Abstract
Background
Point‐of‐care (POC) tests for diagnosing schistosomiasis include tests based on circulating antigen detection and urine reagent strip tests. If they had sufficient diagnostic accuracy they could replace conventional microscopy as they provide a quicker answer and are easier to use.
Objectives
To summarise the diagnostic accuracy of: a) urine reagent strip tests in detecting active Schistosoma haematobium infection, with microscopy as the reference standard; and b) circulating antigen tests for detecting active Schistosoma infection in geographical regions endemic for Schistosoma mansoni or S. haematobium or both, with microscopy as the reference standard.
Search methods
We searched the electronic databases MEDLINE, EMBASE, BIOSIS, MEDION, and Health Technology Assessment (HTA) without language restriction up to 30 June 2014.
Selection criteria
We included studies that used microscopy as the reference standard: for S. haematobium, microscopy of urine prepared by filtration, centrifugation, or sedimentation methods; and for S. mansoni, microscopy of stool by Kato‐Katz thick smear. We included studies on participants residing in endemic areas only.
Data collection and analysis
Two review authors independently extracted data, assessed quality of the data using QUADAS‐2, and performed meta‐analysis where appropriate. Using the variability of test thresholds, we used the hierarchical summary receiver operating characteristic (HSROC) model for all eligible tests (except the circulating cathodic antigen (CCA) POC for S. mansoni, where the bivariate random‐effects model was more appropriate). We investigated heterogeneity, and carried out indirect comparisons where data were sufficient. Results for sensitivity and specificity are presented as percentages with 95% confidence intervals (CI).
Main results
We included 90 studies; 88 from field settings in Africa. The median S. haematobium infection prevalence was 41% (range 1% to 89%) and 36% for S. mansoni (range 8% to 95%). Study design and conduct were poorly reported against current standards.
Tests for S. haematobium
Urine reagent test strips versus microscopy
Compared to microscopy, the detection of microhaematuria on test strips had the highest sensitivity and specificity (sensitivity 75%, 95% CI 71% to 79%; specificity 87%, 95% CI 84% to 90%; 74 studies, 102,447 participants). For proteinuria, sensitivity was 61% and specificity was 82% (82,113 participants); and for leukocyturia, sensitivity was 58% and specificity 61% (1532 participants). However, the difference in overall test accuracy between the urine reagent strips for microhaematuria and proteinuria was not found to be different when we compared separate populations (P = 0.25), or when direct comparisons within the same individuals were performed (paired studies; P = 0.21).
When tests were evaluated against the higher quality reference standard (when multiple samples were analysed), sensitivity was marginally lower for microhaematuria (71% vs 75%) and for proteinuria (49% vs 61%). The specificity of these tests was comparable.
Antigen assay
Compared to microscopy, the CCA test showed considerable heterogeneity; meta‐analytic sensitivity estimate was 39%, 95% CI 6% to 73%; specificity 78%, 95% CI 55% to 100% (four studies, 901 participants).
Tests for S. mansoni
Compared to microscopy, the CCA test meta‐analytic estimates for detecting S. mansoni at a single threshold of trace positive were: sensitivity 89% (95% CI 86% to 92%); and specificity 55% (95% CI 46% to 65%; 15 studies, 6091 participants) Against a higher quality reference standard, the sensitivity results were comparable (89% vs 88%) but specificity was higher (66% vs 55%). For the CAA test, sensitivity ranged from 47% to 94%, and specificity from 8% to 100% (four studies, 1583 participants).
Authors' conclusions
Among the evaluated tests for S. haematobium infection, microhaematuria correctly detected the largest proportions of infections and non‐infections identified by microscopy.
The CCA POC test for S. mansoni detects a very large proportion of infections identified by microscopy, but it misclassifies a large proportion of microscopy negatives as positives in endemic areas with a moderate to high prevalence of infection, possibly because the test is potentially more sensitive than microscopy.
23 April 2019
No update planned
Other
Reliable evidence with clear conclusions. All eligible published studies found in the last search (30 Jun, 2014) were included.
Keywords: Adult; Animals; Child; Female; Humans; Male; Reagent Strips; Schistosoma haematobium; Schistosoma haematobium/immunology; Schistosoma mansoni; Schistosoma mansoni/immunology; Antigens, Helminth; Antigens, Helminth/blood; Cross‐Sectional Studies; Hematuria; Hematuria/diagnosis; Microscopy; Prevalence; Proteinuria; Proteinuria/diagnosis; Reference Standards; Schistosomiasis haematobia; Schistosomiasis haematobia/blood; Schistosomiasis haematobia/diagnosis; Schistosomiasis haematobia/immunology; Schistosomiasis haematobia/urine; Schistosomiasis mansoni; Schistosomiasis mansoni/blood; Schistosomiasis mansoni/diagnosis; Schistosomiasis mansoni/immunology; Schistosomiasis mansoni/urine; Sensitivity and Specificity
Plain language summary
How well do point‐of‐care tests detect Schistosoma infections in people living inendemic areas?
Schistosomiasis, also known as bilharzia, is a parasitic disease common in the tropical and subtropics. Point‐of‐care tests and urine reagent strip tests are quicker and easier to use than microscopy. We estimate how well these point‐of‐care tests are able to detect schistosomiasis infections compared with microscopy.
We searched for studies published in any language up to 30 June 2014, and we considered the study’s risk of providing biased results.
What do the results say?
We included 90 studies involving almost 200,000 people, with 88 of these studies carried out in Africa in field settings. Study design and conduct were poorly reported against current expectations. Based on our statistical model, we found:
• Among the urine strips for detecting urinary schistosomiasis, the strips for detecting blood were better than those detecting protein or white cells (sensitivity and specificity for blood 75% and 87%; for protein 61% and 82%; and for white cells 58% and 61%, respectively). • For urinary schistosomiasis, the parasite antigen test performance was worse (sensitivity, 39% and specificity, 78%) than urine strips for detecting blood. • For intestinal schistosomiasis, the parasite antigen urine test, detected many infections identified by microscopy but wrongly labelled many uninfected people as sick (sensitivity, 89% and specificity, 55%).
What are the consequences of using these tests?
If we take 1000 people, of which 410 have urinary schistosomiasis on microscopy testing, then using the strip detecting blood in the urine would misclassify 77 uninfected people as infected, and thus may receive unnecessary treatment; and it would wrongly classify 102 infected people as uninfected, who thus may not receive treatment.
If we take 1000 people, of which 360 have intestinal schistosomiasis on microscopy testing, then the antigen test would misclassify 288 uninfected people as infected. These people may be given unnecessary treatment. This test also would wrongly classify 40 infected people as uninfected who thus may not receive treatment.
Conclusion of review
For urinary schistosomiasis, the urine strip for detecting blood leads to some infected people being missed and some non‐infected people being diagnosed with the condition, but is better than the protein or white cell tests. The parasite antigen test is not accurate.
For intestinal schistosomiasis, the parasite antigen urine test classifies many microscopy negative people as being infected. This finding may be explained by the low sensitivity of microscopy.
Summary of findings
Summary of findings 1. Summary of findings table for tests to detect S. haematobium.
| What is the diagnostic accuracy of circulating antigen tests and biochemical urine reagent strips in detecting S. haematobium infection? | ||||||
| Patients/Population | People residing in areas endemic for S. haematobium infection (74 out of 90 studies) | |||||
| Prior treatment with praziquantel before baseline study | Yes (6 studies), No (11 studies), Unclear (57 studies) | |||||
| Prior testing | None | |||||
| Settings | Field settings (villages and schools) and 1 outpatient clinic in Africa | |||||
| Index tests | Circulating cathodic antigen test (CCA) Circulating anodic antigen test (CAA)a Urine reagent strips to detect microhaematuria, proteinuria, and leukocyturia |
|||||
| Reference standard | Urine microscopy | |||||
| Importance | These tests are being used as replacements for conventional microscopy in disease control programmes for schistosomiasis, as they are rapid, are easier to use and interpret, and may have comparable sensitivity to microscopy. As control programmes gain impetus and infection intensities decrease, higher sensitivities become a prerequisite for future diagnostics | |||||
| Studies | Cross‐sectional (n = 62), cohort (n = 6), and case‐control studies with controls from same population (n = 3) | |||||
| Quality concerns | Poor reporting of participant characteristics, index test and reference standard methods, and intensity of infection were common concerns. The risk of bias assessment for most included studies was largely unclear for the QUADAS domains Patient Selection, Index Tests, and Reference Tests | |||||
| Test types | Number of evaluations | Summary estimates (95% CI) | In 1000 people tested | |||
| Infected cases S. haematobium |
Missed cases (FNs) |
False‐ positives (FPs) |
All positives (TPs + FPs) |
|||
| Biochemical urine reagent strips | ||||||
| For microhaematuria | 74 | Sens = 75% (71% to 79%) Spec = 87% (84% to 90%) |
410 | 102 | 77 | 384 |
| For proteinuria | 46 | Sens = 61% (53% to 68%) Spec = 82% (77% to 88%) |
410 | 160 | 106 | 356 |
| For leukocyturia | 5 | Sens = 58% (44% to 71%) Spec = 61% (34% to 88%) |
410 | 172 | 230 | 468 |
| Circulating cathodic antigen test (CCA) | ||||||
| Urine POC test | 4 | Sens = 39% (6% to 73%) Spec = 78% (55% to 100%) |
410 | 250 | 94 | 254 |
| Comparisons | ||||||
| Comparison | Comparison type | Number of evaluations and differences in overall accuracy | Explanation | |||
| Microhaematuria vs proteinuria | All studies | 74 microhaematuria vs proteinuria, difference in accuracy (P = 0.25) | We found no evidence of a statistically significant difference in overall accuracy when microhaematuria and proteinuria are carried out and compared in different individuals | Proteinuria would be expected to miss 14% more cases than microhaematuria | Proteinuria would be expected to falsely identify 5% more cases than microhaematuria | |
| Paired studies (tests done in the same individuals) |
44 microhaematuria vs proteinuria, differences in accuracy (P = 0.21) | We found no evidence of a statistically significant difference in overall accuracy when microhaematuria and proteinuria are carried out and compared in the same individuals | ||||
a Studies were insufficient to provide summary estimates for the CAA tests.
When the tests were evaluated against the higher‐quality reference standard (ie when multiple samples were analyzed), sensitivity was lower for microhaematuria (71% vs 76%) and proteinuria (49% vs 61%) in comparison with a lower‐quality reference standard. The specificity of these tests was comparable.
In light‐intensity settings, sensitivity was slightly lower for microhaematuria (73% vs 76%) and specificity was slightly higher (88% vs 86%) compared with results of the overall analysis. In contrast, sensitivity (60% vs 61%) and specificity (83% vs 83%) for proteinuria were comparable.
Microhaematuria and proteinuria had higher sensitivity (77% vs 73% and 67% vs 56%) in children than in mixed populations of adults and children. Specificity was higher for microhaematuria (91% vs 82%) but specificity was comparable for proteinuria (81% vs 82%) in children compared with mixed populations of adults and children.
For the effects of risk of bias, sensitivities and specificities of microhaematuria were comparable when limited to studies with low risk of bias for the participant flow domain. Sensitivity of proteinuria was higher when limited to studies with low risk of bias for the participant selection domain (64%) and the participant flow domain (67%). Specificity on the other hand was comparable for these 2 domains.
Abbreviations: TPs (true‐positives), FPs (false‐positives), FNs (false‐negatives).
Summary of findings 2. Summary of findings table for tests to detect S. mansoni.
| What is the diagnostic accuracy of circulating antigen tests for S. mansoni infection? | ||||||
| Patients/Population | People residing in areas endemic for S. mansoni infection (16 out of 90 studies) | |||||
| Prior treatment with praziquantel before baseline study | Yes (1 study), No (5 studies), Unclear (10 studies) | |||||
| Prior testing | None | |||||
| Settings | Field settings (villages, schools, and military camp) in Africa and South America | |||||
| Index tests | Circulating cathodic antigen test (CCA) Circulating anodic antigen test (CAA)a |
|||||
| Reference standard | Stool microscopy | |||||
| Importance | These tests are being used as replacements for conventional microscopy in disease control programmes for schistosomiasis, as they are rapid, are easier to use and interpret, and may have comparable sensitivity to microscopy. As control programmes gain impetus and infection intensities decrease, higher sensitivities become a prerequisite for future diagnostics | |||||
| Studies | Cross‐sectional studies | |||||
| Quality concerns | Poor reporting of participant characteristics, index test and reference standard methods, and intensity of infection were common concerns. The risk of bias assessment for most included studies was largely unclear for the QUADAS domains Patient Selection, Index Tests, and Reference Tests | |||||
| Test types | Number of evaluations | Summary estimates (95% CI) | In 1000 people tested | |||
| Infected cases S. mansoni |
Missed cases (FNs) |
False‐positives (FPs) |
All positives (TPs + FPs) | |||
| Circulating cathodic antigen test (CCA) | ||||||
| Urine POC test | 15 | Sens = 89% (86% to 92%); Spec = 55% (46% to 65%) | 360 | 40 | 288 | 608 |
a Studies were insufficient to provide summary estimates for CAA tests.
When measured against a higher‐quality reference standard, sensitivity of CCA POC for S. mansoni was comparable (88% vs 88%) but specificity was higher (66% vs 55%) than when measured against a lower‐quality reference standard.
At a positivity threshold ≥ 1, sensitivity of CCA POC for S. mansoni was lower (72% vs 87%) and specificity higher (85% vs 61%) than at a positivity threshold of trace‐positive.
Data were insufficient to estimate the sensitivity of CCA POC for S. mansoni in light‐intensity settings.
For the effects of risk of bias, sensitivity and specificity of CCA POC for S. mansoni were comparable when limited to studies with low risk of bias for the participant flow domain.
Abbreviations: TPs (true‐positives), FPs (false‐positives), FNs (false‐negatives).
Background
Target condition being diagnosed
Schistosomiasis, also known as bilharzia, is the second major parasitic disease affecting tropical and subtropical regions after malaria. It is caused by trematode worms of the genus Schistosoma (Gryseels 2012). The latest estimates show that schistosomiasis is endemic in 76 countries, with 779 million people at risk of infection and approximately 207 million people currently infected. Sub‐Saharan Africa accounts for more than 90% of current cases of schistosomiasis (Engels 2002; WHO 2010; Gryseels 2012). The global burden of disease in 2004 was estimated at 13 to 15 million disability‐adjusted life‐years (DALYs) lost as the result of schistosomiasis (King 2010a). These estimates could be an underestimate resulting from the low sensitivity of routinely used diagnostic tests (King 2010a; King 2010b).
Five main schistosome species are known to infect man (Schistosoma mansoni, Schistosoma haematobium,Schistosoma japonicum, Schistosoma intercalatum, and Schistosoma mekongi), of which S. mansoni, S. haematobium, and S. japonicum have the greatest impact on morbidity (Gryseels 2006). The focus of this review will be on diagnosing infection caused by S. mansoni and S. haematobium, as they are more widespread globally and account for most infections and associated morbidity worldwide. These species cause intestinal schistosomiasis and urogenital schistosomiasis, respectively. As outlined in Appendix 1, urogenital schistosomiasis presents with blood in urine (haematuria), proteins in urine (proteinuria), or white blood cells in urine (leukocyturia). In its chronic form, it presents with major bladder, kidney, and genital pathologies including chronic renal failure. Intestinal schistosomiasis presents with abdominal pain and in its chronic and severe forms can present with enlarged liver (hepatomegaly), abdomen distended with fluid (ascites), and liver failure.
Currently, no vaccine is available to protect against schistosomal infection (Rollinson 2009; Bethony 2011). If left untreated, schistosomal infection may result in chronic disease. The current drug of choice is praziquantel, which is cheap (costing less than USD 0.15 per treatment) and safe and causes few side effects. Praziquantel however is ineffective against the eggs and larval forms of schistosome worms (Gryseels 2012; Rollinson 2013). Mass praziquantel treatment of populations at risk of infection is now routine in many endemic areas (WHO 2010; Rollinson 2013). Reinfections rapidly occur as the result of recurrent direct contact with water bodies infected with schistosomal parasites (WHO/TDR 2006; Rollinson 2009; Rollinson 2013). No strong evidence of clinically relevant drug resistance is available (Geerts 2001; Doenhoff 2002; Fenwick 2003; Doenhoff 2009; Greenberg 2013). However reports have described heterogeneities in egg reduction rates and in systematic non‐clearers of infection after treatment with praziquantel (Black 2009; Melman 2009; Ahmed 2012). In the long run, mass treatment has limitations related to cost‐effectiveness (French 2010), poor sustainability (Utzinger 2009), poor drug compliance by individuals (Guo 2005; Croce 2010), and increased drug selection pressure (Greenberg 2013).
Accurate and affordable diagnostic tools are essential for providing targeted treatment and for maximizing the success of control of schistosomiasis in endemic areas; they are required for monitoring drug efficacy as well. Diagnosis of schistosomiasis can be performed directly or indirectly. Direct methods include detection of schistosome eggs in urine or stool by microscopy, detection of schistosome antigens in serum or urine samples, and detection of Schistosoma‐specific DNA in urine, stool, or blood. Indirect methods include questionnaires, biochemical tests (urine reagent strips for microhaematuria/proteinuria/leukocyturia), antibody tests, ultrasonography, computed tomography (CT) scan, magnetic resonance imaging (MRI) scan, endoscopy, and cystoscopy (Feldmeier 1993; Rabello 1997; Doenhoff 2004; Bichler 2006; Gryseels 2012; Cavalcanti 2013).
Currently no gold standard is recommended for the detection of schistosomiasis. Microscopy is the most widely used test for diagnosing schistosomiasis and, although imperfect, it is commonly used as the reference standard in practice. Its sensitivity has been shown to vary with intensity of infection, prevalence of infection, sample preparation techniques, stool consistency, and circadian and day‐to‐day variation of egg counts in stool and/or urine (Doehring 1983; Doehring 1985a; Rabello 1992; Feldmeier 1993; Rabello 1997; van Lieshout 2000; Knopp 2008). This becomes particularly pertinent as control programmes progress and sensitivity of microscopy decreases as the result of reduced infection intensity. Repeated measurements over multiple days from multiple samples and/or multiple smears/slides taken from each sample has been shown to increase sensitivity (Knopp 2008; da Frota 2011; Siqueira 2011; Deelder 2012); however this task increases the time taken to perform the survey and therefore becomes logistically expensive (van Lieshout 2000; Legesse 2007).
Index test(s)
Urine reagent strips and circulating antigen tests are used as alternatives to microscopy for diagnosis of schistosomiasis. Compared with microscopy, urine reagent strips used to detect microhaematuria or proteinuria as a proxy for S. haematobium infection are cheap, quick, and easy to use (Mott 1985; Brooker 2009); have no technical requirements; and are less influenced by the circadian production of schistosome eggs (Murare 1987; Lengeler 1991b). Furthermore, some studies have shown that the sensitivity of these strips is higher than that of urine filtration (French 2007; Robinson 2009), and that a single test with microhaematuria strips is more sensitive than a single test with urine filtration (Taylor 1990)—features that make these strips suitable for screening of urogenital schistosomiasis in the field. However, results should be interpreted against the background of risk for schistosomiasis, as well as any other signs and symptoms that could be indicative of other diseases. Microhaematuria and proteinuria are non‐specific signs that could also result from other ailments such as urogenital infection, malignancy, immune system disorders, metabolic disorders, and trauma.
Circulating antigen tests (circulating anodic antigen (CAA) and circulating cathodic antigen (CCA)) have also been evaluated as replacements for microscopy in the diagnosis of infection due to S. haematobium or S. mansoni. These tests can differentiate between active and past infections, as the circulating antigens are probably present only when there is active infection (Doenhoff 2004). As circulating antigens are released from living worms, antigen levels may correlate directly with parasite load, whilst microscopy does not. This may make the CCA POC test useful in monitoring the dynamics of worm burdens and clearance of worms after treatment (Cavalcanti 2013; Rollinson 2013). However, the sensitivity of these tests has been shown to vary with prevalence of disease and intensity of infection (De Jonge 1988; De Jonge 1989; van Lieshout 1992; De Clerq 1997; Stothard 2006; Ayele 2008; Obeng 2008; Midzi 2009; Colley 2013).
This review evaluates the urine CCA POC test, urine CCA and CAA enzyme‐linked immunosorbent assay (ELISA), and serum CCA and CAA ELISA. The urine CCA POC test is a lateral flow assay that uses a nitrocellulose strip with a monoclonal antibody–coated test line to detect the presence of Schistosoma‐specific CCA antigen in urine. When urine from an infected individual flows through the strip, the antigen will bind to the test line, which becomes visible with the binding of added labelled monoclonal antibodies (van Dam 2004). Of note, the urine CCA POC test was developed based on the performance of the ELISA format (Brooker 2009). The urine CCA ELISA was found to have the best diagnostic performance, followed by the serum CAA assay for S. mansoni (Polman 1995; van Lieshout 1995; van Lieshout 2000). Therefore, although they are not rapid tests, the accuracy measures of ELISA tests will be systematically assessed, as the summary measures obtained may guide the ongoing development of improved POC tests.
So far, a range of accuracy measures have been reported for urine reagent tests and for circulating antigen tests. Diagnostic and treatment strategies in endemic areas vary with results of these tests (Appendix 2) and depend on financial and human resource capacity.
Clinical pathway
Patients suspected of having active S. haematobium or S. mansoni infection in endemic settings.
Prior test(s)
As outlined in Appendix 2, current practice in endemic settings is to use urine reagent strips as a replacement for microscopy or as a triage test (before microscopy), or circulating antigen tests as a replacement for microscopy. In line with practice in disease control programmes, we focus on the role of these tests as alternatives to microscopy. We will not consider prior testing with other tests, as this is rarely done in public health programmes.
Role of index test(s)
We are interested in the following purposes for testing.
Reagent strips to detect microhaematuria, proteinuria, or leukocyturia as a replacement test for microscopy for S. haematobium infection.
CCA point‐of‐care test as a replacement test for microscopy for S. haematobium or S. mansoni infection.
Alternative test(s)
Apart from the two test types mentioned above, a range of other tests can be used to screen for schistosomiasis. However, all are used in different situations and in different circumstances than the tests mentioned above.
Questionnaires have been used for the initial rapid screening for urinary schistosomiasis in high‐risk communities in endemic areas (Lengeler 1991a; Feldmeier 1993; Chitsulo 1995). These questionnaires rely on self‐reporting of blood in urine. Studies have shown that questionnaires demonstrate moderate to high sensitivities and specificities when used to screen individuals for urogenital schistosomiasis in high‐prevalence areas but low sensitivity and specificity in low‐prevalence areas (Lengeler 1991a; Lengeler 1991b; Brooker 2009). Questionnaires for intestinal schistosomiasis have been shown to be less sensitive and specific than those for urogenital schistosomiasis (WHO/TDR 2006; Brooker 2009). Symptoms of intestinal schistosomiasis are associated with many other diseases, which often overlap in range. As co‐infection is the norm rather than a rare occurrence, the questionnaires are less specific. The accuracy of questionnaires has been shown to be influenced by age and gender. When questionnaires are used repeatedly in the same area, respondents are prone to give biased answers, as they know the consequences of the answers they give. Thus, recall bias may interfere with the accuracy of the test. Consequently, relying on questionnaires may become ineffective, making this screening method unsuitable even for follow‐up of patients after treatment (Ansell 1997; Guyatt 1999; Lengeler 2002). As questionnaires are recommended mainly for initial rapid screening and not for routine screening for schistosomiasis, they will not be evaluated in this review.
Serology tests are alternative tests for the diagnosis of schistosomiasis. These tests detect antibodies against worm antigens, egg antigens (soluble egg antigens (SEAs)), or eosinophil cationic proteins (ECPs) (Reimert 1991; Feldmeier 1993; ITM 2007). Available methods include ELISA, indirect immunofluorescence assay (IFA), and indirect haemagglutination assay (IHA). Antibody tests demonstrate high sensitivity even in areas with light infection and therefore can be used in areas with low endemicity. However these tests fall short in distinguishing current active infection from past infection, have low specificity in endemic areas because of cross‐reactivity with antigens of other helminths, and often show antibody levels that remain elevated after treatment; therefore they yield many false‐positive results (Doenhoff 2004; Cavalcanti 2013). Antibody tests may have a role in checking for maintained exposure to schistosomiasis in areas that are moving towards elimination (Rollinson 2013).
The ECP test is an indirect marker of S. haematobium infection and related morbidity (Reimert 2000; Vennervald 2004). Other test examples include rectal biopsy (ITM 2007), cystoscopy and endoscopy, radiological methods (Bichler 2006), FLOTAC (a novel faecal egg count technique) (Knopp 2009; Glinz 2010), and molecular tests using polymerase chain reaction (PCR) (Ten Hove 2008; Oliveira 2010; Knopp 2011). However these tests may be expensive or may require trained laboratory personnel and an elaborate laboratory infrastructure.
Rationale
For improved mapping to ensure effective selective (or targeted) treatment and for accurate data on treatment success with praziquantel, appropriate diagnostic tests are urgently required. When a test for diagnosing schistosomiasis is considered, a test with high sensitivity is paramount, especially when infection is being monitored within a disease control programme. False‐negative results lead to missed treatment and subsequently to more advanced disease or, if occurring after praziquantel treatment, may lead to overestimated cure rates and potentially undetected cases of praziquantel resistance and the spread of the disease. High specificity is also required, as unnecessary treatment due to false‐positive results could reduce cost‐effectiveness in current control programme strategies through potentially inaccurate classification of prevalence levels or in future targeted treatment control programmes (WHO/TDR 2006). On the other hand, a test for mapping of disease (to get an estimation of disease prevalence in an endemic area) may not need sensitivity and specificity as high as those required for monitoring of disease.
There is currently no recommended gold standard for the detection of active schistosomiasis. However, because microscopy is the most commonly used test in practice and is often used as the reference test in studies, we selected it for use as the reference standard within this review to detect S. haematobium and S. mansoni. The primary concern with microscopy is the possibility of missing infected cases (because of its low and varied sensitivity), especially in areas with low intensity of infection. This means that truly infected cases may be missed and misclassified as non‐infected by microscopy. Therefore when comparing an index test against microscopy, the number of false‐positives (potentially true cases classified as positive by the index test and classified as negative by the reference test) may be high, and the index test may present with low specificity. Increasing the sensitivity of microscopy by taking multiple measurements may reduce the number of true cases wrongly classified as non‐infected by microscopy. An index test compared against a more sensitive reference test (microscopy with multiple measurements) may have higher specificity because the number of false‐positives will be low. Our review will therefore also investigate the effect of the quality of the reference standard on the sensitivity and specificity of the index tests being evaluated.
In this case, a test considered as a replacement for microscopy should have comparable sensitivity or should be less costly, portable, faster, and easier to use or interpret, and it should be less demanding logistically. Point‐of‐care tests based on circulating antigen detection and biochemical urine reagent strips in particular are being included (or developed) in disease control strategies, as they are easy to use and interpret, require minimal laboratory infrastructure, are cost‐effective, reduce patient waiting time and potentially therefore reduce loss to follow‐up, and may have comparable or higher sensitivity to microscopy (Loubiere 2010). The results of this review may guide policy makers on appropriate diagnostic tests to use and may help identify research gaps in diagnostic testing for schistosomiasis in endemic areas.
Objectives
With the goals of making recommendations and informing policy makers on which tests to use and identifying research gaps, these were our primary objectives:
To obtain summary estimates of the diagnostic accuracy of urine reagent strip tests for microhaematuria, proteinuria, and leukocyturia in detecting active S. haematobium infection, with microscopy of urine as the reference standard.
To obtain summary estimates of the diagnostic accuracy of circulating antigen tests—a urine POC circulating cathodic antigen (CCA) test, a urine and serum CCA enzyme‐linked immunosorbent assay (ELISA) test, and a urine and serum circulating anodic antigen (CAA) test—for detection of active Schistosoma infection in geographical regions endemic for S. mansoni or S. haematobium or both, with microscopy as the reference standard.
To compare the accuracy of the above index tests.
To investigate potential sources of heterogeneity in the diagnostic accuracy of the tests listed above.
Secondary objectives
To investigate whether age and gender of participants, positivity thresholds, prevalence of infection, intensity of infection, quality of the reference standard, effects of praziquantel treatment, infection stage, mixed infections, and the methodological quality of included studies can explain observed heterogeneity in estimates of test accuracy.
Methods
Criteria for considering studies for this review
Types of studies
We included primary observational studies that compared the results of one or more of the index tests versus the reference standard. These studies could be cross‐sectional in design, cohort studies, or diagnostic case‐control studies with cases and controls sampled from the same patient population.
We included studies that provide participant data. Only studies in which true‐positives (TPs), true‐negatives (TNs), false‐positives (FPs), and false‐negatives (FNs) were reported or could be extracted from the data were included.
We excluded case‐control studies with healthy controls, controls from non‐endemic areas, or controls with alternative diagnoses (patients with diseases similar to schistosomiasis), as specificity may be overestimated (Rutjes 2005). False‐positive test results may occur when an alternative disease produces the same pathophysiological changes as the target condition. We also excluded studies that enrolled only participants with proven schistosomiasis, as sensitivity may be overestimated.
Participants
Participants had to be individuals residing in regions where S. haematobium and S. mansoni infections were endemic. We excluded articles that studied travelers originating from non‐endemic countries, as they were typically screened with other tests such as antibody tests.
Index tests
We included studies that evaluated the following tests.
Urine reagent strip tests
A urine reagent strip test is a biochemical semiquantitative test. It is regarded as an indirect indicator of S. haematobium infection or morbidity, as it detects microhaematuria, proteinuria, or leukocyturia (white blood cells in urine) that can develop as a consequence of schistosomal infection (Doehring 1985b;Doehring 1988). This test is cheap and easy to use for rapid screening of urinary schistosomiasis (Feldmeier 1993; Gryseels 2006; Gryseels 2012).
The results of urine reagent tests used to measure haematuria are scored as 0 (negative), trace‐positive (tr), 1+ (5 to 10 erythrocytes/μL), 2++ (10 to 50 erythrocytes/μL), or 3+++ (50 to 250 erythrocytes/μL). For proteinuria, results are scored as 0 (negative), trace‐positive (tr), 1+ (30 mg protein/dL), 2++ (100 mg protein/dL), or 3+++ (500 mg protein/dL) (Murare 1987).
Antigen tests
Antigen tests are based on detection of schistosome antigens in the serum and urine of individuals (Gryseels 2006; WHO/TDR 2006; Gryseels 2012). The main circulating antigens are adult worm gut–associated circulating antigens, and CAA and CCA are the main focus of research.
The CCA dipstick is scored according to test band reaction intensity as negative (‐), trace‐positive (tr), single‐positive (+), double‐positive (++), and triple‐positive (+++) (Stothard 2006). ELISA results are continuous, and positivity thresholds may vary. To estimate the accuracy of ELISA tests, ELISA must have been evaluated against the reference standard only.
Target conditions
Active infection with S. haematobium.
Active infection with S. mansoni.
Reference standards
S. haematobium
For diagnosis of S. haematobium infection, the reference standard is microscopy of urine for examination of schistosome eggs. To increase sensitivity, urine samples can be concentrated by sedimentation, filtration, or centrifugation techniques (Gryseels 2006), or more samples can be examined (Feldmeier 1993). We therefore included studies that use all of these concentration techniques, and to estimate the effect of the quality of the reference standard, we accepted studies using microscopy on a single urine sample (lower‐quality reference standard) and studies performing microscopy on multiple urine samples (higher‐quality reference standard).
S. mansoni
For diagnosis of S. mansoni infection, microscopic examination of schistosome eggs in stool is the reference standard. Sensitivity is increased by preparing a faecal thick smear using the Kato‐Katz (KK) method (Gryseels 2006) or by examining multiple stool samples (Feldmeier 1993). To estimate the effect of the quality of the reference standard, we accepted studies using microscopy on a single stool sample (lower‐quality reference standard) and studies performing microscopy on multiple stool samples (higher‐quality reference standard).
