanding of the trial procedures by parent or guardian and willing to participate by signing written informed consent forms | |
| Exclusion criteria: • Haemoglobin below 9 g/dL • Inability to take drugs orally • Known hypersensitivity to any of the drugs given • Reported treatment with antimalarial chemotherapy in the past two weeks • Evidence of chronic disease or acute infection other than malaria • Domicile outside the trial area • Signs of severe malaria (such as respiratory distress, altered consciousness deep breathing, anaemia) • Participating in other malaria studies conducted in the region • Mixed malaria parasite species infection • Positive pregnant test by urine (UPT) if participant is female aged above 12 years • G6PD deficient using the fluorescence spot test | ||
| Interventions | Group 1: AL 6 dose regime orally Group 2: AL 6 dose regime plus single dose PQ (0.75/kg) on day 0 Group 3: AL 6 dose regimen plus single dose PQ (0.75/kg) on day 2 | |
| Outcomes | Primary: Gametocyte prevalence and density by microscopy and QT-NASBA on day 14 Secondary: • Haemoglobin level on days 3, 7, 10 and 14 • Proportion of infected mosquitoes on day 7 after initiation of treatment and the intensity of infection (oocyst burden) by membrane feeding assay | |
| Starting date | May 2013; October 2013 (final data collection date for primary outcome measure) | |
| Contact information | Seif Shekalaghe, MD, PhD sshekalaghe@ihi.or.tz +255 755 470472 | |
| Notes | ClinicalTrials.gov identifier: NCT01906788 Tanzania KCMC and Ifakara | |
Sirima SAFEPRIM (started 2014)
| Trial name or title | Evaluation of the Safety of Primaquine in Combination With Artemether-lumefantrine in Glucose-6-phosphate Dehydrogynase Deficient Males With an Asymptomatic Malaria Infection in Burkina Faso (SAFEPRIM) | |
| Methods | Phase 2/3 randomized, safety study, parallel assignment, open label. Primary purpose: treatment | |
| Participants | Target sample size (N = 70) in Burkina Faso Inclusion criteria: 1. Male gender 2. Aged ≥ 18 years to ≤ 45 years 3. BMI ≥ 16 4. P. falciparum parasitaemia at any density 5. G6PD deficiency by Beutler Fluorescent Spot test for intervention groups and control group receiving AL only (N = 50) 6. G6PD normal activity by Beutler Fluorescent Spot test for control groups (N = 20) 7. Informed consent by participant | |
| Exclusion criteria: 1. Enrolled in another clinical trial 2. Fever = 37.5°C (tympanic) or history of fever in the last 24 hours 3. Evidence of severe illness/danger signs or active infection other than malaria 4. Known allergy to study medications 5. Hb < 11 g/dL 6. Antimalarials taken within the last 2 weeks 7. PQ taken within the last 4 weeks and blood transfusion within the last 90 days 8. Non-falciparum malaria co-infection 9. Current use of tuberculosis or anti-retroviral medication, sulphonamides, dapsone, nitrofurantoin, nalidixic acid, ciprofloxacin, methylene blue, toluidine blue phenazopyridine and co-trimoxazole. 10. History of severe chronic illness | ||
| Interventions | 1. AL + 0.25 mg/kg PQ 2. AL + 0.4 mg/kg PQ 3. AL combination | |
| Outcomes | Primary outcome: Haemoglobin concentration relative to baseline value (time frame: 28 days) Secondary outcome: Gametocyte clearance time (time frame: 14 days) | |
| Starting date | Date of registration 11 June 2014. Date of first enrolment October 2014 | |
| Contact information | s.sirima.cnlp@fasonet.bf + 226 50 32 46 95/96 (Sodiomon Sirima, MD, PhD) teun.bousema@lshtm.ac.uk +31243613663 (Teun Bousema, PhD) | |
| Notes | ClinicalTrials.gov identifier: NCT02174900 Primary sponsor: London School of Hygiene and Tropical Medicine Secondary sponsors: Centre National de Recherche et de Formation sur le Paludisme, Burkina Faso; University Medical Center Nijmegen | |
Spring (recruitment ended 2014)
| Trial name or title | Evaluation of DHAP with and without single dose primaquine: an open-label randomized, controlled trial in anlong Veng, Cambodia |
| Methods | Randomized, open-label clinical trial to evaluate the safety and therapeutic efficacy of DP, and transmission blocking potential in combination with single dose primaquine (PQ) in northern Cambodia. |
| Participants | Up to 150 volunteers, including those with mild-moderate G6PD deficiency with uncomplicated P. falciparum or mixed malaria infections |
| Interventions | Consecutive daily oral doses of 120/960 mg of DP with or without a single 45 mg dose of PQ |
| Outcomes | Mosquito membrane-feeding assay Serial electrocardiograms and time-matched drug levels |
| Starting date | Not known - trial registration not yet located. |
| Contact information | M Spring |
| Notes | Identifed from preliminary results in 63rd Annual Meeting of the American Society of Tropical Medicine and Hygiene, ASTMH 2013 New Orleans |
Thailand (started 2012)
| Trial name or title | Pharmacokinetic Study of Primaquine and Dihydroartemisinin-Piperaquine in Healthy Subjects | |
| Methods | Phase 1 Randomized, crossover, open label safety/efficacy study. Primary purpose: treatment | |
| Participants | Target sample size (N = 16) Inclusion criteria: 1. Healthy as judged by a responsible physician with no abnormality identified on a medical evaluation including medical history and physical examination. 2. Male and female non-smokers aged between 18 years to 60 years. 3. Males and females weight between 36 to 75 kg. 4. A female is eligible to enter and participate in this study if she is: of non-childbearing potential including pre-menopausal females with documented (medical report verification) hysterectomy or double oophorectomy; or postmenopausal defined as 12 months of spontaneous amenorrhoea or 6 months of spontaneous amenorrhoea with serum follicle stimulating hormone levels = 40 mIU/mL or 6 weeks postsurgical bilateral oophorectomy with or without hysterectomy; or of childbearing potential, has a negative serum pregnancy test at screening and prior to start the study drug in each period, and abstain from sexual intercourse or agrees to using effective contraceptive methods (for example, intrauterine device, hormonal contraceptive drug, tubal ligation or female barrier method with spermicide) during the study until completion of the follow-up procedures. 5. A male is eligible to enter and participate in this study if he: agrees to abstain from (or uses a condom during) sexual intercourse with females of childbearing potential or lactating females; or is willing to use a condom/spermicide, during the study until completion of the follow-up procedures. 6. Provide a signed and dated written informed consent prior to study participation. 7. Normal electrocardiogram (ECG) with QTc < 450 msec. 8. Willingness and ability to comply with the study protocol for the duration of the trial. | |
| Exclusion criteria: 1. Females who are pregnant, trying to get pregnant, or are lactating. 2. The subject has evidence of active substance abuse that may compromise safety, pharmacokinetics or ability to adhere with protocol instructions. 3. A positive pre-study hepatitis B surface antigen, positive hepatitis C antibody, or positive human immunodeficiency virus-1 (HIV-1) antibody result at screening. 4. Subjects with a personal history of cardiac disease, symptomatic or asymptomatic arrhythmias, syncopal episodes or additional risk factors for torsades de points (heart failure, hypokalaemia) or with a family history of sudden cardiac death. 5. A creatinine clearance < 70 mL/min as determined by Cockcroft-Gault equation: CLcr (mL/min) = (140 - age) * Wt/(72 * Scr) (multiply answer by 0.85 for females) where age is in years, weight (wt) is in kg, and serum creatinine (Scr) is in units of mg/dL (Cockroft 1976). 6. History of alcohol or substance abuse or dependence within 6 months of the study. 7. Use of prescription or non-prescription drugs except paracetamol at doses of up to 2 g/day, including vitamins, herbal and dietary supplements (including St. John's Wort) within 7 days (or 14 days if the drug is a potential enzyme inducer) or five times the drug half-life (whichever is longer) prior to the first dose of study medication until the completion of the follow-up procedure, unless in the opinion of investigator, the medication will not interfere with the study procedures or compromise subject safety; the investigator will take advice from the manufacturer representative as necessary. 8. The subject has participated in a clinical trial and has received a drug or a new chemical entity within 30 days, or 5 half-lives, or twice the duration of the biological effect of any drug (whichever is longer) prior to the first dose of study medication. 9. The subject is unwilling to abstain from ingesting alcohol within 48 hours prior to the first dose of study medication until collection of the final pharmacokinetic sample during each regimen. 10. Subjects who have donated blood to the extent that participation in the study would result in more than 300 mL blood donated within a 30-day period. Note: This does not include plasma donation. 11. Subjects who have a history of allergy to the study drug or drugs of this class, or a history of drug or other allergy that, in the opinion of the investigator, contraindicates participation in the trial. In addition, if heparin is used during pharmacokinetic sampling, subjects with a history of sensitivity to heparin or heparin-induced thrombocytopenia should not be enrolled. 12. Lack of suitability for participation in this study, including but not limited to, unstable medical conditions, systemic disease manifested by tendency to granulocytopenia for example rheumatoid arthritis and lupus erythematosus that in the opinion of the investigator would compromise their participation in the trial. 13. AST or ALT = 1.5 upper limit of normal (ULN). 14. Subjects with history of renal disease, hepatic disease or cholecystectomy or both. 15. G6PD deficient detected by Beutler's dye test. 16. Abnormal methaemoglobin level. 17. History of antimalarial drugs use including but not limited to MQ, chloroquine, PQ, artesunate, piperaquine and pyronaridine treatment within 12 months. 18. Subject who received quinacrine in last 30 days. | ||
| Interventions | This study is planned to evaluate potential pharmacokinetic interaction of orally administered PQ and dihydroartemisinin-piperaquine (DHA-PQP) in healthy adult subjects. The results of these interaction studies are important in order to provide clinical guidance for the optimum combination of PQ and DHA-PQP treatment regimens in malaria infections. | |
