ABSTRACT
Many bacteria produce secreted iron chelators called siderophores, which can be shared among cells with specific siderophore uptake systems regardless of whether the cell produces siderophores. Sharing secreted products allows freeloading, where individuals use resources without bearing the cost of production. Here we show that the Escherichia coli siderophore enterochelin is not evenly shared between producers and nonproducers. Wild-type Escherichia coli grows well in low-iron minimal medium, and an isogenic enterochelin synthesis mutant (ΔentF) grows very poorly. The enterochelin mutant grows well in low-iron medium supplemented with enterochelin. At high cell densities the ΔentF mutant can compete equally with the wild type in low-iron medium. At low cell densities the ΔentF mutant cannot compete. Furthermore, the growth rate of the wild type is unaffected by cell density. The wild type grows well in low-iron medium even at very low starting densities. Our experiments support a model where at least some enterochelin remains associated with the cells that produce it, and the cell-associated enterochelin enables iron acquisition even at very low cell density. Enterochelin that is not retained by producing cells at low density is lost to dilution. At high cell densities, cell-free enterochelin can accumulate and be shared by all cells in the group. Partial privatization is a solution to the problem of iron acquisition in low-iron, low-cell-density habitats. Cell-free enterochelin allows for iron scavenging at a distance at higher population densities. Our findings shed light on the conditions under which freeloaders might benefit from enterochelin uptake systems.
IMPORTANCE Sociality in microbes has become a topic of great interest. One facet of sociality is the sharing of secreted products, such as the iron-scavenging siderophores. We present evidence that the Escherichia coli siderophore enterochelin is relatively inexpensive to produce and is partially privatized such that it can be efficiently shared only at high producer cell densities. At low cell densities, cell-free enterochelin is scarce and only enterochelin producers are able to grow in low-iron medium. Because freely shared products can be exploited by freeloaders, this partial privatization may help explain how enterochelin production is stabilized in E. coli and may provide insight into when enterochelin is available for freeloaders.
INTRODUCTION
Iron is an essential nutrient for the vast majority of bacteria (1, 2). However, under aerobic conditions at neutral pH, iron is found predominantly in insoluble Fe3+ compounds, resulting in extremely low iron concentrations in water (about 10−18 M) (3). Within a host, available iron is also limited, as many host organisms produce iron-binding molecules, which sequester iron away from bacteria (4). Thus, bacteria have evolved a variety of mechanisms to acquire this resource. One mechanism is to secrete molecules, called siderophores, that bind and solubilize extracellular iron. Different bacteria produce different high-affinity secreted siderophores and there are specific high-affinity uptake systems for the siderophores (5, 6).
Siderophores, by necessity, are secreted, and there has been interest in and debate over the cooperative nature of siderophore production (7–17). In the simplest case, a siderophore, once secreted, is available to all members of a community. Consequently, a freeloading cell, which uses siderophores but does not produce them, may have a fitness advantage over a producing cell, as it does not incur the metabolic cost of siderophore production. Freeloading should destabilize siderophore production. However, competition for siderophores is often much more complex, as evidenced by numerous studies and the continued existence of siderophore-producing bacteria. The availability of siderophores and other extracellular products for use by freeloaders depends on siderophore diffusion as well as population density and structure (18–20). Some bacteria have also evolved mechanisms that appear to prevent or minimize siderophore freeloading, including maintaining variability in structure of siderophore-receptor pairs (diversifying selection) (21, 22) and privatizing siderophores through high hydrophobicity or association with membrane-bound receptors (23–26). We are interested in siderophore maintenance through privatization, for which there is little direct evidence in microbes (24).
The Escherichia coli siderophore enterochelin (Ent) has been studied in detail. It is a catecholate produced not only by E. coli but also by several other Enterobacteriaceae (27–29). Ent synthesis and uptake genes are under the control of the ferric uptake regulator (Fur) protein (30, 31). Ent is produced in the cytoplasm by-products of the ent genes via nonribosomal peptide synthesis (32–35). Following synthesis, Ent is secreted via EntS (36) and released from the cell. Upon binding Fe3+, ferric enterochelin (FeEnt) enters the periplasm via the TonB-dependent receptor FepA (37, 38). In the periplasm, FeEnt binds FepB, a periplasmic binding protein (39), and then moves into the cytoplasm through an ABC transporter (40, 41). Finally, FeEnt is hydrolyzed by the cytoplasmic enterochelin esterase (Fes) and free iron is released (42). Each Ent molecule can only be used once; however, the linear Ent breakdown products can be repurposed as lower-affinity siderophores (43).
It is of interest that Pseudomonas aeruginosa possesses the complete machinery for FeEnt uptake and utilization (44) but it cannot produce Ent itself. The general belief is that this enables P. aeruginosa to steal iron from Ent producers; it is capable of freeloading on enteric bacteria like E. coli. In fact, a variety of microbes possess Ent uptake systems but not ent biosynthesis genes (45). Here we present evidence that Ent is a partially privatized E. coli product. Although Ent mutants are unable to grow independently in iron-limited media, they can grow when cocultured with Ent producers. However, growth of Ent mutants in these cocultures is dependent on the overall frequency and density of Ent producers. Additionally, when an Ent producer is alone, its growth in low-iron media is independent of cell density. Our results are consistent with a model in which some fraction of Ent must remain associated with the cells that produce it.
MATERIALS AND METHODS
Bacterial strains and strain construction.