It is important to note that some regions experience mixed infections of S. haematobium and S. mansoni. In such situations, microscopy of both stool and urine samples must be carried out to confirm infection.
Search methods for identification of studies
Electronic searches
We searched the electronic databases MEDLINE, EMBASE, BIOSIS, MEDION, and HTA (Health Technology Assessment). The MEDLINE search strategy is outlined in Appendix 3. We further translated the MEDLINE search to EMBASE and BIOSIS databases to identify additional records. To avoid missing studies, we did not use a diagnostic search filter. We performed the searches on 12 January 2012 and repeated them on 16 November 2012, 29 August 2013, and 30 June 2014.
Searching other resources
We looked through reference lists of relevant reviews and studies and websites of the World Health Organization (WHO), the Schistosomiasis Control Initiative (SCI), and the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE). When possible, we contacted study authors to request extra information.
Data collection and analysis
Selection of studies
Two independent review authors first looked through titles and abstracts to identify potentially eligible studies. Full‐text articles of these studies were obtained and assessed for study eligibility by two independent review authors using the predefined inclusion and exclusion criteria. Disagreements were resolved through discussion and by consultation with a third review author when necessary.
Data extraction and management
Two independent review authors extracted data onto a data extraction form.
The following data were extracted.
Study authors, publication year, and journal.
Study design.
Study participants—age, sex.
Prevalence of schistosomiasis.
Treatment status of participants with praziquantel—treatment status before study or post treatment.
Reference standard (microscopy), including number of samples per individual and exact volume of stool/urine examined.
Index tests—urine and serum circulating antigen tests (CCA and CAA) and urine reagent strips.
Urine reagent strips—signs measured (microhaematuria, proteinuria, leukocyturia).
Sample preparation techniques—time of day urine/stool sample was taken, intensity of infection—egg counts in urine and stool by microscopy.
Presence of missing or unavailable test results.
Numbers of TPs, FNs, FPs, and FNs.
Assessment of methodological quality
We used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool to assess risk of bias and concerns for applicability of the included studies (Whiting 2011) (Appendix 4). Disagreements were resolved through consensus or by consultation with a third review author. We extracted data using signalling questions and scored for risk of bias and concerns for applicability under the four main domains: participant selection, index test, reference standard, and participant flow.
Statistical analysis and data synthesis
Comparisons of index test versus the reference standard
We analyzed data for the two target conditions (S. haematobium andS. mansoni) separately. Only one included study (Ashton 2011) evaluated the ability of a test to detect S. haematobium and/orS. mansoni in an area of mixed infection.
Among studies reporting sufficient data for calculating sensitivity and specificity, we plotted their sensitivity and specificity in both forest plots and receiver operating characteristic (ROC) space using the software Review Manager 5.2. We performed a meta‐analysis using the statistical software SAS version 9.2 for test types that had sufficient data points (four or more data points) to be pooled by the statistical models and those that did not demonstrate substantial heterogeneity in ROC space (Macaskill 2010). These tests included the reagent strip for microhaematuria, the reagent strip for proteinuria, the reagent strip for leukocyturia, the CCA POC test for S. haematobium, and the CCA POC test for S. mansoni.
The statistical model selected to perform the overall meta‐analysis depended on the variability of the positivity thresholds, as discussed below. Data for urine reagent strips and urine CCA POC tests were ordinal. These tests are typically scored as 0, trace, 1+, 2+, and 3+, or as 0, 1+, 2+, and 3+.
When data from a test had multiple thresholds, we used the hierarchical summary receiver operating characteristic model (HSROC) to perform the overall meta‐analysis. This model estimates the underlying ROC curve, which describes how sensitivity and specificity of the included studies trade off with each other as thresholds vary. It allows for variation in the parameters of accuracy, thresholds between studies, and the shape of the underlying ROC curve (Rutter 2001; Macaskill 2010). Because this method models sensitivity and specificity indirectly, we calculated average sensitivities and average specificities from the output of the model.
When data from a test had one or a common threshold, we used the bivariate random‐effects model to perform the overall meta‐analysis. This method models sensitivity and specificity directly at a common threshold (Reitsma 2005; Macaskill 2010).
We included all studies in the overall meta‐analysis, whether or not a positivity threshold was included. We assumed that different thresholds were used for the studies that did not report their thresholds, and we used the HSROC model to perform the overall meta‐analysis. For urine reagent strips for microhaematuria and proteinuria, many studies did not report a positivity threshold (n = 41 for microhaematuria and n = 25 for proteinuria). Some studies (n = 2) provided data points at both thresholds of trace and +1. When data points were provided at both thresholds, we selected the data point at threshold trace for the overall analysis; we selected the first stipulated positivity threshold. Leukocyturia had five overall data points, with four data points at threshold trace and one at +1. The CCA POC for S. haematobium had four overall data points, with two at threshold trace and two at +1.
All studies evaluating CCA POC for S. mansoni reported positivity thresholds; five provided data points at both thresholds trace and +1. When data points were provided at both thresholds, we selected the data point at threshold trace for the overall analysis; we selected the first stipulated positivity threshold. The overall analysis therefore contained 15 data points with threshold ≥ trace, for which we used the bivariate model for meta‐analysis.
Comparisons of index tests
We compared the accuracy of the reagent strips for microhaematuria in detecting S. haematobium versus the accuracy of the reagent strips for proteinuria. These were the only tests with sufficient data to enable comparisons between different types of tests. Tests were compared by adding the co‐variate test type to the HSROC model and allowing this to have an effect on the accuracy, threshold, and shape parameters. We performed indirect comparisons and direct comparisons; in the latter, we included only studies that applied both index tests in the same individuals.
Investigations of heterogeneity
We investigated heterogeneity by examining the forest plots and statistically by including co‐variates in the HSROC or bivariate model, by conducting subgroup analysis, and by performing sensitivity analysis. In the HSROC model, we investigated whether these co‐variates affect the parameters of this model—accuracy, threshold, and shape—whereas in the bivariate model, we investigated whether these co‐variates affect sensitivity and specificity.
We did not investigate the effects of infection stage and mixed infection caused by poor reporting and insufficient data for these items.
We investigated the following sources of heterogeneity: quality of the reference standard, positivity threshold, age, gender (proportion of female participation), intensity of infection, prevalence of infection, effect of praziquantel treatment, and QUADAS‐2 risk of bias domains. Of these, the co‐variates gender (proportion of female participation) and prevalence of infection were analyzed as a continuous co‐variate. The rest were analyzed as categorical co‐variates.
We classified studies that used single‐measurement microscopy (one stool and/or one slide or smear) and those that did not report how the reference standard was conducted as using lower‐quality reference standards because single measurements are more likely to miss diseased individuals. We assumed that studies that used multiple measurements of microscopy were likely to report this, given the relevance of this additional effort. Reference standards that used multiple urine or stool samples or multiple slides or smears were classified as higher‐quality reference standards.
For the age co‐variate, many mixed adult/children studies did not state the proportions of adults or children. Some did not state the age of participants. As accuracy data were not provided for age subgroups in most studies, we dichotomized the age co‐variate into the groups 'all ages' and 'children only'. We assumed that studies that did not state the age had included participants of all ages.
Because the proportions of female and male participants were poorly reported at the test level and at the level of the 2 × 2 tables, we analyzed the co‐variate of gender as a continuous variable at the study level. For this co‐variate, gender indicated the proportion of female participation. We focused on females because gender may influence accuracy estimates through factors associated with females, such as menstruation and genitourinary tract infection (Hall 1999; French 2007; Brooker 2009).
The World Health Organization (WHO) recommendations (WHO 2002) categorize intensity of infection for S. haematobium as follows: < 50 eggs/10 mL (light) and ≥ 50 eggs/10 mL (heavy) and intensity of S. mansoni as follows: 1 to 99 eggs per gram (epg) (light), 100 to 399 epg (moderate), and ≥ 400 epg (heavy). In our review, the intensity of infection was reported in different ways (arithmetic mean or range of infection, or geometric mean or range of infection, or proportions of participants with light/moderate/heavy infection) and for most included studies was not reported at all (63% and 65% for microhaematuria and proteinuria, respectively). We used the reported estimates of mean (arithmetic/geometric) or median intensity of infection to classify our studies according to WHO recommendations. We classified as unclear studies that reported only proportions of participants with light/moderate/heavy infections or did not report estimates of intensity of infection.
We examined the effects of treatment with praziquantel on the sensitivity and specificity of the testtype microhaematuria because it was the only test with sufficient data to investigate this. Nine studies provided data on praziquantel treatment; seven were follow‐up studies with praziquantel given at variable intervals (King 1988_a (one year), NGoran 1989 (one month), Kitange 1993 (one year), Lengeler 1993 (one month), Shaw 1998 (six weeks), Magnussen 2001 (one year), French 2007 (one year)), and two indicated that praziquantel had been given before the baseline study was performed (Abdel‐Wahab 1992 (two years), Bogoch 2012 (two years)). When multiple follow‐up studies were performed, we selected data for the first follow‐up evaluation (Shaw 1998; French 2007). However, pooling of results of all studies with varying time intervals would likely introduce a lot of heterogeneity, bias our summary estimates, and lead to overestimates of sensitivity, because studies with long time intervals were likely to have a greater number of participants reinfected compared with studies done at shorter time intervals. We opted to present estimates of sensitivity and specificity of individual studies evaluating the performance of microhaematuria post treatment in the ROC space.
We added the following co‐variates one by one to the HSROC model for microhaematuria and proteinuria and to the bivariate model for CCA POC for S. mansoni: quality of the reference standard, age, gender, and prevalence of infection. We then performed a subgroup analysis for the co‐variates—quality of the reference standard, age, positivity threshold, and intensity of infection—for all three index tests.
Sensitivity analyses
We performed a sensitivity analysis to check the robustness of results when filtration was used as a concentration for urine microscopy for S. haematobium, and to estimate sensitivity and specificity for studies with low risk of bias according to the QUADAS domains, along with participant selection, participant flow, and the reference standard.
Assessment of reporting bias
We did not assess reporting bias. Methods of assessing reporting bias for diagnostic accuracy studies are still being refined. For instance, the Deeks test, a test that has been proposed for use in diagnostic accuracy studies, has low power to detect funnel plot asymmetry, especially when a lot of heterogeneity is present (Macaskill 2010). The studies included in our review showed a lot of heterogeneity; therefore assessments for reporting bias may not yield conclusive results.
Results
Results of the search
Our search yielded 17,477 hits. After the titles and abstracts were screened, 152 full texts were retrieved, and after full texts were assessed, 90 articles were deemed suitable for inclusion; 62 were excluded. One study author whom we contacted responded to our request for information, but the data submitted did not meet our eligibility criteria. No additional eligible studies were found through additional searches. This review contains results derived from 90 articles. The search results can be seen in Figure 1.
1.
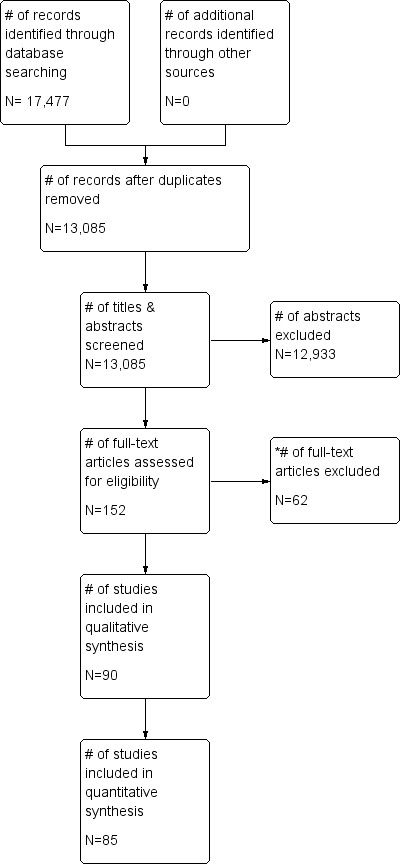
Study flow diagram.
* Reasons for exclusion can be found in the table of Characteristics of excluded studies.
Included studies
Details of included studies can be found in the Characteristics of included studies table. We included 90 studies containing 197,411 participants. Of these included studies, 88 were carried out in Africa, one in South America (Surinam), and one in Asia (Yemen). Only one study was conducted in a hospital setting (antenatal clinic, outpatient setting). The other tests were performed in a field setting (village/school/military camp). S. haematobium was evaluated in most studies (n = 74); 16 evaluated S. mansoni. One study evaluated both species. Eighty studies reported the age of study participants; most of these were conducted in children (n = 50; 62.5%). Median prevalence of S. haematobium infection was 41% (range 1% to 89%), and that of S. mansoni infection was 36% (range 8% to 95%). Median female participation was 50% (Q1 46; Q3 53) for studies that reported gender (n = 46; 51%). Most of the included studies (n = 73; 81%) did not report on the status of praziquantel treatment in the study setting before the baseline study was performed. Eighty‐one studies used a cross‐sectional design; six were cohort studies (longitudinal studies with follow‐up), and three were case‐control studies with controls from the same population (nested case‐control studies). We included 84 English studies and six French studies. One study (Colley 2013), which was retrieved through an updated search, provided recent data for studies retrieved previously (Coulibaly 2011; Shane 2011; Tchuente 2012). In this case, we gathered data for the 2 × 2 tables from the most recent publication (Colley 2013).
Excluded studies
Full details of excluded studies can be found in the Characteristics of excluded studies table. We excluded 62 articles after reading the full texts. We excluded 17 case‐control studies with healthy controls or with controls from non‐endemic areas of schistosomiasis. We could not extract data from 2 × 2 tables for 16 studies. Twelve studies were not test accuracy studies, and four studies enrolled only patients proven to have schistosomiasis. Six studies used reference standards other than microscopy, four studies used other index tests to diagnose schistosomiasis that did not fulfil our inclusion criteria, and three studies performed similar tests on the same population as those reported by other already included studies.
Methodological quality of included studies
Figure 2 and Appendix 5 show results of the quality appraisal of the 60 included studies. Using the QUADAS‐2 tool, we evaluated these studies for risk of bias in the following domains: participant selection, index test, reference standard, and participant flow. In general, poor reporting of quality items hindered our evaluation of quality. We therefore rated the risk of bias for these domains largely as unclear. In the participant selection domain, about 75% of studies were rated as having unclear risk of bias. For index tests, unclear risk of bias ranged from 80% to about 98% (about 98% for reagent strips for microhaematuria, about 95% for reagent strips for proteinuria, and about 80% for CCA POC testing). None of the studies had high risk of bias in the index test domain. For the reference standard, about 50% of the studies had high risk of bias, whereas the other half had unclear risk of bias. For the participant flow domain, about 75% of the studies had low risk of bias, and the remaining studies had unclear risk. Concerns for applicability for all four domains were predominantly low.
2.
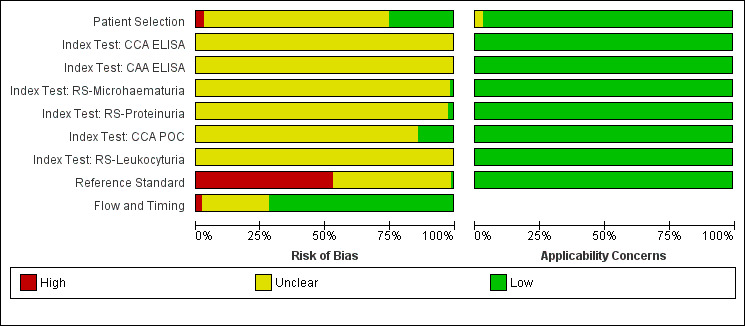
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
Findings
A summary of the main findings can be found in Table 1 and Table 2. Below we present in detail the overall findings for each index test.
Urine reagent strips
For microhaematuria
A total of 74 evaluations of the reagent strip for microhaematuria were performed with a total of 102,447 individuals. All evaluations were conducted in Africa. Median prevalence of S. haematobium was 42% (range 1% to 87%), and median female participation was 49% (Q1 49; Q3 53). Most of these evaluations were conducted with a lower‐quality reference standard of only one slide/person (n = 63; 85%), and most evaluations were carried out in mixed populations of adults and children (n = 40; 54%). These evaluations were described in articles published between the years 1979 and 2014; a large proportion (n = 43; 58%) were published between 1979 and 1999. Over these four decades, no clear pattern was evident for effects of year of study on sensitivity and specificity of microhaematuria (see forest plot in Appendix 6). However, the forest plot shows greater heterogeneity for sensitivity compared with specificity.
A large range of test brands were used to estimate the sensitivity and specificity of microhaematuria, as shown in Appendix 7. Most evaluations (n = 25; 34%) were performed with the brand from the manufacturer Ames.
The forest plot (Figure 3) and the HSROC curve (Figure 4) for the reagent strip for microhaematuria reveal heterogeneity for estimates of both sensitivity and specificity.
3.
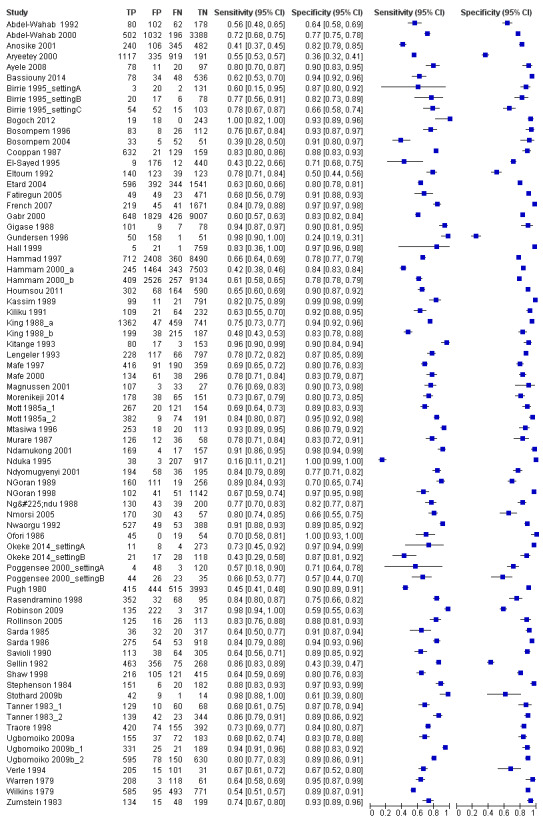
Forest plot of sensitivity and specificity of the urine reagent strip for microhaematuria.
Squares represent sensitivity and specificity of one study, the black line its confidence interval.
4.
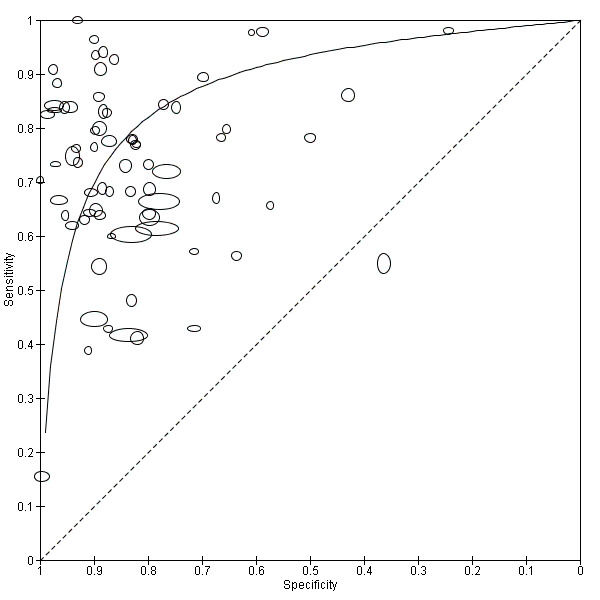
Summary ROC plot of sensitivity versus specificity of the urine reagent strip for microhaematuria.
The size of the points is proportional to the study sample size. The solid line shows the summary ROC curve.
Meta‐analytical sensitivity and specificity (95% confidence interval (CI)) of data at mixed thresholds were 75% (71% to 79%) and 87% (84% to 90%).
For proteinuria
A total of 46 evaluations of the reagent strip for proteinuria were performed with a total of 82,113 individuals. All evaluations were conducted in Africa. Median prevalence of S. haematobium was 51% (range 4% to 89%), and median female participation was 50% (Q1 46; Q3 53). Most of these evaluations were conducted with a lower‐quality reference standard (n = 36; 78%), and most were carried out in mixed populations of adults and children (n = 28; 61%). These evaluations were described in articles published between the years 1979 and 2014; the largest proportion (n = 27; 59%) were published before the year 2000. Over these four decades, no clear pattern was evident for effects of year of study on sensitivity and specificity of proteinuria (see forest plot in Appendix 8).
A large range of test brands were used to estimate the sensitivity and specificity of proteinuria, as shown in Appendix 9. Most evaluations (n = 17; 37%) were performed using the brand from the manufacturer Ames.
The forest plot (Figure 5) and the HSROC plot (Figure 6) for the reagent strip for proteinuria reveal greater heterogeneity for estimates of sensitivity than specificity. Meta‐analytical sensitivity and specificity (95% CI) of data at mixed thresholds were 61% (53% to 68%) and 82% (77% to 88%).
5.
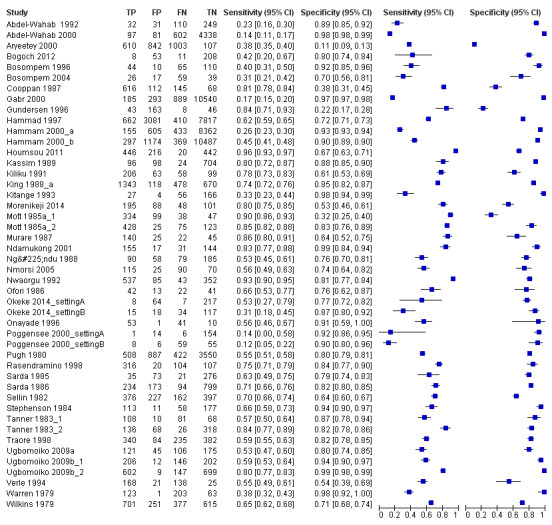
Forest plot of sensitivity and specificity of the urine reagent strip for proteinuria.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
6.
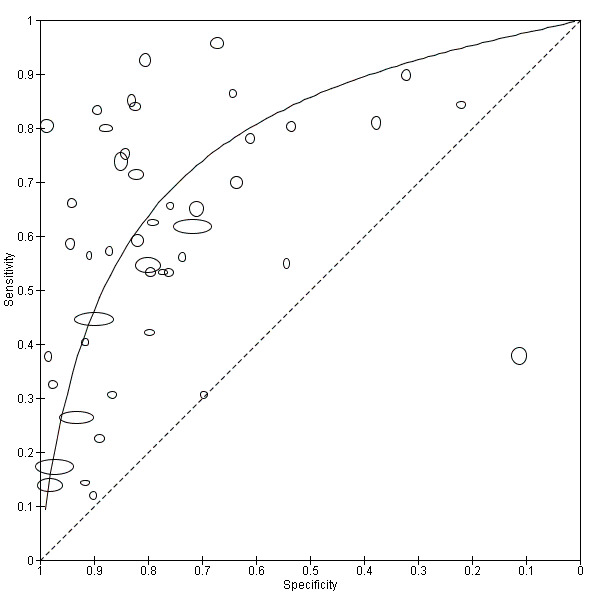
Summary ROC plot of sensitivity versus specificity of the urine reagent strip for proteinuria.
The size of the points is proportional to the study sample size. The solid line shows the summary ROC curve.
For leukocyturia
A total of five evaluations of the reagent strip for leukocyturia were performed with data from four publications and a total of 1532 individuals. Of these evaluations, two were carried out with a higher‐quality reference standard (40%). Median prevalence of S. haematobium was 34% (range 4% to 77%), and median female participation was 100% (Q1 68; Q3 100). All evaluations except one were conducted in Africa in mixed populations of adults and children. These evaluations were described in articles published between the years 1992 and 2000; most (n = 3) were published before the year 2000. Two different test brands were evaluated. Most evaluations (n = 3; 60%) were done using the Nephur‐test from Boehringer Mannheim.
The forest plot (Figure 7) and the HSROC plot (Figure 8) for the reagent strip for leukocyturia reveal greater heterogeneity for estimates of specificity than sensitivity. The ROC plot also reveals poor accuracy of the test, as most study points lie close to the diagonal line. Meta‐analytical sensitivity and specificity (95% CI) of data at mixed thresholds were 58% (44% to 71%) and 61% (34% to 88%).
7.
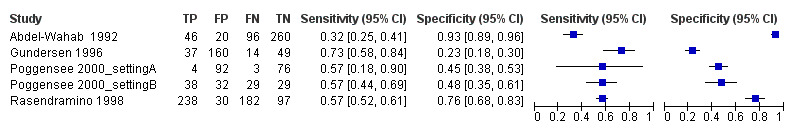
Forest plot of sensitivity and specificity of the urine reagent strip for leukocyturia.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
8.
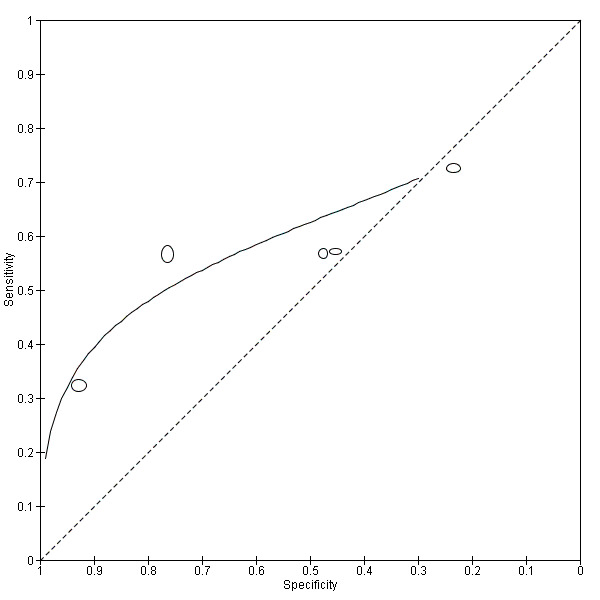
Summary ROC plot of sensitivity versus specificity of the urine reagent strip for leukocyturia.
The size of the points is proportional to the study sample size. The solid line shows the summary ROC curve.
Urine CCA POC test
For S. haematobium
A total of four evaluations of the CCA POC test for S. haematobium were performed on data derived from four publications with a total population of 901 individuals. Median prevalence of S. haematobium was 40% (range 31% to 48%), and median female participation was 47% (Q1 40; Q3 51). Most of these evaluations were conducted with a lower‐quality reference standard (n = 3; 75%). All evaluations were conducted in Africa. All evaluations included data from children only. These evaluations were described in articles published between the years 2008 and 2011. Four different test brands were evaluated.
Forest plots (Figure 9) and ROC plots (Figure 10) for this test reveal a high degree of heterogeneity for estimates of both sensitivity and specificity. The ROC plot also reveals poor accuracy of the test, as the study points lie close to the diagonal line. Meta‐analytical sensitivity and specificity (95% CI) of data at mixed thresholds were 39% (6% to 73%) and 78% (55% to 100%).
9.
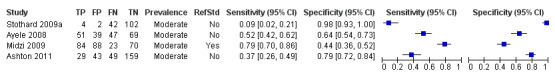
Forest plot of the sensitivity and specificity of the urine CCA POC test for S. haematobium.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
10.
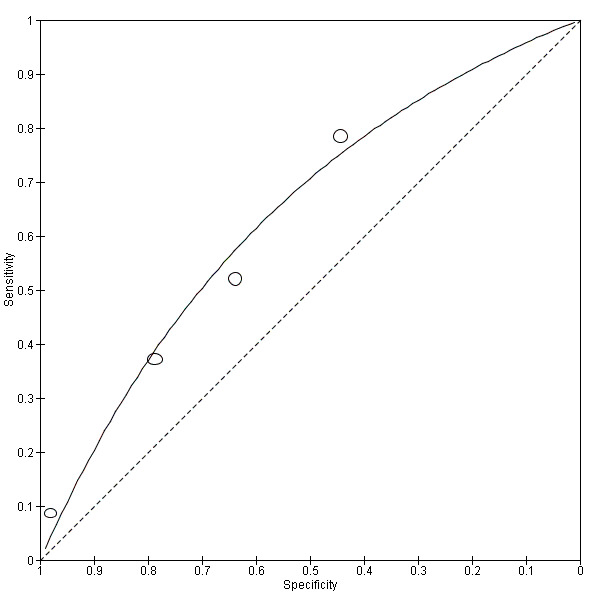
Summary ROC plot of sensitivity versus specificity of the urine CCA POC test for S. haematobium.
The size of the points is proportional to the study sample size. The solid line shows the summary ROC curve.
For S. mansoni
A total of 15 evaluations of the CCA POC test for S. mansoni were performed on data derived from 13 publications with a total population of 6091 individuals. Median prevalence of S. mansoni was 36% (range 8% to 68%), and median female participation was 49% (Q1 48; Q3 51). Most of these evaluations were conducted with a lower‐quality reference standard (n = 10; 67%). All evaluations were conducted in Africa, and all except one included data from children only. These 15 evaluations were described in articles published between the years 2007 and 2014. Two different test brands were evaluated: Rapid Diagnostic Tests from Pretoria South Africa and Schistosomiasis One Step Test from EVL Holland, as shown in Appendix 10. Most evaluations (n = 9) were performed using the Rapid Diagnostic Tests from South Africa.
The forest plot for this test reveals greater heterogeneity for estimates of specificity versus estimates of sensitivity (Figure 11). Meta‐analytical sensitivity and specificity (95% CI) of data at a threshold ≥ trace positive were 89% (86% to 92%) and 55% (46% to 65%) (Figure 12).
11.
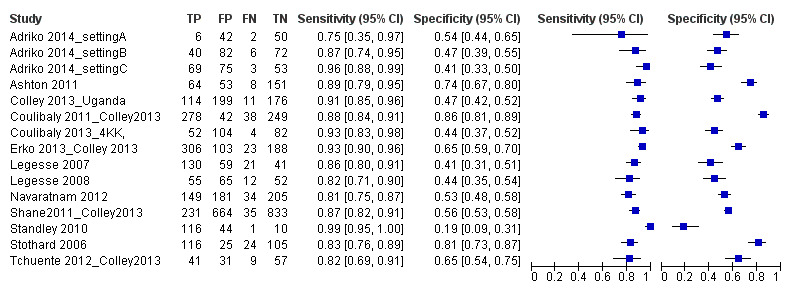
Forest plot of sensitivity and specificity of the urine CCA POC test for S. mansoni.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval. Colley 2013 was a study that included data for 5 studies (done in different countries). Some of the studies had been published earlier (Coulibaly 2011, Erko 2013, Shane 2011, Tchuente 2012). In this case, we used data from Colley 2013, which provided the most recent and updated data.
12.
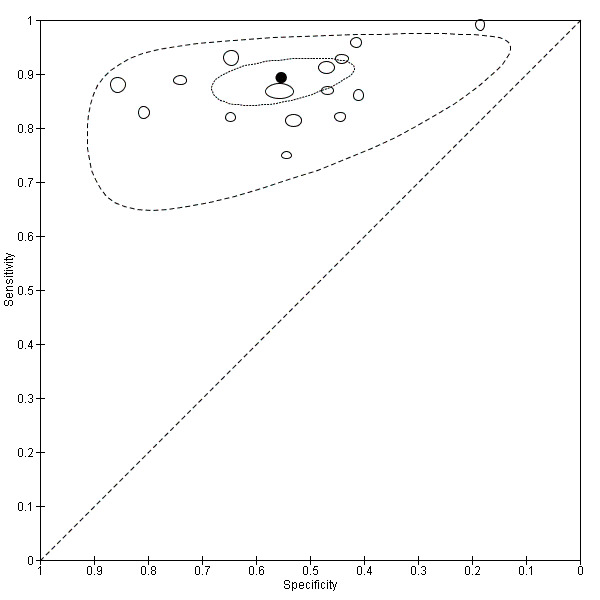
Summary ROC plot of sensitivity versus specificity of the urine CCA POC test for S. mansoni.