| Outcomes | Primary • AUC for PQ (Time frame: 36 days; designated as safety issue: no). Area under the concentration-time curve [(AUC(0-∞) and AUC(0-last)] and maximal concentration (Cmax) for PQ and metabolites when given alone or together with DHA-PQP. • AUC for dihydroartemisinin (DHA) and piperaquine (PQP) (Time frame: 36 days; designated as safety issue: no). Area under the concentration-time curve [(AUC(0-∞) and AUC(0-last)] and maximal concentration (Cmax) for piperaquine and dihydroartemisinin when given alone as DHA-PQP or together with PQ. | |
| Secondary • Clearance rate and half life of PQ and its metabolites (Time frame: 36 days; designated as safety issue: no). PQ, carboxyprimaquine (and other detectable major metabolites) elimination clearance rate (CL/F), terminal elimination half-life (t1/2) and apparent volume of distribution (Vd) • Dihydroartemisinin and piperaquine elimination clearance rate (CL/F), terminal elimination half-life (t1/2) and apparent volume of distribution (Vd). Safety of dihydroartemisinin-piperaquine (DHA-PQP) (Time frame: 36 days; designated as safety issue: yes). Safety and tolerability parameters, including adverse events, clinical laboratory, and vital signs assessments, in particular QTc prolongation for DHA-PQP. • Pharmacogenetic polymorphisms (Time frame: 36 days; designated as safety issue: yes) in the case of unusually high or low drug levels. | ||
| Starting date | June 2012 | |
| Contact information | Sasithon Pukrittayakamee, MD, Principal Investigator, Mahidol University Salwaluk Panapipat, MBA salwaluk@tropmedres.ac | |
| Notes | ClinicalTrials.gov identifier: NCT01525511 University of Oxford | |
Tiono (started 2013)
| Trial name or title | Low Dose Primaquine for Clearance of Gametocytes: LOPRIM-1 | |
| Methods | Randomized, parallel assignment, double blind (subject, caregiver, investigator, outcomes assessor); safety/efficacy study | |
| Participants | Target sample size 360 participants Inclusion criteria • Age = 2 and < 15 years • P. falciparum parasitaemia = 1000 and < 200,000 parasites/µL • P. falciparum gametocytes • Normal G6PD • Informed consent by legally acceptable representative | |
| Exclusion criteria • Enrolled in another study • Fever or history of fever in last 24 hours • Evidence of severe illness/danger signs • Known allergy to study medications • Hb < 8 g/dL • Started menstruation • Pregnancy or breastfeeding • Antimalarials taken within last 2 days • PQ taken within last 4 weeks • Blood transfusion within the last 90 days • Non-falciparum malaria co-infection | ||
| Interventions | Group 1: AL Group 2: AL with a single dose of 0.25 mg/kg PQ Group 3: AL with a single dose of 0.4 mg/kg PQ | |
| Outcomes | Primary: Gametocyte carriage (time frame: 14 days during follow-up) Secondary: • Haematological recovery (time frame: 14 days during follow-up) • Transmission to An. gambiae mosquitoes (time frame: day 1, 3, and 7 | |
| Starting date | September 2013 | |
| Contact information | t.alfred@fasonet.bf; teun.bousema@lshtm.ac.uk | |
| Notes | ClinicalTrials.gov identifier: NCT01935882 Burkina Faso, Centre National de Recherche et de Formation sur le Paludisme, Ougadougou, Burkina Faso. | |
DATA AND ANALYSES
Comparison 1.
Non-artemisinin treatment regimen: PQ versus no PQ
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with gametocytes | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Day 2 | 2 | 96 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.46, 1.12] |
| 1.2 Day 3 | 1 | 83 | Risk Ratio (M-H, Fixed, 95% CI) | 0.75 [0.45, 1.27] |
| 1.3 Day 4 or 5 | 3 | 273 | Risk Ratio (M-H, Fixed, 95% CI) | 0.84 [0.64, 1.09] |
| 1.4 Day 8 | 5 | 402 | Risk Ratio (M-H, Fixed, 95% CI) | 0.54 [0.44, 0.65] |
| 1.5 Day 15 | 4 | 366 | Risk Ratio (M-H, Fixed, 95% CI) | 0.31 [0.22, 0.43] |
| 1.6 Day 22 | 4 | 323 | Risk Ratio (M-H, Fixed, 95% CI) | 0.30 [0.20, 0.46] |
| 1.7 Day 29 | 4 | 290 | Risk Ratio (M-H, Fixed, 95% CI) | 0.34 [0.19, 0.60] |
| 1.8 Day 36 | 1 | 81 | Risk Ratio (M-H, Fixed, 95% CI) | 0.37 [0.15, 0.94] |
| 1.9 Day 43 | 1 | 73 | Risk Ratio (M-H, Fixed, 95% CI) | 0.27 [0.04, 1.71] |
| 2 Gametocyte clearance time (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Participants infectious | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Day 5 | 2 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.06 [0.01, 0.42] |
| 3.2 Day 8 | 2 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.07 [0.01, 0.45] |
| 3.3 Day 15 | 2 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.14 [0.02, 1.04] |
| 3.4 Day 22 | 2 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.02, 7.24] |
| 4 Mosquitoes infected | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Day 5 | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Day 8 | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Day 15 | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Day 22 | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Participants with asexual parasites at day 29 | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Not PCR adjusted | 1 | 209 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.78, 1.15] |
| 5.2 PCR adjusted | 1 | 209 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
| 6 Asexual parasite clearance time (hrs) | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | -1.68 [-9.60, 6.25] |
| 7 Adverse effects | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Nausea | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Vomiting | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Dizziness | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Any adverse effect | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 By dose: Participants with gametocytes at day 8 (microscopy) | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 8.1 < 0.4 mg/kg PQ per day | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 ≥ 0.4 to < 0.6 mg/kg PQ per day | 1 | 216 | Risk Ratio (M-H, Fixed, 95% CI) | 0.60 [0.49, 0.75] |
| 8.3 ≥ 0.6 mg/kg PQ per day | 4 | 186 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.25, 0.62] |
| 9 By schedule: Participants with gametocytes at day 8 | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Single dose day 1 or 2 | 4 | 191 | Risk Ratio (M-H, Fixed, 95% CI) | 0.64 [0.49, 0.84] |
| 9.2 Single dose day 3 or 4 | 3 | 243 | Risk Ratio (M-H, Fixed, 95% CI) | 0.45 [0.33, 0.60] |
| 9.3 Multiple dose days 1 to 7 | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Artemisinin treatment regimen: PQ versus no PQ
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with gametocytes (microscopy) | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 4 | 4 | 971 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 1.2 Day 8 | 5 | 1071 | Risk Ratio (M-H, Random, 95% CI) | 0.21 [0.08, 0.54] |
| 1.3 Day 15 | 4 | 995 | Risk Ratio (M-H, Random, 95% CI) | 0.09 [0.04, 0.19] |
| 1.4 Day 22 | 3 | 858 | Risk Ratio (M-H, Random, 95% CI) | 0.10 [0.03, 0.32] |
| 1.5 Day 29 | 4 | 945 | Risk Ratio (M-H, Random, 95% CI) | 0.17 [0.04, 0.72] |
| 1.6 Day 36 | 3 | 838 | Risk Ratio (M-H, Random, 95% CI) | 0.21 [0.01, 4.32] |
| 1.7 Day 43 | 4 | 917 | Risk Ratio (M-H, Random, 95% CI) | 0.41 [0.04, 3.89] |
| 2 Participants with gametocytes (PCR) | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Day 8 | 3 | 627 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.26, 0.69] |
| 2.2 Day 15 | 3 | 609 | Risk Ratio (M-H, Random, 95% CI) | 0.27 [0.11, 0.70] |
| 2.3 Day 29 | 1 | 90 | Risk Ratio (M-H, Random, 95% CI) | 0.23 [0.08, 0.62] |
| 2.4 Day 43 | 1 | 79 | Risk Ratio (M-H, Random, 95% CI) | 0.44 [0.17, 1.16] |
| 3 Participants with asexual parasites | 5 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Day 8 | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 1.28 [0.30, 5.40] |
| 3.2 Day 15 | 2 | 198 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.23, 4.15] |
| 3.3 Day 29 | 3 | 747 | Risk Ratio (M-H, Fixed, 95% CI) | 0.54 [0.33, 0.88] |
| 3.4 Day 43 | 2 | 178 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.59, 1.81] |
| 4 Asexual parasite clearance time (hrs) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | -6.0 [-16.31, 4.31] |
| 5 Haemoglobin concentration | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Day 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Day 15 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Day 29 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Day 43 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 % change in haemoglobin concentration | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Day 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Day 15 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Day 29 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Day 43 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Other adverse effects | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Headache | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Fatigue | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Nausea | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Vomiting | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Abdominal pain | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Diarrhea | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 Pruritis | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 Paresthesia | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.9 Unspecified | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 By dose: Participants with gametocytes at day 8 (microscopy or PCR) | 7 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 8.1 < 0.4 mg/kg PQ per day | 1 | 223 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.44, 1.02] |
| 8.2 ≥ 0.4 to < 0.6 mg/kg PQ per day | 1 | 219 | Risk Ratio (M-H, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 8.3 ≥ 0.6 mg/kg PQ per day | 7 | 1380 | Risk Ratio (M-H, Fixed, 95% CI) | 0.29 [0.22, 0.37] |
| 9 By schedule: Participants with gametocytes at day 8 (microscopy or PCR) | 7 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Single dose day 1 or 2 | 2 | 843 | Risk Ratio (M-H, Fixed, 95% CI) | 0.13 [0.07, 0.22] |
| 9.2 Single dose day 3 or 4 | 5 | 748 | Risk Ratio (M-H, Fixed, 95% CI) | 0.47 [0.37, 0.59] |
| 9.3 Multiple dose days 1 to 7 | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3.