All bacteria used were E. coli BW25113 (46) derivatives. We obtained an entF::kan mutant from the Keio collection (47) and moved the mutation into E. coli BW25113 by using P1 transduction. The kanamycin resistance marker was removed by using Flp recombinase methods (48) to create E. coli RS100, which has a nonpolar scar in place of entF. We constructed a ΔentCEBAH mutant by using the method of Datsenko and Wanner (46). Briefly, a kanamycin marker flanked by Flp recombination target (FRT) sites was PCR amplified from pKD4. The amplification primers included DNA homologous to the regions upstream of entC and downstream of entH. The PCR product was introduced into the E. coli BW25113 chromosome by λ red recombination to yield a ΔentCEBAH operon mutant with a deletion extending from the entC translation start codon to the entH stop codon. We used pKD46 to express the λ red genes and removed the kanamycin marker as described above. Where noted, strains also carried either kanamycin or chloramphenicol resistance markers. These markers were PCR amplified from pKD4 or pKD3, respectively, before insertion into the arsenate transporter (arsB) gene in the opposite orientation as described elsewhere (46). Again, we used pKD46 to express the λ red genes. The markers replaced most of arsB, leaving only the start codon, codons for the 6 C-terminal residues, and the stop codon. Previous work has shown that inserting a marker into this gene does not noticeably affect growth under normal laboratory conditions (47, 49, 50).
Bacterial growth media.
We use Luria-Bertani broth (LB) with added kanamycin sulfate (30 μg/ml), chloramphenicol (25 μg/ml), or ampicillin sodium salt (100 μg/ml) as appropriate for routine bacterial growth. The low-iron minimal medium for our experiments consisted of 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS; pH 7), 50 mM NaCl, 40 mM NH4Cl, 5 mM KH2PO4, 5 mM Na2HPO4, 2 mM MgSO4, 0.1 mM CaCl2, and 1% glycerol. Where noted, FeCl3 was added to a final concentration of 5 or 50 μM, apoenterochelin to 5 μg/ml (Sigma Chemicals or Genaxxon BioScience), human apotransferrin to 100 μg/ml (Sigma Chemicals), and sodium bicarbonate to 20 mM. In some experiments we included siderophore extracts of E. coli culture fluid prepared as described below.
Preparation of siderophore extracts.
Extracts from E. coli BW25113 growth medium were prepared as described previously (36). Briefly, spent medium from stationary-phase cultures grown in low-iron medium was centrifuged and filtered to remove cells. The pH of the culture fluid was then reduced to about 2.5 with 12 N hydrochloric acid, and the siderophores were extracted with two equal volumes of ethyl acetate. The ethyl acetate extract was dried under a steady stream of nitrogen, and the residue was dissolved in ethyl acetate. This extraction method should result in a solution containing Ent and recycled Ent degradation products (36, 51). Extracted siderophores were added to growth medium as follows: the ethyl acetate preparation was added to sterile 18-mm tubes at the desired level, and the ethyl acetate was removed by evaporation under a stream of nitrogen gas. After removal of ethyl acetate, the siderophores were dissolved in low-iron medium.
Growth and competition experiments.
For starter cultures, bacteria were first grown overnight in LB broth, washed once in an equal volume of low-iron medium, and then used as an inoculum (1%, vol/vol) in low-iron medium containing siderophore extract prepared as described above. The extract used was equivalent to the material extracted from a 1% volume of E. coli BW25113 culture fluid. The bacteria were then grown in 5 ml of medium in 18-mm tubes with shaking at 37°C. When cultures reached mid-logarithmic phase (optical density at 600 nm of 0.2 to 0.3), cells were washed twice in low-iron medium, diluted to an optical density at 600 nm of 0.1, mixed at desired starting ratios for competition experiments, and then used as inocula for growth or competition experiments. The addition of siderophore extract allows equal growth of the ΔentF mutant and the wild type in the starter cultures. For growth curves, bacteria were grown in 2.5 ml of medium in 18-mm tubes with shaking at 37°C.
To determine cell numbers in single-strain growth experiments, we performed plate counts on LB agar. For competition experiments, cells were grown for 14 to 16 h. The frequencies of competing strains were measured before and after growth by plate counting on LB agar with kanamycin or chloramphenicol, and total cells per milliliter were calculated as the sum of the number of cells resistant to each antibiotic.
Our detection threshold for colony counting was 102 cells per ml. The counts for the ΔentF mutant at the lowest starting density in the density-dependent fitness experiments occasionally fell below this threshold. When this happened, we assigned a value equal to one-half of the detection threshold, or 50 cells per ml, to these cultures. The competitive index (CI) is defined as the ratio of mutant to wild type in the output divided by the ratio of mutant to wild type in the input (52). To calculate generations, we used the following formula: number of generations = ln(Nfinal/Ninitial), where N is the measured cells per milliliter. We used GraphPad Prism software to generate graphs and nonlinear semilog fit curves for the wild type-to-wild type competitions. To test whether slopes were significantly different from 0, we used a sum-of-squares F test with a P value of <0.05.
RESULTS
Growth of the ΔentF mutant in low-iron medium with and without added enterochelin.