The size of the points is proportional to the study sample size. The thick black point shows the average value for sensitivity and specificity. The inner ellipse around the black spot represents the 95% confidence regions around the summary estimates. The outer ellipse represents the prediction region.
For mixed infection
One study assessed the capability of the POC test to detect schistosomiasis in an area of mixed S. haematobium and S. mansoni infection. This evaluation was conducted in Africa (Southern Sudan) in children only and was published in 2011. The brand used was Rapid Diagnostic Tests from Pretoria, South Africa. The sensitivity of the test was 66%, and the specificity was 79%. No meta‐analysis was performed for this test because of insufficient data.
CAA ELISA test
Serum
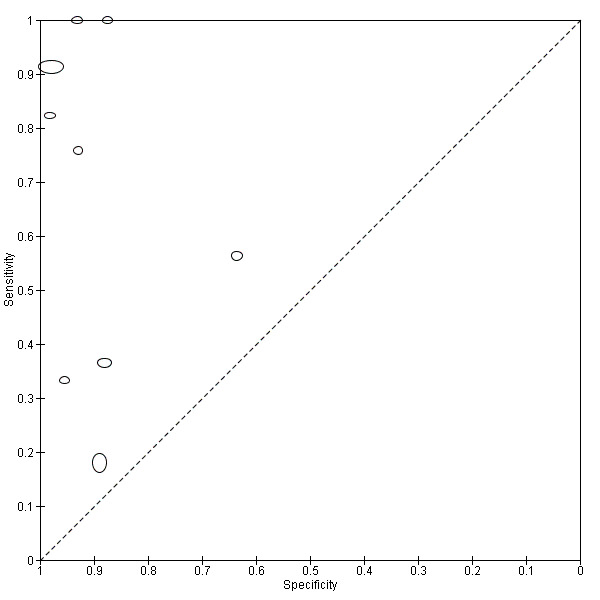
A total of five evaluations of the serum CAA test for S. mansoni were performed on data derived from four publications (total population 1583, years of publication 1995 to 1998). Median prevalence of S. mansoni was 93% (range 28% to 96%), and median female participation was 49% (Q1 49; Q3 51). All of these evaluations were conducted using relatively higher‐quality reference standards (n = 5; 100%). All were in‐house assays, and one study involved only children. Sensitivity of the serum CAA ELISA for S. mansoni ranged from 47% to 94%, and specificity ranged from 8% to 100% (Appendix 11). The ROC plot (Appendix 12) reveals a lot of scatter of the estimates of sensitivity and specificity provided by the included studies.
A total of three evaluations of the serum CAA test forS. haematobium were performed on data derived from three publications (total population 990, years of publication 1995 to 1999). Median prevalence of S. haematobium was 38% (range 18% to 57%). Only one study provided data on gender proportions (female participation was 54%). Two of the three evaluations were conducted using a higher‐quality reference standard (67%). All were in‐house assays, and all were carried out in mixed populations of adults and children. Sensitivity of the serum CAA test for S. haematobium ranged from 55% to 97%, and specificity ranged from 24% to 57% (Appendix 13; Appendix 14).
Urine
Only one evaluation of the urine CAA test for S. mansoni was performed on data derived from one publication (total population 204, year of publication 1995).. This was an in‐house assay and was done on data obtained from a mixed population of adults and children. Sensitivity of this test was 10%, and specificity was 99%.
Only one evaluation of the urine CAA test for S. haematobium was performed on data derived from one publication (total population 370, year of publication 1999). This in‐house assay was performed on data obtained from a mixed population of adults and children. Sensitivity of this test was 16%, and specificity was 94%.
CCA ELISA test
Serum
Two evaluations of the urine CCA test for S. mansoni were performed on data derived from two publications (total population 569, year of publication 1995). Both were in‐house assays performed on data obtained from a mixed population of adults and children. Sensitivity of this test ranged from 36% to 85%, and specificity was 50% to 93% (Appendix 15).
Only one evaluation of the urine CCA test for S. haematobium was performed on data derived from one publication (total population 370, year of publication 1999). This in‐house assay was performed on data obtained from a mixed population of adults and children. Sensitivity of this test was 3%, and specificity was 90%.
Urine
Two evaluations of the urine CCA test for S. mansoni were performed on data derived from two publications (total population 560, year of publication 1995). Both were in‐house assays, and neither involved children only. Sensitivity of this test ranged from 62% to 97%, and specificity from 27% to 84% (Appendix 16).
Only one evaluation of the urine CCA test for S. haematobium was performed on data derived from one publication (total population 370, year of publication 1999). This in‐house assay did not involve children only. Sensitivity of this test was 78%, and specificity was 70%.
Comparisons of accuracy between reagent strips for microhaematuria and proteinuria
Results of comparisons between microhaematuria and proteinuria are outlined in the Table 1. We first compared accuracy in all studies (indirect comparisons); we then limited the comparison to paired studies (direct comparisons). No statistically significant difference between the accuracy of microhaematuria and that of proteinuria was observed when the tests were compared in different populations using all studies (P = 0.25) (Figure 13). This can be demonstrated in the ROC curve showing the curves of tests as close together and crossing. The difference in accuracy also was not statistically significant when the tests were directly compared in the same individuals (P = 0.21) (Figure 14). A statistically significant difference in the threshold parameter was noted when the tests were compared in different populations using all studies (P < 0.0001), and when the tests were directly compared in the same individuals (P = 0.0009). This could imply that one test has a different operating threshold when compared with the other, and although overall accuracy is not statistically significantly different, sensitivity and specificity may be different under field circumstances.
13.
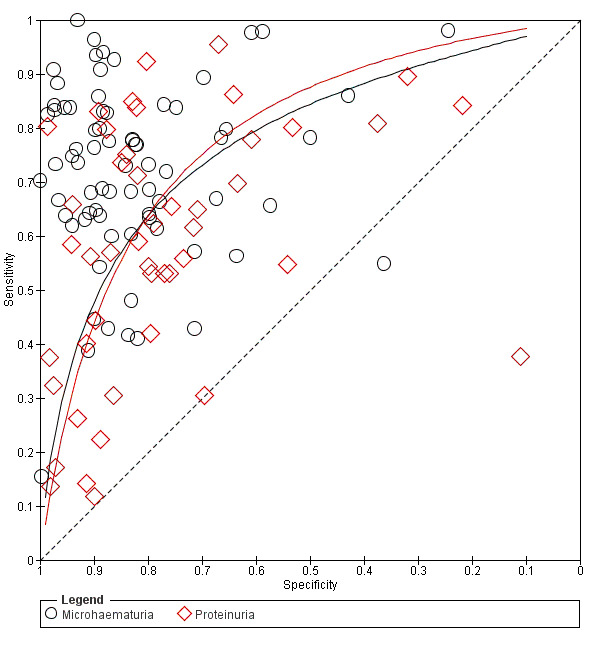
Summary ROC plot of sensitivity versus specificity showing the indirect comparison between microhaematuria and proteinuria (all studies). The solid lines show the summary ROC curves.
14.
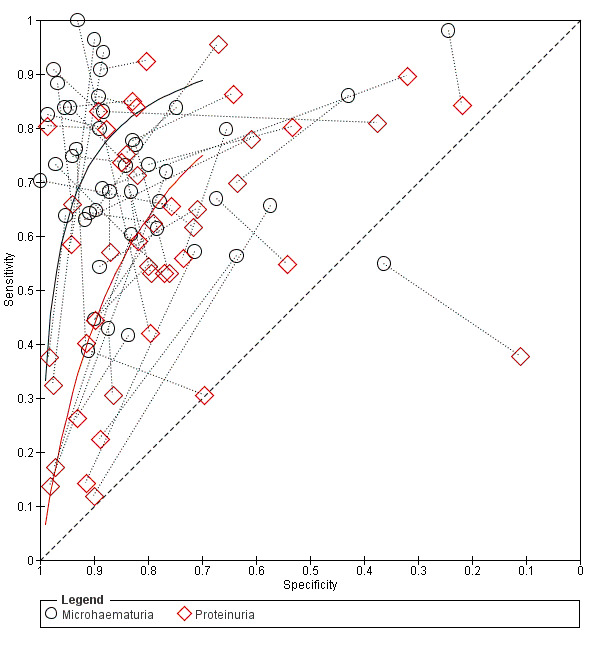
Summary ROC plot of sensitivity and specificity showing the direct comparison between microhaematuria and proteinuria (paired studies). Study points of microhaematuria and proteinuria from the same study are joined by a dotted line. The solid lines show the summary ROC curves.
Investigations of heterogeneity
Co‐variates in the models
The co‐variates quality of reference standard, age, gender (% female participation), prevalence of infection, and intensity of infection were added to the HSROC model. We investigated whether these co‐variates affect the parameters of the HSROC model, that is, accuracy, threshold, and shape.
For the reagent strip for microhaematuria, the co‐variates age (P = 0.002) and gender (% female participation) (P = 0.02) had statistically significant effects only on the threshold parameter of the HSROC model.
For the reagent strip for proteinuria, the co‐variates quality of reference standard (P = 0.01) and prevalence of infection (P value 0.007) had statistically significant effects on the accuracy parameter. Accuracy was higher with the higher‐quality reference standard and in settings with higher prevalence. Other co‐variates did not have a statistically significant effect on any of the other parameters of the HSROC model.
For CCA POC used to detect S. mansoni, no co‐variate had a statistically significant effect on sensitivity or on specificity.
Subgroup analysis
Table 3, Table 4, and Table 5 outline the results of subgroup analyses on the tests microhaematuria, proteinuria, and CCA POC for S. mansoni. When these tests were evaluated against the higher‐quality reference standard (ie when multiple samples were analyzed), sensitivity was lower for microhaematuria (71% vs 76%) and proteinuria (49% vs 68%) than with a lower‐quality reference standard. Specificity of these tests was lower for microhaematuria (85% vs 87%) but higher for proteinuria (83% vs 78%). In contrast, sensitivity was similar (88%) and specificity was higher for the CCA POC test for S. mansoni (66% vs 55%) when measured against a higher‐quality reference standard in comparison with a lower‐quality reference standard.
1. Sources of heterogeneity for urine reagent strip for microhaematuria.
| Group | Co‐variate | Subgroup | n (N = 74) | Sensitivity (95% CI) | Specificity (95% CI) |
| Overall | 0.75 (0.71‐0.79) | 0.87 (0.84‐0.90) | |||
| Subgroup analysis | Reference standard | Higher quality (> 1 sample) | 10 | 0.71 (0.62‐0.80) | 0.85 (0.78‐0.93) |
| Lower quality (1 sample) | 64 | 0.76 (0.71‐0.80) | 0.87 (0.84‐0.90) | ||
| Threshold | ≥ +1 | 23 | 0.80 (0.73‐0.85) | 0.85 (0.78‐0.92) | |
| Age | Children | 34 | 0.77 (0.71‐0.82) | 0.91 (0.87‐0.93) | |
| Intensity of infection | Light | 28 | 0.73 (0.66‐0.79) | 0.88 (0.84‐0.92) | |
| Sensitivity analysis | Concentration | Filtration only | 62 | 0.73 (0.69‐0.78) | 0.86 (0.82‐0.89) |
| QUADAS Patient Selection | Low risk of bias | 16 | 0.77 (0.70‐0.86) | 0.86 (0.79‐0.92) | |
| QUADAS Reference Standard | Low risk of biasa | 1 | ‐ | ‐ | |
| QUADAS Flow and Timing | Low risk of bias | 43 | 0.77 (0.72‐0.82) | 0.87 (0.83‐0.90) |
aInsufficient data for synthesis.
2. Sources of heterogeneity for urine reagent strip for proteinuria.
| Group | Co‐variate | Subgroup | n (N = 46) | Sensitivity (95% CI) | Specificity (95% CI) |
| Overall | 0.61 (0.53‐0.68) | 0.82 (0.77‐0.88) | |||
| Subgroup analysis | Reference standard | Higher quality (> 1 sample) | 9 | 0.49 (0.28‐0.70) | 0.83 (0.76‐0.90) |
| Lower quality (1 sample) | 37 | 0.68 (0.60‐0.76) | 0.78 (0.69‐0.87) | ||
| Threshold | ≥ +1 | 13 | 0.69 (0.56‐0.81) | 0.72 (0.54‐0.90) | |
| Age | Children | 18 | 0.67 (0.56‐0.76) | 0.81 (0.74‐0.87) | |
| Intensity of infection | Light | 15 | 0.60 (0.43‐0.77) | 0.83 (0.73‐0.93) | |
| Sensitivity analysis | Concentration | Filtration only | 35 | 0.62 (0.52‐0.71) | 0.80 (0.73‐0.86) |
| QUADAS Patient Selection | Low risk of bias | 11 | 0.64 (0.50‐0.79) | 0.81 (0.70‐0.93) | |
| QUADAS Reference Standard | Low risk of biasa | 1 | ‐ | ||
| QUADAS Flow and Timing | Low risk of bias | 36 | 0.67 (0.59‐0.76) | 0.82 (0.73‐0.88) |
aInsufficient data for synthesis.
3. Sources of heterogeneity for CCA POC test for S. mansoni.
| Group | Co‐variate | Subgroup | n (N = 15) | Sensitivity (95% CI) | Specificity (95% CI) |
| Overall | 0.89 (0.86‐0.92) | 0.55 (0.46‐0.65) | |||
| Subgroup analysis | Reference standarda | ||||
| Higher quality (> 1 sample) | 5 | 0.88 (0.82‐0.92) | 0.66 (0.46‐0.82) | ||
| Lower quality (1 sample) | 13 | 0.88 (0.85‐0.91) | 0.55 (0.45‐0.66) | ||
| Positivity thresholdb | > +1 | 5 | 0.72 (0.60‐0.82) | 0.85 (0.71‐0.93) | |
| Age | Children | 14 | 0.90 (0.86‐0.92) | 0.56 (0.46‐0.66) | |
| Intensity of infection | Lightc | 3 | ‐ | ‐ | |
| Sensitivity analysis | QUADAS Patient Selection | Low risk of biasc | 3 | ‐ | ‐ |
| QUADAS Reference Standard | Low risk of biasc | 0 | ‐ | ‐ | |
| QUADAS Flow and Timing | Low risk of bias | 11 | 0.87 (0.84‐0.90) | 0.57 (0.49‐0.65) |
aThree studies had data points for evaluations with both a lower‐ and a higher‐quality reference standard.
bFive studies had data points at both thresholds: trace and +1.
cInsufficient data for synthesis.
Microhaematuria and proteinuria had higher sensitivity (77% vs 73% and 67% vs 56%) in children than in mixed populations of adults and children. Specificity was higher for microhaematuria (91% vs 82%) but was comparable for proteinuria (81% vs 82%) in children compared with mixed populations of adults and children. All except one study of CCA POC for S. mansoni were carried out with children. At a positivity threshold ≥ 1, sensitivity of CCA POC for S. mansoni was lower (72% vs 89%) and specificity higher (85% vs 55%) than at a positivity threshold of trace positive. In the light‐intensity subgroup, sensitivity was slightly lower for microhaematuria (73% vs 75%) and specificity was slightly higher (88% vs 87%) compared with results of the overall analysis. In contrast, sensitivity (60% vs 61%) and specificity (83% vs 82%) for proteinuria were comparable. Data were insufficient to permit estimation of the sensitivity and specificity of CCA POC for S. mansoni in light‐intensity settings.
The forest plot (Figure 15) and the ROC plot (Figure 16) demonstrating sensitivity and specificity for microhaematuria after praziquantel treatment show a lot of variation in the estimates (predominantly for sensitivity) of the individual studies.
15.
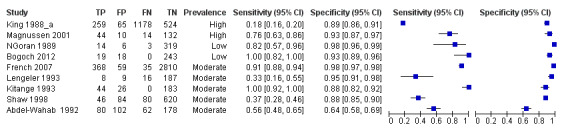
Forest plot of sensitivity and specificity of the urine reagent strip for microhaematuria for studies done after treatment with praziquantel.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
16.
Summary ROC plot of sensitivity and specificity of the urine reagent strip for microhaematuria for studies done after treatment with praziquantel.
The size of the points is proportional to the study sample size
Sensitivity analysis
For microhaematuria, when the analysis was limited to studies that used filtration only as the concentration method for urine microscopy, sensitivity (73% (69% to 78%) vs 76% (72% to 80%)) was lower and specificity was comparable (86% (82% to 89%) vs 86% (82% to 89%)) with those produced by the overall analysis. For proteinuria, when the analysis was limited to studies that used filtration only as the concentration method for urine microscopy, sensitivity was comparable (62% (52% to 71%) vs 61% (53% to 69%) and specificity was lower (80% (73% to 86%) than those produced by the overall analysis (83% (77% to 88%)) (Table 3; Table 4; Table 5).
Sensitivities and specificities of microhaematuria were comparable when analysis was limited to studies with low risk of bias for the participant flow domain. Sensitivity of proteinuria was higher when limited to studies with low risk of bias for the participant selection domain (64%) and the participant flow domain (67%). Specificity on the other hand was comparable for these two domains. Sensitivity and specificity of CCA POC for S. mansoni were comparable when limited to studies with low risk of bias for the participant flow domain (Table 3; Table 4; Table 5). Data were insufficient to allow estimation of sensitivity and specificity for studies with low risk of bias in the other domains—reference standard and participant selection—for the CCA POC test for S. mansoni.
As part of post hoc analyses, we noted that three evaluations showed substantial heterogeneity for the tests microhaematuria (Aryeetey 2000; sensitivity 55%, specificity 36%), proteinuria (Aryeetey 2000; sensitivity 38%, specificity 11%), and CCA POC for S.mansoni (Standley 2010; sensitivity 99%, specificity 19%). We excluded these evaluations in sensitivity analyses for the respective tests and found the following results. Results for microhaematuria (sensitivity 75%, specificity 87%) and proteinuria (sensitivity 61%, specificity 82%) were similar to those of the overall analysis. For CCA POC for S. mansoni, sensitivity was comparable (88% vs 89%) and specificity was slightly higher (58% vs 55%) compared with those of the overall analysis.
Discussion
Summary of main results
This review focused on analyzing the accuracy of urine reagent strips for the diagnosis of S. haematobium and of circulating antigen tests for the detection of S. haematobium and S. mansoni infections. Microscopy was used as the reference standard, and 90 studies were found to fit our inclusion criteria; data from these studies were used in this review. The main results, including average sensitivities and specificities for tests included in the meta‐analyses, are reported in Table 1 and Table 2.
Most of the studies included in our overall meta‐analyses used a ‘lower‐quality reference test’: microhaematuria 81%, proteinuria 73%, leukocyturia 60%, circulating cathodic antigen point‐of‐care (CCA POC) for S. haematobium 75%, and CCA POC for S. mansoni 81%. This implies that infections missed by single‐sample microscopy may have increased the number of false‐positives identified by the index tests, consequently leading to lower estimates of specificity.
Our overall analyses suggest that among the tests used to detect S. haematobium, the urine reagent strip for microhaematuria detects the largest proportion of schistosome infections identified by microscopy (sensitivity 75%); it also detects the largest proportion of non‐infections identified by microscopy (specificity 87%). Proteinuria follows suit, with sensitivity of 61% and specificity of 82%.
The superior performance of microhaematuria over proteinuria was not statistically significant when the comparison was performed both indirectly (using all studies) and directly (using paired studies) within the HSROC model. When measured against a higher‐quality reference standard (multiple measurements), microhaematuria had both lower sensitivity (71% vs 75%) and lower specificity (85% vs 87%) than were seen with a lower‐quality reference standard. Proteinuria on the other hand, when measured against a higher‐quality reference standard, had lower sensitivity (49% vs 61%) and higher specificity (82% vs 78%) versus a lower‐quality reference standard. Increasing the sensitivity of microscopy by taking multiple measurements may reduce the number of true cases wrongly classified as non‐infected by microscopy. An index test compared against a more sensitive reference test (higher quality) may have higher specificity because the number of false‐positives will be low. The lower specificity for microhaematuria may be due in part to poor reporting of how the reference standard was conducted in some studies.
Our results suggest that the urine reagent strip when used to detect leukocyturia is limited by low sensitivity (58%) and specificity (61%) and is not useful in practice. The low sensitivity for leukocyturia could be explained by the variations in morbidity caused by S. haematobium. Not all infected people have leukocyturia; therefore the proportion of false‐negatives is higher. The CCA POC test has very low sensitivity (39%) to detect S. haematobium and specificity of 78% and may not be suitable for mapping or estimation of infection, because it misses very many infections identified by microscopy.
The CCA POC test for S. mansoni detected a large proportion of infections identified by microscopy (sensitivity 89%). However, it also detected a lower proportion of the non‐infected cases identified by microscopy (specificity 55%). The low specificity can be explained by the fact that most studies in the overall analyses were measured against a lower‐quality reference standard. When compared with a higher‐quality reference standard, the CCA POC test had comparable sensitivity (88%) but higher specificity (66%). Arguably, if the reference standard had been even better, this specificity might have increased further.
As studies were insufficient, we were unable to generate summary estimates for the circulating antigen enzyme‐linked immunosorbent assay (ELISA) tests (CCA and circulating anodic antigen (CAA)). Estimates of sensitivity and specificity from the included studies evaluating these tests ranged widely.
Results of our assessment of risk of bias of the included studies were largely unclear because of poor reporting of items in these studies.
Application of the meta‐analysis to a hypothetical cohort
Table 1 and Table 2 apply the results of the meta‐analyses to a hypothetical cohort of 1000 individuals suspected of having active S. haematobium and/or active S. mansoni infection in a field setting. We illustrate the impact of using microhaematuria, proteinuria, leukocyturia, and CCA POC for S. haematobium in a setting with a prevalence of S. haematobium infection of 41%, and the impact of using CCA POC for S. mansoni in a setting with a prevalence of S. mansoni infection of 36%. These are the estimates of median prevalence of infection obtained from all studies included in this review.
Delivery of population‐based control programmes such as treatment with praziquantel requires knowledge of prevalence estimates of schistosomal infections (Colley 2014). This helps the clinician in determining whether mass drug treatment should be administered in settings of very high prevalence, or targeted treatment in settings of low prevalence. We have included descriptions of the performance of these tests in estimating the prevalence (index test positives (TP + FP)) of S. haematobium and S. mansoni infections.
S. haematobium infection
If the point estimates of the tests for S. haematobium are applied to a hypothetical cohort of 1000 individuals suspected of having active S. haematobium infection, among whom 410 actually have the infection, the strip for microhaematuria would be expected to miss (102) and falsely identify (77) the least number of cases. This test would identify 384 positive cases in total.
For the other tests (in increasing order of missed cases): The strip for proteinuria would be expected to miss 160 cases and to falsely identify 106 cases; proteinuria would be expected to miss 14% more cases than microhaematuria and to falsely identify 5% more cases than microhaematuria; leukocyturia would be expected to miss 172 cases and to falsely identify 230 cases; and the CCA POC test would be expected to miss 250 cases and to falsely identify 130 cases. In total, the strips for proteinuria, leukocyturia, and the CCA POC test would identify 356, 468, and 254 positive cases, respectively.
Overall, when infection is mapped, the prevalence of microhaematuria would seem to be 38%—close to the true prevalence of 41%. The prevalence of proteinuria would seem to be 36%, that of leukocyturia 47%, and that of CCA POC 25%. In cases of mass treatment, the ultimate consequences of these numbers would depend on the minimal prevalence needed to start mass treatment.
S. mansoni infection
If the point estimates for the CCA POC test are applied to the same hypothetical cohort of 1000 individuals suspected of having active S. mansoni infection, among whom 360 actually have the infection, the CCA POC test would be expected to miss 40 cases and to falsely identify 288 cases. In total, the test would identify 608 positive cases (for an observed prevalence of 61%).
Comparison with other reports
The absence of a suitable gold standard for active schistosomiasis is reflected in the existing literature, where different reference standards are used with subsequent variation in accuracy (especially with specificity) of the index test (Koukounari 2009; Coulibaly 2011; Tchuente 2012; Colley 2013; Erko 2013; King 2013;Lodh 2013; Sousa‐Figueiredo 2013).
A meta‐analysis was recently published that assessed the accuracy of urine reagent strips for microhaematuria against conventional microscopy as a reference standard (King 2013). Unlike King’s review, our review also estimated the accuracy of other urine reagent strips for proteinuria and leukocyturia. To guide decision making, it is important to show which of these tests fares better. Our analyses suggest that microhaematuria has higher sensitivity than proteinuria and leukocyturia.
Compared with results from King’s meta‐analysis (King 2013), our estimate of sensitivity for microhaematuria was lower (75% vs 81%) but specificity was comparable (87% vs 89%). This difference may be attributed to the method of meta‐analysis used. King used the HSROC regression following a Bayesian Monte Carlo Markov chain approach (Dendukuri 2012), and we used the HSROC model recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Macaskill 2010). With regard to sources of heterogeneity, some of our results are also comparable with those of King 2013. For instance, King found through multi‐variable regression modelling that the urine heme dipstick performed better in children than in mixed populations of adults and children (Relative diagnostic odds ratio = 3.16). In our review, we found that sensitivity and specificity were higher in studies on children compared with studies on mixed populations of adults and children. We strongly confirm that this test is therefore highly suitable for mass mapping of school‐aged children in endemic areas. Again our analyses show that sensitivity of the urine heme dipstick was slightly lower in settings of low intensity (73%) compared with that of the overall estimate (75%). This finding was similar to the findings of King, which showed that sensitivity of the urine heme dipstick was lower in settings of lower infection intensity (65%) in the subgroup analysis than in the overall analysis (81%). However it should be noted that our definition of light intensity differed from that used by King. We selected the more commonly used World Health Organization (WHO) recommended cutoff of < 50 eggs per 10 mL, whereas King defined low intensity as ≤ 100 eggs/10 mL. This could explain in part why our sensitivity estimates were higher than those of King in settings of light intensity.
A key difference between our review and that of King 2013 concerned the effects of treatment on the estimate of sensitivity of the heme dipstick. In a subgroup of eight studies with mixed post‐treatment evaluations of one year (n = 6), six months (n = 1), and one month (n = 1), King’s review produced a lower summary estimate of sensitivity (72%) in the subgroup of treated populations as compared with the overall analysis (81%). King considered treatment evaluations with praziquantel and metrifonate, whereas we focused on studies that evaluated the effects of praziquantel treatment, as this is the current drug of choice. Because studies reported varied time intervals between treatment and retesting, we opted not to pool the estimates of studies, as this would likely produce biased overestimates of sensitivity and specificity. Studies with long time intervals were likely to include greater numbers of participants reinfected compared with studies carried out at shorter time intervals, and their results may be confounded by repeated treatments provided by national programmes.
A recently published multi‐centre evaluation of CCA POC tests done in five African countries (Colley 2013) recommended that the CCA POC test for S. mansoni (evaluated with a positivity threshold ≥ trace positive) was a sufficiently sensitive and specific tool for mapping intestinal schistosomiasis in moderate‐ to high‐prevalence areas, and therefore it was a viable alternative to microscopy (Colley 2013). After acknowledging the absence of a gold standard, this multi‐centre study used latent class analysis (modelling results from CCA POC, Kato‐Katz, and PCR) to generate an overall estimate of 86% sensitivity and 72% specificity of the CCA POC based on data from 4405 school‐age children. Using microscopy only (KK) as the reference standard, our review, which incorporated all include study results along with findings of additional studies, produced a comparable summary estimate of 89% sensitivity but a lower summary estimate of 55% specificity at a threshold of trace positive. Differences in specificity could be explained by the reference standard and indicate that some of the false‐positives identified by CCA POC are indeed likely to be true infections that are not detected by standard microscopy.
Few studies have fully evaluated the accuracy of the circulating antigen ELISA tests (CCA and CAA). The serum CAA ELISA test is currently being converted to a point‐of‐care format for S. mansoni (Corstjens 2008) andS. haematobium (van Dam 2013) with promising results of analytical sensitivity and specificity. In our review, sensitivity of the included studies evaluating the serum CAA ELISA test for S. mansoni ranged widely from 47% to 94%, and specificity ranged widely from 8% to 100%. Sensitivity of the included studies evaluating the serum CAA ELISA test for S. haematobium ranged from 55% to 97%, and specificity was low, ranging from 24% to 57%. However, the studies included in our review were carried out before the year 2000 with in‐house tests. The tests currently being developed are most likely improved versions; therefore additional studies analyzing the clinical sensitivity and specificity of the serum ELISA tests are needed for conclusive determination of whether they are suitable for the diagnosis of active schistosomiasis.
Strengths and weaknesses of the review
Strengths
We have evaluated the accuracy of POC tests currently in use and tests that have recently been transformed into POC tests for detection of active schistosomiasis in endemic areas. This makes our review relevant to current practice. To avoid missing studies, we did not use a search filter, and we did not limit our search by publication year or language; also to limit bias, data extraction was performed by two people independently.
Weaknesses
Choice of the reference standard
In light of the absence of a suitable gold standard for active schistosomiasis and the presence of other proposed alternative reference standards, evaluation of index tests with only microscopy as the reference standard may be considered a shortcoming of our review. However because microscopy remains the most commonly used test and therefore reference test, we wanted our review to be applicable to current practice. Our review provides better insight into the proportion of cases detected and the proportion of cases misclassified by urine reagent strips and CCA POC tests when microscopy is used as the reference standard. A more reliable way of evaluating whether an index test can replace microscopy would be to compare the accuracy of microscopy, urine reagent strips, and circulating antigen tests against other proposed reference standards in the same set of participants (direct comparison studies). A few studies have compared the accuracy of one or more KK smears and CCA POC against a reference standard comprising six or more KK smears (Coulibaly 2011; Tchuente 2012; Erko 2013) or against PCR as the reference test (Lodh 2013) (see comparisons in Appendix 17). All of these studies have shown the CCA POC test to be more sensitive but less specific than single or double KK. More direct comparative studies and reviews are needed to reliably confirm this finding and to identify sources of variation in results.
Quality of included studies
Poor and inconsistent reporting of participant characteristics such as clinical status of participants, intensity of infection, administration of praziquantel treatment, and conduct of the study limited our investigations of sources of heterogeneity and risk of bias assessment.
In our review, the reporting of intensity of infection was unclear (reported in different ways (arithmetic mean or range of infection or geometric mean or range of infection or proportions with light/moderate/heavy infections) or not reported at all) for a large proportion of the included studies (microhaematuria 44%, proteinuria 42%, and CCA POC 45%). It was therefore difficult to effectively investigate its influence on the accuracy of the evaluated tests. It was also a challenge to fully investigate the effects of praziquantel treatment on the accuracy of the evaluated tests because 82% of the studies did not report the treatment status of participants before the start of the study. The effects of intensity of infection and the effects of praziquantel treatment on the accuracy of diagnostic tests for schistosomiasis are currently an important concern for national control programmes, particularly as praziquantel treatments progress, with subsequent decreases in infection intensities. Indeed, in areas where the force of infection and associated morbidity have been greatly reduced, some programmes are beginning to focus on elimination. It is therefore of vital importance that highly sensitive tests are used for monitoring, and that highly sensitive and specific tests are used in efficacy studies before and after treatment.