PQ versus no PQ; gametocytes at day 8 (microscopy or PCR); stratified by non-artemisinin versus artemisinin regimen
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with gametocytes at day 8 (microscopy or PCR) | 11 | 1994 | Risk Ratio (M-H, Random, 95% CI) | 0.38 [0.27, 0.52] |
| 1.1 Non-artemisinin-based partner drug | 5 | 402 | Risk Ratio (M-H, Random, 95% CI) | 0.54 [0.38, 0.77] |
| 1.2 Artemisinin-based partner drug | 7 | 1592 | Risk Ratio (M-H, Random, 95% CI) | 0.32 [0.20, 0.51] |
Comparison 4.
PQ versus other 8AQ
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with gametocytes on day 8 | 2 | 112 | Risk Ratio (M-H, Fixed, 95% CI) | 0.41 [0.26, 0.66] |
Analysis 1.1.
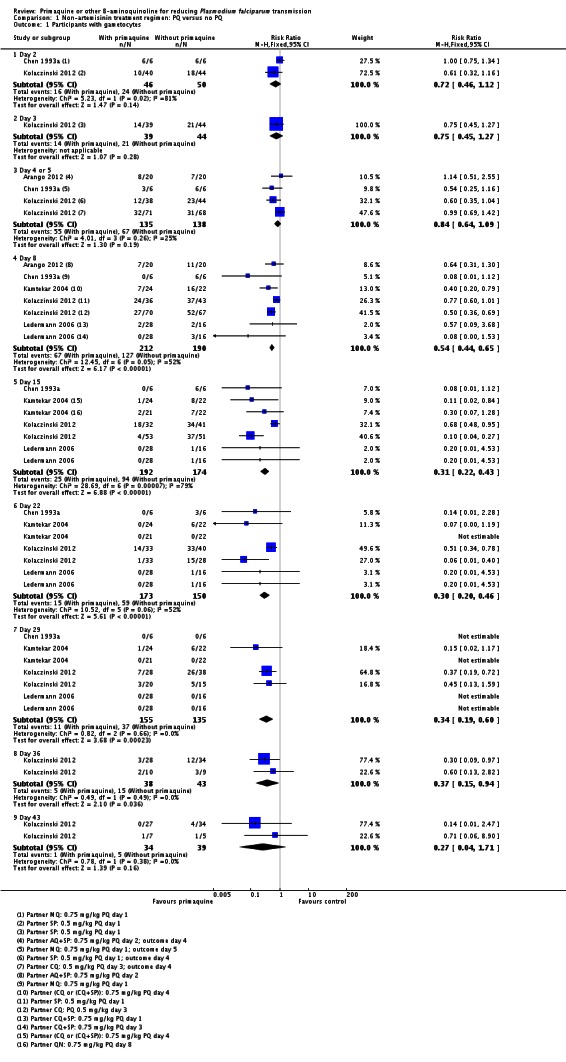
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 1 Participants with gametocytes.
Analysis 1.2.

Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 2 Gametocyte clearance time (days).
Analysis 1.3.
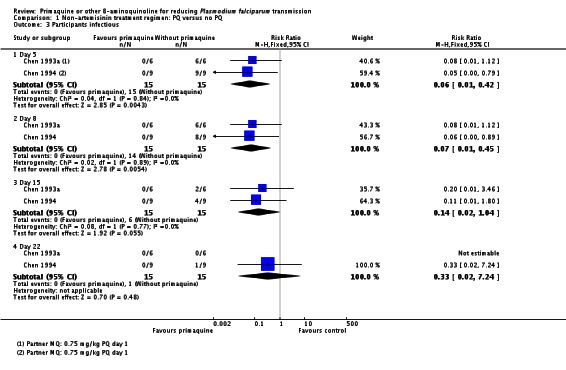
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 3 Participants infectious.
Analysis 1.4.
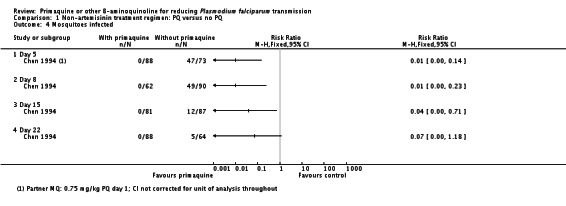
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 4 Mosquitoes infected.
Analysis 1.5.
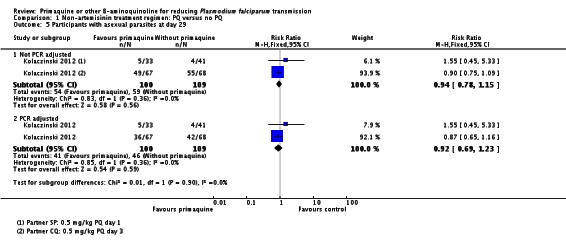
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 5 Participants with asexual parasites at day 29.
Analysis 1.6.
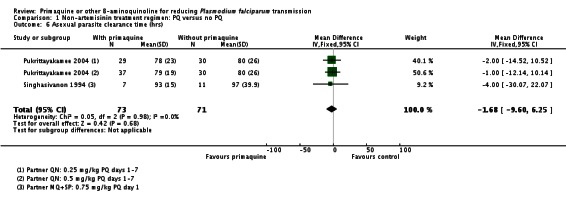
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 6 Asexual parasite clearance time (hrs).
Analysis 1.7.
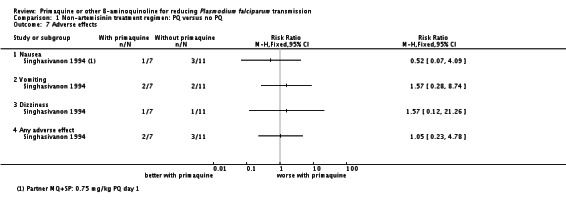
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 7 Adverse effects.
Analysis 1.8.
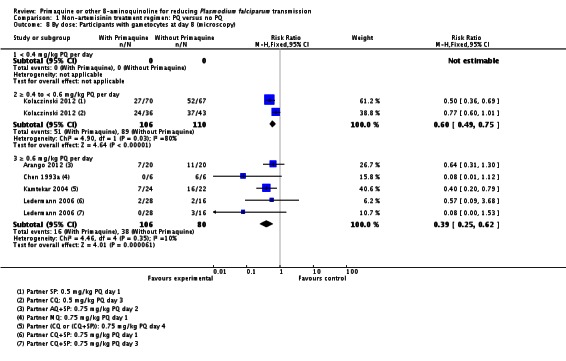
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 8 By dose: Participants with gametocytes at day 8 (microscopy).
Analysis 1.9.
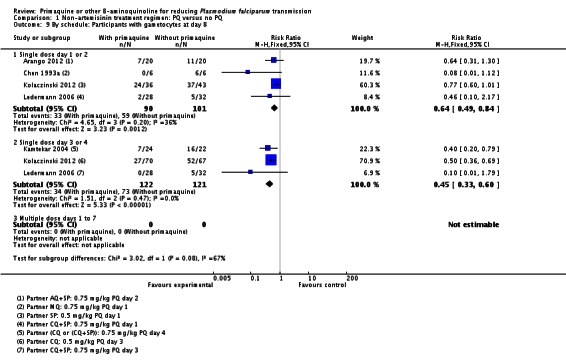
Comparison 1 Non-artemisinin treatment regimen: PQ versus no PQ, Outcome 9 By schedule: Participants with gametocytes at day 8.
Analysis 2.1.