As expected, our ΔentF mutant showed very limited growth in low-iron medium compared to that of the parent (Fig. 1). The final growth yield was <1% of the parent strain. The addition of 5 or 50 μM FeCl3 to cultures failed to rescue ΔentF mutant growth. In the presence of added apo-Ent (5 μg/ml), however, growth of the ΔentF mutant was equivalent to growth of the wild type (Fig. 1). This is consistent with the general view that Ent can serve as a shared resource, which can be used by any cell with an Ent receptor system whether or not that cell produced Ent. To show that iron limits growth in unsupplemented low-iron medium, we measured growth yields. Without added enterochelin, yields were 2.6 × 109 cells per ml without added iron and 6.7 × 109 cells per ml with 50 μM FeCl3. With enterochelin, yields were 2.3 × 109 cells per ml without added FeCl3 and 8.6 × 109cells per ml with 50 μM FeCl3. Wild-type growth rates, however, were indistinguishable between the conditions of no addition of iron and addition of 50 μM FeCl3 (0.45 ± 0.02 generations per hour). These growth curve data indicate that Ent production is relatively inexpensive.
FIG 1.
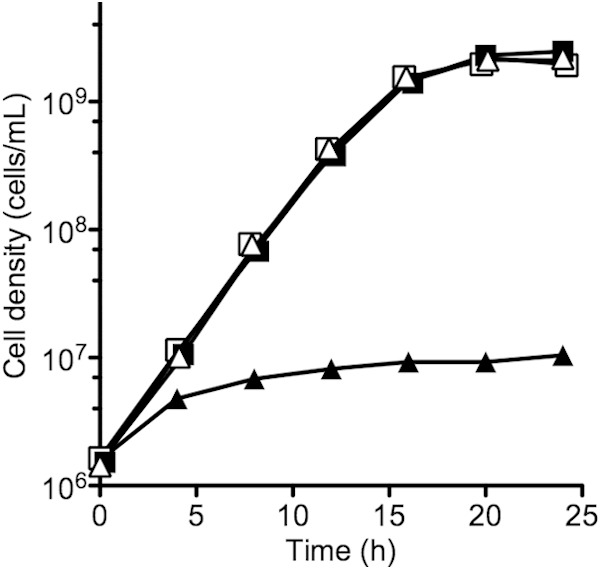
Growth curves of wild-type E. coli BW25113 grown with (open squares) or without (closed squares) added Ent and the ΔentF mutant grown with (open triangles) and without (closed triangles) added Ent. We used low-iron medium for these experiments and supplemented with 5 μg/ml of Ent where indicated. Cultures were run in duplicate, and ranges are within the symbols.
The ΔentF mutant can grow in low-iron medium when cocultured with the Ent-producing wild type.
Coculture with wild-type E. coli BW25113 supported growth of the ΔentF mutant (Fig. 2). When the starting ratio of ΔentF mutant to wild type was 1:1 and the starting density was 105 cells per ml, the two strains grew equally well: the final cell yield was comparable to that of the wild type in pure culture, and the CI was about 1. These findings indicate that the ΔentF mutant can freeload; it can use Ent produced by the wild type to acquire iron. The CI is a measure of the relative fitness of a mutant strain, with a CI of >1 indicating an advantage and a CI of <1 indicating a disadvantage. A CI of about 1 indicates that the cost of Ent production is low under the conditions of this experiment.
FIG 2.
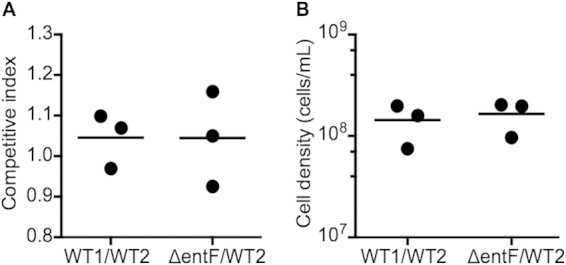
The ΔentF mutant can grow in low-iron medium when in coculture with the wild type. E. coli BW25113 arsB::kan (WT1) or ΔentF arsB::kan (ΔentF) was mixed at a 1:1 ratio with E. coli BW25113 arsB::cat (WT2). (A) Competitive index; (B) final cell density. The initial cell density was about 105 cells per ml. Total cells per milliliter and strain frequencies were measured at the start of the experiment and after 14 h. The competitive index was calculated as described in Materials and Methods. Results from three independent experiments are shown, and the bars indicate the means.
Enterochelin mutants have a negative frequency-dependent fitness in competition with the wild type.
To more thoroughly investigate whether production of Ent confers a burden to the wild type in coculture with the ΔentF mutant, we measured the CI in experiments started at a wide range of ΔentF mutant-to-wild type ratios. Previous work with P. aeruginosa showed that a siderophore mutant can have negative frequency-dependent fitness in competition with a wild type: the siderophore mutant showed greater fitness when at low relative abundance than it did at higher relative abundance (11). We tested whether initial frequencies affect the competitive fitness of the ΔentF mutant. The starting cell densities for these experiments were about 106 cells per ml of low-iron medium, and the starting ratios ranged from 1,000:1 to 1:1,000 for the ΔentF mutant to the wild type. As a control, we performed a similar experiment in which the wild type with a kanamycin resistance marker was cocultured with the wild type with a chloramphenicol resistance marker. The control provides a measure of any burden from the antibiotic resistance marker. The control showed little effect of the antibiotic resistance markers and did not show frequency dependence. At low initial frequencies of the ΔentF mutant, the CI was about 1, indicating that neither strain has a significant growth advantage over the other. This supports the view that Ent production is inexpensive and therefore does not significantly reduce the growth rate of the Ent producers (Fig. 3A). In contrast, at high ΔentF mutant starting frequencies, the CI was <1. That is, the wild type was more fit than the ΔentF mutant. The antibiotic markers are not responsible for this result, as the CIs in wild-type control experiments remained about 1.