Applicability of findings to the review question
Our concern about the applicability of the included studies to our review question was low, as assessed by QUADAS‐2. As all but one study were carried out in Africa, and all but one study were conducted in field settings, our results are highly applicable for use in endemic communities for which disease control programmes are often targeted. However, one area that may limit the applicability of our findings to the review question is our investigation into sources of heterogeneity such as effects of praziquantel treatment and risk of bias assessment on the accuracy estimates of evaluated tests. As discussed earlier, poor and inconsistent reporting limited this investigation. In light of the ongoing disease control programmes, fully showing any variation in test accuracy associated with effects of praziquantel treatment would be useful for policy makers. Knowing the risk of bias of included studies would also help in objective assessments of the strength of the evidence. Study authors therefore are encouraged to use the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines (Bossuyt 2003) in reporting the design and conduct of their studies.
Authors' conclusions
Implications for practice.
Among the tests evaluated for S. haematobium infection, microhaematuria has detected the largest proportion of infections and non‐infections identified by microscopy. This test could continue to serve as a replacement test for microscopy for initial mapping or estimation of S. haematobium infection, particularly in endemic areas with moderate to high prevalence of infection.
The CCA POC test for S. mansoni detects a very large proportion of infections identified by microscopy but misclassifies many microscopy‐negatives as ‐positives in endemic areas with moderate to high prevalence of infection. This may occur because the test is potentially more sensitive than microscopy. Nevertheless, healthcare workers should interpret the results with care when using this test for initial mapping or estimation of S. mansoni infection, as some of the positives may still be false‐positives, in particular when trace‐positive is used as the threshold.
Besides assessment of the accuracy of a test, the choice of a suitable diagnostic test should be made in light of cost and logistical considerations. Costs for microscopy (USD per examination, 0.3 for a single thick KK smear) (Cavalcanti 2013) and for reagent strips for microhaematuria (USD 0.32) (Legesse 2008) are comparable, but the strips are easier to use and interpret and therefore are not logistically challenging in field settings. The CCA POC tests are more costly (USD 2.6 per examination) (Cavalcanti 2013) but are rapid and easy to use and interpret, are highly portable, and require fewer technical personnel than microscopy; they are also suitable for field screening and diagnosis.
Implications for research.
As control programmes progress with expected subsequent decreases in prevalence and intensity of infection, we highlight the importance of additional primary research conducted to identify a suitable clinical reference standard for active schistosomiasis.
Additional studies comparing the accuracy of microscopy, circulating antigen tests, and urine reagent strips versus other proposed reference standards are needed if a suitable replacement for microscopy in practice is to be reliably recommended.
Further studies to identify other sensitive tests to detect active S. haematobium and S. mansoni infections and further evaluations of the CAA test as a future POC test for serum or urine are also needed.
For suitable tests to be reliably recommended for monitoring effects of praziquantel treatment in disease control programmes, additional follow‐up studies are required to evaluate the effects of praziquantel treatment on intensity of infection and accuracy of urine reagent strips and circulating antigen tests.
Further research on cost‐effectiveness of diagnostic tests in areas of different endemicity is also needed, as cost is a key deciding factor in resource‐limited settings.
Finally, authors of primary test accuracy studies should be encouraged to use the STARD guidelines when reporting the design and conduct of their studies. This will enable systematic reviewers to better synthesize the data and to draw conclusions on risk of bias in studies of test accuracy.
Feedback
Feedback from Dr Charles King, 17 March 2015
Summary
Point 1:
I feel that the current review's results and conclusions are misleading. The inappropriate analysis used in the HSROC estimation results in incorrect conclusions about the diagnostic performance of both antigen tests and dipsticks. The main objection I have is to the use of microscopic detection of eggs as the reference standard for the diagnosis of Schistosoma infection. Microscopy to detect S. mansoni or S. japonicum eggs in stool or S. haematobium eggs in filtered urine has long been known to be poorly sensitive for moderate and low intensity infections. When subjects are repeatedly tested for 7‐15 days in a row, single day egg visualization has a sensitivity of 40‐60%. The poor performance of microscopy for S. mansoni has been well documented by de Vlas and colleagues [1, 2] for S. japonicum by Carabin, et al.[3] and Hubbard, et al. [4] and for S. haematobium by Savioli et al.[5] and Warren, et al [6], among others.
Point 2:
Given the lack of a true ‘gold standard’ and a sensitivity by microscopy of ˜50%, a more appropriate approach for the review would have been Latent Class Analysis (LCA), in which results from two or more imperfect tests are used together to estimate an unmeasured ‘true’ infection status. In stating that the antigen test 'misclassify' (i.e., have poor specificity), the review claims that a person with a positive POC CCA and negative stool examination is not infected. In fact, several lines of evidence appear to indicate that many if not most of those who have negative stool examinations but positive POC CCA results are, in fact, infected. [7, 8, 9, 10, 11]
Point 3:
I would also encourage the authors to include results from populations or areas without significant Schistosoma risk. Measuring results among persons with very low pre‐test probability of infection can contribute greatly to assessing the specificity of new tests.
Point 4:
Could the authors revisit the data using the LCA approach of Dendukuri, et al., 2012 [12] for situations in which there is no gold standard? Their SAS code is available online, and the reanalysis could be done in a matter of a day. A revised review, reflecting the LCA approach, would do much to remove the confusion about these tests in policy circles.
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
References
1. de Vlas SJ, Engels D, Rabello AL, Oostburg BF, Van Lieshout L, Polderman AM, Van Oortmarssen GJ, Habbema JD, Gryseels B, 1997. Validation of a chart to estimate true Schistosoma mansoni prevalences from simple egg counts. Parasitology 114 ( Pt 2): 113‐21.
2. de Vlas SJ, Gryseels B, 1992. Underestimation of Schistosoma mansoni prevalences. Parasitol Today 8: 274‐277.
3. Carabin H, Marshall CM, Joseph L, Riley S, Olveda R, McGarvey ST, 2005. Estimating the intensity of infection with Schistosoma japonicum in villagers of Leyte, Philippines. Part I: A Bayesian cumulative logit model. The Schistosomiasis Transmission & Ecology Project (STEP). Am J Trop Med Hyg 72: 745‐753.
4. Hubbard A, Liang S, Maszle D, Qiu D, Gu X, Spear RC, 2002. Estimating the distribution of worm burden and egg excretion of Schistosoma japonicum by risk group in Sichuan Province, China. Parasitology 125: 221‐31.
5. Savioli L, Hatz C, Dixon H, Kisumku UM, Mott KE, 1990. Control of morbidity due to Schistosoma haematobium on Pemba Island: egg excretion and hematuria as indicators of infection. Am J Trop Med Hyg 43: 289‐295.
6. Warren KS, Arap Siongok TK, Hauser HB, Ouma JH, Peters PAS, 1978. Quantification of infection with Schistosoma haematobium in relation to epidemiology and selective population chemotherapy. I. Minimal number of daily egg counts in urine necessary to establish intensity of infection. Journal of Infectious Diseases 138: 849‐55.
7. Tchuem Tchuente, LA, Kuete Fouodo, CJ, Kamwa Ngassam, RI, Sumo, L, Dongmo Noumedem, C, Kenfack, CM, Gipwe, NF, Nana, ED, Stothard, JR, Rollinson, D. Evaluation of circulating cathodic antigen (CCA) urine‐tests for diagnosis of Schistosoma mansoni infection in Cameroon. 2012. PLoS Negl Trop Dis. 6(7):e1758.
8. Colley, DG, Binder S, Campbell C, King CH, Tchuem Tchuenté LA, N'Goran EK, Erko B, Karanja DM, Kabatereine NB, van Lieshout L, Rathbun S. A five‐country evaluation of a point‐of‐care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. 2013. Am J Trop Med Hyg. 88(3):426‐32.
9. Lamberton, PH, Kabatereine NB, Oguttu DW, Fenwick A, Webster JP. 2014. Sensitivity and specificity of multiple Kato‐Katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre‐ and post‐repeated‐praziquantel treatment. PLoS Negl Trop Dis. 8(9):e3139.
10. Adriko, M, Standley CJ, Tinkitina B, Tukahebwa EM, Fenwick A, Fleming FM, Sousa‐Figueiredo JC, Stothard JR, Kabatereine NB. 2014. Evaluation of circulating cathodic antigen (CCA) urine‐cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta Trop (2014) 136:50‐7.
11. Mwinzi, P, Kittur, N, Ochola, E, Cooper, PJ, Campbell, CH, Jr., King, CH, Colley, DG. 2015. Additional evaluation of the Point‐of‐Contact Circulating Cathodic Antigen assay for Schistosoma mansoni infection. Front. Public Health. doi: 10.3389/fpubh.2015.00048
12. Dendukuri N, Schiller I, Joseph L, Pai M, 2012. Bayesian meta‐analysis of the accuracy of a test for tuberculous pleuritis in the absence of a gold standard reference. Biometrics 68: 1285‐1293.
Reply
Point 1:
We would like to thank Professor King for his comment, although we do believe that the analysis used was appropriate. The limitations of microscopy as a reference standard have been acknowledged several times in our review. In the main text, we interpret the sensitivity of all tests as percentage of microscopy positives retrieved by the index test; and the specificity as microscopy negatives found negative by the index test. We therefore believe that our review gives better insight in the proportion of cases detected and missed by microscopy, which is still a commonly used tool in practice. Our discussion and conclusion within the main text and abstract reflect this. However we agree that the final line of the Plain Language Summary may be misleading, and we have therefore corrected this, incorporating the likely low sensitivity of egg counts (see below).
Moreover, attempts have been made by researchers to improve the quality of the microscopy (by increasing the number of samples or slides used) as the reference standard. A higher quality reference standard may be expected to detect more of the lower intensity infections. We showed how this affects the index test’s estimates. For S. mansoni, in studies with a higher quality reference standard the specificity of the POC‐CCA increased. This strongly supports our, and your, conclusion that the apparent low specificity of POC‐CCA is due to low sensitivity of the microscopy reference standard. POC‐CCA may be more sensitive than Kato‐Katz, particularly in low endemicity areas. Conversely, for S.haematobium the sensitivity of microhaematuria was lower in studies using a higher quality reference standard. The extra infections found by the higher quality reference standard were not picked up by microhaematuria dipsticks.
Point 2:
The proposed latent class analysis (LCA) approach for meta‐analysis of diagnostic accuracy data takes into account the imperfect nature of the reference standard to come to a ‘true’ sensitivity and specificity. However, in latent class models, the target condition is a statistical entity and is not defined in a clinical way. The interpretation and use of accuracy results based on latent class models may therefore be challenging in practice, as clinicians are unclear about the target condition or what the results stand for. This target condition may reflect infection status, but there may also be another, unknown underlying latent patient status that does not necessarily correlate with infection. At least in our meta‐analyses, we know what the limitations are and we know how to interpret the results.
We agree that ‘misclassify’ may not be the appropriate term and we will replace it in the abstract of the review with the first update. We have corrected the Plain Language Summary, incorporating the likely low sensitivity of egg counts. The end of the plain language summary now states
"For intestinal schistosomiasis, the parasite antigen urine test classifies many microscopy negative people as being infected. This finding may be explained by the low sensitivity of microscopy."
Point 3:
We understand the value of assessing the accuracy of these tests in non‐endemic areas. However, we wanted to focus our review to endemic populations where disease control programs are mostly based and where diagnostic methods and control interventions are mostly applied. Yet, in the discussion we have included comments on the high specificity of POC‐CCA tests in non‐endemic areas. This was to strengthen our argument that the low specificity calculated from our meta analyses is likely due to low sensitivity of the commonly used reference standard (i.e. microscopy).
Point 4:
As explained above, the interpretation of LCA results may not be as straightforward as indicated. Moreover, the validity of results produced by LCA models depends on the specifications of the statistical model and the assumptions made when modelling the data. Especially determining the appropriate levels of dependence between tests complicates interpretation and the actual conduct of the models.
In summary, we whole heartedly agree on the potential benefits of LCA, but would like to see more research done on the validity, variability and interpretation of the models before using it at a regular basis and accepting it as the true gold standard approach for these meta‐analyses in infectious diseases.
Contributors
All authors contributed to drafting this response.
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2015 | Feedback has been incorporated | Feedback from Dr Charles King and responses from authors incorporated into the review. |
| 8 July 2015 | Amended | Review amended to incorporate small change in Plain Language Summary and feedback from contributor. |
Acknowledgements
We thank René Spijker, MSc (Dutch Cochrane Centre, University of Amsterdam), for assisting in the development of the search strategy of this project.
Appendices
Appendix 1. Geographical distribution, infection, and morbidity of S. haematobium and S. mansoni
| Species | Geographical distributiona | Number infected (millions) | Morbidity (millions) | |
| S. haematobium | Africa, Middle East | In SSA (112)b | Urogenital schistosomiasisa Signs and symptoms: Haematuria (blood in urine), proteinuria (proteins in urine), leukocyturia (white blood cells in urine), urinary obstruction, hydronephrosis, chronic renal failure, bladder cancer, genital lesions, vaginal bleeding, pain during sexual intercourse, nodules in the vulva, infertility, pathology in prostrate and seminal vesicles |
In SSAb: Haematuria (71) Dysuria (32) Minor bladder pathology (76) Major bladder pathology (24) Major hydronephrosis (9.6) |
| S. mansoni | Africa, Middle East, the Caribbean, South America | In SSA (54)b | Intestinal schistosomiasisa Signs and symptoms: Abdominal pain, blood in stool, portal hypertension, ascites |
In SSAb: Diarrhoea (0.78) Blood in stool (4.4) Hepatomegaly (8.5) |
Abbreviations: SSA = sub‐Saharan Africa.
aWHO 2010.
Appendix 2. Diagnostic and treatment strategies
17.
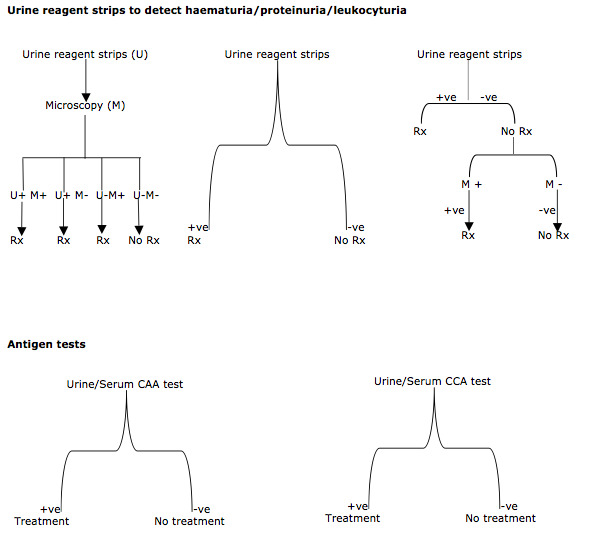
Diagnostic and treatment strategies. Abbreviations: +ve = positive; ‐ve = negative; CAA = circulating anodic antigen; CCA = circulating cathodic antigen; M+ = microscopy positive; M‐ = microscopy negative; U+ = urine reagent strips positive; U‐ = urine reagent strips negative; Rx = treatment; No Rx = no treatment.
Abbreviations: +ve = positive; ‐ve = negative; CAA = circulating anodic antigen; CCA = circulating cathodic antigen; M+ = microscopy positive; M‐ = microscopy negative; U+ = urine reagent strips positive; U‐ = urine reagent strips negative; Rx = treatment; No Rx = no treatment.
Appendix 3. MEDLINE search strategy via Ovid SP platform
Limits: limited to human studies
| Line # | Term |
| 1 | (anodic adj3 antigen*).ti,ab. |
| 2 | (cathodic adj3 antigen*).ti,ab. |
| 3 | exp Enzyme‐Linked Immunosorbent Assay/ |
| 4 | exp Immunoenzyme Techniques/ |
| 5 | hematuria/ or exp proteinuria/ |
| 6 | leukocyturia.ti,ab. |
| 7 | leucocyturia.ti,ab. |
| 8 | h?ematuria.ti,ab. |
| 9 | proteinuria.ti,ab. |
| 10 | albuminuria.ti,ab. |
| 11 | CCA.ti,ab. |
| 12 | CAA.ti,ab. |
| 13 | urinalysis.ti,ab. |
| 14 | elisa.ti,ab. |
| 15 | eia.ti,ab. |
| 16 | exp Reagent Strips/ or dipstick.mp. |
| 17 | (reagent adj3 strip*).ti,ab. |
| 18 | (test adj3 strip*).ti,ab. |
| 19 | haemastix.ti,ab. |
| 20 | "schistosoma mansoni".ti,ab. or "schistosoma haematobium".ti,ab. |
| 21 | exp Glycoproteins/ |
| 22 | exp Antigens, Helminth/ |
| 23 | exp Helminth Proteins/ |
| 24 | exp Schistosoma haematobium/ |
| 25 | exp Antibodies, Monoclonal/ |
| 26 | exp Schistosoma mansoni/ |
| 27 | or/1‐26 |
| 28 | schistosomiasis/ or schistosomiasis haematobia/ or schistosomiasis mansoni/ |
| 29 | schistosomiasis.ti,ab. |
| 30 | bilharzia*.ti,ab. |
| 31 | or/28‐30 |
| 32 | animals/ not humans/ |
| 33 | exp Letter/ |
| 34 | exp Case Reports/ |
| 35 | or/32‐34 |
| 36 | 27 and 31 |
| 37 | 36 not 35 |
With use of the Ovid platform, this MEDLINE search was translated automatically to suit the EMBASE and BIOSIS databases to identify additional records. In the search interface, under 'resource selected,' with the link 'change,' one can select the desired database.
Appendix 4. QUADAS tool
We used the QUADAS‐2 tool. The signalling questions under the four recommended domains are outlined in questions 7 to 10 on the data extraction form.
The scoring guidance for these questions was as follows.
Flow diagram
For questions 7 and 8, drawing a flow diagram of the study may be helpful (this is not mandatory). Flow charts of patients display how many patients were eligible for the study, how many were actually recruited, how many received the index test, how many received the reference standard, etc. In addition, the numbers of true‐ and false‐positives and true‐ and false‐negatives are displayed. If necessary, please draw a flow diagram for the primary study in the space provided on page 8 of the extraction form.
7. Patient selection (patient selection domain)
These questions will help assess risks of bias in the study design.
a. Please cite here the selection criteria
Please list in the space provided the selection criteria used to recruit patients into the study. You can also cite the page number in the article on which the selection criterion was written.
If no criteria were reported, indicate “Not reported/NR” in the space provided. If the criterion was unclear, please indicate “Unclear,” and explain your answer.
b. Stage of disease
Participants recruited into the study may be without symptoms or with symptoms. Please indicate the disease stage for participants. If the study clearly reports that both asymptomatic and symptomatic cases were evaluated, please tick the appropriate box provided (both A and S). If the study does not clearly report the clinical status of the participants, please tick the box ‘Unclear.’ A box N/A has been provided. If S. m for example was not evaluated in the study, please tick this box. The same applies to S. h. A comment box is provided for any comments that you may have.
c. What was the study design?
Please indicate the design of the study by ticking one of the choices provided.
We will not include case‐control studies that incorporate healthy controls, alternative diagnosis controls, or controls from non‐endemic areas. Research has shown that this type of study overestimates accuracy measures. Healthy controls are those who have been confirmed as disease–free. Alternative diagnosis controls are controls who have symptoms similar to those of the disease under study.
If the design is not stated or is unclear, please tick the appropriate boxes. If necessary, insert comment into the box provided.
d. Was a consecutive or random sample of patients enrolled?
Yes: when the authors report random patient sampling or consecutive enrolment.
No: when patients were selected, for example, based on previous (reference or index) test results.
Unclear: there seems to be no problem, but the study authors do not explicitly state that patients were enrolled consecutively.
e. Did the study avoid inappropriate exclusions?
Yes: No patients were excluded after inclusion.
No: For example, when patients with mild disease were excluded, because they are more difficult to detect.
Unclear: not reported or insufficient information given to permit a decision.
f. Could the selection of patients have introduced bias?
High: if one or more of the questions above (7 d‐e) was answered with ‘no.’
Low: if all questions were answered with ‘yes’ (7 d‐e), or if at most one question was answered with ‘unclear.’
Unclear: for any other combination of answers (eg if two or more questions were unclear and the other(s) was/were answered with ‘yes.’
g. Is there a concern that the included patients do not match the review question?
High concern: when participants are those who do not reside in endemic areas, such as tourists, healthy controls, or controls with alternative diagnoses.
Low concern: when participants in the study are those who reside in schistosomiasis endemic areas. This group will include those at risk of infection, those who are infected but asymptomatic, or those who are infected with symptoms.
Unclear: scored when information is insufficient to permit a decision.
8. Patient flow and timing (Flow and Timing domain)
a. Was there an appropriate interval between index test(s) and reference standard?
Yes: if urine/stool samples are examined by both the reference standard and the index standard at the same time, or if the time period is less than one week.
No: if time period between index and reference standards is longer than one week.
Unclear: if no or insufficient information on time period is provided.
b. Did all patients receive a reference standard? (focus on those included in 2 × 2 table)
Yes: scored when the whole sample or a random selection of the sample or a selection of the sample with consecutive series receive verification using the reference standard.
No: scored when a part of the sample that is non‐randomly or non‐consecutively selected receives verification with the reference standard.
Unclear: scored when no or insufficient information is provided to ascertain whether the whole sample or a random selection of the sample received verification with a reference standard.
c. Did patients receive the same reference standard?
Yes: scored when study participants are tested with the same reference standard, urine/stool microscopy, regardless of index test result.
No: scored when microscopy is used with different urine concentration techniques depending on index test results for S. haematobium.
Unclear: scored when no or insufficient information is provided on the different reference standards used.
d. Were all patients included in the analysis?
Yes: scored when the patients who were included in the study were also included in the analysis.
No: scored when some patients/results are missing.
Unclear: scored when no or insufficient information is provided to permit a judgement.
e. Could the conduct or interpretation of the flow and timing have introduced bias?
High: if two or more questions above (8 a‐d) were answered with ‘no.’
Low: if all questions were answered with ‘yes’; or at least three and the other one with unclear.
Unclear: for any other combination of answers (eg all questions were unclear; three were unclear and the last one was ‘yes’).
Please state the tests under evaluation in the study.
Indicate the tests that have been evaluated for S. mansoni and/or S. haematobium in the study by ticking the appropriate boxes for the respective species. If a species was not evaluated, please tick the box ‘not applicable.’
9. Index tests (Index test domain)
a. Was quality control done?
To ensure reliability or good quality of results, a sample of slides may be cross‐checked by a second person, by an expert, or by a reference laboratory. Please indicate whether this was done in the study. If information given is unclear, please tick the box ‘unclear.’ If not reported, tick the box ‘not stated.’ If necessary, provide comment in the box provided.
b. Were the index test results interpreted without knowledge of the results of the reference standard?
Yes: when results of the index tests are interpreted without knowledge of reference test results, or when index tests are done before the reference standard.
No: when results of the index tests are interpreted with knowledge of reference test results in cases when reference tests were used before the index tests.
Unclear: when information on when the index and reference tests were interpreted is insufficient.
Not stated: when no information was reported on this item.
c. If a threshold was used, was it prespecified?
Yes: when the study authors report the use of one prespecified cutoff value. A prespecified threshold also includes statements such as, “the test was scored according to manufacturer’s instructions.”
No: when multiple cutoff values were tested and the best one chosen afterwards.
Unclear: when only one cutoff value was used, but this was not explicitly stated in the Methods section.
Not stated: when no information was reported on this item.
Could the conduct or interpretation of the index test have introduced bias?
High: if two or more questions above (9 a‐c) were answered with ‘no.’
Low: if questions (9 a‐c) were answered with ‘yes.’
Unclear: for any other combination of answers (eg both questions were unclear; one was unclear and one was ‘yes’).
10. Reference test (Reference Test domain)
The reference test for S.h that this review will evaluate is urine microscopy.
The following questions (10 A (h‐k)) are part of the QUADAS tool and will be used to assess for risk of bias in how the reference test is carried out.
A. S. haematobium
h. Was quality control done?
To ensure reliability or good quality of results, a sample of slides may be cross‐checked by a second person, by an expert, or by a reference laboratory. Please indicate whether this was done in the study. If information given is unclear, please tick the box ‘unclear.’ If not reported, tick the box ‘not stated.’ If necessary, insert comment into the box provided.
i. Is the reference standard likely to correctly classify the target condition?
Yes: if measures to increase sensitivity are used (eg concentration techniques, multiple slides examined, stool sampled over a number of days. The recommended reference std for microscopy is one carried out on 3 stools or 3 urine samples (grading as follows: 1 sample; poor; 2 samples; moderate; 3 samples; good).
No: for example, if only ill children are sampled for the reference standard, or if stool samples with blood are thrown away to avoid contaminating technicians
Unclear: scored when information on the reference standard used or sample preparation technique used was insufficient.
j. Were the reference standard results interpreted without knowledge of results of the index test?
Yes: when results of the reference tests are interpreted without knowledge of index test results in cases when reference tests are used before the index standard.
No: when results of the reference tests are interpreted with knowledge of the index test results in cases in which index tests are used before reference tests.
Unclear: when information on when the index and reference tests were interpreted is insufficient.
Not stated: when no information on this item was reported.
k. Could the conduct or interpretation of the reference standard have introduced bias?
High: if one or both questions above (a‐b) were answered with ‘no.’
Low: if both questions were answered with ‘yes.’
Unclear: if both questions were unclear; or one was unclear and one was ‘yes.’
B. S. mansoni
Tick the appropriate box for the index tests used to detect S. m in the article.
These questions for S.m should be tackled in a similar fashion to those for S. haematobium.
a. Reference standard
The reference test for S.m that this review will evaluate is microscopy of stool that is prepared by the Kato‐Katz method.
b. Was quality control done?
To ensure reliability or good quality of results, a sample of slides may be cross‐checked by a second person, by an expert, or by a reference laboratory. Please indicate whether this was done in the study. If information given is unclear, please tick the box ‘unclear.’ If not reported, tick the box ‘not stated.’ If necessary, insert comment into the box provided.
The questions 10 B (i‐l) are part of the QUADAS tool and will be used to assess for risk of bias in how the reference test is carried out. Instructions for these questions are similar to those for S. haematobium given above.
Appendix 5. Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
18.
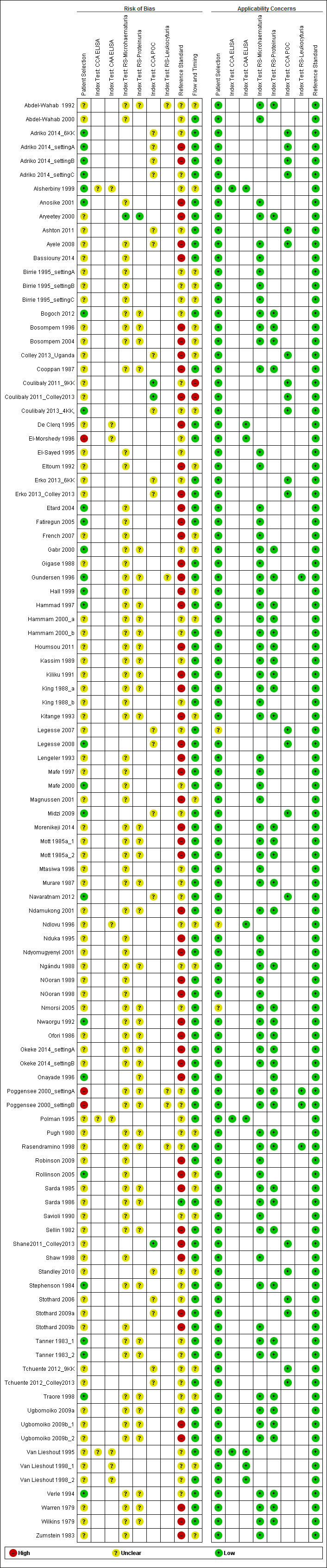
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study. The blank cells refer to information that is not applicable to the stated study.
Appendix 6. Effect of year of study on the accuracy of microhaematuria
19.
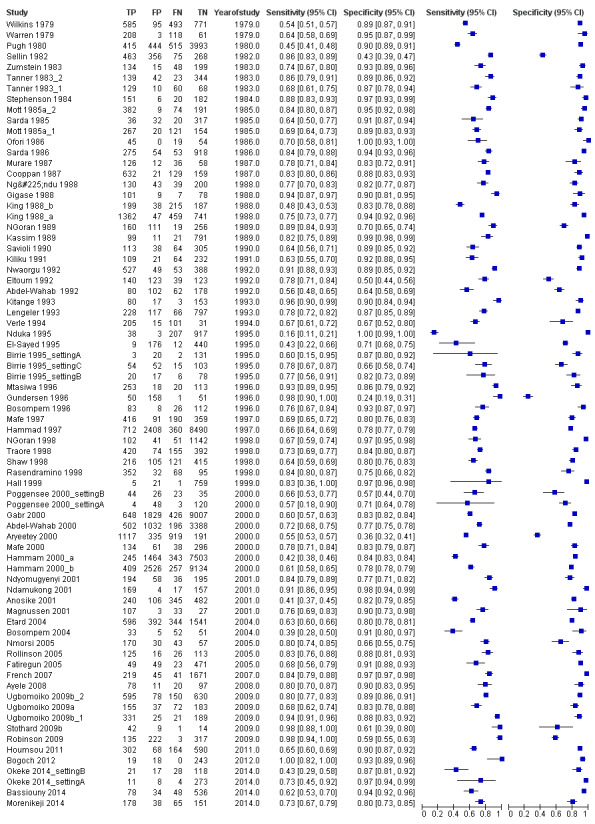
Forest plot showing effect of year of study on sensitivity and specificity of microhaematuria.
Appendix 7. Effect of test brand on accuracy of microhaematuria
20.
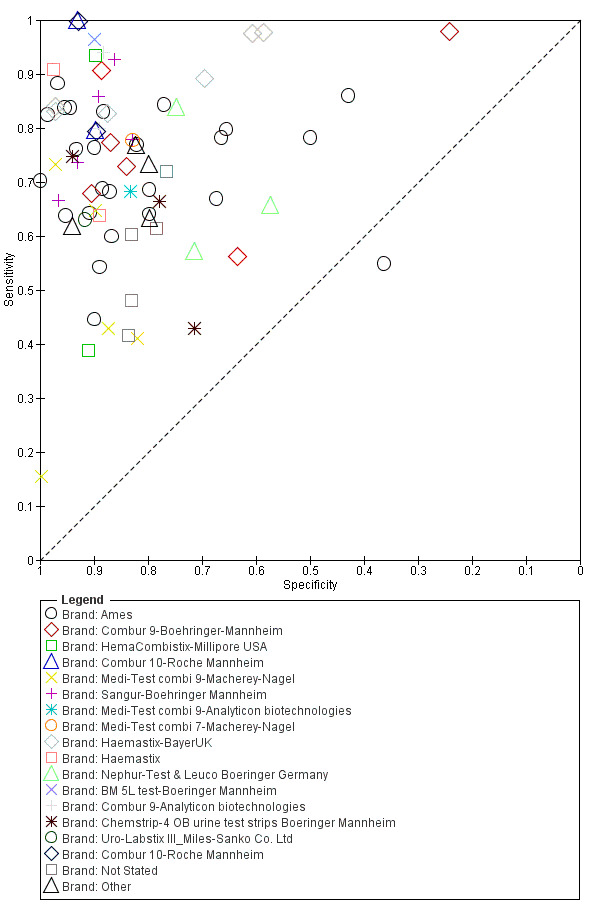
Summary ROC plot showing effect of test brand on sensitivity and specificity of microhaematuria.
Appendix 8. Effect of year of study on the accuracy of proteinuria
21.