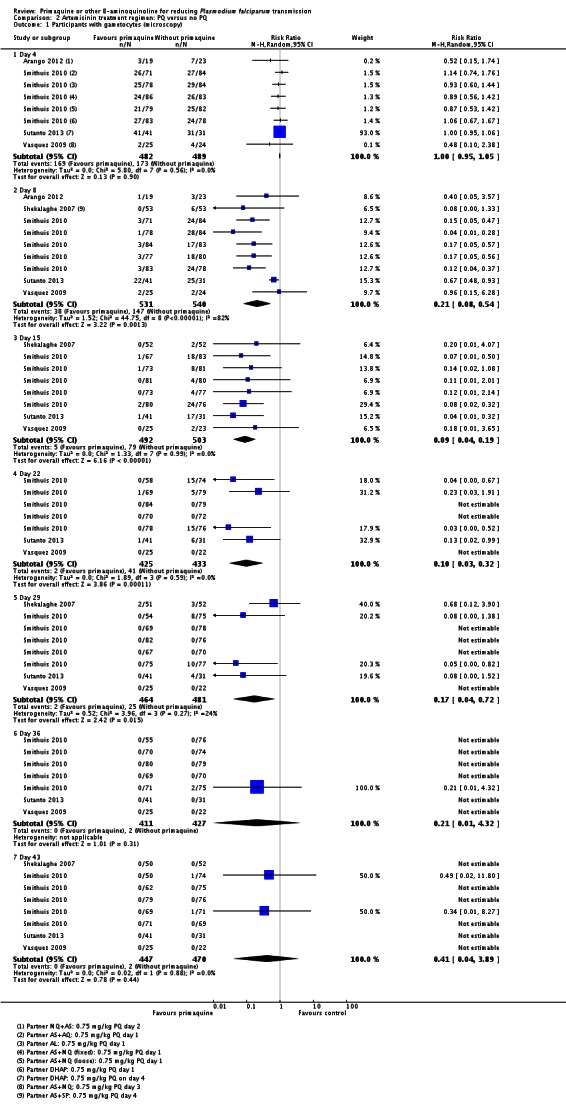
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 1 Participants with gametocytes (microscopy).
Analysis 2.2.
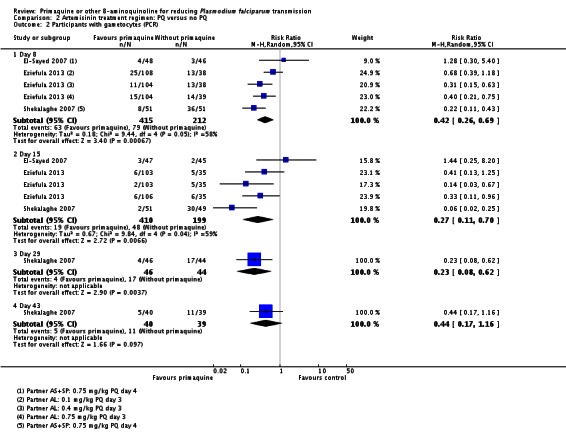
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 2 Participants with gametocytes (PCR).
Analysis 2.3.
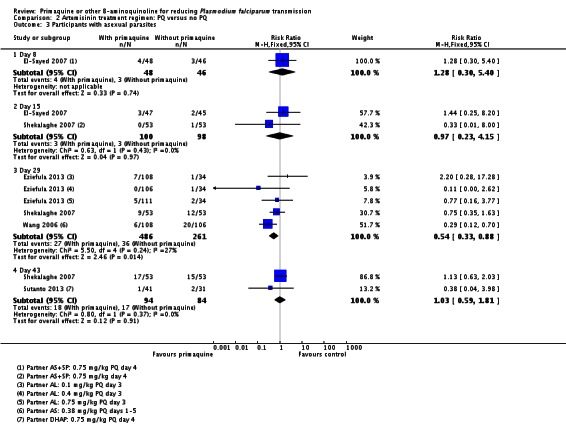
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 3 Participants with asexual parasites.
Analysis 2.4.
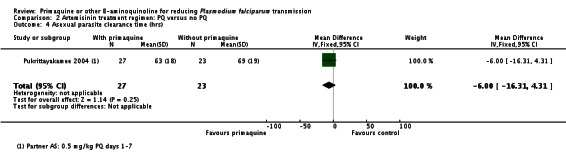
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 4 Asexual parasite clearance time (hrs).
Analysis 2.5.
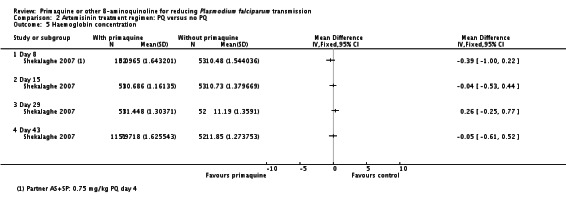
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 5 Haemoglobin concentration.
Analysis 2.6.
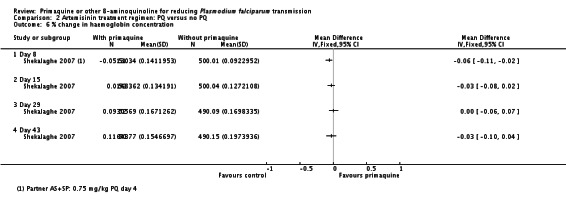
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 6 % change in haemoglobin concentration.
Analysis 2.7.
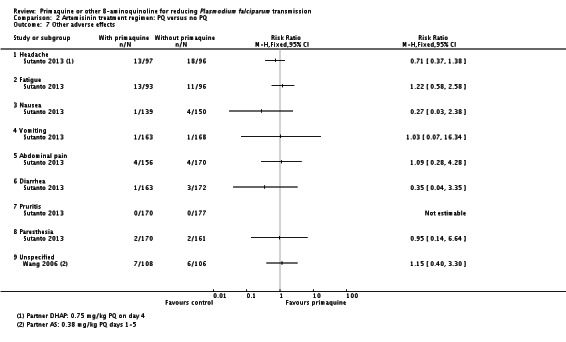
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 7 Other adverse effects.
Analysis 2.8.
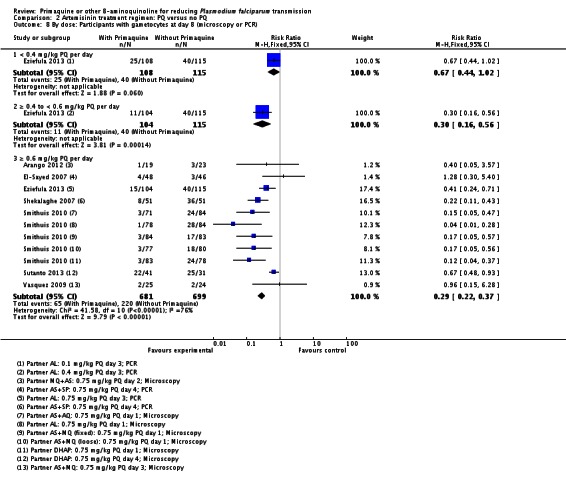
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 8 By dose: Participants with gametocytes at day 8 (microscopy or PCR).
Analysis 2.9.
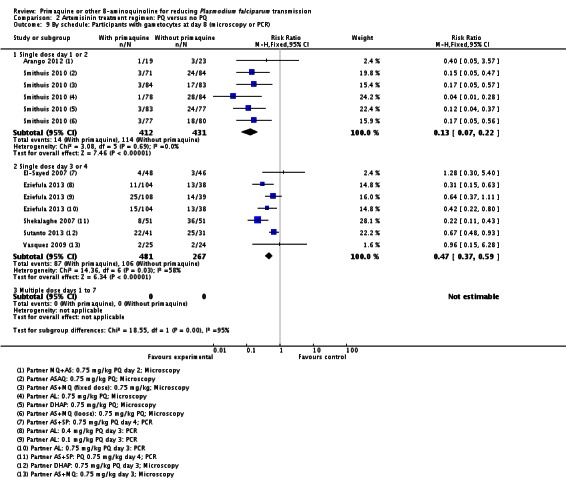
Comparison 2 Artemisinin treatment regimen: PQ versus no PQ, Outcome 9 By schedule: Participants with gametocytes at day 8 (microscopy or PCR).
Analysis 3.1.
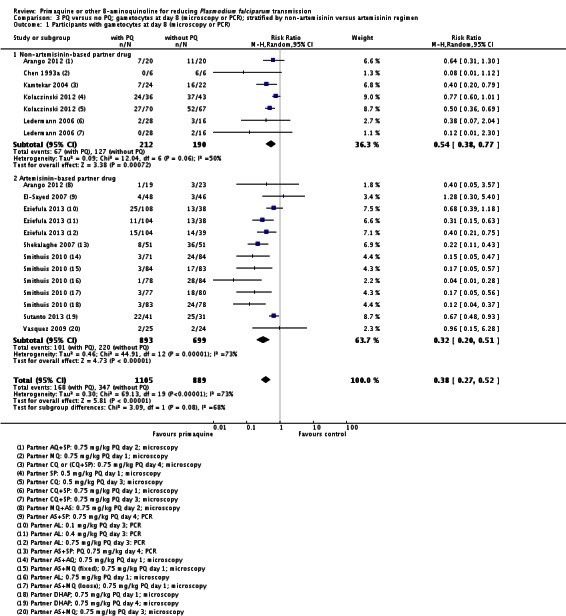
Comparison 3 PQ versus no PQ; gametocytes at day 8 (microscopy or PCR); stratified by non-artemisinin versus artemisinin regimen, Outcome 1 Participants with gametocytes at day 8 (microscopy or PCR).
Analysis 4.1.