FIG 3.
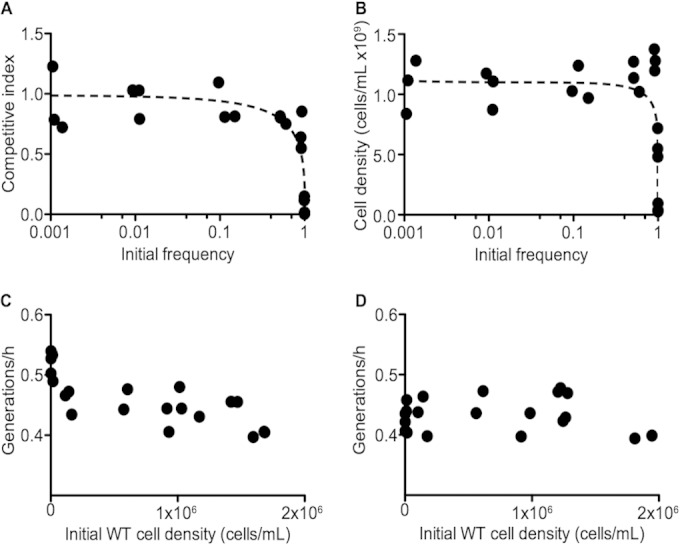
Negative frequency-dependent fitness of the ΔentF mutant. Competition experiments were conducted with WT1 or ΔentF against WT2. Initial ratios ranged from 1,000:1 to 1:1,000, and initial cell densities were about 106 cells per ml. Cells were grown in low-iron medium. (A) CI of competitions between the ΔentF mutant and WT2; (B) total yield of the cultures. At high frequencies of the ΔentF mutant, its fitness and total culture yield fall off. Controls with WT1 and WT2 show a CI of about 1 regardless of initial frequency, and total yields also remain constant. Frequencies were measured at the start of the experiment and after 14 to 16 h of growth. Also shown are growth of WT2 in culture with the ΔentF mutant (C) or with WT1 (D). Generations per hour were calculated as generations over the growth period divided by the hours of growth.
We performed similar experiments with a ΔentCEBAH mutant. This mutant is defective in conversion of chorismic acid to the aromatic substrate for Ent synthesis, whereas EntF is required for incorporation of serine in Ent biosynthesis. Our results with the ΔentCEBAH mutant were consistent with those obtained with the ΔentF mutant. For the ΔentCEBAH mutant, the competitive index is close to 1 at high wild-type frequencies and rapidly decreases as the starting mutant frequency increases above 10%.
Additionally, we found that although the overall culture growth was reduced with high ΔentF mutant starting frequencies (Fig. 3B), the approximate generations of the competing wild type per hour were unaffected by ΔentF mutant frequency (Fig. 3C and D). Therefore, the reduced growth yield in these cultures is due solely to the reduced growth of the ΔentF mutant. We believe that the results of the frequency dependence experiments indicate that Ent is not a freely shared public good. Rather, it is partially privatized by the wild type, and when wild-type cells are scarce, they make sufficient Ent to support themselves but there is insufficient free Ent for iron acquisition by the ΔentF mutant.
Fitness of the ΔentF mutant depends on the initial cell density.
Privatization of bacterial extracellular products has been demonstrated by showing density-dependent fitness during competition, although previous work has been done on surfaces where population structure plays a large role (12). By measuring density-dependent fitness, we can determine how wild-type cell density affects fitness of the ΔentF mutant. For these experiments, the initial ratios of the wild type to the ΔentF mutant were 1:1, and the initial cell densities were varied between 101 and 105 per ml (Fig. 4). The ΔentF mutant showed density-dependent fitness in low-iron medium. The greater the initial cell density, the greater the fitness of the ΔentF mutant. These data, in agreement with the data on frequency-dependent fitness, indicate that Ent is partially privatized by Ent-producing E. coli. We interpret the results to mean that at sufficiently high densities of wild-type E. coli, the fraction of Ent in the culture fluid is sufficient for growth of the mutant. Cell-free Ent concentrations are too low to be of value at lower cell densities where only the wild type with cell-associated Ent is capable of growth in low-iron medium. When Ent is added to the culture medium, the mutant and wild type grow well together regardless of the starting cell density. Thus, the inability of the Ent mutant to compete with the wild type when the initial cell density is low is a direct consequence of the lack of sufficient cell-free Ent.
FIG 4.
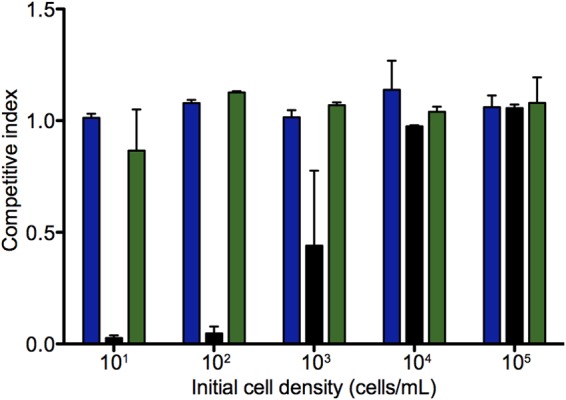
Fitness of the ΔentF mutant versus the wild type depends on initial cell density. The E. coli BW25113 arsB::cat strain was grown together with the E. coli BW25113 arsB::kan strain or the ΔentF mutant (E. coli ΔentF arsB::kan) in low-iron medium with or without supplements as indicated. Starting ratios were 1:1, and initial cell densities are shown. Blue bars represent the two Ent wild-type strains in unsupplemented low-iron medium. The CI was about 1 regardless of inoculum size. Black bars represent the ΔentF mutant and the wild type in unsupplemented medium. The ΔentF mutant is competitive only at initial cell densities of 103 to 104 or greater. Green bars represent the ΔentF mutant and wild type in medium supplemented with Ent (5 μg/ml). The mutant is competitive regardless of inoculum size. Cell numbers and frequencies were determined immediately after inoculation and at 14 h. The bars represent results from two independent experiments, and error bars show ranges.