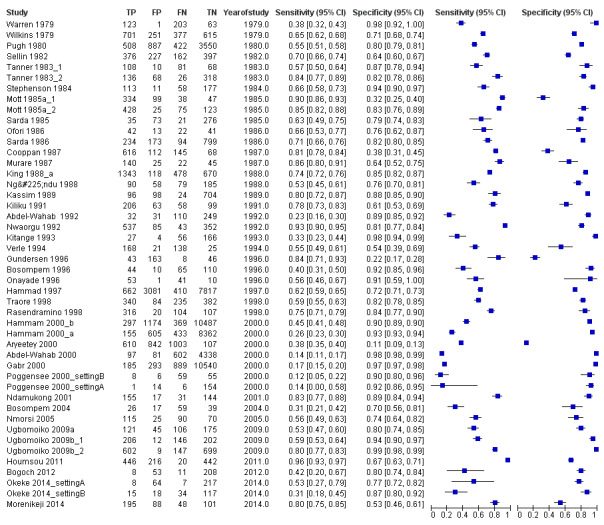
Forest plot showing effect of year of study on sensitivity and specificity of proteinuria.
Appendix 9. Effect of test brand on accuracy of proteinuria
22.
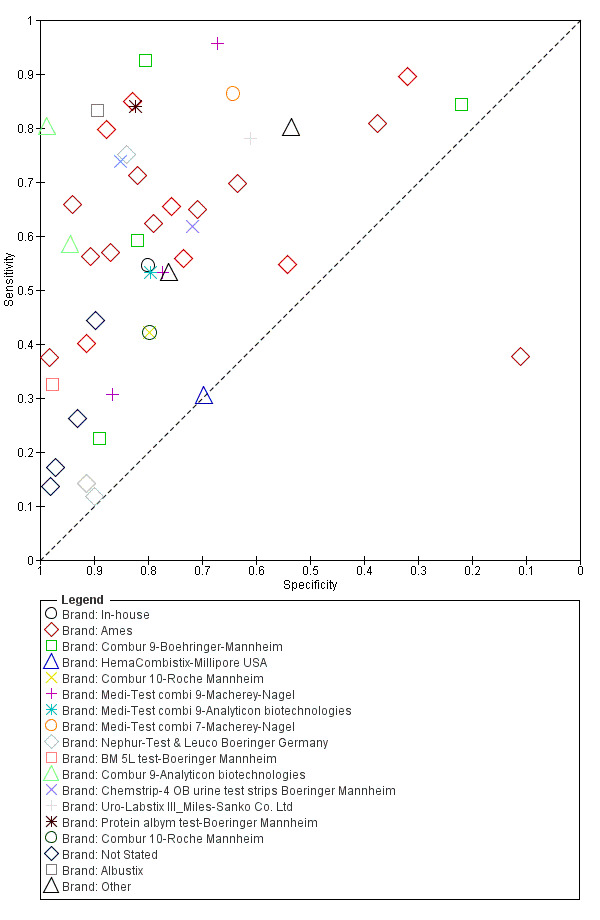
Summary ROC plot showing effect of test brand on sensitivity and specificity of proteinuria.
Appendix 10. Effect of test brand on accuracy of CCA POC S. mansoni
23.
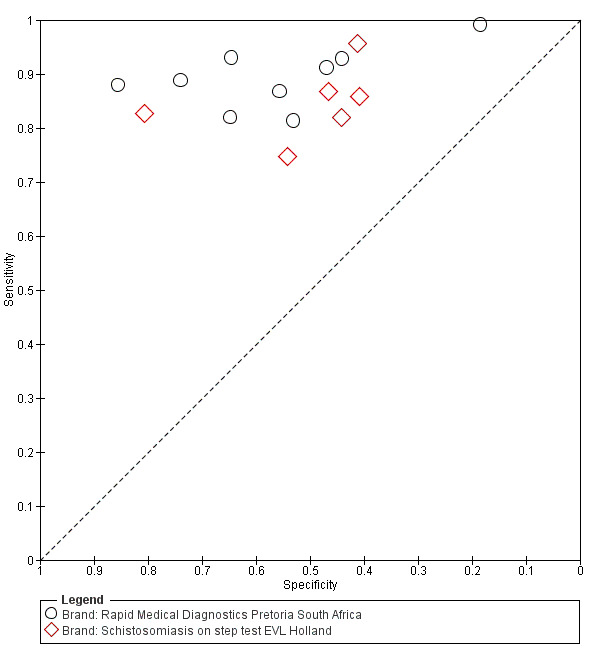
Summary ROC plot showing effect of test brand on sensitivity and specificity of CCA POC mansoni.
Appendix 11. Forest plot of sensitivity and specificity of serum CAA ELISA for S. mansoni
24.
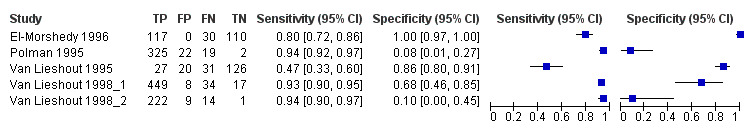
Forest plot of sensitivity and specificity of serum CAA ELISA for S. mansoni.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
Squares represent the sensitivity and specificity of one study. The black line shows its confidence interval.
Appendix 12. Summary ROC plot of sensitivity versus specificity of serum CAA ELISA for S. mansoni
25.
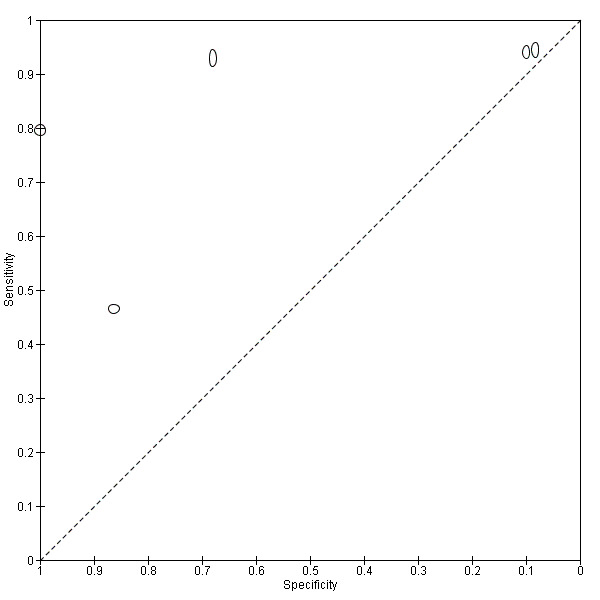
Summary ROC plot of sensitivity versus specificity of serum CAA ELISA for S. mansoni.
The size of the points is proportional to the study sample size.
The size of the points is proportional to the study sample size.
Appendix 13. Forest plot of sensitivity and specificity of serum CAA ELISA for S. haematobium
26.
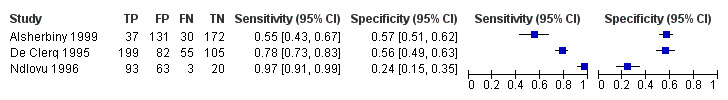
Forest plot of sensitivity and specificity of serum CAA ELISA for S. haematobium.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
Squares represent sensitivity and specificity of one study. The black line shows its confidence interval.
Appendix 14. Summary ROC plot of sensitivity versus specificity of serum CAA ELISA for S. haematobium
27.
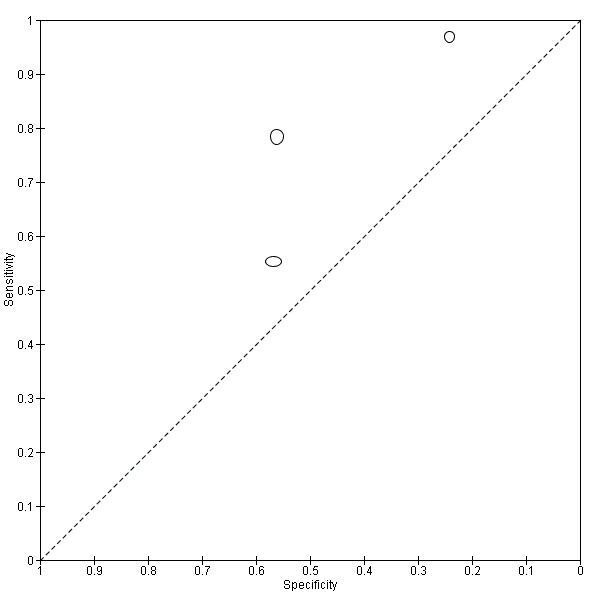
Summary ROC plot of sensitivity versus specificity of serum CAA ELISA for S. haematobium.
The size of the points is proportional to the study sample size
The size of the points is proportional to the study sample size.
Appendix 15. Forest plot of sensitivity and specificity of serum CCA ELISA for S. mansoni
28.

Forest plot of sensitivity and specificity of serum CCA ELISA for S. mansoni.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
Squares represent sensitivity and specificity of one study. The black line shows its confidence interval.
Appendix 16. Forest plot of sensitivity and specificity of urine CCA ELISA for S. mansoni
29.
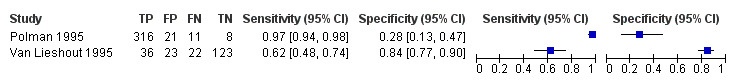
Forest plot of sensitivity and specificity of urine CCA ELISA for S. mansoni.
Squares represent the sensitivity and specificity of one study, the black line its confidence interval.
Squares represent sensitivity and specificity of one study. The black line shows its confidence interval.
Appendix 17. Comparison of KK smears and CCA POC against other reference standards (as reported by study authors)
| Study | Ref std | Index test | ||||
| KK | 1 CCA | |||||
| Sensitivity | Specificity | Sensitivity | Specificity | |||
| Coulibaly 2011_Setting C | 9KK | 1 KK | 83 (76‐88) | 100 (77‐100) | 90 (83‐94) | 85 (55‐99) |
| 2 KK | 86 (80‐91) | 100 (77‐100) | ||||
| 3 KK | 94 (89‐97) | 100 (77‐100) | ||||
| Tchuente 2012 | 9KK | 1 KK | 54 (49‐59) | 100 | 84 (81‐88) | 61 (55‐68) |
| 3 KK | 68 (64‐74) | 100 | ||||
| Erko 2013 | 6KK | 1 KK | 70 (65‐75) | 100 | 93 (90‐96) | 65 (59‐70) |
| 2 KK | 81 (77‐85) | 100 | ||||
| Lodh 2013 | PCR | 1 KK | 57 (47‐68) | 100 (69‐100) | 67 (56‐77) | 60 (26‐88) |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Microhaematuria | 74 | 102447 |
| 2 Microhaematuria after treatment | 9 | 7845 |
| 3 CCA POC mansoni trace threshold | 15 | 6091 |
| 4 Proteinuria | 46 | 82113 |
| 5 Leukocyturia | 5 | 1532 |
| 6 CCA POC mansoni +1 threshold | 5 | 1404 |
| 7 CCA POC mansoni with good reference standard | 5 | 2399 |
| 8 CCA POC haematobium | 4 | 901 |
| 10 CCA POC mixed species | 1 | 373 |
| 11 Serum CAA ELISA mansoni | 5 | 1583 |
| 12 Serum CAA ELISA haematobium | 3 | 990 |
| 13 Urine CAA ELISA mansoni | 1 | 204 |
| 14 Urine CAA ELISA haematobium | 1 | 370 |
| 15 Serum CCA ELISA mansoni | 2 | 569 |
| 16 Serum CCA ELISA haematobium | 1 | 370 |
| 17 Urine CCA ELISA mansoni | 2 | 560 |
| 19 Urine CCA ELISA haematobium | 1 | 370 |
1. Test.
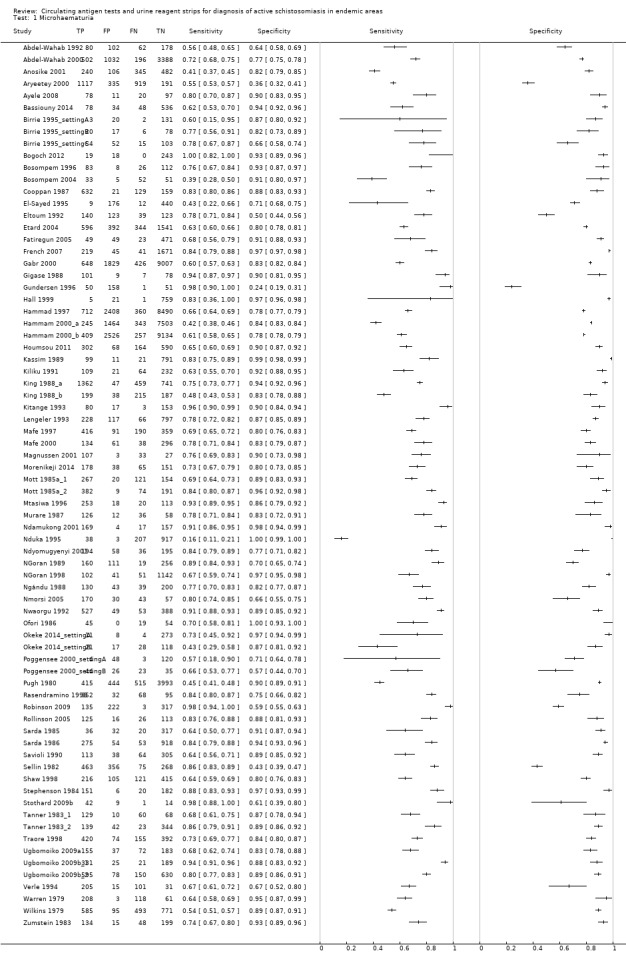
Microhaematuria.
2. Test.
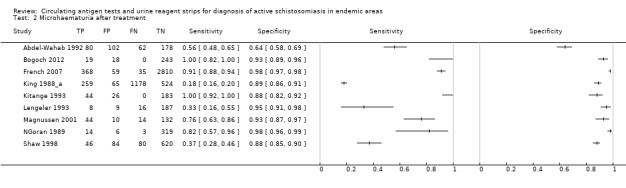
Microhaematuria after treatment.
3. Test.
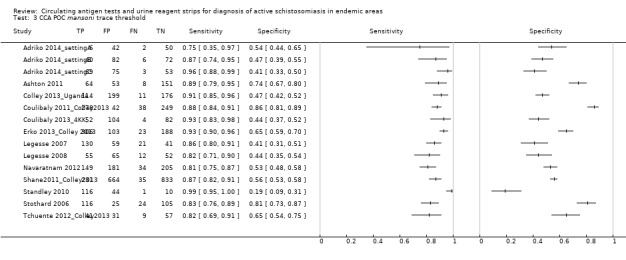
CCA POC mansoni trace threshold.
4. Test.
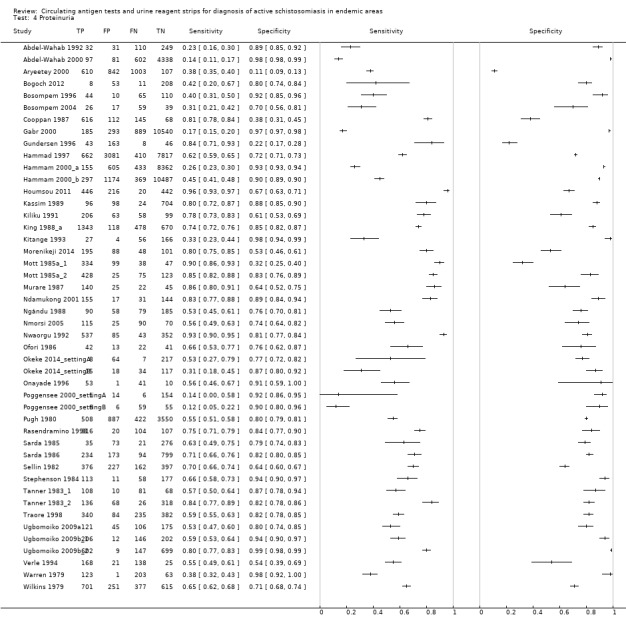
Proteinuria.
5. Test.
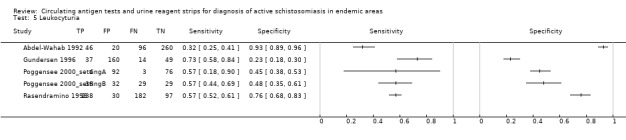
Leukocyturia.
6. Test.
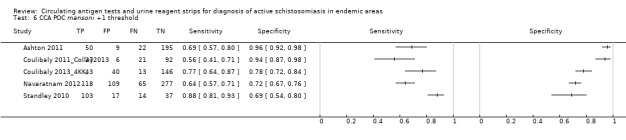
CCA POC mansoni +1 threshold.
7. Test.
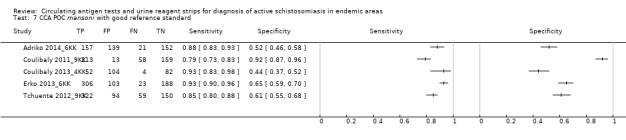
CCA POC mansoni with good reference standard.
8. Test.
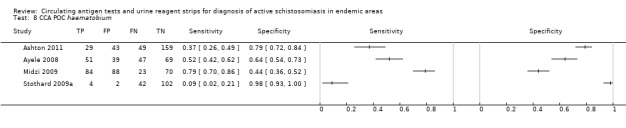
CCA POC haematobium.
10. Test.

CCA POC mixed species.
11. Test.
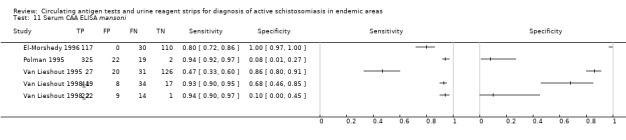
Serum CAA ELISA mansoni.
12. Test.

Serum CAA ELISA haematobium.
13. Test.

Urine CAA ELISA mansoni.
14. Test.

Urine CAA ELISA haematobium.
15. Test.
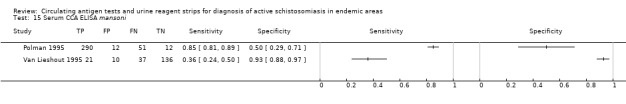
Serum CCA ELISA mansoni.
16. Test.

Serum CCA ELISA haematobium.
17. Test.

Urine CCA ELISA mansoni.
19. Test.

Urine CCA ELISA haematobium.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Wahab 1992.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 422 Age range: 12 to 16 years Participants: school children whose parents gave consent Setting: field study Praziquantel status before study: About half of the included children gave a history of receiving PZQ in past 2 years |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria, RS‐Leukocyturia (Combur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Leukocyturia | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Abdel‐Wahab 2000.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; multi‐stage stratified random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 5214 Age range: 5 to 25 years Participants: residents from villages in Fayoum Governorate Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Adriko 2014_6KK.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Uganda Sample size: 469 Age range: 7 to 13 years Participants: children from 5 schools categorized into 3 settings Setting: field study Praziquantel status before study: Annual mass treatment had been administered 5 years before study began |
||
| Index tests | CCA POC test | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (6 Kato‐Katz smears) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Adriko 2014_settingA.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Uganda Sample size: 100 Age range: 7 to 13 years Participants: children from 1 school from low endemic setting (setting A) Setting: field study Praziquantel status before study: Annual mass treatment had been administered 5 years before study began |
||
| Index tests | CCA POC test | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (2 Kato‐Katz smears from 1 stool sample) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Adriko 2014_settingB.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Uganda Sample size: 200 Age range: 7 to 13 years Participants: children from 2 schools from moderate endemic setting (setting B) Setting: field study Praziquantel status before study: Annual mass treatment had been administered 5 years before study began |
||
| Index tests | CCA POC test | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (2 Kato‐Katz smears from 1 stool sample) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Adriko 2014_settingC.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Uganda Sample size: 200 Age range: 7 to 13 years Participants: children from 2 schools from high endemic setting (setting C) Setting: field study Praziquantel status before study: Annual mass treatment had been administered 5 years before study began |
||
| Index tests | CCA POC test | ||
| Target condition and reference standard(s) | S. mansoni measured by stool microscopy (2 Kato‐Katz smears from 1 stool sample) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Alsherbiny 1999.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive enrolment | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 370 Age range: 5 to 75 years Participants: Occupants > 5 years of age living in Behbeet Village willing to provide a stool, urine, and blood sample Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CAA ELISA‐Serum and Urine; CCA ELISA‐Serum and Urine (in‐house assays) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Anosike 2001.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 1173 Age range: not reported Participants: all participating households in 7 communities Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Medi‐Test Combi‐9, Macherey Nagel, Düren, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Aryeetey 2000.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ghana Sample size: 370 Age range: > 5 years Participants: All participants aged 5 years and above from the 3 study areas Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Hema‐Combi‐Stix, Bayer Diagnostics, Sudbury, UK) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Low | |||
Ashton 2011.
| Study characteristics | |||
| Patient sampling | Nested case‐control design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium and S. mansoni Country: Ivory Coast Sample size: 370 Age range: 5 to 16 years Participants: enrolled children within a study, rapid mapping for soil‐transmitted helminthiasis Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) and S. mansoni infection by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ayele 2008.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ethiopia Sample size: 206 Age range: 4 to 21 years Participants: school children from 1 school, born and grown up in the area, and not moved since birth Setting: field Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhamaturia (Combur 10 test, Roche GmbH, Mannheim, Germany); CCA POC test (European Veterinary Laboratory (EVL), Woerden, Holland) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Bassiouny 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Yemen Sample size: 696 Age range: 10 to 16 years Participants: primary school children from fifth and sixth grades and first and second grades of preparatory education Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Urocolor 9, Standard Diagnostics Inc., Suwon City, Kyonggi Province, Korea) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (sedimentation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Birrie 1995_settingA.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ethiopia Sample size: 156 Age range: 0 to > 40 years Participants: all residents invited for checkup (low endemic area) Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Multistix Reagent Strips, Ames‐Miles, Elkhart, IN, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Birrie 1995_settingB.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ethiopia Sample size: 121 Age range: 0 to > 40 years Participants: all residents invited for checkup (moderate endemic area) Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Multistix Reagent Strips, Ames‐Miles, Elkhart, IN, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Birrie 1995_settingC.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ethiopia Sample size: 224 Age range: 0 to > 40 years Participants: all residents invited for checkup (high endemic area) Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Multistix Reagent Strips, Ames‐Miles, Elkhart, IN, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Bogoch 2012.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ghana Sample size: 280 Age range: 1 to 77 years Participants: all willing to participate in voluntary screening and treatment Setting: field study Praziquantel status before study: 2 years before study |
||
| Index tests | RS‐Microhaematuria (Combur 10 Test, Roche GmbH, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (centrifugation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Bosompem 1996.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ghana Sample size: 229 Age range: 1 to 86 years Participants: volunteers Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Ames‐Miles, Tokyo, Japan) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (centrifugation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Bosompem 2004.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ghana Sample size: 141 Age range: not reported Participants: Urine samples were collected from 90 individuals with symptoms and 51 asymptomatic individuals Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Haemacombrix Strips, Millipore Corp., Billerica, MA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Colley 2013_Uganda.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | |||
| Index tests | CCA POC cassette test (Rapid Medical Diagnostics; Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni as measured by stool microscopy (1 KK smear) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Cooppan 1987.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: South Africa Sample size: 941 Age range: 4 to 20 years Participants: school children belonging to most infected age group were examined at selected localities Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Labstix, Ames, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Coulibaly 2011_9KK.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Ivory Coast Sample size: 146 Age range: 8 to 12 years Participants: children from grades 3 to 5 attending the schools selected for participation in the study Setting: field study (low endemic area) Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | In Coulibaly 2011_9KK, the index test was measured against a higher‐quality reference standard (9 Kato‐Katz smears) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Coulibaly 2011_Colley2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Ivory Coast Sample size: 146 Age range: 8 to 12 years Participants: children from grades 3 to 5 attending the schools selected for participation in the study Setting: field study (low endemic area) Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | This article describes part of a multi‐centre study (Colley 2013). This was similar to Coulibaly 2011_9KK, but this article presented 2‐by‐2 tables of the CCA POC measured against the first daily stool specimen (triplicate KK smears on 1 stool sample) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Coulibaly 2013_4KK,
| Study characteristics | |||
| Patient sampling | Cohort design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Cote D'ivoire Sample size: 367 Age range: < 6 years Participants: all preschool children from 2 villages Setting: field study Praziquantel status before study: reported that there had been no treatment in the area |
||
| Index tests | CCAPOC cassette test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni as measured by stool microscopy (4 Kato‐Katz smears) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
De Clerq 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Mali Sample size: 441 Age range: not reported Participants: Blood and urine samples were collected from 182 and 271 people in the villages of Kassa and Boro Setting: field study Praziquantel status before study: no prior drugs |
||
| Index tests | CAA ELISA Serum (in‐house assay) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
El‐Morshedy 1996.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Egypt Sample size: 257 Age range: 20 to 25 years Participants: Cohort consisted of 257 men, treated, infected cases in a military camp Setting: military camp Praziquantel status before study: no prior drugs |
||
| Index tests | CAA ELISA Serum (in‐house assay) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
El‐Sayed 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 280 Age range: 4 to 36 years Participants: permanent settlers who agreed to participate in study Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Chemistrip, Boehringer, Indianapolis, IN, USA) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | |||
| Were all patients included in the analysis? | |||
Eltoum 1992.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Sudan Sample size: 425 Age range: 3 to 39 years Participants: asymptomatic and symptomatic participants randomly selected from population Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Ames‐Miles, Elkhart, IN, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Erko 2013_6KK.
| Study characteristics | |||
| Patient sampling | Cross sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Ethiopia Sample size: 620 Age range: 8 to 12 years Participants: children from a village in Western Kenya Setting: field study Praziquantel status before study: reported that there had been no treatment in the area |
||
| Index tests | CCA POC cassette test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni as measured by stool microscopy (3 Kato‐Katz smears on 3 stool samples (6KK)) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Erko 2013_Colley 2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Ethiopia Sample size: 620 Age range: 8 to 12 years Participants: children from a village in Western Kenya Setting: field study Praziquantel status before study: reported that there had been no treatment in the area |
||
| Index tests | CCA POC cassette test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni as measured by stool microscopy (3 Kato‐Katz smears on 1 stool sample) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | This article describes part of a multi‐centre study (Colley 2013). This was similar to Erko 2013_6KK, but in this article 2 × 2 tables of the CCA POC measured against the first daily stool specimen (triplicate KK smears on 1 stool sample) were presented | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Etard 2004.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Mali Sample size: 2873 Age range: 10 to 22 years Participants: families from 14 villages Setting: field study Praziquantel status before study: Half of the villages had received mass treatment |
||
| Index tests | RS‐Microhaematuria (Ecur test, Boehringer‐ Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured with urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Fatiregun 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 592 Age range: 11 to 20 years Participants: all students of junior classes Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Combi‐9 Multi‐Strip, Macherey Nagel, Düren, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
French 2007.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 1976 Age range: 6 to 19 years Participants: school children from 24 sentinel schools Setting: field study Praziquantel status before study: Participants were already receiving praziquantel as part of a World Health Organization (WHO) programme, but no time interval was provided |
||
| Index tests | RS‐Microhaematuria (Haemastix, Bayer, Glasgow, UK) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Gabr 2000.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 12,134 Age range: 0 to > 55years Participants: Randomization took place at village and household levels Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Combur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Gigase 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Chad Sample size: 195 Age range: 7 to 19 years Participants: children from a village Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Hema‐Combi‐Stix) (Combur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (centrifugation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Gundersen 1996.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Malawi Sample size: 260 Age range: 6 to 19 years Participants: all women of childbearing age (range 15 to 47 years) willing to provide samples, irrespective of complaints Setting: outpatient department, hospital Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Combur Test 9, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Leukocyturia | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hall 1999.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ghana Sample size: 786 Age range: 6 to 16 years Participants: school‐age children from 10 communities Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Hemastix, Bayer, Glasgow, UK) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Hammad 1997.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 11,970 Age range: not reported Participants: participants interviewed and willing to participate in study Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Chemstrip‐4 OB, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hammam 2000_a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 9555 Age range: 0 > 55 years Participants: residents from villages and households in Assiut Governorate Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Combur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Hammam 2000_b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; multi‐stage stratified cluster sample | ||
| Patient characteristics and setting | Species: S. haematobium Country: Egypt Sample size: 12,327 Age range: 0 to > 55years Participants: residents from villages and households in Qena Governorate Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Combur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Houmsou 2011.
| Study characteristics | |||
| Patient sampling | Cross‐sectional; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 1124 Age range: 3 to 27 years Participants: those interviewed and willing to participate in study Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Medi‐Test Combi 9, Macherey‐Nagel, Düren, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kassim 1989.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 922 Age range: 5 to 14 years Participants: school children from Epe and surrounding communities in SW Nigeria Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Labstix, Ames, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (centrifugation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kiliku 1991.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species:S. haematobium Country: Kenya Sample size: 426 Age range: not reported Participants: sample of all participants in Kwale District Setting: field study Praziquantel status before study: no prior drug given |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Uro‐Labstix III, Miles‐Sanko Co., Ltd., Osaka, Japan) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
King 1988_a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Kenya Sample size: 2628 Age range: 4 to 21 years Participants: students registered at 5 local primary and secondary schools Setting: field study Praziquantel status before study: before and after study; follow‐up evaluation 1 year after PZQ and metrifonate given |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Chemstrip 5 Indicator Dipsticks, Roche Diagnostics, Montreal, Quebec Canada) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
King 1988_b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Kenya Sample size: 639 Age range: 0 to 60+ years Participants: residents of a village who submitted urine samples Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Combur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kitange 1993.
| Study characteristics | |||
| Patient sampling | Cohort design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 253 Age range: not reported Participants: children in classes 1 to 7 in Melela primary school Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (BM Test 5L, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (centrifugation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Legesse 2007.
| Study characteristics | |||
| Patient sampling | Cross sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Ethiopia Sample size: 251 Age range: 5 to 75 years Participants: those > 5 years recruited through house‐to‐house visits Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Schistosomiasis One Step Test, BV European, Veterinary Laboratory, Woerden, The Netherlands) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Legesse 2008.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Ethiopia Sample size: 184 Age range: 5 to 22 years Participants: primary school children Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Schistosomiasis One Step Test, BV European, Veterinary Laboratory, Woerden, The Netherlands) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Lengeler 1993.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 1208 Age range: 11 to 15 years Participants: school children who were willing to participate and provided a urine sample Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Combur 9 Multistix, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Mafe 1997.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 1056 Age range: 5 to > 60 years Participants: individuals residing in 4 lakeside villages Setting: field study Praziquantel status before study: no prior drugs given |
||
| Index tests | RS‐Microhaematuria (Ames Chemical Reagent Strip, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Mafe 2000.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 529 Age range: mean 11 years Participants: school children in Borgo local government area Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Sangur Sticks, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Magnussen 2001.
| Study characteristics | |||
| Patient sampling | Cohort design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 170 Age range: 11 to 17 years Participants: All children in class 5 in each school in the district were selected Setting: field study Praziquantel status before study: given prior, but time interval not stated |
||
| Index tests | RS‐Microhaematuria (Haemastix, Ames Labs, Ames, IA, USA; Bayer Diagnostics, Sudbury, UK) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Midzi 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Zimbabwe Sample size: 265 Age range: 2 to 19 years Participants: preschool and primary school children Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Van Dam version) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Morenikeji 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Uganda Sample size: 432 Age range: 7 to 13 years Participants: primary school children Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Medi‐Test Combi 10, Standard Diagnostics Inc., Suwon City, Kyonggi Province, Korea) | ||
| Target condition and reference standard(s) | S. haematobium infection measured bu urine microscopy (centrifugation) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Mott 1985a_1.
| Study characteristics | |||
| Patient sampling | Cohort design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ghana Sample size: 562 Age range: 5 to 64 years Participants: those from 5 settlements interviewed and samples collected Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Neostix‐3, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Mott 1985a_2.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Zambia Sample size: 656 Age range: 0 to 64 years Participants: those in Mutenda Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Neostix‐3, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Mtasiwa 1996.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 404 Age range: 7 to 15 years Participants: Urine samples were drawn from 404 pupils, including those with frank haematuria Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Sangur Reagent Sticks, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Murare 1987.