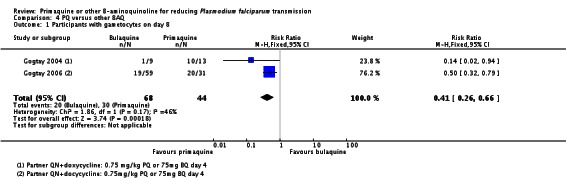
Comparison 4 PQ versus other 8AQ, Outcome 1 Participants with gametocytes on day 8.
CONTRIBUTIONS OF AUTHORS
2015 edition: PMG, HG and PG contributed to adjusting the data and updating the text.
Graves 2014: PMG and HG added the new studies. PG helped rewrite the review. All review authors contributed to the interpretation of the results and the conclusions drawn.
Graves 2012: Two review authors (PMG and HG) independently screened all abstracts, applied inclusion criteria and extracted data. PG helped structure the review and contributed to the logic framework of the 'Summary of findings' tables. All review authors contributed to the writing of the review, the interpretation of the results and the conclusions drawn.
DECLARATIONS OF INTEREST
We have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of the review (for example, employment, consultancy, stock ownership, honoraria or expert testimony).
This review and the salary of PG is supported by a DFID grant aimed at ensuring the best possible systematic reviews, particularly Cochrane Reviews, are completed on topics relevant to the poor in low- and middle-income countries. DFID does not participate in the selection of topics, in the conduct of the review or in the interpretation of findings.
PMG is a member of the WHO Malaria Policy Advisory Committee, to provide independent strategic advice in forming WHO policies in malaria. PG is a member of the WHO Guidelines for the Treatment of Malaria Group that made the recommendation for PQ to reduce P. falciparum malaria transmission.
None of the review authors are investigators on any of the included trials.
SOURCES OF SUPPORT
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
1. After reading the trials, we added several new outcomes and modified some outcomes; we deleted two outcomes.
Changes to primary outcomes:
Proportion of participants with gametocytes: we added: by microscopy and PCR;
We added: Proportion of participants infectious;
We included: Gametocyte density (by microscopy and PCR);
We added: Gametocyte clearance time and duration of gametocyte carriage.
We arranged the primary outcomes to capture the three categories: transmission intensity, infectiousness and potential infectiousness.
Changes to secondary outcomes:
We deleted AUC of asexual parasite density over time. We did not identify any relevant data;
We added asexual clearance time.
Changes to Adverse events:
We deleted: All adverse events (data reported was minimal and not in a form that was easily summarised. The main question is whether there are serious adverse events);
We modified haemolysis or drop in haemoglobin or PCV (as assessed/defined in each trial) by deleting reference to G6PD since these outcomes occur in non-G6PD people too. We also added PCV since this was used in some trials as a measure of anaemia.
2. In the first version of the review, we deleted the objective: "To compare the effects of different doses and schedules of PQ given to reduce infectiousness" and we modified the definition of control in comparisons accordingly. We only included controls without PQ. We deleted the comparison of different doses of PQ with identical other treatment regimens since it does not answer the important question of whether adding PQ is effective. We included one trial with two arms using different doses of PQ with same other treatment regimens as two separate arms within the same comparison.
In the June 2014 update, we reversed this decision.
3. We planned to use the following comparisons described in the protocol:
CQ (with and without PQ, or with different doses of PQ);
SP (with and without PQ, or with different doses of PQ);
CQ plus sulfadoxine + pyrimethamine (with and without PQ, or with different doses of PQ);
Artemisinin derivatives (with and without PQ, or with different doses of PQ);
Other drugs (with and without PQ, or with different doses of PQ).
In the review, we changed the groups, added some, and combined some for the following reasons:
a. some trials combined two types of malaria treatment regimens, not distinguishing the patients who received each one (for example, CQ or CQ plus SP);
b. there were many different artemisinin derivatives and combinations tested, with few trials of each, so these were grouped within the same comparison. We also grouped combinations of an artemisinin derivative with SP here.
3. There were no eligible cluster-RCTs so we deleted how we would manage them from the Methods section. If we include any cluster-RCTs in future editions, we will check that trials have correctly adjusted for clustering and, if not, attempt to make this adjustment. When the analyses have not adjusted for clustering, we will attempt to adjust the results for clustering by multiplying the standard errors of the estimates by the square root of the design effect, where the design effect is calculated as DEff=1+(m-1)*ICC. This assumes that the necessary information is reported, the average cluster size (m) and the intra-cluster correlation coefficient (ICC).
4. We intended a sensitivity analysis to investigate the robustness of the results to the quality (risk of bias) components, but were unable to do so as there were insufficient trials. If appropriate and necessary, we will conduct sensitivity analysis on cluster-RCTs using a range of estimates for the ICC to see if clustering could influence the individual trial's result.
NOTES
We received comments from Professor Nick White, who has published extensively on using PQ to prevent transmission. Professor White sent some helpful comments on the use of the data and its interpretation. These were considered by the authorship team and disaggregated into key points that needed to be addressed by the review. The Cochrane Contact Editor moderated the process. The main points raised and addressed were:
The lack of effect in low dose categories of PQ does not mean there is no effect and the data suggests a dose response relationship. Response: we have adjusted the wording within the review.
Data from Pukrittayakamee 2004 has been incorrectly extracted/interpreted. Response: Two review authors working independently assumed "after treatment" meant after the seven day course, and it was helpful to have it clarified that this was not the case. Therefore we excluded the data for day 8 gametocyte prevalence from this analysis. We inserted additional text on the gametocyte clearance time and duration of gametocyte carriage to the Results section.
Professor White's comments subsequently appeared in a publication about the topic (White 2014). We have corrected all points of factual detail in this current review version.
INDEX TERMS
Medical Subject Headings (MeSH)
Antimalarials [*administration & dosage]; Artemisinins [therapeutic use]; Chloroquine [therapeutic use]; Drug Combinations; Glucosephosphate Dehydrogenase Deficiency [diagnosis]; Malaria, Falciparum [*prevention & control; transmission]; Mefloquine [therapeutic use]; Plasmodium falciparum [*drug effects]; Primaquine [*administration & dosage]; Pyrimethamine; Quinine [therapeutic use]; Randomized Controlled Trials as Topic; Sulfadoxine
MeSH check words
Humans
References
References to studies included in this review
- Arango EA, Upegui UA, Carmona-Fonseca J. Efficacy of different primaquine-based antimalarial regimens againstPlasmodium falciparum gametocytemia. Acta Tropica. 2012;122((2012)):177–82. doi: 10.1016/j.actatropica.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Chen PQ, Li GQ, Guo XB, Fu YX, He KR, Fu LC, et al. A double blind study on the infectivity of gametocytes of P. falciparum in patients treated with mefloquine and Fansimef. Journal of Guangzhou College of Traditional Chinese Medicine. 1993;10((1)):1–5. [Google Scholar]
- Chen PQ, Li GQ, Guo XB. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chinese Medical Journal. 1994;74((4)):209-10, 253-4. [PubMed] [Google Scholar]
- Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, et al. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chinese Medical Journal. 1994;107((9)):709–11. [PubMed] [Google Scholar]
- El-Sayed B, El-Zaki SE, Babiker H, Gadalla N, Ageep T, Mansour F, et al. A randomized open-label trial of artesunate-sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS ONE. 2007;2((12)):e1311. doi: 10.1371/journal.pone.0001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infectious Diseases. 2013;14((2)):130–9. doi: 10.1016/S1473-3099(13)70268-8. [DOI] [PubMed] [Google Scholar]
- Eziefula AC, Staedke SG, Yeung S, Webb E, Kamya M, White NJ, et al. Study protocol for a randomised controlled double-blinded trial of the dose-dependent efficacy and safety of primaquine for clearance of gametocytes in children with uncomplicated falciparum malaria in Uganda. BMJ Open. 2013;3((3)):e002759. doi: 10.1136/bmjopen-2013-002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay NJ, Kamtekar KD, Dalvi SS, Chogle AR, Aigal U, Kshirsagar N. Preliminary report of the evaluation of the gametocytocidal action of bulaquine, in adult patients with acute, Plasmodium falciparum malaria. Annals of Tropical Medicine and Parasitology. 2004;98((5)):525–8. doi: 10.1179/000349804225003541. [DOI] [PubMed] [Google Scholar]