One possible explanation for our results is that a significant fraction of iron-free Ent partitions to the periplasm of producing cells and diffusion of Fe3+ into the periplasm allows growth on low-iron medium when cell density is low. We do not have conclusive evidence for or against this hypothesis, but it is worth noting that inclusion of the iron chelator transferrin (with bicarbonate) in the low-iron medium restricts the growth of the wild type at low starting cell densities but not at higher starting densities (Fig. 5). Exogenous enterochelin can support wild-type growth in the presence of transferrin even at low starting cell densities, and the ΔentF mutant does not grow in the presence of transferrin regardless of starting density. One interpretation of these findings is that cell-associated Ent is within the periplasm, where it cannot obtain iron bound to transferrin. At high cell densities there is sufficient cell-free Ent for iron acquisition from transferrin.
FIG 5.
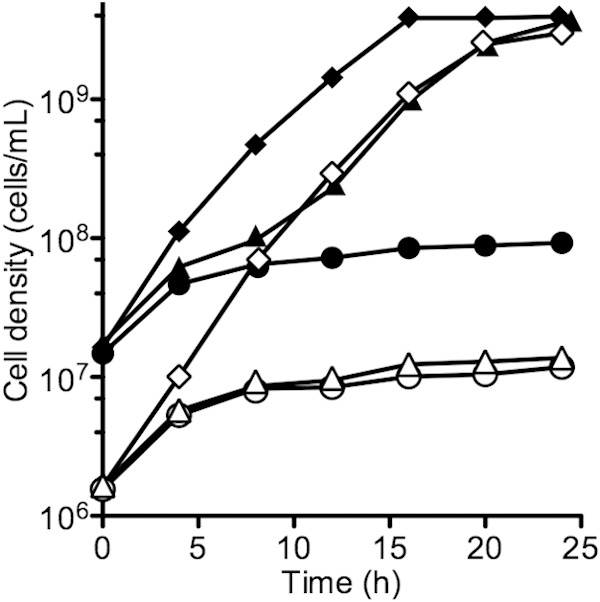
Growth of wild-type E. coli in the presence of transferrin is dependent on inoculum size. E. coli BW25113 and the ΔentF mutant were grown in low-iron medium with human apotransferrin and sodium bicarbonate. Open circles represent the ΔentF mutant started from low density and closed circles the ΔentF mutant started from high density. Open triangles represent the wild type started from low density and closed triangles the wild type started from high density. Open diamonds represent the wild type started from low density with Ent added, and closed diamonds represent the wild type started from high density with Ent added. Cultures were run in duplicate, and ranges are within the symbols.
Growth of Ent-producing wild-type E. coli in low-iron medium is not influenced by cell density.
The ability of Ent-producing wild-type E. coli to grow at low cell density and the inability of the ΔentF mutant to grow together with the wild type at low cell density indicate that some fraction of Ent remains with producing cells. At low cell density there is insufficient public Ent for cell growth. To obtain further evidence that the growth rates of the wild type are similar at very low and higher cell densities, we serially diluted the wild type in low-iron medium and followed growth. Growth was observed even from starting densities of one or a few cells per milliliter (Fig. 6). Previous work on yeast invertase has shown that for predominantly shared products, growth rate is severely limited at low starting densities, as products rapidly diffuse away from producers (53). Therefore, our finding that growth of E. coli in low-iron medium is unaffected by cell density is further evidence that a fraction of this siderophore remains bound to producing cells.
FIG 6.
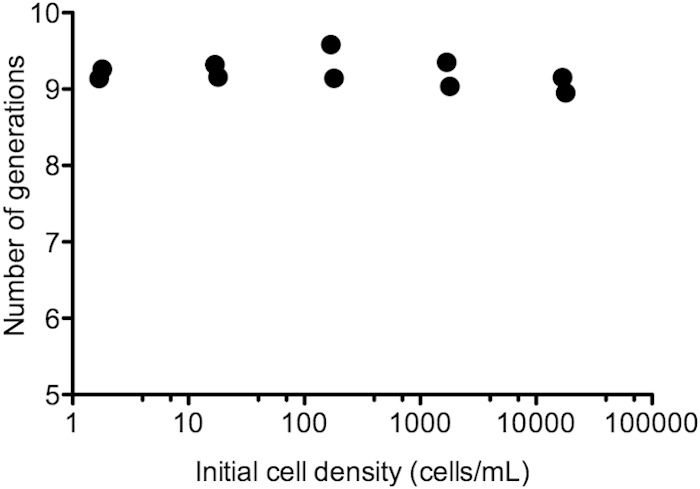
Growth rate of wild-type E. coli in low-iron medium is not affected by inoculum size. E. coli BW25113 was grown in low-iron medium starting at various densities as indicated (initial cell density). Cell numbers were determined by plate counting at the time of inoculation and at 18 h. In all cases, growth was in logarithmic phase at 18 h. The number of generations is the number of doublings in 18 h. Cultures were run in triplicate. One of the lowest-density starting cultures failed to grow, presumably because this culture did not receive any cells during inoculation; this culture was excluded from analysis.