| Study characteristics | |||
| Patient sampling | Cohort design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Zimbabwe Sample size: 232 Age range: 9 to 14 years Participants: school children from a school chosen on basis of previous studies Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Medi‐Test Combi‐7, Macherey‐Nagel, Düren, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Navaratnam 2012.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Uganda Sample size: 569 Age range: 1 to 5 years Participants: preschool children living in 4 villages in Buliisa District Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ndamukong 2001.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Cameroon Sample size: 347 Age range: 5 to 16 years Participants: primary school children attending 6 primary schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Haemastix and Albustix, Bayer, Pittsburgh, PA, USA) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ndlovu 1996.
| Study characteristics | |||
| Patient sampling | Nested case‐control design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Zimbabawe Sample size: 179 Age range: > 5 years Participants: egg‐positives and egg‐negatives, resulting in 96 cases and 83 controls from same population Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CAA ELISA Serum (in‐house assay) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Nduka 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 1165 Age range: 6 to 21 years Participants: school children from a rural town Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Medi‐Test Combi‐9, Macherey Nagel, Düren, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ndyomugyenyi 2001.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 483 Age range: 5 to 19 years Participants: children from 3 primary schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Multistix, Ames Labs, Ames, IA, USA; Bayer Diagnostics, Tarrytown, NY, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
NGoran 1989.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ivory Coast Sample size: 1059 Age range: not reported Participants: inhabitants of village of Nguessan Pokoukro, present on the day of examination Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Hemastix) (Combur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
NGoran 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ivory Coast Sample size: 1336 Age range: 12.2 +/‐ 1.6 years Participants: school children from 14 schools in town of Toumoudi Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Sangur‐Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ngándu 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Zambia Sample size: 412 Age range: 6 to 19 years Participants: school children from 9 primary schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Bili‐Labstix, Miles, Bridgend, UK) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Nmorsi 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 300 Age range: 5 to 60 years Participants: volunteers; excluded were patients with allergy and skin infections Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Haemastix, Ames Laboratories, Ames, IA, USA), RS‐Proteinuria (Albustix, Ames Laboratories) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (centrifugation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Nwaorgu 1992.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 437 Age range: 0 to 35+ years Participants: permanent settlers who agreed to participate in study Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (L‐Combur, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ofori 1986.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Ghana Sample size: 118 Age range: not reported Participants: urine specimens collected from 118 pupils Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (N‐Multistix SG, Ames, Glasgow, England) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Okeke 2014_settingA.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 296 Age range: 5 to 13 years Participants: primary school children from Niger Lake, a low endemic setting (setting A) Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Medi‐Test Combi‐9, Macherey‐Nagel, Düren, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (sedimentation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Okeke 2014_settingB.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 184 Age range: 5 to 13 years Participants: primary school children from Nigercem, a moderate endemic setting (setting B) Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Medi‐Test Combi‐9, Macherey‐Nagel, Düren, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (sedimentation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Onayade 1996.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 105 Age range: 8 to 16 years Participants: all grade 4 to 6 pupils with minimum age of 4 Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Proteinuria (N‐Multistix, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (sedimentation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Poggensee 2000_settingA.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; non–probability‐based sampling procedure | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 175 Age range: 15 to 60 years Participants: women of childbearing age Setting: field study (low endemic setting) Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria, RS‐Leukocyturia (Nephur‐Test + Leuco, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Leukocyturia | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Poggensee 2000_settingB.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; non–probability‐based sampling procedure | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 128 Age range: 15 to 60 years Participants: women of childbearing age Setting: field study (high endemic setting) Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria, RS‐Leukocyturia (Nephur‐Test + Leuco, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Leukocyturia | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Polman 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Senegal Sample size: 422 Age range: 0 to 77 years Participants: 10% of the households (all members) from an updated census list Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CAA ELISA Serum; CCA ELISA Serum and Urine (in‐house) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Low | |||
Pugh 1980.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 5367 Age range: 5 to > 36 years Participants: males 5 to 25 years of age from 3 villages and all participants over 4 years from 2 study areas Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria; RS‐Proteinuria (Labstix, Ames Labs, Berlin, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Rasendramino 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Madagascar Sample size: 574 Age range: > 5 years Participants: all inhabitants of a village > 5 years Setting: field study Praziquantel status before study: Study reports that no praziquantel was administered before the study |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria, RS‐Leukocyturia (Nephur 7 test, Roche Diagnostics, Montreal, Quebec, Canada) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Leukocyturia | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Robinson 2009.
| Study characteristics | |||
| Patient sampling | Nested case‐control design; quasi‐random 2‐stage cluster sampling method | ||
| Patient characteristics and setting | Species: S. haematobium Country: Sudan Sample size: 677 Age range: 5 to 16 years Participants: In each selected household, children were asked to provide a urine sample Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Hemastix Bayer Diagnostics, Bridgend, UK) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Rollinson 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 280 Age range: 10 to 22 years Participants: children from 2 schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Hemastix, Bayer, Pittsburgh, PA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Sarda 1985.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 2418 Age range: 7 to 19 years Participants: children from 12 schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (N‐Multistix, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy(filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Sarda 1986.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Kenya Sample size: 1300 Age range: 6 to 19 years Participants: school children from various schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (N‐Multistix Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was quality control done? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Savioli 1990.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 879 Age range: 5 to 19 years Participants: children in a village Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Hemastix, Ames‐Miles Laboratories, Elkhart, IN, USA) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Sellin 1982.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Burkina Faso Sample size: 1162 Age range: not reported Participants: people from a high endemic village in Upper Volta Setting: field study Praziquantel status before study: treatment given after baseline study and follow‐up accuracy study done 1 year later |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Laboratoires Ames, Paris, France) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Shane2011_Colley2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Kenya Sample size: 1845 (updated from Colley 2013) Age range: 1 to 15 years Participants: children from a village in Western Kenya Setting: field study Praziquantel status before study: reported that there had been no treatment in the area |
||
| Index tests | CCA POC cassette (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz smears) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | This article was part of a multi‐centre study (Colley 2013). In this article, 2‐by‐2 tables of the CCA POC measured against the first daily stool specimen (duplicate KK smears on 1 stool sample) were presented | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was quality control done? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Shaw 1998.
| Study characteristics | |||
| Patient sampling | Cohort design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Senegal Sample size: 857 Age range: 4 to > 40 Participants: individuals in households invited to participate Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Ames Labs, Ames, IA, USA; Bayer Diagnostics, Gent, Belgium) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Standley 2010.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Eastern Lake Victoria (Tanzania and Kenya) Sample size: 171 Age range: 6 to 17 years Participants: school children selected in 11 schools by headmaster Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Stephenson 1984.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Kenya Sample size: 359 Age range: 6 to 16 years Participants: Children from 2 primary schools not previously tested were examined Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Ames N‐Multistix, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Stothard 2006.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Uganda Sample size: 270 Age range: 11 years Participants: children from 9 sentinel schools of matched sexes Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Schistosomiasis One Step Test, EVL, Woerden, Holland) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Stothard 2009a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 150 Age range: 8 to 14 years Participants: children from 5 schools Setting: field study Praziquantel status before study: annual MDA 11 months before the study |
||
| Index tests | CCA POC test (Leiden University Medical Centre, Leiden, The Netherlands) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Stothard 2009b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 66 Age range: 9 to 15 years Participants: school children Setting: field study Praziquantel status before study: Likely, children enrolled were already part of a 'kick out schistosomiasis' campaign |
||
| Index tests | RS‐Microhaematuria (Hemastix, Bayer, Sudbury, UK) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Tanner 1983_1.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Liberia Sample size: 267 Age range: 0 to 15 years Participants: school children from 3 villages Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Labstix, Ames, Glasgow, England) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Tanner 1983_2.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; random sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 548 Age range: 0 to 15 years Participants: children from 1 village and river plain Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Blood Sangur Test, Boehringer, Mannheim FRG), RS‐Proteinuria (Protein Albym Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Tchuente 2012_9KK.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Cameroon Sample size: 138 Age range: 7 to 15 years Participants: children who provided all 3 samples Setting: field study (low endemicity) Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Tchuente 2012_Colley2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Cameroon Sample size: 138 Age range: 7 to 15 years Participants: children who provided all 3 samples Setting: field study (low endemicity) Praziquantel status before study: not reported |
||
| Index tests | CCA POC test (Rapid Medical Diagnostics, Pretoria, South Africa) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | This article describes part of a multi‐centre study (Colley 2013), which was similar to Tchuente 2012_9KK, but in this article, 2‐by‐2 tables of the CCA POC measured against the first daily stool specimen (triplicate KK smears on 1 stool sample) were presented | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA POC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Traore 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Mali Sample size: 1041 Age range: 2 to 25+ years Participants: all inhabitants in a village older than 2 years Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Combur‐9, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured with urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Ugbomoiko 2009a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 447 Age range: 3 to 17 years Participants: all school children except girls who had menstruated within 5 days of sample collection Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Medi‐Test Combi‐9, Analyticon Biotechnologies, Rosbach vor der Höhe, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ugbomoiko 2009b_1.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 566 Age range: > 1 year Participants: consenting individuals at household level in 5 communities Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (5L test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (sedimentation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ugbomoiko 2009b_2.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Nigeria Sample size: 1457 Age range: > 1 year Participants: consenting participants at central locations in 5 communities Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Combur‐9 test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (sedimentation method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Van Lieshout 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Surinam Sample size: 389 Age range: 1 to 85 years Participants: all inhabitants of a village except those younger than 1 year of age Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CAA and CCA ELISA_Serum (in‐house assays) | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CCA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Van Lieshout 1998_1.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Zaire Sample size: 508 Age range: 1 to 66 years Participants: data set populations living in Maniema—area with intense transmission Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CAA ELISA Serum test | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Van Lieshout 1998_2.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. mansoni Country: Senegal Sample size: 246 Age range: 1 to 77 years Participants: data set of populations living in Ndombo—area with intense transmission Setting: field study Praziquantel status before study: not reported |
||
| Index tests | CAA ELISA Serum test | ||
| Target condition and reference standard(s) | S. mansoni infection measured by stool microscopy (Kato‐Katz) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test CAA ELISA | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Verle 1994.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; consecutive sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Senegal Sample size: 352 Age range: 0 to > 50 years Participants: registered village inhabitants invited to participate Setting: field study Praziquantel status before study: not given previously |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Multistix, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium infection measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Warren 1979.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Kenya Sample size: 390 Age range: 5 to 18 years Participants: school children from 2 schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria, RS‐Proteinuria (Bili‐Lab‐Stix, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wilkins 1979.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Gambia Sample size: 1944 Age range: ≥ 2 years Participants: study based on specimens collected from earlier study Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Lab‐Stix, Ames Labs, Ames, IA, USA) | ||
| Target condition and reference standard(s) | S. haematobium measured by urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Proteinuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Zumstein 1983.
| Study characteristics | |||
| Patient sampling | Cross‐sectional design; unclear sampling | ||
| Patient characteristics and setting | Species: S. haematobium Country: Tanzania Sample size: 3478 Age range: 6 to 19 years Participants: school children form 15 schools Setting: field study Praziquantel status before study: not reported |
||
| Index tests | RS‐Microhaematuria (Sangur Test, Boehringer, Mannheim, Germany) | ||
| Target condition and reference standard(s) | S. haematobium measured with urine microscopy (filtration method) | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test RS‐Microhaematuria | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was quality control done? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was quality control done? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Deelder 1981 | Not a test accuracy study |
| Feldmeier 1982 | Case‐control study with healthy controls |
| Kassim 1983 | Case‐control study with healthy controls |
| Mott 1983 | Accuracy study carried out with similar tests and populations as another included paper |
| Doehring 1985 | Not a test accuracy study |
| Mott 1985 | Accuracy study carried out with similar tests and populations as another included paper |
| Feldmeier 1986 | Case series with healthy individuals from “same endemic area” |
| Madwar 1988 | Not a test accuracy study |
| de Jonge 1988 | Case‐control study with healthy controls |
| de Jonge 1989_a | Only proven cases included in study |
| Deelder 1989 | Not a test accuracy study |
| Savioli 1989 | Not a test accuracy study |
| de Jonge 1989_b | Only proven cases included in study |
| de Jonge 1990_1 | Case‐control study with controls from non‐endemic areas |
| de Jonge 1990_2 | Cannot extract 2‐by‐2 tables |
| Taylor 1990 | Cannot extract 2‐by‐2 tables |
| Lengeler 1991 | Cannot extract 2‐by‐2 tables |
| Eltoum 1992_b | Accuracy study carried out with similar tests and populations as another included paper |
| van Lieshout 1992 | Case‐control study with controls from non‐endemic areas |
| Hassan 1992 | Ineligible index test |
| Kaiser 1992 | Ineligible reference standard |
| Gundersen 1992 | Case‐control study with healthy controls |
| Krijger 1994 | Case‐control study with healthy controls |
| Kremsner 1994 | Cannot extract 2‐by‐2 tables |
| van Etten 1994 | Case‐control study with healthy controls |
| Hassan 1994 | Cannot extract 2‐by‐2 tables |
| Fillie 1994 | Case‐control study with healthy controls |
| Jemaneh 1994 | Cannot extract 2‐by‐2 tables |
| van Lieshout 1995 | Case‐control study with controls from non‐endemic areas |
| Hakangard 1996 | Case‐control study with controls from non‐endemic areas |
| van Etten 1997 | Ineligible reference standard |
| Lwambo 1997 | Cannot extract 2‐by‐2 tables |
| de Clerq 1997 | Cannot extract 2‐by‐2 tables |
| Tiemersma 1997 | Cannot extract 2‐by‐2 tables |
| Disch 1997 | Only proven cases included in study |
| Polman 1998 | Not a test accuracy study |
| Kahama 1998 | Cannot extract 2‐by‐2 tables |
| Nibbeling 1998 | Ineligible index test |
| Poggensee 1998 | Cannot extract 2‐by‐2 tables |
| Pereira 1999 | Case‐control study with controls from non‐endemic areas |
| Kahama 1999 | Not a test accuracy study |
| Hassan 1999 | Only proven cases included in study |
| Polman 2000 | Case‐control study with healthy controls |
| van Dam 2004 | Case‐control study with controls from non‐endemic areas |
| Brouwer 2004 | Cannot extract 2‐by‐2 tables |
| Takougang 2004 | Cannot extract 2‐by‐2 tables |
| Obeng 2008 | Case‐control study with controls from non‐endemic areas |
| Leutscher 2008 | Case‐control study with healthy controls |
| Koukounari 2009 | Ineligible reference standard |
| Stothard 2011 | Ineligible reference standard |
| Verani 2011 | Cannot extract 2‐by‐2 tables |
| Kosinski 2011 | Cannot extract 2‐by‐2 tables |
| Coulibaly 2012 | Not a test accuracy study |
| Adesola 2012 | Cannot extract 2‐by‐2 tables |
| Eyo 2012 | Not a test accuracy study |
| Coulibaly 2013_2 | Not a test accuracy study |
| Lodh 2013 | Ineligible reference standard |
| Grenfell 2013 | Not a test accuracy study |
| Coulibaly 2013_3 | Ineligible index test |
| Sousa‐Figueiredo 2013 | Ineligible reference standard |
| Degarege 2014 | Not a test accuracy study |
| Melchers 2014 | Ineligible index test |
Differences between protocol and review
Title of the review: To make the title of the review more specific to the tests that we evaluated, we have changed the title from "Rapid diagnostic tests for human schistosomiasis in endemic areas" to "Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas."
We used QUADAS‐2 to assess the methodological quality of studies included in the review. In the protocol, we stated that we would use the original QUADAS tool to assess quality and planned to perform a sensitivity analysis of the individual quality (QUADAS) items 4, 7, 8, 10, and 11, to explore whether the results that we found are robust for methodological challenges. Items 10 and 11 are not included in QUADAS‐2. We instead assessed whether reference tests could classify the target condition as a co‐variate.
In the protocol, we stated that we would analyze the intensity of infection as numerical co‐variates. Because of poor reporting, we converted the data into categorical co‐variates, including intensity of infection (light, moderate, heavy, unclear).
In the protocol, we also stated that we would estimate the sensitivity of urine reagent strips and urine CCA POC at positivity thresholds of +1 and ≥ +1. Instead we estimated the accuracy at thresholds > trace and > +1, as these data were most commonly provided.
As part of the post hoc analyses, we noted that three evaluations had substantial heterogeneity for the tests microhaematuria (Aryeetey 2000; sensitivity 55%, specificity 36%), proteinuria (Aryeetey 2000; sensitivity 38%, specificity 11%), and CCA POC for S. mansoni (Standley 2010; sensitivity 99%, specificity 19%). We excluded these evaluations in sensitivity analyses for the respective tests, as shown in the Results section.
Contributions of authors
Writing of first draft of review: Eleanor Ochodo.
Methodological advice: Mariska Leeflang, Johannes Reitsma, Patrick Bossuyt.
Content advice: Lisette Van Lieshout, Katja Polman, Poppy Lamberton.
Data collection: Eleanor Ochodo, Gowri Gopalakrishna, Bea Spek, Mariska Leeflang, Lisette Van Lieshout, Katja Polman, Poppy Lamberton.
Data analysis: Eleanor Ochodo, Mariska Leeflang, Johannes Reitsma.
Contributions to manuscript drafts: Eleanor Ochodo, Mariska Leeflang, Johannes Reitsma, Patrick Bossuyt, Lisette Van Lieshout, Katja Polman, Poppy Lamberton, Gowri Gopalakrishna, Bea Spek.
Agreement with final draft of review: Eleanor Ochodo, Gowri Gopalakrishna, Bea Spek, Poppy Lamberton, Lisette Van Lieshout, Katja Polman, Johannes Reitsma, Patrick Bossuyt, Mariska Leeflang.
Sources of support
Internal sources
-
Academic Medical Centre Medical Research; University of Amsterdam, Netherlands.
Funding PhD project of EAO
-
Dutch Cochrane Centre, Netherlands.
Technical support
External sources
No sources of support supplied
Declarations of interest
The review authors have reported no conflicts of interest.
Unchanged, comment added to review
References
References to studies included in this review
Abdel‐Wahab 1992 {published data only}
- Abdel‐Wahab MF, Esmat G, Ramzy I, Fouad R, Abdel‐Rahman M, Yosery A, et al. [Schistosoma haematobium infection in Egyptian schoolchildren: demonstration of both hepatic and urinary tract morbidity by ultrasonography]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1992;86:406‐9. [DOI] [PubMed] [Google Scholar]
Abdel‐Wahab 2000 {published data only}
- Abdel‐Wahab MF, Esmat G, Ramzy I, Narooz S, Medhat E, Ibrahim M, et al. [The epidemiology of schistosomiasis in Egypt: Fayoum Governorate]. American Journal of Tropical Medicine and Hygiene 2000;62(2):55‐64. [DOI] [PubMed] [Google Scholar]
Adriko 2014_6KK {published data only}
- Adriko M, Standley CJ, Tinkitina B, Tukahebwa EM, Fenwick A, Fleming FM, et al. [Evaluation of circulating cathodic antigen (CCA) urine‐cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda]. Acta Tropica 2014;136:50‐7. [DOI] [PubMed] [Google Scholar]
Adriko 2014_settingA {published data only}
- Adriko M, Standley CJ, Tinkitina EM, Fenwick A, Fleming FM, Sousa‐Figueiredo JC, et al. [Evaluation of circulating cathodic antigen (CCA) urine‐cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda]. Acta Tropica 2014;136:50‐7. [DOI] [PubMed] [Google Scholar]
Adriko 2014_settingB {published data only}
- Adriko M, Standley CJ, Tinkitina EM, Fenwick A, Fleming FM, Sousa‐Figueiredo JC, et al. [Evaluation of circulating cathodic antigen (CCA) urine‐cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda]. Acta Tropica 2014;136:50‐7. [DOI] [PubMed] [Google Scholar]
Adriko 2014_settingC {published data only}
- Adriko M, Standley CJ, Tinkitina EM, Fenwick A, Fleming FM, Sousa‐Figueiredo JC, et al. [Evaluation of circulating cathodic antigen (CCA) urine‐cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda]. Acta Tropica 2014;136:50‐7. [DOI] [PubMed] [Google Scholar]
Alsherbiny 1999 {published data only}
- Al‐Sherbiny MM, Osman AM, Hancock K, Deelder AM, Tsang VC. [Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings]. American Journal of Tropical Medicine and Hygiene 1999;60(6):960‐6. [DOI] [PubMed] [Google Scholar]
Anosike 2001 {published data only}
- Anosike JC, Nwoke BEB, Njoku AJ. [The validity of haematuria in the community diagnosis of urinary schistosomiasis infections]. Journal of Helminthology 2001;75(3):223‐5. [PubMed] [Google Scholar]
Aryeetey 2000 {published data only}
- Aryeetey ME, Wagatsuma Y, Yeboah G, Asante M, Mensah G, Nkrumah FK, et al. [Urinary schistosomiasis in southern Ghana: 1. Prevalence and morbidity assessment in three (defined) rural areas drained by the Densu river]. Parasitology International 2000;49(2):155‐63. [DOI] [PubMed] [Google Scholar]
Ashton 2011 {published data only}
- Ashton RA, Stewart BT, Petty N, Lado M, Finn T, Brooker S, et al. [Accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in Southern Sudan]. Tropical Medicine and International Health 2011;16(9):1099‐103. [DOI] [PubMed] [Google Scholar]
Ayele 2008 {published data only}
- Ayele B, Erko B, Legesse M, Hailu A, Medhin G. [Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar, Ethiopia]. Parasite 2008;15(1):69‐75. [DOI] [PubMed] [Google Scholar]
Bassiouny 2014 {published data only}
- Bassiouny HK, Hasab AA, El‐Nimr NA, Al‐Shibani LA, Al‐Waleedi AA. Rapid diagnosis of schistosomiasis in Yemen using a simple questionnaire and urine reagent strips [Diagnostic rapide de la schistosomiase au Yemen a l'aide d'un questionnaire simple et de bandelettes urinaires reactives]. Eastern Mediterranean Health Journal 2014;20(4):242‐9. [PubMed] [Google Scholar]
Birrie 1995_settingA {published data only}
- Birrie H, Medhin G, Jemaneh L. [Comparison of urine filtration and a chemical reagent strip in the diagnosis of urinary schistosomiasis in Ethiopia]. East African Medical Journal 1995;72(3):180‐5. [PubMed] [Google Scholar]
Birrie 1995_settingB {published data only}
- Birrie H, Medhin G, Jemaneh L. [Comparison of urine filtration and a chemical reagent strip in the diagnosis of urinary schistosomiasis in Ethiopia]. East African Medical Journal 1995;72(3):180‐5. [PubMed] [Google Scholar]
Birrie 1995_settingC {published data only}
- Birrie H, Medhin G, Jemaneh L. [Comparison of urine filtration and a chemical reagent strip in the diagnosis of urinary schistosomiasis in Ethiopia]. East African Medical Journal 1995;72(3):180‐5. [PubMed] [Google Scholar]
Bogoch 2012 {published data only}
- Bogoch II, Andrews JR, Dadzie Ephraim RK, Utzinger J. [Simple questionnaire and urine reagent strips compared to microscopy for the diagnosis of Schistosoma haematobium in a community in northern Ghana]. Tropical Medicine and International Health 2012;17(10):1217‐21. [DOI] [PubMed] [Google Scholar]
Bosompem 1996 {published data only}
- Bosompem KM, Ayi I, Anyan WK, Nkrumah FK, Kojima S. [Limited field evaluation of a rapid monoclonal antibody‐based dipstick assay for urinary schistosomiasis]. Hybridoma 1996;15(6):443‐7. [DOI] [PubMed] [Google Scholar]
Bosompem 2004 {published data only}
- Bosompem KM, Owusu O, Okanla EO, Kojima S. [Applicability of a monoclonal antibody‐based dipstick in diagnosis of urinary schistosomiasis in the Central Region of Ghana]. Tropical Medicine and International Health 2004;9(9):991‐6. [DOI] [PubMed] [Google Scholar]
Colley 2013_Uganda {published data only}
- Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuente LA, N'goran EK, et al. [A five‐country evaluation of a point‐of‐care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni]. American Journal of Tropical Medicine and Hygiene 2013;88(3):426‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooppan 1987 {published data only}
- Cooppan RM, Schutte CH, Dingle CE, Deventer JM, Becker PJ. [Urinalysis reagent strips in the screening of children for urinary schistosomiasis in the RSA]. South African Medical Journal Suid‐Afrikaanse Tydskrif Vir Geneeskunde 1987;72(7):459‐62. [PubMed] [Google Scholar]
Coulibaly 2011_9KK {published data only}
- Coulibaly JT, Knopp S, N'Guessan NA, Silue KD, Furst T, Lohourignon LK, et al. [Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Cote d'Ivoire]. PLoS Neglected Tropical Diseases 2011;5(11):e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulibaly 2011_Colley2013 {published data only}
- Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuente LA, N'goran EK, et al. [A five‐country evaluation of a point‐of‐care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni]. American Journal of Tropical Medicine and Hygiene 2013;88(3):426‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulibaly 2013_4KK, {published data only}
- Coulibaly JT, N'Gbesso YK, Knopp S, N'Guessan NA, Silue KD, Dam GJ, et al. [Accuracy of urine circulating cathodic antigen test for the diagnosis of Schistosoma mansoni in preschool‐aged children before and after treatment]. PLoS Neglected Tropical Diseases 2013;7(3):e2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Clerq 1995 {published data only}
- De CD, Sacko M, Vercruysse J, Diarra A, Landoure A, vanden BV, et al. [Comparison of the circulating anodic antigen detection assay and urine filtration to diagnose Schistosoma haematobium infections in Mali]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(4):395‐7. [DOI] [PubMed] [Google Scholar]
El‐Morshedy 1996 {published data only}
- El‐Morshedy H, Kinosien B, Barakat R, Omer E, Khamis N, Deelder AM, et al. [Circulating anodic antigen for detection of Schistosoma mansoni infection in Egyptian patients]. American Journal of Tropical Medicine and Hygiene 1996;54(2):149‐53. [DOI] [PubMed] [Google Scholar]