- Gogtay NJ, Kamtekar KD, Dalvi SS, Mehta SS, Chogle AR, Aigal U, et al. A randomized, parallel study of the safety and efficacy of 45 mg primaquine versus 75 mg bulaquine as gametocytocidal agents in adults with blood schizonticide-responsive uncomplicated falciparum malaria [ISCRTN50134587] BMC Infectious Diseases. 2006;6:16. doi: 10.1186/1471-2334-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamtekar KD, Gogtay NJ, Dalvi SS, Karnad DR, Chogle AR, Aigal U, et al. A prospective study evaluating the efficacy of a single, 45-mg dose of primaquine, as a gametocytocidal agent, in patients with Plasmodium falciparum malaria in Mumbai, India. Annals of Tropical Medicine and Parasitology. 2004;98((5)):453–8. doi: 10.1179/000349804225003550. [DOI] [PubMed] [Google Scholar]
- Khoo KK. The treatment of malaria in glucose-6-phosphate dehydrogenase deficient patients in Sabah. Annals of Tropical Medicine and Parasitology. 1981;75((6)):591–5. doi: 10.1080/00034983.1981.11687489. [DOI] [PubMed] [Google Scholar]
- Kolaczinski K, Leslie T, Ali I, Durrani N, Lee S, Barends M, et al. Defining Plasmodium falciparum treatment in South West Asia: a randomized trial comparing artesunate or primaquine combined with chloroquine or SP. PLoS One. 2012;7((1)):e28957. doi: 10.1371/journal.pone.0028957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman ER, Maguire JD, Sumawinata IW, Chand K, Elyazar I, Estiana L, et al. Combined chloroquine, sulfadoxine/pyrimethamine and primaquine against Plasmodium falciparum in Central Java, Indonesia. Malaria Journal. 2006;5:108. doi: 10.1186/1475-2875-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrittayakamee S, Chotivanich K, Chantra A, Clemens R, Looareesuwan S, White NJ. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrobial Agents and Chemotherapy. 2004;48((4)):1329–34. doi: 10.1128/AAC.48.4.1329-1334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malaria Journal. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekalaghe S, Drakeley C, Gosling R, Ndaro A, van Meegeren M, Enevold A, et al. Primaquine clears submicroscopicPlasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS ONE. 2007;2((10)):e1023. doi: 10.1371/journal.pone.0001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhasivanon V, Chongsuphajaisiddhi T, Sabchareon A, Attanath P, Webster HK, Edstein MD, et al. Pharmacokinetic study of mefloquine in Thai children aged 5-12 years suffering from uncomplicated falciparum malaria treated with MSP or MSP plus primaquine. European Journal of Drug Metabolism and Pharmacokinetics. 1994;19((1)):27–32. doi: 10.1007/BF03188819. [DOI] [PubMed] [Google Scholar]
- Smithuis F, Kyaw MK, Phe O, Win T, Aung PP, Oo AP, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infectious Diseases. 2010;10((10)):673–81. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutanto I, Suprijanto S, Kosasih A, Dahlan MS, Syafruddin D, Kusriastuti R, et al. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in South Sumatra, Western Indonesia: an open-label, randomized, controlled trial. Clinical Infectious Diseases. 2013;56((5)):685–93. doi: 10.1093/cid/cis959. [DOI] [PubMed] [Google Scholar]
- Vásquez AM, Sanín F, Alvarez LG, Tobón A, Ríos A, Blair S. Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development. Biomedica. 2009;29((2)):307–19. [Estudio piloto de la eficacia y de los efectos sobre los gametocitos del esquema artesunato-mefloquina-primaquina para la malaria por Plasmodium falciparum] [PubMed] [Google Scholar]
- Wang YS, Brown PP. Clinical study on artemether combined with Primaquine for Pf cases treatment. Tianjin Medical Journal. 2006;34((8)):538. [Chinese] [Google Scholar]
References to studies excluded from this review
- Baird JK, Wiady I, Sutanihardja A, Suradi, Purnomo, Basri H, et al. Short report: therapeutic efficacy of chloroquine combined with primaquine against Plasmodium falciparum in northeastern Papua, Indonesia. 2002;66(6):659–60. doi: 10.4269/ajtmh.2002.66.659. American Journal of Tropical Medicine and Hygiene. [DOI] [PubMed] [Google Scholar]
- Barber MA, Komp WHW, Newman BM. The effect of small doses of plasmochin on the viability of gametocytes of malaria as measured by mosquito infection experiments. Public Health Reports. 1929;44((24)):1409–20. [Google Scholar]
- Barber MA, Rice JB, Brown JY. Malaria studies on the Firestone Rubber Plantation in Liberia, West Africa. American Journal of Hygiene. 1932;15((3)):601–33. [Google Scholar]
- Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. American Journal of Tropical Medicine & Hygiene. 1998;58((5)):645–9. doi: 10.4269/ajtmh.1998.58.645. [DOI] [PubMed] [Google Scholar]
- Bunnag D, Harinasuta T, Pinichpongse D, Suntharasamai P. Effect of primaquine on gametocytes of Plasmodium falciparum in Thailand. Lancet. 1980;2((8185)):91. doi: 10.1016/s0140-6736(80)92970-0. [DOI] [PubMed] [Google Scholar]
- Burgess RW, Bray RS. The effect of a single dose of primaquine upon the gametocytes, gametogony and sporogony of Laverania (=Plasmodium) falciparum. 1960. pp. 1–10. World Health Organization WHO/MAL/271. [PMC free article] [PubMed]
- Burgess RW, Bray RS. The effect of a single dose of primaquine upon the gametocytes, gametogony and sporogony of Laverania falciparum. Bulletin of the World Health Organization. 1961;24:451–6. [PMC free article] [PubMed] [Google Scholar]
- Cai X, Yang X, He X, Zhan W, Zhan X, Ye B. The combined use of artemether, SP & PQ in the treatment of CQ-resistant Pf. Chinese Journal of Parasitology and Parasitic Diseases. 1985;3((2)):81–4. [Chinese] [PubMed] [Google Scholar]
- Carter N, Pamba A, Duparc S, Waitumbi JN. Frequency of glucose-6-phosphate dehydrogenase deficiency in malaria patients from six African countries enrolled in two randomized anti-malarial clinical trials. Malaria Journal. 2011;10:241. doi: 10.1186/1475-2875-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che LG, Huang KG, Yang HL, Yu L, Lin ZL, Huang R. Combined use of pyronaridine, sulfadoxine and primaquine in areas with chloroquine-resistant falciparum malaria. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi (Chinese Journal of Parasitology and Parasitic Diseases) 1987;5((3)):194–6. [PubMed] [Google Scholar]
- Che L, Huang K, Dong Y, Yang H, Yang P. Efficacy of two combined therapies for treatment of chloroquine-resistant P. falciparum. Chinese Journal of Parasitic Disease Control. 1990;3((1)):24–6. [Chinese] [Google Scholar]
- Chevalley S, Coste A, Lopez A, Pipy B, Valentin A. Flow cytometry for the evaluation of anti-plasmodial activity of drugs on Plasmodium falciparum gametocytes. Malaria Journal. 2010;9:49. doi: 10.1186/1475-2875-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde DF. Mass administration of an antimalarial drug containing 4-aminoquinoline and 8-aminoquinoline in Tanganyika. Bulletin of the World Health Organization. 1962;27:203–12. [PMC free article] [PubMed] [Google Scholar]
- Clyde DF, DuPont HL, Miller RM, McCarthy VC. Prophylactic and sporontocidal treatment of chloroquine resistant Plasmodium falciparum from Malaya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1970;64((6)):834–8. doi: 10.1016/0035-9203(70)90102-1. [DOI] [PubMed] [Google Scholar]
- Clyde DF, Miller RM, Music SI, McCarthy VC. Prophylactic and sporontocidal treatment of chloroquine-resistant Plasmodium falciparum from Vietnam. American Journal of Tropical Medicine and Hygiene. 1971;20((1)):1–5. doi: 10.4269/ajtmh.1971.20.1. [DOI] [PubMed] [Google Scholar]
- da Silva AR, Carneiro EW, dos Santos HJ. Response of human Plasmodium to antimalarials on the island of Saint Louis, State of Maranhão, Brazil. Revista do Instituto de Medecina Tropical de São Paulo. 1984;26((3)):139–46. doi: 10.1590/s0036-46651984000300003. [DOI] [PubMed] [Google Scholar]
- Degowin RL, Eppes RB, Powell RD, Carson PE. The haemolytic effects of diaphenylsulfone (DDS) in normal subjects and in those with glucose-6-phosphate-dehydrogenase deficiency. Bulletin of the World Health Organization. 1966;35((2)):165–79. [PMC free article] [PubMed] [Google Scholar]
- Doi H, Kaneko A, Panjaitan W, Ishii A. Chemotherapeutic malaria control operation by single dose of Fansidar plus primaquine in North Sumatra, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health. 1989;20((3)):341–9. [PubMed] [Google Scholar]
- Giao PT, de Vries PJ, Hung le Q, Binh TQ, Nam NV, Kager PA. CV8, a new combination of dihydroartemisinin, piperaquine, trimethoprim and primaquine, compared with atovaquone-proguanil against falciparum malaria in Vietnam. Tropical Medicine and International Health. 2004;9((2)):209–16. doi: 10.1046/j.1365-3156.2003.01180.x. [DOI] [PubMed] [Google Scholar]
- Gogtay NJ, Chogle AR, Sorabjee JS, Marathe SN, Kshirsagar NA. Poor gametocytocidal activity of 45 mg primaquine in chloroquine-treated patients with acute, uncomplicated, Plasmodium falciparum malaria in Mumbai (Bombay): an issue of public-health importance. Annals of Tropical Medicine and Parasitology. 1999;93((8)):813–6. doi: 10.1080/00034989957808. [DOI] [PubMed] [Google Scholar]