DISCUSSION
We tested the hypothesis that the E. coli siderophore Ent is a secreted public good. Our results indicate that sufficient Ent or Ent breakdown products are released from cells to support the growth of non-siderophore-producing mutants, but only at relatively high cell densities. At low densities Ent producers grow well, but they cannot support growth of nonproducers. This leads to an alternate hypothesis that only a fraction of the Ent produced by wild-type cells is public, and the public Ent is not sufficient to support growth in low-iron medium when E. coli is at low population densities. Furthermore, we did not find that Ent mutants were cheating. Under any condition we tested, they did not have a fitness advantage over the wild type. Thus, we conclude that EntF expression and Ent production are relatively inexpensive.
The apparent partial privatization of Ent contrasts with findings for the P. aeruginosa siderophore pyoverdine. Pyoverdine nonproducers arise both in vivo and in vitro, and they are social cheats; they can have a fitness advantage over pyoverdine producers in low-iron media (7, 13, 54). However, there are indications that, as we have found with Ent, pyoverdine too is somewhat privatized (14, 24). Mathematical models indicate that social cheating can occur even in the face of significant privatization, so the differences in competition between siderophore-producing and nonproducing E. coli and P. aeruginosa may be due to siderophore cost and degree of privatization (15, 18–20).
Although it has long been suspected that many siderophores might remain cell associated after secretion (25, 55–57), evidence for this notion is limited and restricted to a few bacterial species. Furthermore, aside from the few studies on amphiphilic siderophores and pyoverdine, the mechanisms for privatization appear to be largely unexplored (23, 24, 26). Apo-Ent might be retained in the periplasm, it might bind to FepA, and it might adhere to the cell surface nonspecifically. Evidence indicates that adherence of FeEnt to the surface of E. coli is almost entirely mediated by FepA (58), but the mechanism for apo-Ent adherence is unclear, as studies typically measure adsorbance and transport of radiolabeled iron-siderophore complexes into cells, not aposiderophores (59). Due to the hydrophobicity of apo-Ent, it is possible that it remains associated with the cell surface after secretion (25, 56). We have not addressed the mechanism of Ent privatization, but we have found that iron chelated with transferrin supports growth of E. coli only when cells are at sufficient population densities or if exogenous enterochelin is added. This result suggests that Ent may be sequestered in the periplasm. By studying salmochelins, which are a family of glycosylated enterochelins, it may be possible to assess the importance of siderophore hydrophobicity to privatization. Salmochelin glycosylation results in siderophores that are more hydrophilic than enterochelin (60).
Many microbes produce FeEnt uptake receptors despite their lack of Ent biosynthesis genes. The general view is that this allows these microbes to acquire iron at the expense of Ent-producing enteric bacteria. Our experiments suggest that this occurs only when the density of Ent-producing cells is sufficiently high. Thus, Ent production is stabilized in at least two ways: Ent production is inexpensive and nonproducers should have little to no growth advantage over producers, and Ent producers will always have an initial head start over nonproducing cells when growing from low densities, such as after a dispersal event. What might be the advantage of making Ent publicly available at high cell densities? One might imagine that as cell density rises, local iron concentrations become increasingly limited. In this situation iron will come in contact with cells less often than at low cell densities. Here iron scavenging by diffusible Ent might provide a benefit to any cell in the group with an Ent uptake system. Ent may also serve purposes other than iron acquisition. For example, it has been suggested that cell-free siderophores may be beneficial to producers because they can sequester iron from competitors or possibly to support mutualists (27, 61, 62).
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service (USPHS) grant GM59026 (to E.P.G.). R.L.S. was supported in part by Public Health Service, National Research Service, award T32GM007270 from the National Institute of General Medical Sciences. We also acknowledge funding from the National Science Foundation under cooperative agreement no. DBI-0939454.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
We thank Beth Traxler and S. Brook Peterson for providing strains and plasmids for mutant construction. We also thank several members of our laboratory and department for helpful discussion and technical assistance.