El‐Sayed 1995 {published data only}
- El‐Sayed HF, Rizkalla NH, Mehanna S, Abaza SM, Winch PJ. [Prevalence and epidemiology of Schistosoma mansoni and S. haematobium infection in two areas of Egypt recently reclaimed from the desert]. American Journal of Tropical Medicine and Hygiene 1995;52(2):194‐8. [DOI] [PubMed] [Google Scholar]
Eltoum 1992 {published data only}
- Eltoum IA, Sulaiman S, Ismail BM, Ali MM, Elfatih M, Homeida MM. [Evaluation of haematuria as an indirect screening test for schistosomiasis haematobium: a population‐based study in the White Nile province, Sudan]. Acta Tropica 1992;51(2):151‐7. [DOI] [PubMed] [Google Scholar]
Erko 2013_6KK {published data only}
- Erko B, Medhin G, Teklehaymanot T, Degarege A, Legesse M. [Evaluation of urine‐circulating cathodic antigen (Urine‐CCA) cassette test for the detection ofSchistosoma mansoni infection in areas of moderate prevalence in Ethiopia]. Tropical Medicine and International Health 2013;18(8):1029‐35. [DOI] [PubMed] [Google Scholar]
Erko 2013_Colley 2013 {published data only}
- Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuente LA, N'goran EK, et al. [A five‐country evaluation of a point‐of‐care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni]. American Journal of Tropical Medicine and Hygiene 2013;88(3):426‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etard 2004 {published data only}
- Etard JE. [Modelling sensitivity, specificity and predictive values of hematuria testing using reagent sticks in the diagnosis of Schistosoma haematobium infection]. Bulletin de la Societe de Pathologie Exotique 204;97(1):24‐8. [PubMed] [Google Scholar]
Fatiregun 2005 {published data only}
- Fatiregun AA, Osungbade KO, Olumide EA. [Diagnostic performance of screening methods for urinary schistosomiasis in a school‐based control programme, in Ibadan, Nigeria]. Journal of Community Medicine and Primary Health Care 2005;17(1):24‐7. [DOI] [PubMed] [Google Scholar]
French 2007 {published data only}
- French MD, Rollinson D, Basanez M‐G, Mgeni AF, Khamis IS, Stothard JR. [School‐based control of urinary schistosomiasis on Zanzibar, Tanzania: monitoring micro‐haematuria with reagent strips as a rapid urological assessment]. Journal of Pediatric Urology 2007;3(5):364‐8. [DOI] [PubMed] [Google Scholar]
Gabr 2000 {published data only}
- Gabr NS, Hammad TA, Orieby A, Shawky E, Khattab MA, Strickland GT. [The epidemiology of schistosomiasis in Egypt: Minya Governorate]. American Journal of Tropical Medicine and Hygiene 2000;62(2):65‐72. [DOI] [PubMed] [Google Scholar]
Gigase 1988 {published data only}
- Gigase PL, Mangelschots E, Bockaert R, Autier Ph, Kestens L. [Indicateurs simples de la prevalence et de líntesite de la bilharziose urinaire au tchad]. Annales de la Societe Belge de Medecine Tropicale 1988;68:123‐32. [PubMed] [Google Scholar]
Gundersen 1996 {published data only}
- Gundersen SG, Kjetland EF, Poggensee G, Helling‐Giese G, Richter J, Chitsulo L, et al. [Urine reagent strips for diagnosis of Schistosomiasis haematobium in women of fertile age]. Acta Tropica 1996;62(4):281‐7. [DOI] [PubMed] [Google Scholar]
Hall 1999 {published data only}
- Hall A, Fentiman A. [Blood in the urine of adolescent girls in an area of Ghana with a low prevalence of infection with Schistosoma haematobium]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(4):411‐2. [DOI] [PubMed] [Google Scholar]
Hammad 1997 {published data only}
- Hammad TA, Gabr NS, Talaat MM, Orieby A, Shawky E, Strickland GT. [Hematuria and proteinuria as predictors of Schistosoma haematobium infection]. American Journal of Tropical Medicine and Hygiene 1997;57(3):363‐7. [DOI] [PubMed] [Google Scholar]
Hammam 2000_a {published data only}
- Hammam HM, Allam FAM, Moftah FM, Abdel‐Aty MA, Hany AH, Abd‐El‐Motagaly, et al. [The epidemiology of schistosomiasis in Egypt: Assiut Governorate]. American Journal of Tropical Medicine and Hygiene 2000;62(2):73‐9. [DOI] [PubMed] [Google Scholar]
Hammam 2000_b {published data only}
- Hammam HM, Zarzour AH, Moftah FM, Abdel‐Aty MA, Hany AH, El‐Kady AY, et al. [The epidemiology of schistosomiasis in Egypt: Qena Governorate]. American Journal of Tropical Medicine and Hygiene 2000;62(2):80‐7. [DOI] [PubMed] [Google Scholar]
Houmsou 2011 {published data only}
- Houmsou RS, Kela SL, Suleiman MM. [Performance of microhaematuria and proteinuria as measured by urine reagent strips in estimating intensity and prevalence of Schistosoma haematobium infection in Nigeria.]. Asian Pacific Journal of Tropical Medicine 2011;4(12):997‐1000. [DOI] [PubMed] [Google Scholar]
Kassim 1989 {published data only}
- Kassim OO. [Proteinuria and haematuria as predictors of schistosomiasis in children]. Annals of Tropical Paediatrics 1989;9(3):156‐60. [DOI] [PubMed] [Google Scholar]
Kiliku 1991 {published data only}
- Kiliku FM, Kimura E, Muhoho N, Migwi DK, Katsumata T. [The usefulness of urinalysis reagent strips in selecting Schistosoma haematobium egg positives before and after treatment with praziquantel]. Journal of Tropical Medicine and Hygiene 1991;94(6):401‐6. [PubMed] [Google Scholar]
King 1988_a {published data only}
- King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, et al. [Chemotherapy‐based control of schistosomiasis haematobia. I. Metrifonate versus praziquantel in control of intensity and prevalence of infection]. American Journal of Tropical Medicine and Hygiene 1988;39(3):295‐305. [DOI] [PubMed] [Google Scholar]
King 1988_b {published data only}
- King CH, Keating CE, Muruka JF, Ouma JH, Houser H, Arap Siongok TK, et al. [Urinary tract morbidity in schistosomiasis haematobia: associations with age and intensity of infection in an endemic area of Coast Province, Kenya]. American Journal of Tropical Medicine and Hygiene 1988;39(4):361‐8. [DOI] [PubMed] [Google Scholar]
Kitange 1993 {published data only}
- Kitange HM, Swai AB, McLarty DG, Alberti KG. [Schistosomiasis prevalence after administration of praziquantel to school children in Melela village, Morogoro region, Tanzania]. East African Medical Journal 1993;70(12):782‐6. [PubMed] [Google Scholar]
Legesse 2007 {published data only}
- Legesse M, Erko B. [Field‐based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(7):668‐73. [DOI] [PubMed] [Google Scholar]
Legesse 2008 {published data only}
- Legesse M, Erko B. [Field‐based evaluation of a reagent strip test for diagnosis of schistosomiasis mansoni by detecting circulating cathodic antigen (CCA) in urine in low endemic area in Ethiopia]. Parasite 2008;15(2):151‐5. [DOI] [PubMed] [Google Scholar]
Lengeler 1993 {published data only}
- Lengeler C, Mshinda H, Morona D, deSavigny D. [Urinary schistosomiasis: testing with urine filtration and reagent sticks for haematuria provides a comparable prevalence estimate]. Acta Tropica 1993;53(1):39‐50. [DOI] [PubMed] [Google Scholar]
Mafe 1997 {published data only}
- Mafe MA. [The diagnostic potential of three indirect tests for urinary schistosomiasis in Nigeria]. Acta Tropica 1997;68(3):277‐84. [DOI] [PubMed] [Google Scholar]
Mafe 2000 {published data only}
- Mafe MA, Stamm T, Utzinger J, N'Goran EK. [Control of urinary schistosomiasis: an investigation into the effective use of questionnaires to identify high‐risk communities and individuals in Niger State, Nigeria]. Tropical Medicine and International Health 2000;5(1):53‐63. [DOI] [PubMed] [Google Scholar]
Magnussen 2001 {published data only}
- Magnussen P, Ndawi B, Sheshe AK, Byskov J, Mbwana K, Christensen NO. [The impact of a school health programme on the prevalence and morbidity of urinary schistosomiasis in Mwera Division, Pangani District, Tanzania]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(1):58‐64. [DOI] [PubMed] [Google Scholar]
Midzi 2009 {published data only}
- Midzi N, Butterworth AE, Mduluza T, Munyati S, Deelder AM, Dam GJ. [Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(1):45‐51. [DOI] [PubMed] [Google Scholar]
Morenikeji 2014 {published data only}
- Morenikeji O, Quazim J, Omoregie C, Hassan A, Nwuba R, Anumudu C, et al. [A cross‐sectional study on urogenital schistosomiasis in children; haematuria and proteinuria as diagnostic indicators in an endemic rural area of Nigeria]. African Health Sciences 2014;14(2):390‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mott 1985a_1 {published data only}
- Mott KE, Dixon H, Osei‐Tutu E, England EC, Ekue K, Tekle A. [Indirect screening for Schistosoma haematobium infection: a comparative study in Ghana and Zambia]. Bulletin of the World Health Organization 1985;63(1):135‐42. [PMC free article] [PubMed] [Google Scholar]
Mott 1985a_2 {published data only}
- Mott KE, Dixon H, Osei‐Tutu E, England EC, Ekue K, Tekle A. [Indirect screening for Schistosoma haematobium infection: a comparative study in Ghana and Zambia]. Bulletin of the World Health Organization 1985;63(1):135‐42. [PMC free article] [PubMed] [Google Scholar]
Mtasiwa 1996 {published data only}
- Mtasiwa D, Mayombana C, Kilima P, Tanner M. [Validation of reagent sticks in diagnosing urinary schistosomiasis in an urban setting]. East African Medical Journal 1996;73(3):198‐200. [PubMed] [Google Scholar]
Murare 1987 {published data only}
- Murare HM, Taylor P. [Haematuria and proteinuria during Schistosoma haematobium infection: relationship to intensity of infection and the value of chemical reagent strips for pre‐ and post‐treatment diagnosis]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;81(3):426‐30. [DOI] [PubMed] [Google Scholar]
Navaratnam 2012 {published data only}
- Navaratnam AM, Mutumba‐Nakalembe MJ, Stothard JR, Kabatereine NB, Fenwick A, Sousa‐Figueiredo JC. [Notes on the use of urine‐CCA dipsticks for detection of intestinal schistosomiasis in preschool children.]. Transactions of the Royal Society of Tropical Medicine & Hygiene 2012;106(10):619‐22. [DOI] [PubMed] [Google Scholar]
Ndamukong 2001 {published data only}
- Ndamukong KJ, Ayuk MA, Dinga JS, Akenji TN. [Prevalance and intensity of urinary schistosomiasis in primary school children of the Kotto Barombi]. East African Medical Journal 2001;78(6):287‐9. [DOI] [PubMed] [Google Scholar]
Ndlovu 1996 {published data only}
- Ndhlovu P, Cadman H, Gundersen S, Vennervald BJ, Friis H, Christensen NO, et al. [Circulating anodic antigen (CAA) levels in different age groups in a Zimbabwean rural community endemic for Schistosoma haematobium determined using the magnetic beads antigen‐capture enzyme‐linked immunoassay]. American Journal of Tropical Medicine and Hygiene 1996;54(5):537‐42. [DOI] [PubMed] [Google Scholar]
Nduka 1995 {published data only}
- Nduka FO, Ajaero CM, Nwoke BE. [Urinary schistosomiasis among school children in an endemic community in south‐eastern Nigeria]. Applied Parasitology 1995;36:34‐40. [PubMed] [Google Scholar]
Ndyomugyenyi 2001 {published data only}
- Ndyomugyenyi R, Minjas JN. [Urinary schistosomiasis in schoolchildren in Dar‐es‐Salaam, Tanzania, and the factors influencing its transmission]. Annals of Tropical Medicine and Parasitology 2001;95(7):697‐706. [DOI] [PubMed] [Google Scholar]
Ngándu 1988 {published data only}
- Ngándu NH. [The use of Baye's theorem and other indices of agreement in evaluating the use of reagent strips in screening rural school children for Schistosoma haematobium in Zambia]. International Journal of Epidemiology 1988;17(1):202‐8. [DOI] [PubMed] [Google Scholar]
NGoran 1989 {published data only}
- N'Goran KE, Yapi YG, Rey J‐L, Soro B, Coulibaly A, Bellec C. Screening of urinary schistosomiasis by sticks reactive to haematuria study in Ivory Coast [Depistage de la schistosomose urinaire par bandelettes reactives a l'hematurie. Evaluation en zones de moyenne et faible endemie de cote‐d'ivoire]. Bulletin de la Societe de Pathologie Exotique et de Ses Filiales 1989;82(2):236‐42. [PubMed] [Google Scholar]
NGoran 1998 {published data only}
Nmorsi 2005 {published data only}
- Nmorsi OPG, Egwunyenga OA, Ukwandu NCD, Nwokolo NQ. [Urinary schistosomiasis in a rural community in Edo state, Nigeria: eosinophiluria as a diagnostic marker]. African Journal of Biotechnology 2005;4(2):183‐6. [Google Scholar]
Nwaorgu 1992 {published data only}
- Nwaorgu OC, Anigbo EU. [The diagnostic value of haematuria and proteinuria in Schistosoma haematobium infection in southern Nigeria]. Journal of Helminthology 1992;66(3):177‐85. [DOI] [PubMed] [Google Scholar]
Ofori 1986 {published data only}
- Ofori‐Adjei D, Adjepon‐Yamoah KK, Ashitey GA, Osei‐Tutu E. [Screening methods for urinary schistosomiasis in an endemic area (the Kraboa/Coaltar district of Ghana)]. Annals of Tropical Medicine and Parasitology 1986;80(3):365‐6. [DOI] [PubMed] [Google Scholar]
Okeke 2014_settingA {published data only}
- Okeke OC, Obachukwu PO. [Performance of three rapid screening methods in the detection of Schistosoma haematobium infection in school‐age children in Southeastern Nigeria]. Pathogens and Global Health 2014;108(2):111‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okeke 2014_settingB {published data only}
- Okeke OC, Obachukwu PO. [Performance of three rapid screening methods in the detection of Schistosoma haematobium infection in school‐age children in Southeastern Nigeria]. Pathogens and Global Health 2014;108(2):111‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onayade 1996 {published data only}
- Onayade AA, Abayomi IO, Fabiyi AK. [Urinary schistosomiasis: options for control within endemic rural communities. A case study in south‐west Nigeria]. Public Health 1996;110(4):221‐7. [DOI] [PubMed] [Google Scholar]
Poggensee 2000_settingA {published data only}
- Poggensee G, Krantz I, Kiwelu I, Feldmeier H. [Screening of Tanzanian women of childbearing age for urinary schistosomiasis: validity of urine reagent strip readings and self‐reported symptoms]. Bulletin of the World Health Organization 2000;78(4):542‐8. [PMC free article] [PubMed] [Google Scholar]
Poggensee 2000_settingB {published data only}
- Poggensee G, Krantz I, Kiwelu I, Feldmeier H. [Screening of Tanzanian women of childbearing age for urinary schistosomiasis: validity of urine reagent strip readings and self‐reported symptoms]. Bulletin of the World Health Organization 2000;78(4):542‐8. [PMC free article] [PubMed] [Google Scholar]
Polman 1995 {published data only}
- Polman K, Stelma FF, Gryseels B, Dam GJ, Talla I, Niang M, et al. [Epidemiologic application of circulating antigen detection in a recent Schistosoma mansoni focus in Northern Senegal]. American Journal of Tropical Medicine and Hygiene 1995;53(2):152‐7. [DOI] [PubMed] [Google Scholar]
Pugh 1980 {published data only}
- Pugh RNH, Bell DR, Gilles HM. [The potential medical importance of bilharzia in northern Nigeria: a suggested rapid, cheap and effective solution for control of Schistosoma haematobium infection]. Annals of Tropical Medicine and Parasitology 1980;74(6):597‐613. [PubMed] [Google Scholar]
Rasendramino 1998 {published data only}
- Rasendramino MH, Rajaona HR, Ramarokoto CE, Ravaoalimalala VE, Leutscher P, Cordonnier D, et al. [Prevalence of uro‐nephrologic complications of urinary bilharziasis in hyperendemic focus in Madagascar]. Nephrologie 1998;19(6). [PubMed] [Google Scholar]
Robinson 2009 {published data only}
- Robinson E, Picon D, Sturrock HJ, Sabasio A, Lado M, Kolaczinski J, et al. [The performance of haematuria reagent strips for the rapid mapping of urinary schistosomiasis: field experience from Southern Sudan]. Tropical Medicine and International Health 2009;14(12):1484‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rollinson 2005 {published data only}
- Rollinson D, Klinger EV, Mgeni AF, Khamis IS, Stothard JR. [Urinary schistosomiasis on Zanzibar: application of two novel assays for the detection of excreted albumin and haemoglobin in urine]. Journal of Helminthology 2005;79(3):199‐206. [DOI] [PubMed] [Google Scholar]
Sarda 1985 {published data only}
- Sarda RK, Simonsen PE, Mahikwano LF. [Urban transmission of urinary schistosomaisis in Dar es Salaam, Tanzania]. Acta Tropica 1985;42:71‐8. [PubMed] [Google Scholar]
Sarda 1986 {published data only}
- Sarda RK. [Frequency of haematuria and proteinuria in relation to prevalence and intensity of Schistosoma haematobium infection in Dar es Salaam, Tanzania]. East African Medical Journal 1986;63(2):105‐8. [PubMed] [Google Scholar]
Savioli 1990 {published data only}
- Savioli L, Hatz C, Dixon H, Kisumku UM, Mott KE. [Control of morbidity due to Schistosoma haematobium on Pemba Island:Egg excretion and hematuria as indicators of infection]. American Journal of Tropical Medicine and Hygiene 1990;43(3):289‐95. [DOI] [PubMed] [Google Scholar]
Sellin 1982 {published data only}
- Sellin B, Simonkovich E, Ovazza L, Sellin E, Desfontaine M, Rey JL. Value of macroscopic urine examination and reagent strips for the detection of hematuria and proteinuria in the mass diagnosis of urinary schistosomiasis, before and after treatment [Valeur de l'examen macroscopique des urines et des bandelettes reactives pour la detection de l'hematurie et de la proteinurie dans le diagnostic de masse de la schistosomiase urinaire, avant et apres traitement]. Medecine Tropicale 1982;42(5):521‐6. [PubMed] [Google Scholar]
Shane2011_Colley2013 {published data only}
- Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuente LA, N'goran EK, et al. [A five‐country evaluation of a point‐of‐care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni]. American Journal of Tropical Medicine and Hygiene 2013;88(3):426‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 1998 {published data only}
- Shaw DJ, Picquet M, Ly A, Sambou B, Vercruysse J. [Evaluation of dipsticks in Schistosoma haematobium infections in four villages in the middle valley of the Senegal River Basin, Senegal]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(6):634‐5. [DOI] [PubMed] [Google Scholar]
Standley 2010 {published data only}
- Standley CJ, Lwambo NJS, Lange CN, Kariuki HC, Adriko M, Stothard JR. [Performance of circulating cathodic antigen (CCA) urine‐dipsticks for rapid detection of intestinal schistosomiasis in schoolchildren from shoreline communities of Lake Victoria]. Parasites and Vectors 2010;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephenson 1984 {published data only}
- Stephenson LS, Latham MC, Kinoti SN, Oduori ML. [Sensitivity and specificity of reagent strips in screening of Kenyan children for Schistosoma haematobium infection]. American Journal of Tropical Medicine and Hygiene 1984;33(5):862‐71. [DOI] [PubMed] [Google Scholar]
Stothard 2006 {published data only}
- Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, et al. [Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis]. Acta Tropica 2006;97(2):219‐28. [DOI] [PubMed] [Google Scholar]
Stothard 2009a {published data only}
- Stothard JR, Sousa‐Figueiredo JC, Standley C, Dam GJ, Knopp S, Utzinger J, et al. [An evaluation of urine‐CCA strip test and fingerprick blood SEA‐ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar]. Acta Tropica 2009;111(1):64‐70. [DOI] [PubMed] [Google Scholar]
Stothard 2009b {published data only}
- Russell SJ, Sousa‐Figueiredo JC, Simba KI, Garba A, Rollinson D. [Urinary schistosomiasis‐associated morbidity in schoolchildren detected with urine albumin‐to‐creatinine ratio (UACR) reagent strips]. Journal of Pediatric Urology 2009;5(4):287‐91. [DOI] [PubMed] [Google Scholar]
Tanner 1983_1 {published data only}
- Tanner M, Holzer B, Marti HP, Saladin B, Degremont AA. [Frequency of haematuria and proteinuria among Schistosoma haematobium infected children of two communities from Liberia and Tanzania]. Acta Tropica 1983;40(3):231‐7. [PubMed] [Google Scholar]
Tanner 1983_2 {published data only}
- Tanner M, Holzer B, Marti HP, Saladin B, Degremont AA. [Frequency of haematuria and proteinuria among Schistosoma haematobium infected children of two communities from Liberia and Tanzania]. Acta Tropica 1983;40(3):231‐7. [PubMed] [Google Scholar]
Tchuente 2012_9KK {published data only}
- Tchuem Tchuente LA, Kuete Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo NC, Kenfack CM, et al. [Evaluation of circulating cathodic antigen (CCA) urine‐tests for diagnosis of Schistosoma mansoni infection in Cameroon]. PLoS Neglected Tropical Diseases 2012;6(7):e1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tchuente 2012_Colley2013 {published data only}
- Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuente LA, N'goran EK, et al. [A five‐country evaluation of a point‐of‐care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni]. American Journal of Tropical Medicine and Hygiene 2013;88(3):426‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Traore 1998 {published data only}
- Traore M, Traore HA, Kardorff R, Diarra A, Landoure Vester U, Doehring E, et al. [The public health significance of urinary schistosomiasis as a cause of morbidity in 2 districts in Mali]. American Journal of Tropical Medicine and Hygiene 1998;59(3):407‐13. [DOI] [PubMed] [Google Scholar]
Ugbomoiko 2009a {published data only}
- Ugbomoiko U.S, Dalumo V, Ariza L, Bezerra FSM, Heukelbach J. [A simple approach improving the performance of urine reagent strips for rapid diagnosis of urinary schistosomiasis in Nigerian schoolchildren]. Memorias do Instituto Oswaldo Cruz 2009;104(3):456‐61. [DOI] [PubMed] [Google Scholar]
Ugbomoiko 2009b_1 {published data only}
- Ugbomoiko US, Obiezue RNN, Ogunniyi TAB, Ofoezie IE. [Diagnostic accuracy of different urine dipsticks to detect urinary schistosomiasis: a comparative study in five endemic communities in Osun and Ogun States, Nigeria]. Journal of Helminthology 2009;83(3):203‐9. [DOI] [PubMed] [Google Scholar]
Ugbomoiko 2009b_2 {published data only}
- Ugbomoiko US, Obiezue RNN, Ogunniyi TAB, Ofoezie IE. [Diagnostic accuracy of different urine dipsticks to detect urinary schistosomiasis: a comparative study in five endemic communities in Osun and Ogun States, Nigeria]. Journal of Helminthology 2009;83(3):203‐9. [DOI] [PubMed] [Google Scholar]
Van Lieshout 1995 {published data only}
- Lieshout L, Panday UG, Jonge N, Krijger FW, Oostburg BF, Polderman AM, et al. [Immunodiagnosis of schistosomiasis mansoni in a low endemic area in Surinam by determination of the circulating antigens CAA and CCA]. Acta Tropica 1995;59(1):19‐29. [DOI] [PubMed] [Google Scholar]
Van Lieshout 1998_1 {published data only}
- Lieshout L, Polman K, Gryseels B, Deelder AM. [Circulating anodic antigen levels in two areas endemic for schistosomiasis mansoni indicate differences in worm fecundity]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(1):115‐9. [DOI] [PubMed] [Google Scholar]
Van Lieshout 1998_2 {published data only}
- Lieshout L, Polman K, Gryseels B, Deelder AM. [Circulating anodic antigen levels in two areas endemic for schistosomiasis mansoni indicate differences in worm fecundity]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(1):115‐9. [DOI] [PubMed] [Google Scholar]
Verle 1994 {published data only}
- Verle P, Stelma F, Desreumaux P, Dieng A, Diaw O, Kongs A, et al. [Preliminary study of urinary schistosomiasis in a village in the delta of the Senegal river basin, Senegal]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(4):401‐5. [DOI] [PubMed] [Google Scholar]
Warren 1979 {published data only}
- Warren KS, Mahmoud AAF, Muruka JF, Whittaker LR, Ouma JH, Arap Siongok TK. [Schistosomaisis haematobia in Coast province Kenya]. American Journal of Tropical Medicine and Hygiene 1979;28(5):864‐70. [PubMed] [Google Scholar]
Wilkins 1979 {published data only}
- Wilkins HA, Goll P, Marshall TF, Moore P. [The significance of proteinuria and haematuria in Schistosoma haematobium infection]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1979;73(1):74‐80. [DOI] [PubMed] [Google Scholar]
Zumstein 1983 {published data only}
- Zumstein A. [A study of some factors influencing the epidemiology of urinary schistosomiasis at Ifakara (Kilombero Districy, Morogoro Region, Tanzania)]. Acta Tropica 1983;40:187‐204. [PubMed] [Google Scholar]
References to studies excluded from this review
Adesola 2012 {published data only}
- Adesola H, Uduak N, Olajumoke M, Roseangela N, Chiaka A, Sunday A, et al. [Urine turbidity and microhaematuria as rapid assessment indicators for Schistosoma haematobium infection among school children in endemic areas]. American Journal of Infectious Diseases 2012;8(1). [Google Scholar]
Brouwer 2004 {published data only}
- Brouwer KC, Munatsi A, Ndhlovu PD, Wagatsuma Y, Shiff CJ. [Urinary schistosomiasis in Zimbabwean school children: predictors of morbidity]. African Health Sciences 2004;4(2):115‐8. [PMC free article] [PubMed] [Google Scholar]
Coulibaly 2012 {published data only}
- Coulibaly JT, N'Gbesso YK, Knopp S, Keiser J, N'goran EK, Utzinger J. [Efficacy and safety of praziquantel in preschool‐aged children in an area co‐endemic for Schistosoma mansoni and S. haematobium]. PLoS Neglected Tropical Diseases 2012;6(12):e1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulibaly 2013_2 {published data only}
- Coulibaly JT, N'Gbesso YK, Knopp S, Keiser J, N'goran EK, Utzinger J. [Efficacy and safety of praziquantel in preschool‐aged children in an area co‐endemic for Schistosoma mansoni and S. haematobium]. PLoS Neglected Tropical Diseases 2013;6(12):e1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulibaly 2013_3 {published data only}
- Coulibaly JT, N'goran EK, Utzinger J, Doenhoff MJ, Dawson EM. [A new rapid diagnostic test for detection of anti‐Schistosoma mansoni and anti‐Schistosoma haematobium antibodies]. Parasites and Vectors 2013;6(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
de Clerq 1997 {published data only}
- Clerq D, Sacko M, Vercruysse J, vanden BV, Landoure A, Diarra A, et al. [Circulating anodic and cathodic antigen in serum and urine of mixed Schistosoma haematobium and S. mansoni infections in Office du Niger, Mali]. Tropical Medicine and International Health 1997;2(7):680‐5. [DOI] [PubMed] [Google Scholar]
Deelder 1981 {published data only}
- Deelder AM, Berge W. [Detection of antibodies against circulating cathodic antigen of Schistosoma mansoni using the enzyme‐linked immunosorbent assay]. Zeitschrift fur Parasitenkunde 1981;64(2):179‐86. [DOI] [PubMed] [Google Scholar]
Deelder 1989 {published data only}
- Deelder AM, Jonge N, Fillie YE, Kornelis D, Helaha D, Qian ZL, et al. [Quantitative determination of circulating antigens in human schistosomiasis mansoni using an indirect hemagglutination assay]. American Journal of Tropical Medicine and Hygiene 1989;40(1):50‐4. [DOI] [PubMed] [Google Scholar]
Degarege 2014 {published data only}
- Degarege A, Legesse M, Medhin G, Teklehaymanot T, Erko B. [Day‐to‐day fluctuation of point‐of‐care circulating antigen test scores and faecal egg counts in children infected with Schistosoma mansoni in Ethiopia]. BMC Infectious Diseases 2014;14(210). [DOI] [PMC free article] [PubMed] [Google Scholar]
de Jonge 1988 {published data only}
- Jonge N, Gryseels B, Hilberath GW, Polderman AM, Deelder AM. [Detection of circulating anodic antigen by ELISA for seroepidemiology of schistosomiasis mansoni]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(4):591‐4. [DOI] [PubMed] [Google Scholar]
de Jonge 1989_a {published data only}
- Jonge N, Caluwe P, Hilberath GW, Krijger FW, Polderman AM, Deelder AM. [Circulating anodic antigen levels in serum before and after chemotherapy with praziquantel in schistosomiasis mansoni]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1989;83(3):368‐72. [DOI] [PubMed] [Google Scholar]
de Jonge 1989_b {published data only}
- Jonge N, Fillie YE, Hilberath GW, Krijger FW, Lengeler C, Savigny DH, et al. [Presence of the schistosome circulating anodic antigen (CAA) in urine of patients with Schistosoma mansoni or S. haematobium infections]. American Journal of Tropical Medicine and Hygiene 1989;41(5):563‐9. [DOI] [PubMed] [Google Scholar]
de Jonge 1990_1 {published data only}
- Jonge N, Schommer G, Feldmeier H, Krijger FW, Dafalla AA, Bienzle U, et al. [Mixed Schistosoma haematobium and S. mansoni infection: effect of different treatments on the serum level of circulating anodic antigen (CAA)]. Acta Tropica 1990;48(1):25‐35. [DOI] [PubMed] [Google Scholar]
de Jonge 1990_2 {published data only}
- Jonge N, Kremsner PG, Krijger FW, Schommer G, Fillie YE, Kornelis D, et al. [Detection of the schistosome circulating cathodic antigen by enzyme immunoassay using biotinylated monoclonal antibodies]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84(6):815‐8. [DOI] [PubMed] [Google Scholar]
Disch 1997 {published data only}
- Disch J, Garcia MMA, Krijger GW, Amorim MN, Katz N, Deelder AM, et al. [Daily fluctuation of levels of circulating cathodic antigen in urine of children infected with Schistosoma mansoni in Brazil]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(2):222‐5. [DOI] [PubMed] [Google Scholar]
Doehring 1985 {published data only}
- Doehring E, Ehrich JH, Vester U, Feldmeier H, Poggensee U, Brodehl J. [Proteinuria, hematuria, and leukocyturia in children with mixed urinary and intestinal schistosomiasis]. Kidney International 1985;28(3):520‐5. [DOI] [PubMed] [Google Scholar]
Eltoum 1992_b {published data only}
- Eltoum IA, Suliaman SM, Ismail BM, Ismail AIA, Ali MMM, Homeida MMA. [Evaluation of eosinophiluria in the diagnosis of schistosomiasis haematobium: a field study]. American Journal of Tropical Medicine and Hygiene 1992;46(6):732‐6. [DOI] [PubMed] [Google Scholar]
Eyo 2012 {published data only}
- Eyo JE, Onyishi GC, Okafor FC. [Urinary schistosomiasis among pregnant women in some endemic tropical semi‐urban communities of Anambra State, Nigeria]. Tropical Biomedicine 2012;29(4):575‐9. [PubMed] [Google Scholar]
Feldmeier 1982 {published data only}
- Feldmeier H, Doehring E, Daffalla AA. [Simultaneous use of a sensitive filtration technique and reagent strips in urinary schistosomiasis]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1982;76(3):416‐21. [DOI] [PubMed] [Google Scholar]
Feldmeier 1986 {published data only}
- Feldmeier H, Nogueira‐Queiroz JA, Peixoto‐Queiroz MA, Doehring E, Dessaint JP, Alencar JE, et al. [Detection and quantification of circulating antigen in schistosomiasis by monoclonal antibody. II. The quantification of circulating antigens in human schistosomiasis mansoni and haematobium: relationship to intensity of infection and disease status]. Clinical and Experimental Immunology 1986;65(2):232‐43. [PMC free article] [PubMed] [Google Scholar]
Fillie 1994 {published data only}
- Fillie YE, Lieshout L, Kornelis D, Deelder AM. [Evaluation of an ELISA for combined measurement of CAA and CCA in schistosomiasis mansoni]. Acta Tropica 1994;57(4):279‐87. [DOI] [PubMed] [Google Scholar]
Grenfell 2013 {published data only}
- Grenfell R, Harn DA, Tundup S, Da'dara A, Siqueira L, Coelho PM. [New approaches with different types of circulating cathodic antigen for the diagnosis of patients with low Schistosoma mansoni load]. PLoS Neglected Tropical Diseases 2013;7(2):e2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gundersen 1992 {published data only}
- Gundersen SG, Haagensen I, Jonassen TO, Figenschau KJ, Jonge N, Deelder AM. [Magnetic bead antigen capture enzyme‐linked immunoassay in microtitre trays for rapid detection of schistosomal circulating anodic antigen]. Journal of Immunological Methods 1992;148(1‐2):1‐8. [DOI] [PubMed] [Google Scholar]
Hakangard 1996 {published data only}
- Hakangard C, Deelder AM, Gabone RM, Nilsson LA, Ouchterlony O. [A comparative study on specific antibodies and circulating antigen (CAA) in serum and parasitological findings for diagnosis of schistosomiasis mansoni in an endemic area in Tanzania]. Acta Tropica 1996;61(3):213‐22. [DOI] [PubMed] [Google Scholar]
Hassan 1992 {published data only}
- Hassan MM, Badawi MA, Strand M. [Circulating schistosomal antigen in diagnosis and assessment of cure in individuals infected with Schistosoma mansoni]. American Journal of Tropical Medicine and Hygiene 1992;46(6):737‐44. [DOI] [PubMed] [Google Scholar]
Hassan 1994 {published data only}
- Hassan SI, Talaat M, Attar GM. [Evaluation of urinalysis reagent strips versus microscopical examination of urine for Schistosoma haematobium]. Journal of the Egyptian Society of Parasitology 1994;24(3):603‐9. [PubMed] [Google Scholar]
Hassan 1999 {published data only}
- Hassan MM, Hegab MH, Soliman SZ, Gaber OA, Shalaby MM, Kamel FM. [Relationship between circulating antigen level and morbidity in Schistosoma mansoni‐infected children evaluated by ultrasonography]. American Journal of Tropical Medicine and Hygiene 1999;61(4):635‐8. [DOI] [PubMed] [Google Scholar]
Jemaneh 1994 {published data only}
- Jemaneh L, Tedla S, Birrie H. [The use of reagent strips for detection of urinary schistosomiasis infection in the middle Awash Valley, Ethiopia]. East African Medical Journal 1994;71(10):679‐83. [PubMed] [Google Scholar]
Kahama 1998 {published data only}