- Gunders AE. The effect of a single dose of pyrimethamine and primaquine in combination upon gametocytes and sporogony of Laverania falciparum; Plasmodium falciparum in Liberia. Bulletin of the World Health Organization. 1961;24:650–3. [PMC free article] [PubMed] [Google Scholar]
- Hii JL, Vun YS, Chin KF, Chua R, Tambakau S, Binisol ES, et al. The influence of permethrin-impregnated bednets and mass drug administration on the incidence of Plasmodium falciparum malaria in children in Sabah, Malaysia. Medical and Veterinary Entomology. 1987;1((4)):397–407. doi: 10.1111/j.1365-2915.1987.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Huang ZS, Fu SG, Cai XZh. Combined use of pyronaridine/SP with primaquine for P. falciparum treatment. Journal of Hainan Medicine. 1993;4((1)):10–2. [Chinese] [Google Scholar]
- Huang Z, Meng F, Fu S. Comparative studies on the treatment of drug-resistant P. falciparum with pyronaridine/SP and primaquine. Chinese Journal Parasitology and Parasitic Disease. 1996;14((4)):314–7. [Chinese] [Google Scholar]
- Huang JR, Gao YQ, Elie N. A study of artemether combined with primaquine in the treatment of falciparum malaria. Chinese Journal of Parasitology and Parasitic Diseases. 2001;19((5)):308–9. [Chinese] [PubMed] [Google Scholar]
- Jeffery GM, Young MD, Eyles DE. The treatment of Plasmodium falciparum infection with chloroquine, with a note on infectivity to mosquitoes of primaquine- and pyrimethamine- treated cases. American Journal of Hygiene. 1956;64((1)):1–11. doi: 10.1093/oxfordjournals.aje.a119818. [DOI] [PubMed] [Google Scholar]
- Jeffery GM, Collins WE, Skinner JC. Antimalarial drug trials on a multiresistant strain of Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene. 1963;12:844–50. doi: 10.4269/ajtmh.1963.12.844. [DOI] [PubMed] [Google Scholar]
- Jerace F, Giovannola A. The sterilizing action of plasmochina on gametocytes of malaria parasites and its prophylactic importance. Rivista di Malariaologia. 1933;12:475. [L'azzione sterilizzante della plasmochina sui gametociti di parassiti malarigeni e sua importanza profilattica] [Google Scholar]
- Kaneko A, Kamei K, Suzuki T, Ishii A, Siagian R, Panjaitan W. Gametocytocidal effect of primaquine in a chemotherapeutic malaria control trial in North Sumatra, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health. 1989;20((3)):351–9. [PubMed] [Google Scholar]
- Karbwang J, Molunto P, Bunnag D, Harinasuta T. Plasma quinine levels in patients with falciparum malaria when given alone or in combination with tetracycline with or without primaquine. Southeast Asian Journal of Tropical Medicine and Public Health. 1991;22((1)):72–6. [PubMed] [Google Scholar]
- Karbwang J, Na Bangchang K, Thanavibul A, Back DJ, Bunnag D. Pharmacokinetics of mefloquine in the presence of primaquine. European Journal of Clinical Pharmacology. 1992;42((5)):559–60. doi: 10.1007/BF00314870. [DOI] [PubMed] [Google Scholar]
- Myat-Phone-Kyaw, Myint-Oo, Aung-Naing, Aye-Lwin-Htwe The use of primaquine in malaria infected patients with red cell glucose phosphate (G6PD) deficiency in Myanmar. Southeast Asian Journal of Tropical Medicine and Public Health. 1994;25((4)):170–13. [PubMed] [Google Scholar]
- Li J, Xiao H, Wang W, Ma W, Rao B. Artemether combined with primaquine for P.falciparum cases treatment. Clinical Medical Journal of China. 2007;14((5)):736–7. [Chinese] [Google Scholar]
- Li WJ, Li XL. Observation of curative effect of falciparum malaria treatment with artemether/lumefantrine and primaquine. Journal of Modern Preventive Medicine. 2010;37((5)):973–5. [Chinese] [Google Scholar]
- Lin L, Wang D-L, Liu B, Hu G. Artemether combined with primaquine for treatment of malaria cases from UN peace force. Chinese Journal of Parasitic Disease Control. 2004;17((5)):5–6. [Chinese] [Google Scholar]
- Mackerras MJ, Ercole QN. Observations on the action of quinine, atebrine and plasmoquine on the gametocytes of Plasmodium falciparum. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1949;42((5)):455–63. doi: 10.1016/0035-9203(49)90051-6. [DOI] [PubMed] [Google Scholar]
- Rieckmann KH, McNamara JV, Frischer H, Stockert TA, Carson PE, Powell RD. Gametocytocidal and sporontocidal effects of primaquine and of sulfadiazine with pyrimethamine in a chloroquine-resistant strain of Plasmodium falciparum. Bulletin of the World Health Organization. 1968;38((4)):625–32. [PMC free article] [PubMed] [Google Scholar]
- Rieckmann KH, McNamara JV, Kass L, Powell RD. Gametocytocidal and sporontocidal effects of primaquine upon two strains of Plasmodium falciparum. Military Medicine. 1969;134((10)):802–19. [PubMed] [Google Scholar]
- Santana MS, da Rocha MA, Arcanjo AR, Sardinha JF, Alecrim WD, Alecrim Md. Association of methemoglobinemia and glucose-6-phosphate dehydrogenase deficiency in malaria patients treated with primaquine. Revista da Sociedade de Brasileira de Medicina Tropical. 2007;40((5)):533–6. doi: 10.1590/s0037-86822007000500008. [Associação de metemoglobinemia e deficiência de glicose-6-fosfato desidrogenase em pacientes com malária tratados com primaquina] [DOI] [PubMed] [Google Scholar]
- Shah NK, Schapira A, Juliano JJ, Srivastava B, MacDonald PD, Poole C, et al. Nonrandomized controlled trial of artesunate plus sulfadoxine-pyrimethamine with or without primaquine for preventing posttreatment circulation of Plasmodium falciparum gametocytes. Antimicrobial Agents and Chemotherapy. 2013;57((7)):2948–54. doi: 10.1128/AAC.00139-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekalaghe SA, ter Braak R, Daou M, Kavishe R, vanden Bijllaardt W, vanden Bosch S, et al. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A-) individuals. Antimicrobial Agents and Chemotherapy. 2010;54((5)):1762–8. doi: 10.1128/AAC.01135-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skekalaghe SA, Drakeley C, vanden Bosch S, ter Braak R, vanden Bijllhaardt W, Mwanziva C, et al. A cluster-randomized trial of mass drug administration with a gametocytocidal drug combination to interrupt malaria transmission in a low endemic area in Tanzania. Malaria Journal. 2011;10:247. doi: 10.1186/1475-2875-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W-H, Traore A. Artesunate combined with primaquine in treatment study on delay or relapse of malaria. Chinese Journal of Clinical Pharmacology and Therapeutics. 2011;1:98–100. [Chinese] [Google Scholar]
- Suputtamongkol Y, Chindarat S, Silpasakorn S, Chaikachonpatd S, Lim K, Chanthapakajee K, et al. The efficacy of combined mefloquine-artesunate versus mefloquine-primaquine on subsequent development of Plasmodium falciparum gametocytemia. American Journal of Tropical Medicine and Hygiene. 2003;68((5)):620–3. doi: 10.4269/ajtmh.2003.68.620. [DOI] [PubMed] [Google Scholar]
- Tangpukdee N, Krudsood S, Srivilairit S, Phophak N, Chonsawat P, Yanpanich W, et al. Gametocyte clearance in uncomplicated and severe Plasmodium falciparum malaria after artesunate-mefloquine treatment in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2008;46((2)):65–70. doi: 10.3347/kjp.2008.46.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Che L, Huang K, Yang P, Dong Y, Lin Z, et al. The effect of combinations of pyronaridine, sufadoxine and primaquine on chloroquine-resistant P. falciparum. Chinese Journal of Parasitic Disease Control. 1989;2((1)):7–10. [Google Scholar]
- Yeramian P, Meshnick SR, Krudsood S, Chalermrut, K, Silachamroon U, Tangpukdee N, et al. Efficacy of DB289 in Thai patients with Plasmodium vivax or acute, uncomplicated Plasmodium falciparum infections. Journal of Infectious Diseases. 2005;192((2)):319–22. doi: 10.1086/430928. [DOI] [PubMed] [Google Scholar]
- Young MD. The effect of small doses of primaquine upon malaria infections. Indian Journal of Malariology. 1959;13:69–74. [Google Scholar]
References to studies awaiting assessment
- Chen L. Efficacy of artemether/primaquine against drug resistant P. falciparum. Journal of Applied Medicine. 1993;1((1)):31–3. [Chinese] [Google Scholar]
- Ishii A, Ohta N, Owhashi M, Kawabata M, Chung D, Bobogare A, et al. Trials of transmission blocking of P. falciparum with single dose primaquine in villages of Solomon Islands. MIM conference October 2009 MIM 16723361.
- Li J, et al. Artemether combined with primaquine for treatment of 50 Pf cases. Journal of Applied Medicine. 2006;22((19)):2299–300. [Chinese] [Google Scholar]
References to ongoing studies
- Primaquine's Gametocytocidal Efficacy in Malaria Asymptomatic Carriers Treated With Dihydroartemisinin-piperaquine in The Gambia. Ongoing study August 2013; December 2014 (final data collection date for primary outcome measure)
- Phase 2a Dose Escalation Study of the Efficacy, Safety, and Pharmacokinetics of Low Dose Primaquine for Gametocytocidal Activity Against P. Falciparum in Sub-Saharan Africa and South East Asia. Ongoing study September 2014 (final data collection date for primary outcome measure)
- Surveillance and Treatment With Dihydroartemisinin-piperaquine Plus Primaquine (MTC Belu)Sub-title: Impact of Mass Screening and Selective Treatment With Dihydroartemisinin-piperaquine Plus Primaquine on Malaria Transmission in High Endemic Area, Belu Regency, Nusa Tenggara Timur Province, Indonesia: a Randomized Cluster Trial. Ongoing study June 2013.