REFERENCES
- 1.Guerinot ML. 1994. Microbial iron transport. Annu Rev Microbiol 48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 2.Neilands JB. 1995. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 3.Neilands JB, Konopka K, Schwyn B, Coy M, Francis RT, Paw BH, Bagg A. 1987. Comparative biochemistry of microbial iron assimilation, p 3–33. In Winkelmann G, Van der Helm D, Neilands JB (ed), Iron transport in microbes, plants, and animals. VCH, Weinheim, Federal Republic of Germany. [Google Scholar]
- 4.Weinberg ED. 1978. Iron and infection. Microbiol Rev 42:45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lankford CE, Byers BR. 1973. Bacterial assimilation of iron. Crit Rev Microbiol 2:273–331. doi: 10.3109/10408417309108388. [DOI] [Google Scholar]
- 6.Neilands JB. 1981. Microbial iron compounds. Annu Rev Biochem 50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 7.Griffin AS, West SA, Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 8.Kümmerli R, Gardner A, West SA, Griffin AS. 2009. Limited dispersal, budding dispersal, and cooperation: an experimental study. Evolution 63:939–949. doi: 10.1111/j.1558-5646.2008.00548.x. [DOI] [PubMed] [Google Scholar]
- 9.Kümmerli R, Jiricny N, Clarke LS, West SA, Griffin AS. 2009. Phenotypic plasticity of a cooperative behaviour in bacteria. J Evol Biol 22:589–598. doi: 10.1111/j.1420-9101.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 10.Kümmerli R, Griffin AS, West SA, Buckling A, Harrison F. 2009. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc Biol Sci 276:3531–3538. doi: 10.1098/rspb.2009.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross-Gillespie A, Gardner A, West SA, Griffin AS. 2007. Frequency dependence and cooperation: theory and a test with bacteria. Am Nat 170:331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 12.Ross-Gillespie A, Gardner A, Buckling A, West SA, Griffin AS. 2009. Density dependence and cooperation: theory and a test with bacteria. Evolution 63:2315–2325. doi: 10.1111/j.1558-5646.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 13.Jiricny N, Diggle SP, West SA, Evans BA, Ballantyne G, Ross-Gillespie A, Griffin AS. 2010. Fitness correlates with the extent of cheating in a bacterium. J Evol Biol 23:738–747. doi: 10.1111/j.1420-9101.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XX, Rainey PB. 2013. Exploring the sociobiology of pyoverdin-producing Pseudomonas. Evolution 67:3161–3174. doi: 10.1111/evo.12183. [DOI] [PubMed] [Google Scholar]
- 15.Kümmerli R, Ross-Gillespie A. 2014. Explaining the sociobiology of pyoverdin producing Pseudomonas: a comment on Zhang and Rainey (2013). Evolution 68:3337–3343. doi: 10.1111/evo.12311. [DOI] [PubMed] [Google Scholar]
- 16.Ghoul M, West SA, Diggle SP, Griffin AS. 2014. An experimental test of whether cheating is context dependent. J Evol Biol 27:551–556. doi: 10.1111/jeb.12319. [DOI] [PubMed] [Google Scholar]
- 17.Rainey PB, Desprat N, Driscoll WW, Zhang XX. 2014. Microbes are not bound by sociobiology: response to Kümmerli and Ross-Gillespie (2013). Evolution 68:3344–3355. doi: 10.1111/evo.12508. [DOI] [PubMed] [Google Scholar]
- 18.Allison SD. 2005. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol Lett 8:626–635. doi: 10.1111/j.1461-0248.2005.00756.x. [DOI] [Google Scholar]
- 19.Driscoll WW, Pepper JW. 2010. Theory for the evolution of diffusible external goods. Evolution 64:2682–2687. doi: 10.1111/j.1558-5646.2010.01002.x. [DOI] [PubMed] [Google Scholar]
- 20.Nadell CD, Bucci V, Drescher K, Levin SA, Bassler BL, Xavier JB. 2013. Cutting through the complexity of cell collectives. Proc Biol Sci 280:20122770. doi: 10.1098/rspb.2012.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith EE, Sims EH, Spencer DH, Kaul R, Olson MV. 2005. Evidence for diversifying selection at the pyoverdine locus of Pseudomonas aeruginosa. J Bacteriol 187:2138–2147. doi: 10.1128/JB.187.6.2138-2147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee W, van Baalen M, Jansen VA. 2012. An evolutionary mechanism for diversity in siderophore-producing bacteria. Ecol Lett 15:119–125. doi: 10.1111/j.1461-0248.2011.01717.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinez JS, Carter-Franklin JN, Mann EL, Martin JD, Haygood MG, Butler A. 2003. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc Natl Acad Sci U S A 100:3754–3759. doi: 10.1073/pnas.0637444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schalk IJ, Hennard C, Dugave C, Poole K, Abdallah MA, Pattus F. 2001. Iron-free pyoverdin binds to its outer membrane receptor FpvA in Pseudomonas aeruginosa: a new mechanism for membrane iron transport. Mol Microbiol 39:351–360. doi: 10.1046/j.1365-2958.2001.02207.x. [DOI] [PubMed] [Google Scholar]
- 25.Kümmerli R, Schiessl KT, Waldvogel T, McNeill K, Ackermann M. 2014. Habitat structure and the evolution of diffusible siderophores in bacteria. Ecol Lett 17:1536–1544. doi: 10.1111/ele.12371. [DOI] [PubMed] [Google Scholar]
- 26.Xu G, Martinez JS, Groves JT, Butler A. 2002. Membrane affinity of the amphiphilic marinobactin siderophores. J Am Chem Soc 124:13408–13415. doi: 10.1021/ja026768w. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien IG, Gibson F. 1970. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta 215:393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- 28.Pollack JR, Neilands JB. 1970. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun 38:989–992. doi: 10.1016/0006-291X(70)90819-3. [DOI] [PubMed] [Google Scholar]
- 29.Payne SM. 1988. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol 16:81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- 30.Hantke K. 1981. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet 182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 31.Stojiljkovic I, Baumler AJ, Hantke K. 1994. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol 236:531–545. [DOI] [PubMed] [Google Scholar]