- Kahama AI, Nibbeling HAM, Zeyl RJM, Vennervald BJ, Ouma JH, Deelder AM. [Detection and quantification of soluble egg antigen in urine of Schistosoma haematobium‐infected children from Kenya]. American Journal of Tropical Medicine and Hygiene 1998;59(5):769‐74. [DOI] [PubMed] [Google Scholar]
Kahama 1999 {published data only}
- Kahama AI, Odek AE, Kihara RW, Vennervald BJ, Kombe Y, Nkulila T, et al. [Urine circulating soluble egg antigen in relation to egg counts, hematuria, and urinary tract pathology before and after treatment in children infected with Schistosoma haematobium in Kenya]. American Journal of Tropical Medicine and Hygiene 1999;61(2):215‐9. [DOI] [PubMed] [Google Scholar]
Kaiser 1992 {published data only}
- Kaiser C, Bergel F, Doehring‐Schwerdtfeger E, Feldmeier H, Ehrich JH. [Urine test strips: reliability of semi‐quantitative findings under tropical conditions]. Pediatric Nephrology 1992;6(2):145‐8. [DOI] [PubMed] [Google Scholar]
Kassim 1983 {published data only}
- Kassim OO, Stek M. [Bacteriuria and hematuria in Infections due to Schistosoma haematobium]. The Journal of Infectious Diseases 1983;147(5):960. [DOI] [PubMed] [Google Scholar]
Kosinski 2011 {published data only}
- Kosinski KC, Bosompem KM, Stadecker MJ, Wagner AD, Plummer J, Durant JL, et al. [Diagnostic accuracy of urine filtration and dipstick tests for Schistosoma haematobium infection in a lightly infected population of Ghanaian schoolchildren]. Acta Tropica 2011;118(2):123‐7. [DOI] [PubMed] [Google Scholar]
Koukounari 2009 {published data only}
- Koukounari A, Webster JP, Donnelly CA, Bray BC, Naples J, Bosompem K, et al. [Sensitivities and specificities of diagnostic tests and infection prevalence of Schistosoma haematobium estimated from data on adults in villages northwest of Accra, Ghana]. American Journal of Tropical Medicine and Hygiene 2009;80(3):435‐41. [PMC free article] [PubMed] [Google Scholar]
Kremsner 1994 {published data only}
- Kremsner PG, Enyong P, Krijger FW, Jonge N, Zotter GM, Thalhammer F, et al. [Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium‐infected Cameroonian children receiving praziquantel: a longitudinal study]. Clinical Infectious Diseases 1994;18(3):408‐13. [DOI] [PubMed] [Google Scholar]
Krijger 1994 {published data only}
- Krijger FW, Lieshout L, Deelder AM. [A simple technique to pretreat urine and serum samples for quantitation of schistosome circulating anodic and cathodic antigen]. Acta Tropica 1994;56(1):55‐63. [DOI] [PubMed] [Google Scholar]
Lengeler 1991 {published data only}
- Lengeler C, Komba S, Morona D. [Urinary schistosomiasis: influence of the circadian variation of hematuria and proteinuria on reagent stick testing]. Acta Tropica 1991;48(4):313‐7. [DOI] [PubMed] [Google Scholar]
Leutscher 2008 {published data only}
- Leutscher PDC, Dam GTJ, Reimert CM, Ramarakoto C‐E, Deelder AM, Ornbjerg N. [Eosinophil cationic protein, soluble egg antigen, circulating anodic antigen, and egg excretion in male urogenital schistosomiasis]. American Journal of Tropical Medicine and Hygiene 2008;79(3):422‐6. [PubMed] [Google Scholar]
Lodh 2013 {published data only}
- Lodh N, Mwansa JC, Mutengo MM, Shiff CJ. [Diagnosis of Schistosoma mansoni without the stool: comparison of three diagnostic tests to detect Schistosoma [corrected] mansoni infection from filtered urine in Zambia]. American Journal of Tropical Medicine and Hygiene 2013;89(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lwambo 1997 {published data only}
- Lwambo NJ, Savioli L, Kisumku UM, Alawi KS, Bundy DA. [Control of Schistosoma haematobium morbidity on Pemba Island: validity and efficiency of indirect screening tests]. Bulletin of the World Health Organization 1997;75(3):247‐52. [PMC free article] [PubMed] [Google Scholar]
Madwar 1988 {published data only}
- Madwar MA, Hassan MM, Strickland GT. [Circulating antigens for assessing cure in schistosomiasis mansoni]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(6):881‐4. [DOI] [PubMed] [Google Scholar]
Melchers 2014 {published data only}
- Melchers NVS, Dam GJ, Shaproski D, Kahama AI, Brienen EAT, Vennervald BJ, et al. [Diagnostic performance of schistosoma real‐time PCR in urine samples from Kenyan children infected with Schistosoma haematobium: day‐to day variation and follow‐up after praziquantel treatment]. PLoS Neglected Tropical Diseases 2014;8(4):e2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mott 1983 {published data only}
- Mott KE, Dixon H, Osei‐Tutu E, England EC. [Relation between intensity of Schistosoma haematobium infection and clinical haematuria and proteinuria]. Lancet 1983;1(8332):1005‐8. [DOI] [PubMed] [Google Scholar]
Mott 1985 {published data only}
- Mott KE, Dixon H, Osei‐Tutu E, England EC, Ekue K, Tekle A. [Evaluation of reagent strips in urine tests for detection of Schistosoma haematobium infection: a comparative study in Ghana and Zambia]. Bulletin of the World Health Organization 1985;63(1):125‐33. [PMC free article] [PubMed] [Google Scholar]
Nibbeling 1998 {published data only}
- Nibbeling HAM, Lieshout L, Deelder AM. [Levels of circulating soluble egg antigen in urine of individuals infected with Schistosoma mansoni before and after treatment with praziquantel]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(6):675‐7. [DOI] [PubMed] [Google Scholar]
Obeng 2008 {published data only}
- Obeng BB, Aryeetey YA, Dood CJ, Amoah AS, Larbi IA, Deelder AM, et al. [Application of a circulating‐cathodic‐antigen (CCA) strip test and real‐time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana]. Annals of Tropical Medicine and Parasitology 2008;102(7):625‐33. [DOI] [PubMed] [Google Scholar]
Pereira 1999 {published data only}
- Pereira ES, Secor E, Andrade MO, Katz N, Rabello A. [Circulating antigens levels in different clinical forms of the Schistosoma mansoni infection]. Memorias do Instituto Oswaldo Cruz 1999;94(1):83‐6. [DOI] [PubMed] [Google Scholar]
Poggensee 1998 {published data only}
- Poggensee G, Kiwelu I, Saria M, Richter J, Krantz I, Feldmeier H. [Schistosomiasis of the lower reproductive tract without egg excretion in urine]. American Journal of Tropical Medicine and Hygiene 1998;59(5):782‐3. [DOI] [PubMed] [Google Scholar]
Polman 1998 {published data only}
- Polman K, Engels D, Fathers L, Deelder AM, Gryseels B. [Day‐to‐day fluctuation of schistosome circulating antigen levels in serum and urine of humans infected with Schistosoma mansoni in Burundi]. American Journal of Tropical Medicine and Hygiene 1998;59(1):150‐4. [DOI] [PubMed] [Google Scholar]
Polman 2000 {published data only}
- Polman K, Vlas SJ, Gryseels B, Deelder AM. [Relating serum circulating anodic antigens to faecal egg counts in Schistosoma mansoni infections: a modelling approach]. Parasitology 2000;121(6):601‐10. [DOI] [PubMed] [Google Scholar]
Savioli 1989 {published data only}
- Savioli L, Dixon H, Kisumku UM, Mott KE. [Control of morbidity due to Schistosoma haematobium on Pemba Island: programme organization and management]. Tropical Medicine and Parasitology 1989;40(2):189‐94. [PubMed] [Google Scholar]
Sousa‐Figueiredo 2013 {published data only}
- Sousa‐Figueiredo JC, Betson M, Kabatereine NB, Stothard JR. [The urine circulating cathodic antigen (CCA) dipstick: a valid substitute for microscopy for mapping and point‐of‐care diagnosis of intestinal schistosomiasis]. PLoS Neglected Tropical Diseases 2013;7(1):e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stothard 2011 {published data only}
- Stothard JR, Sousa‐Figuereido JC, Betson M, Adriko M, Arinaitwe M, Rowell C, et al. [Schistosoma mansoni infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable?]. PLoS Neglected Tropical Diseases 2011;5(1):e938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takougang 2004 {published data only}
- Takougang I, Meli J, Fotso S, Angwafo F 3rd, Kamajeu R, Ndumbe PM. [Hematuria and dysuria in the self‐diagnosis of urinary schistosomiasis among school‐children in Northern Cameroon]. African Journal of Health Sciences 2004;11(3‐4):121‐7. [PubMed] [Google Scholar]
Taylor 1990 {published data only}
- Taylor P, Chandiwana SK, Matanhire D. [Evaluation of the reagent strip test for haematuria in the control of Schistosoma haematobium infection in schoolchildren]. Acta Tropica 1990;47(2):91‐100. [DOI] [PubMed] [Google Scholar]
Tiemersma 1997 {published data only}
- Tiemersma EW, Hafid S, Boelee E, Khallaayoune K, Gryseels B. [Detection of urinary schistosomiasis in a low prevalence region]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(3):285‐6. [DOI] [PubMed] [Google Scholar]
van Dam 2004 {published data only}
- Dam GJ, Wichers JH, Ferreira TM, Ghati D, van AA, Deelder AM. [Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen]. Journal of Clinical Microbiology 2004;42(12):5458‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Etten 1994 {published data only}
- Van EL, Folman CC, Eggelte TA, Kremsner PG, Deelder AM. [Rapid diagnosis of schistosomiasis by antigen detection in urine with a reagent strip]. Journal of Clinical Microbiology 1994;32(10):2404‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Etten 1997 {published data only}
- Van EL, Lieshout L, Mansour MM, Deelder AM. [A reagent strip antigen capture assay for the assessment of cure of schistosomiasis patients]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(2):154‐5. [DOI] [PubMed] [Google Scholar]
van Lieshout 1992 {published data only}
- Lieshout L, Jonge N, Masry NA, Mansour MM, Krijger FW, Deelder AM. [Improved diagnostic performance of the circulating antigen assay in human schistosomiasis by parallel testing for circulating anodic and cathodic antigens in serum and urine]. American Journal of Tropical Medicine and Hygiene 1992;47(4):463‐9. [DOI] [PubMed] [Google Scholar]
van Lieshout 1995 {published data only}
- Lieshout L, Polderman AM, Vlas SJ, Caluwe P, Krijger FW, Gryseels B, et al. [Analysis of worm burden variation in human Schistosoma mansoni infections by determination of serum levels of circulating anodic antigen and circulating cathodic antigen]. Journal of Infectious Diseases 1995;172(5):1336‐42. [DOI] [PubMed] [Google Scholar]
Verani 2011 {published data only}
- Verani JR, Abudho B, Montgomery SP, Mwinzi PNM, Shane HL, Butler SE, et al. [Schistosomiasis among young children in Usoma, Kenya]. American Journal of Tropical Medicine and Hygiene 2011;84(5):787‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahmed 2012
- Ahmed AM, Tash LA, Mohamed EY, Adam I. [High levels of Schistosoma mansoni infections among schoolchildren in central Sudan one year after treatment with praziquantel]. Journal of Helminthology 2012;86(2):228‐32. [DOI] [PubMed] [Google Scholar]
Ansell 1997
- Ansell J, Guyatt H, Hall A, Kihamia C, Kivugo J, Ntimbwa P, et al. [The reliability of self‐reported blood in urine and schistosomiasis as indicators of Schistosoma haematobium infection in school children: a study in Muheza District, Tanzania]. Tropical Medicine and International Health 1997;2(12):1180‐9. [DOI] [PubMed] [Google Scholar]
Ayele 2008
- Ayele B, Erko B, Legesse M, Hailu A, Medhin G. [Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar, Ethiopia]. Parasite 2008;15(1):69‐75. [DOI] [PubMed] [Google Scholar]
Bethony 2011
- Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, Loukas A, et al. [Vaccines to combat the neglected tropical diseases]. Immunological Reviews 2011;239(1):237‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bichler 2006
- Bichler KH, Savatovsky I, Naber KG, Bischop MC, Bjerklund‐Johansen TE, Botto H, et al. [EAU guidelines for the management of urogenital schistosomiasis]. European Urology 2006;49(6):998‐1003. [DOI] [PubMed] [Google Scholar]
Black 2009
- Black CL, Steinauer ML, Mwinzi PN, Evan SW, Karanja DM, Colley DG. [Impact of intense, longitudinal retreatment with praziquantel on cure rates of schistosomiasis mansoni in a cohort of occupationally exposed adults in western Kenya]. Tropical Medicine and International Health 2009;14(4):450‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bossuyt 2003
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. [Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative]. Annals of Internal Medicine 2003;138(1):40‐4. [DOI] [PubMed] [Google Scholar]
Brooker 2009
- Brooker S, Kabatereine NB, Gyapong JO, Stothard JR, Utzinger J. [Rapid mapping of schistosomiasis and other neglected tropical diseases in the context of integrated control programmes in Africa]. Parasitology 2009;136(13):1707‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavalcanti 2013
- Cavalcanti MG, Silva LF, Peralta RH, Barreto MG, Peralta JM. [Schistosomiasis in areas of low endemicity: a new era in diagnosis]. Trends in Parasitology 2013;29(2):75‐82. [DOI] [PubMed] [Google Scholar]
Chitsulo 1995
- Chitsulo L, Lengeler C, Jenkins J. [The Schistosomiasis Manual]. UNDP/World Bank/ WHO Special Programme for Research Training in Tropical Diseases (TDR), 1995; http://libdoc.who.int/hq/1995/TDR_SER_MSR_95.2.pdf (accessed 10 October 2010).
Colley 2013
- Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuente LA, N'goran EK, et al. [A five‐country evaluation of a point‐of‐care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni]. American Journal of Tropical Medicine and Hygiene 2013;88(3):426‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colley 2014
- Colley DG, Bustinduy AL, Secor WE, King CH. [Human schistosomiasis]. Lancet 2014;383:2253‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corstjens 2008
- Corstjens PLAM, Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, et al. [Up‐converting phosphor technology‐based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum]. Journal of Clinical Microbiology 2008;46(1):171‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulibaly 2011
- Coulibaly JT, Knopp S, N'Guessan NA, Silue KD, Furst T, Lohourignon LK, et al. [Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Cote d'Ivoire]. PLoS Neglected Tropical Diseases 2011;5(11):e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Croce 2010
- Croce D, Porazzi E, Foglia E, Restelli U, Sinuon M, Socheat D, et al. [Cost‐effectiveness of a successful schistosomiasis control programme in Cambodia (1995‐2006)]. Acta Tropica 2010;113(3):279‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
da Frota 2011
- Frota SM, Carneiro TR, Queiroz JA, Alencar LM, Heukelbach J, Bezerra FS. [Combination of Kato‐Katz faecal examinations and ELISA to improve accuracy of diagnosis of intestinal schistosomiasis in a low‐endemic setting in Brazil]. Acta Tropica 2011;120(Suppl 1):S138‐S141. [DOI] [PubMed] [Google Scholar]
De Clerq 1997
- Clerq D, Sacko M, Vercruysse J, vanden Bussche V, Landoure A, Diarra A, et al. [Circulating anodic and cathodic antigen in serum and urine of mixed Schistosoma haematobium and S. mansoni infections in Office du Niger, Mali]. Tropical Medicine and International Health 1997;2(7):680‐5. [DOI] [PubMed] [Google Scholar]
De Jonge 1988
- Jonge N, Gryseels B, Hilberath GW, Polderman AM, Deelder AM. [Detection of circulating anodic antigen by ELISA for seroepidemiology of schistosomiasis mansoni]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(4):591‐4. [DOI] [PubMed] [Google Scholar]
De Jonge 1989
- Jonge N, Fillie YE, Hilberath GW, Krijger FW, Lengeler C, Savigny DH, et al. [Presence of the schistosome circulating anodic antigen (CAA) in urine of patients with Schistosoma mansoni or S. haematobium infections]. American Journal of Tropical Medicine and Hygiene 1989;41(5):563‐9. [DOI] [PubMed] [Google Scholar]
Deelder 2012
- Deelder AM, Dam GJ, Lieshout L. [Response to: accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in Southern Sudan by RA Ashton et al]. Tropical Medicine and International Health 2012;17(3):402‐3. [DOI] [PubMed] [Google Scholar]
Dendukuri 2012
- Dendukuri N, Schiller I, Joseph L, Pai M. [Bayesian meta‐analysis of the accuracy of a test for tuberculous pleuritis in the absence of a gold standard reference]. Biometrics 2012;68:1285‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doehring 1983
- Doehring E, Feldmeier H, Daffalla AA. [Day‐to‐day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan]. Annals of Tropical Medicine and Parasitology 1983;77(6):587‐94. [DOI] [PubMed] [Google Scholar]
Doehring 1985a
- Doehring E, Ehrich JH, Vester U, Feldmeier H, Poggensee U, Brodehl J. [Proteinuria, hematuria, and leukocyturia in children with mixed urinary and intestinal schistosomiasis]. Kidney International 1985;28(3):520‐5. [DOI] [PubMed] [Google Scholar]
Doehring 1985b
- Doehring E, Vester U, Ehrich JH, Feldmeier H. [Circadian variation of ova excretion, proteinuria, hematuria, and leukocyturia in urinary schistosomiasis]. Kidney International 1985;27(4):667‐71. [DOI] [PubMed] [Google Scholar]
Doehring 1988
- Doehring E. [Schistosomiasis in childhood]. European Journal of Pediatrics 1988;147:2‐9. [DOI] [PubMed] [Google Scholar]
Doenhoff 2002
- Doenhoff MJ, Kusel JR, Coles GC, Cioli D. [Resistance of Schistosoma mansoni to praziquantel: is there a problem?]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(5):465‐9. [DOI] [PubMed] [Google Scholar]
Doenhoff 2004
- Doenhoff MJ, Chiodini PL, Hamilton JV. [Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies?]. Trends in Parasitology 2004;20(1):35‐9. [DOI] [PubMed] [Google Scholar]
Doenhoff 2009
- Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica‐Mattoccia L, Botros S, et al. [Praziquantel: its use in control of schistosomiasis in sub‐Saharan Africa and current research needs]. Parasitology 2009;136(13):1825‐35. [DOI] [PubMed] [Google Scholar]
Engels 2002
- Engels D, Chitsulo L, Montresor A, Savioli L. [The global epidemiological situation of schistosomiasis and new approaches to control and research]. Acta Tropica 2002;82(2):139‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erko 2013
- Erko B, Medhin G, Teklehaymanot T, Degarege A, Legesse M. [Evaluation of urine‐circulating cathodic antigen (Urine‐CCA) cassette test for the detection of Schistosoma mansoni infection in areas of moderate prevalence in Ethiopia]. Tropical Medicine and International Health 2013;18(8):1029‐35. [DOI] [PubMed] [Google Scholar]
Feldmeier 1993
- Feldmeier H, Poggensee G. [Diagnostic techniques in schistosomiasis control. A review]. Acta Tropica 1993;52:205‐20. [DOI] [PubMed] [Google Scholar]
Fenwick 2003
- Fenwick A, Savioli L, Engels D, Robert BN, Todd MH. [Drugs for the control of parasitic diseases: current status and development in schistosomiasis]. Trends in Parasitology 2003;19(11):509‐15. [DOI] [PubMed] [Google Scholar]
French 2007
- French MD, Rollinson D, Basanez MG, Mgeni AF, Khamis IS, Stothard JR. [School‐based control of urinary schistosomiasis on Zanzibar, Tanzania: monitoring micro‐haematuria with reagent strips as a rapid urological assessment]. Journal of Pediatric Urology 2007;3(5):364‐8. [DOI] [PubMed] [Google Scholar]
French 2010
- French MD, Churcher TS, Gambhir M, Fenwick A, Webster JP, Kabatereine NB, et al. [Observed reductions in Schistosoma mansoni transmission from large‐scale administration of praziquantel in Uganda: a mathematical modelling study]. PLoS Neglected Tropical Diseases 2010;4(11):e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geerts 2001
- Geerts S, Gryseels B. [Anthelmintic resistance in human helminths: a review]. Tropical Medicine and International Health 2001;6(11):915‐21. [DOI] [PubMed] [Google Scholar]
Glinz 2010
- Glinz D, Silue KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, et al. [Comparing diagnostic accuracy of Kato‐Katz, Koga agar plate, ether‐concentration, and FLOTAC for Schistosoma mansoni and soil‐transmitted helminths]. PLoS Neglected Tropical Diseases 2010;4(7):e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 2013
- Greenberg RM. [New approaches for understanding mechanisms of drug resistance in schistosomes]. Parasitology 2013;140(12):1534‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gryseels 2006
- Gryseels B, Polman K, Clerinx J, Kestens L. [Human schistosomiasis]. Lancet 2006;368(9541):1106‐18. [DOI] [PubMed] [Google Scholar]
Gryseels 2012
- Gryseels B. [Schistosomiasis]. Infectious Disease Clinics of North America 2012;26(2):383‐97. [DOI] [PubMed] [Google Scholar]
Guo 2005
- Guo JG, Cao CL, Hu GH, Lin H, Li D, Zhu R, et al. [The role of 'passive chemotherapy' plus health education for schistosomiasis control in China during maintenance and consolidation phase]. Acta Tropica 2005;96(2‐3):177‐83. [DOI] [PubMed] [Google Scholar]
Guyatt 1999
- Guyatt H, Brooker S, Lwambo NJ, Siza JE, Bundy DA. [The performance of school‐based questionnaires of reported blood in urine in diagnosing Schistosoma haematobium infection: patterns by age and sex]. Tropical Medicine and International Health 1999;4(11):751‐7. [DOI] [PubMed] [Google Scholar]
ITM 2007
- ITM (Institute of Tropical Medicine). [Illustrated Lecture Notes on Tropical Medicine, 2007]. http://content‐e.itg.be/content‐e/pub_ITG/Illustrated_lecture_notes_on_Tropical_Medicine_1169817124568/index.htm (accessed 8 March 2011).
King 2010a
- King CH. [Parasites and poverty: the case of schistosomiasis]. Acta Tropica 2010;113(2):95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2010b
- King CH. [Chapter 3 Health metrics for helminthic infections]. Advances in Parasitology 2010;73:51‐69. [DOI] [PubMed] [Google Scholar]
King 2013
- King CH, Bertsch D. [Meta‐analysis of urine heme dipstick diagnosis of Schistosoma haematobium infection, including low‐prevalence and previously treated populations]. PLoS Neglected Tropical Diseases 2013;7(9):e2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knopp 2008
- Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, et al. [Diagnosis of soil‐transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques]. PLoS Neglected Tropical Diseases 2008;2(11):e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knopp 2009
- Knopp S, Glinz D, Rinaldi L, Mohammed KA, N'goran, EK, Stothard JR, et al. [FLOTAC: a promising technique for detecting helminth eggs in human faeces]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(12):1190‐4. [DOI] [PubMed] [Google Scholar]
Knopp 2011
- Knopp S, Speich B, Hattendorf J, Rinaldi L, Mohammed KA, Khamis IS, et al. [Diagnostic accuracy of Kato‐Katz and FLOTAC for assessing anthelmintic drug efficacy]. PLoS Neglected Tropical Diseases 2011;5(4):e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koukounari 2009
- Koukounari A, Webster JP, Donnelly CA, Bray BC, Naples J, Bosompem K, et al. [Sensitivities and specificities of diagnostic tests and infection prevalence of Schistosoma haematobium estimated from data on adults in villages northwest of Accra, Ghana]. American Journal of Tropical Medicine and Hygiene 2009;80(3):435‐41. [PMC free article] [PubMed] [Google Scholar]
Legesse 2007
- Legesse M, Erko B. [Field‐based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(7):668‐73. [DOI] [PubMed] [Google Scholar]
Lengeler 1991a
- Lengeler C, Kilima P, Mshinda H, Morona D, Hatz C, Tanner M. [Rapid, low‐cost, two‐step method to screen for urinary schistosomiasis at the district level: the Kilosa experience]. Bulletin of the World Health Organization 1991;69(2):179‐89. [PMC free article] [PubMed] [Google Scholar]
Lengeler 1991b
- Lengeler C, Utzinger J, Tanner M. [Questionnaires for rapid screening of schistosomiasis in sub‐Saharan Africa]. Bulletin of the World Health Organization 2002;80(3):235‐42. [PMC free article] [PubMed] [Google Scholar]
Lengeler 2002
- Lengeler C, Utzinger J, Tanner M. [Questionnaires for rapid screening of schistosomiasis in sub‐Saharan Africa]. Bulletin of the World Health Organization 2002;80(3):235‐42. [PMC free article] [PubMed] [Google Scholar]
Lodh 2013
- Lodh N, Mwansa JC, Mutengo MM, Shiff CJ. [Diagnosis of Schistosoma mansoni without the stool: comparison of three diagnostic tests to detect Schistosoma [corrected] mansoni infection from filtered urine in Zambia]. American Journal of Tropical Medicine and Hygiene 2013;89(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loubiere 2010
- Loubiere S, Moatti JP. [Economic evaluation of point‐of‐care diagnostic technologies for infectious diseases]. Clinical Microbiology and Infection 2010;16(8):1070‐6. [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10 Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0. http://srdta.cochrane.org/. The Cochrane Collaboration, 2010. [Google Scholar]
Melman 2009
- Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, et al. [Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni]. PLoS Neglected Tropical Diseases 2009;3(8):e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Midzi 2009
- Midzi N, Butterworth AE, Mduluza T, Munyati S, Deelder AM, Dam GJ. [Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(1):45‐51. [DOI] [PubMed] [Google Scholar]
Mott 1985
- Mott KE, Dixon H, Osei‐Tutu E, England EC, Ekue K, Tekle A. [Indirect screening for Schistosoma haematobium infection: a comparative study in Ghana and Zambia]. Bulletin of the World Health Organization 1985;63(1):135‐42. [PMC free article] [PubMed] [Google Scholar]
Murare 1987
- Murare HM, Taylor P. [Haematuria and proteinuria during Schistosoma haematobium infection: relationship to intensity of infection and the value of chemical reagent strips for pre‐ and post‐treatment diagnosis]. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(3):426‐30. [DOI] [PubMed] [Google Scholar]
Obeng 2008
- Obeng BB, Aryeetey YA, Dood CJ, Amoah AS, Larbi IA, Deelder AM, et al. [Application of a circulating‐cathodic‐antigen (CCA) strip test and real‐time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana]. Annals of Tropical Medicine Parasitology 2008;102(7):625‐33. [DOI] [PubMed] [Google Scholar]
Oliveira 2010
- Oliveira LM, Santos HL, Goncalves MM, Barreto MG, Peralta JM. [Evaluation of polymerase chain reaction as an additional tool for the diagnosis of low‐intensity Schistosoma mansoni infection]. Diagnostic Microbiology and Infectious Disease 2010;68(4):416‐21. [DOI] [PubMed] [Google Scholar]
Polman 1995
- Polman K, Stelma FF, Gryseels B, Dam GJ, Talla I, Niang M, et al. Epidemiologic application of circulating antigen detection in a recent Schistosoma mansoni focus in northern Senegal. American Journal of Tropical Medicine and Hygiene 1995;53(2):152‐7. [DOI] [PubMed] [Google Scholar]
Rabello 1992
- Rabello AL. [Parasitological diagnosis of schistosomiasis mansoni: fecal examination and rectal biopsy]. Memorias do Instituto Oswaldo Cruz 1992;87 Suppl 4:325‐31. [DOI] [PubMed] [Google Scholar]
Rabello 1997
- Rabello A. [Diagnosing schistosomiasis]. Memorias do Instituto Oswaldo Cruz 1997;92(5):669‐76. [DOI] [PubMed] [Google Scholar]
Reimert 1991
- Reimert CM, Venge P, Kharazmi A, Bendtzen K. [Detection of eosinophil cationic protein (ECP) by an enzyme‐linked immunosorbent assay]. Journal of Immunological Methods 1991;138(2):285‐90. [DOI] [PubMed] [Google Scholar]
Reimert 2000
- Reimert CM, Mshinda HM, Hatz CF, Kombe Y, Nkulila T, Poulsen LK, et al. [Quantitative assessment of eosinophiluria in Schistosoma haematobium infections: a new marker of infection and bladder morbidity]. American Journal of Tropical Medicine and Hygiene 2000;62(1):19‐28. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
Robinson 2009
- Robinson E, Picon D, Sturrock HJ, Sabasio A, Lado M, Kolaczinski J, et al. [The performance of haematuria reagent strips for the rapid mapping of urinary schistosomiasis: field experience from Southern Sudan]. Tropical Medicine and International Health 2009;14(12):1484‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rollinson 2009
- Rollinson D. [A wake up call for urinary schistosomiasis: reconciling research effort with public health importance]. Parasitology 2009;136(12):1593‐610. [DOI] [PubMed] [Google Scholar]
Rollinson 2013
- Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuente LA, Garba A, et al. [Time to set the agenda for schistosomiasis elimination]. Acta Tropica 2013;128(2):423‐40. [DOI] [PubMed] [Google Scholar]
Rutjes 2005
- Rutjes AWS, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PMM. [Case‐control and two‐gate designs in diagnostic accuracy studies]. Clinical Chemistry 2005;51(8):1335‐41. [DOI] [PubMed] [Google Scholar]
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [DOI] [PubMed] [Google Scholar]
Shane 2011
- Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PNM, et al. [Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya]. PLoS Neglected Tropical Diseases 2011;5(1):e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siqueira 2011
- Siqueira LM, Coelho PM, Oliveira AA, Massara CL, Carneiro NF, Lima AC, et al. [Evaluation of two coproscopic techniques for the diagnosis of schistosomiasis in a low‐transmission area in the state of Minas Gerais, Brazil]. Memorias do Instituto Oswaldo Cruz 2011;106(7):844‐50. [DOI] [PubMed] [Google Scholar]
Sousa‐Figueiredo 2013
- Sousa‐Figueiredo JC, Betson M, Kabatereine NB, Stothard JR. [The urine circulating cathodic antigen (CCA) dipstick: a valid substitute for microscopy for mapping and point‐of‐care diagnosis of intestinal schistosomiasis]. PLoS Neglected Tropical Diseases 2013;7(1):e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stothard 2006
- Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, et al. [Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis]. Acta Tropica 2006;97(2):219‐28. [DOI] [PubMed] [Google Scholar]
Taylor 1990
- Taylor P, Chandiwana SK, Matanhire D. [Evaluation of the reagent strip test for haematuria in the control of Schistosoma haematobium infection in schoolchildren]. Acta Tropica 1990;47(2):91‐100. [DOI] [PubMed] [Google Scholar]
Tchuente 2012
- Tchuem Tchuente LA, Kuete Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo NC, Kenfack CM, et al. [Evaluation of circulating cathodic antigen (CCA) urine‐tests for diagnosis of Schistosoma mansoni infection in Cameroon]. PLoS Neglected Tropical Diseases 2012;6(7):e1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ten Hove 2008
- Hove RJ, Verweij JJ, Vereecken K, Polman K, Dieye L, Lieshout L. [Multiplex real‐time PCR for the detection and quantification of Schistosoma mansoni and S. haematobium infection in stool samples collected in northern Senegal]. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(2):179‐85. [DOI] [PubMed] [Google Scholar]
Utzinger 2009
- Utzinger J, Raso G, Brooker S, Savigny D, Tanner M, Ornbjerg N, et al. [Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution]. Parasitology 2009;136(13):1859‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dam 2004
- Dam GJ, Wichers JH, Ferreira TMF, Ghati D, Amerongen A, Deedler AM. [Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen]. Journal of Clinical Microbiology 2004;42(12):5458‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dam 2013
- Dam GJ, Dood CJ, Lewis M, Deelder AM, Lieshout L, Tanke HJ, et al. [A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen]. Experimental Parasitology 2013;135(2):274‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Werf 2003
- Werf MJ, Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, et al. [Quantification of clinical morbidity associated with schistosome infection in sub‐Saharan Africa]. Acta Tropica 2003;86(2‐3):125‐39. [DOI] [PubMed] [Google Scholar]
van Lieshout 1992
- van Lieshout, Jonge N, Masry NA, Mansour MM, Krijger FW, Deelder AM. [Improved diagnostic performance of the circulating antigen assay in human schistosomiasis by parallel testing for circulating anodic and cathodic antigens in serum and urine]. American Journal of Tropical Medicine and Hygiene 1992;47(4):463‐9. [DOI] [PubMed] [Google Scholar]
van Lieshout 1995
- van Lieshout, Panday UG, Jonge N, Krijger FW, Oostburg BF, Polderman AM, et al. [Immunodiagnosis of schistosomiasis mansoni in a low endemic area in Surinam by determination of the circulating antigens CAA and CCA]. Acta Tropica 1995;59(1):19‐29. [DOI] [PubMed] [Google Scholar]
van Lieshout 2000
- van Lieshout, Polderman AM, Deelder AM. [Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections]. Acta Tropica 2000;77(1):69‐80. [DOI] [PubMed] [Google Scholar]
Vennervald 2004
- Vennervald BJ, Dunne DW. [Morbidity in schistosomiasis: an update]. Current Opinion in Infectious Diseases 2004;17(5):439‐47. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. [QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies]. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
WHO 2002
- WHO Expert Committee on the Control of Schistosomiasis. [Prevention and control of schistosomiasis and soil transmitted helminthiasis: report of a WHO expert committee:Geneva, 8‐14 October 2001]. World Health Organization 2002:1‐57. [PubMed] [Google Scholar]
WHO 2010
- WHO (World Health Organization). [Schistosomiasis fact sheet]. http://www.who.int/mediacentre/factsheets/fs115/en/index.html (accessed 10 October 2010).
WHO/TDR 2006
- WHO/TDR. [Scientific working group on Schistosomiasis; Meeting report 14–16 November 2005, Geneva, Switzerland]. http://apps.who.int/tdr/svc/publications/tdr‐research‐publications/swg‐report‐schistosomiasis (accessed 10 October 2010).