- Active Surveillance for P. falciparum Drug Resistance With Assessment of Transmission Blocking Activity of Single Dose Primaquine in Cambodia. Ongoing study December 2012; December 2014 (final data collection date for primary outcome measure)
- The Optimal Timing of Primaquine to Prevent Malaria Transmission After Artemisinin-Combination Therapy. Ongoing study May 2013; October 2013 (final data collection date for primary outcome measure)
- Pharmacokinetic Study of Primaquine and Dihydroartemisinin-Piperaquine in Healthy Subjects. Ongoing study February 2012.
- Low Dose Primaquine for Clearance of Gametocytes: LOPRIM-1. Ongoing study September 2013.
Additional references
- Abay SM. Blocking malaria transmission to Anopheles mosquitoes using artemisinin derivatives and primaquine: a systematic review and meta-analysis. Parasites and Vectors. 2013;6((1)):278. doi: 10.1186/1756-3305-6-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Alving AS, Hockwald RS, Clayman CB, Dern RJ, Beutler E, et al. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, P-F-6 strain) Journal of Laboratory and Clinical Medicine. 1955;46((3)):391–7. [PubMed] [Google Scholar]
- Barnes, Ki, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, et al. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. Journal of Infectious Diseases. 2008;197((11)):1605–13. doi: 10.1086/587645. [DOI] [PubMed] [Google Scholar]
- Bhasin VK, Trager W. Chapter XI: Gametocytocidal effects in vitro of primaquine and related compounds on Plasmodium falciparum. In: Wernsdorfer WH, Trigg PI, editors. Primaquine: pharmacokinetics, metabolism, toxicity and activity. UNDP/World Bank/WHO; 1984. [Google Scholar]
- Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malaria Journal. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and eradication. Clinical Microbiology Reviews. 2011;24((2)):377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Dinglasan RR, Morlais I, Gouagna LC, van Warmerdam T, Awono-Ambene PH, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One. 2012;7((8)):e42821. doi: 10.1371/journal.pone.0042821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman JG. Resistance to artemisinin based combination therapy. Lancet Infectious Diseases. 2012;12((11)):820–2. doi: 10.1016/S1473-3099(12)70226-8. [DOI] [PubMed] [Google Scholar]
- Carter R, Graves PM. Gametocytes. In: Wernsdorfer, MacGregror, editors. Malaria: principles and practice of malariology. Edinburgh: Churchill Livingstone; 1988. [Google Scholar]
- Chotivanich K, Sattabongkot J, Udomsangpetch U, Looareesuwan S, Day NP, Coleman RE, et al. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrobial Agents and Chemotherapy. 2006;50((6)):1927–30. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, et al. Predicting mosquito infection from Plasmodium falciparum gametocye density and estimating the reservoir of infection. eLife. 2013;2:e00626. doi: 10.7554/eLife.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16((1)):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Drakeley, Sutherland C, Bousema JT, Sauerwein RW, Targett GA. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends in Parasitology. 2006;22((9)):424–30. doi: 10.1016/j.pt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Dunyo S, Milligan P, Edwards T, Sutherland C, Targett G, Pinder M. Gametocytaemia after drug treatment of asymptomatic Plasmodium falciparum. PLoS Clinical Trials. 2006;1((4)):e20. doi: 10.1371/journal.pctr.0010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95((5)):497–501. doi: 10.1016/s0035-9203(01)90016-1. [DOI] [PubMed] [Google Scholar]
- Eziefula A, Gosling R, Hwang J, Hsiang M, Bousema T, von Seidlein L, et al. Rationale for short course primaquine in Africa to interrupt malaria transmission. Malaria Journal. 2012;11:360. doi: 10.1186/1475-2875-11-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll Back Malaria Partnership. Global Malaria Action Plan. Geneva: RBM/WHO; 2008. [Google Scholar]
- Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, et al. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua New Guinea. Parasitology. 1988;96((Pt 2)):251–63. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- Graves PM, Burkot TR, Saul AJ, Hayes RJ, Carter R. Estimation of anopheline survival rate, vectorial capacity and mosquito infection probability from malaria infection rates in villages near Madang, Papua New Guinea. Journal of Applied Ecology. 1990;27:134–47. [Google Scholar]
- Higgins JPT, Altman DH, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2008. Available from http://www.cochrane-handbook.org.
- Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol. 2013;81:133–201. doi: 10.1016/B978-0-12-407826-0.00004-7. [DOI: 10.1016/B978-0-12-407826-0.00004-7] [DOI] [PubMed] [Google Scholar]
- Johnston GL, Gething PW, Hay SI, Smith DL, Fidock DA. Modeling within-host effects of drugs on Plasmodium falciparum transmission and prospects for malaria elimination. PLoS Computational Biology. 2014;10((1)):e1003434. doi: 10.1371/journal.pcbi.1003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Taleo G, Kalkoa M, Yamar S, Kobayakawa T, Björkman A. Malaria eradication on islands. Lancet. 2000;356((9241)):1560–4. doi: 10.1016/S0140-6736(00)03127-5. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Ross A, Smith T. Infectiousness of malaria-endemic human populations to vectors. American Journal of Tropical Medicine and Hygiene. 2006;75((2 Suppl)):38–45. doi: 10.4269/ajtmh.2006.75.2_suppl.0750038. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Chichester: John Wiley & Sons; 2011. [Google Scholar]
- Lines JD, Wilkes TJ, Lyimo EO. Human malaria infectiousness measured by age-specific sporozoite rates in Anopheles gambiae in Tanzania. Parasitology. 1991;102((2)):167–77. doi: 10.1017/s0031182000062454. [DOI] [PubMed] [Google Scholar]
- Méndez F, Muñoz A, Plowe CV. Use of area under the curve to characterize transmission potential after antimalarial treatment. American Journal of Tropical Medicine and Hygiene. 2006;75((4)):640–4. [PubMed] [Google Scholar]
- Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer W. From malaria control to eradication: the WHO perspective. Tropical Medicine and International Health. 2009;14((7)):802–9. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin therapies: a pooled analysis of six randomized trials. Malaria Journal. 2008;7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okell LC, Drakeley CJ, Bousema T, Whitty CJ, Ghani AC. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Medicine. 2008;5((11)):e226. doi: 10.1371/journal.pmed.0050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot E, Skarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J. Mass drug administration for malaria. Cochrane Database of Systematic Reviews. 2013;(12) doi: 10.1002/14651858.CD008846.pub2. [DOI: 10.1002/14651858.CD008846.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R, Nosten F, Luxemburger C, ter Kuile FO, Paiphun L, Chongsuphajaisiddhi T, et al. The effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347((9016)):1654–8. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan) 2011. 5.1. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration.
- Smalley ME, Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977;74((1)):1–8. doi: 10.1017/s0031182000047478. [DOI] [PubMed] [Google Scholar]
- Sturrock HJW, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Medicine. 2013;10((6)):e1001467. doi: 10.1371/journal.pmed.1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Seidlein L, Greenwood BM. Mass administrations of antimalarial drugs. Trends in Parasitology. 2003;19((10)):452–60. doi: 10.1016/j.pt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- White NJ. Antimalarial pharmacokinetics and treatment regimens. British Journal of Clinical Pharmacology. 1992;34((1)):1–10. doi: 10.1111/j.1365-2125.1992.tb04100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. The role of antimalarial drugs in eliminating malaria. Malaria Journal. 2008;7((Suppl 1)):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ, Guo Qiao LG, Qi G, Luzzatto L. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malaria Journal. 2012;11:418. doi: 10.1186/1475-2875-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infectious Diseases. 2013;13((2)):175–81. doi: 10.1016/S1473-3099(12)70198-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Guidelines for the treatment of malaria. 2nd Edition. Geneva: WHO; 2010. Guidelines for the treatment of malaria, second edition. [Google Scholar]
- World Health Organization. WHO Evidence Review Group: The safety and effectiveness of single dose primaquine as a P. falciparium gametoctyocide. Meeting Report. Geneva: World Health Organization; 2012. [Google Scholar]
- World Health Organization. Single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. Updated WHO Policy Recommendation. Geneva: Global Malaria Programme. World Health Organization; October 2012. [Google Scholar]
References to other published versions of this review
- Graves PM, Gelband H, Garner P. Primaquine for reducing Plasmodium falciparum transmission. Cochrane Database of Systematic Reviews. 2012;(9) doi: 10.1002/14651858.CD008152.pub2. [DOI: 10.1002/14651858.CD008152.pub2] [DOI] [PubMed] [Google Scholar]
- Graves PM, Gelband H, Garner P. Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission. 2014;(6) doi: 10.1002/14651858.CD008152.pub3. Cochrane Database of Systematic Reviews [DOI: 10.1002/14651858.CD008152.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