- 32.Young IG, Langman L, Luke RK, Gibson F. 1971. Biosynthesis of the iron-transport compound enterochelin: mutants of Escherichia coli unable to synthesize 2,3-dihydroxybenzoate. J Bacteriol 106:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luke RKJ, Gibson F. 1971. Location of three genes concerned with the conversion of 2, 3-dihydroxybenzoate into enterochelin in Escherichia coli K-12. J Bacteriol 107:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodrow GC, Young IG, Gibson F. 1975. Mu-induced polarity in the Escherichia coli K-12 ent gene cluster: evidence for a gene (entG) involved in the biosynthesis of enterochelin. J Bacteriol 124:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodrow GC, Young IG, Gibson F. 1979. Biosynthesis of enterochelin in Escherichia coli K-12: separation of the polypeptides coded for by the entD, E, F and G genes. Biochim Biophys Acta 582:145–153. doi: 10.1016/0304-4165(79)90297-6. [DOI] [PubMed] [Google Scholar]
- 36.Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA. 2002. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol 44:1225–1234. doi: 10.1046/j.1365-2958.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- 37.Rutz JM, Liu J, Lyons JA, Goranson J, Armstrong SK, McIntosh MA, Feix JB, Klebba PE. 1992. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science 258:471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- 38.Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol 6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 39.Stephens DL, Choe MD, Earhart CF. 1995. Escherichia coli periplasmic protein FepB binds ferrienterobactin. Microbiology 141(Part 7):1647–1654. [DOI] [PubMed] [Google Scholar]
- 40.Shea CM, McIntosh MA. 1991. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other peripiasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol 5:1415–1428. doi: 10.1111/j.1365-2958.1991.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 41.Chenault SS, Earhart CF. 1991. Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol Microbiol 5:1405–1413. doi: 10.1111/j.1365-2958.1991.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 42.Langman L, Young IG, Frost GE, Rosenberg H, Gibson F. 1972. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol 112:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hantke K. 1990. Dihydroxybenzoylserine—a siderophore for E. coli. FEMS Microbiol Lett 55:5–8. [DOI] [PubMed] [Google Scholar]
- 44.Poole K, Young L, Neshat S. 1990. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol 172:6991–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thulasiraman P, Newton SM, Xu J, Raymond KN, Mai C, Hall A, Montague MA, Klebba PE. 1998. Selectivity of ferric enterobactin binding and cooperativity of transport in gram-negative bacteria. J Bacteriol 180:6689–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- 49.Sabri S, Steen JA, Bongers M, Nielsen LK, Vickers CE. 2013. Knock-in/knock-out (KIKO) vectors for rapid integration of large DNA sequences, including whole metabolic pathways, onto the Escherichia coli chromosome at well-characterised loci. Microb Cell Fact 12:60. doi: 10.1186/1475-2859-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlin A, Shi W, Dey S, Rosen BP. 1995. The ars operon of Escherichia coli confers arsenical and antimonial resistance. J Bacteriol 177:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffiths E, Humphreys J. 1980. Isolation of enterochelin from the peritoneal washings of guinea pigs lethally infected with Escherichia coli. Infect Immun 28:286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macho AP, Zumaquero A, Ortiz-Martin I, Beuzon CR. 2007. Competitive index in mixed infections: a sensitive and accurate assay for the genetic analysis of Pseudomonas syringae-plant interactions. Mol Plant Pathol 8:437–450. doi: 10.1111/j.1364-3703.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 53.Gore J, Youk H, van Oudenaarden A. 2009. Snowdrift game dynamics and facultative cheating in yeast. Nature 459:253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Vos D, De Chial M, Cochez C, Jansen S, Tummler B, Meyer JM, Cornelis P. 2001. Study of pyoverdine type and production by Pseudomonas aeruginosa isolated from cystic fibrosis patients: prevalence of type II pyoverdine isolates and accumulation of pyoverdine-negative mutations. Arch Microbiol 175:384–388. doi: 10.1007/s002030100278. [DOI] [PubMed] [Google Scholar]
- 55.Granger J, Price NM. 1999. The importance of siderophores in iron nutrition of heterotrophic marine bacteria. Limnol Oceanogr 44:541–555. doi: 10.4319/lo.1999.44.3.0541. [DOI] [Google Scholar]
- 56.Wilhelm SW, Trick CG. 1994. Iron-limited growth of cyanobacteria—multiple siderophore production is a common response. Limnol Oceanogr 39:1979–1984. doi: 10.4319/lo.1994.39.8.1979. [DOI] [Google Scholar]
- 57.Neilands JB. 1973. Microbial iron transport compounds (siderochromes), p 167–202. In Eichhorn GL. (ed), Inorganic biochemistry. Elsevier Scientific Publishing Co, Amsterdam, NY. [Google Scholar]
- 58.Wagegg W, Braun V. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol 145:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faraldo-Gómez JD, Sansom MS. 2003. Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol 4:105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- 60.Bister B, Bischoff D, Nicholson GJ, Valdebenito M, Schneider K, Winkelmann G, Hantke K, Sussmuth RD. 2004. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals 17:471–481. doi: 10.1023/B:BIOM.0000029432.69418.6a. [DOI] [PubMed] [Google Scholar]
- 61.Johnson LJ, Koulman A, Christensen M, Lane GA, Fraser K, Forester N, Johnson RD, Bryan GT, Rasmussen S. 2013. An extracellular siderophore is required to maintain the mutualistic interaction of Epichloe festucae with Lolium perenne. PLoS Pathog 9:e1003332. doi: 10.1371/journal.ppat.1003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amin SA, Green DH, Hart MC, Kupper FC, Sunda WG, Carrano CJ. 2009. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc Natl Acad Sci U S A 106:17071–17076. doi: 10.1073/pnas.0905512106. [DOI] [PMC free article] [PubMed] [Google Scholar]


