ABSTRACT
Interspecies interactions have been described for numerous bacterial systems, leading to the identification of chemical compounds that impact bacterial physiology and differentiation for processes such as biofilm formation. Here, we identified soil microbes that inhibit biofilm formation and sporulation in the common soil bacterium Bacillus subtilis. We did so by creating a reporter strain that fluoresces when the transcription of a biofilm-specific gene is repressed. Using this reporter in a coculture screen, we identified Pseudomonas putida and Pseudomonas protegens as bacteria that secrete compounds that inhibit biofilm gene expression in B. subtilis. The active compound produced by P. protegens was identified as the antibiotic and antifungal molecule 2,4-diacetylphloroglucinol (DAPG). Colonies of B. subtilis grown adjacent to a DAPG-producing P. protegens strain had altered colony morphologies relative to B. subtilis colonies grown next to a DAPG-null P. protegens strain (phlD strain). Using a subinhibitory concentration of purified DAPG in a pellicle assay, we saw that biofilm-specific gene transcription was delayed relative to transcription in untreated samples. These transcriptional changes also corresponded to phenotypic alterations: both biofilm biomass and spore formation were reduced in B. subtilis liquid cultures treated with subinhibitory concentrations of DAPG. Our results add DAPG to the growing list of antibiotics that impact bacterial development and physiology at subinhibitory concentrations. These findings also demonstrate the utility of using coculture as a means to uncover chemically mediated interspecies interactions between bacteria.
IMPORTANCE Biofilms are communities of bacteria adhered to surfaces by an extracellular matrix; such biofilms can have important effects in both clinical and agricultural settings. To identify chemical compounds that inhibited biofilm formation, we used a fluorescent reporter to screen for bacteria that inhibited biofilm gene expression in Bacillus subtilis. We identified Pseudomonas protegens as one such bacterium and found that the biofilm-inhibiting compound it produces was the antibiotic 2,4-diacetylphloroglucinol (DAPG). We showed that even at subinhibitory concentrations, DAPG inhibits biofilm formation and sporulation in B. subtilis. These findings have potential implications for understanding the interactions between these two microbes in the natural world and support the idea that many compounds considered antibiotics can impact bacterial development at subinhibitory concentrations.
INTRODUCTION
Metabolites secreted by microbes often kill other microbes. Not all bacterial natural products act as antibiotics, however; many microbial metabolites have roles as intraspecific signaling cues. For instance, quorum-sensing compounds such as acyl-homoserine lactones allow microbes to modulate their gene expression in response to cellular density and are frequently involved in regulating biofilm formation (1, 2). Another example is the developmental signaling cue goadsporin, which controls cellular development in Streptomyces species (3, 4). Compounds secreted by microbes can also act as interspecific signaling cues, altering microbial development in other bacterial species (5–8). Some metabolites with antibiotic activity have the ability to simultaneously impact cellular development, whether at subinhibitory concentrations or via other mechanistic pathways (9–13). Here, we explore the identification of interspecies interactions that lead to alterations in bacterial development in the Gram-positive model bacterium Bacillus subtilis.
B. subtilis is a bacterial model system for cellular differentiation: it develops into multiple transcriptionally distinct cell types (for example, cells that are swimming, forming biofilms, competent to take up DNA, or sporulating, among others) (14, 15). These different cell types coexist within genetically identical populations of B. subtilis cells, and many of them are regulated by the activity of the master transcriptional regulator Spo0A (16–18). Spo0A's activity is governed by the extent of its phosphorylation (Spo0A∼P): when there is little Spo0A∼P in the cell, genes involved in swimming (e.g., hag) are expressed; when there is a moderate amount of Spo0A∼P present, genes involved in biofilm formation are expressed (e.g., tapA); and when there is a high level of Spo0A∼P present, genes involved in sporulation are expressed (e.g., sspB) (16, 18). B. subtilis cells progressively transition from swimming to biofilm matrix-producing to sporulating cells as Spo0A∼P accumulates (16, 19–21). The distributions of these different cell types are spatiotemporally regulated within B. subtilis biofilms (19, 22).
Biofilms are communities of microbes living together in a self-produced extracellular matrix that binds the cells to one another and often adheres them to a surface. In B. subtilis this extracellular matrix is composed of an exopolysaccharide and a protein (TasA) that forms amyloid fibrils (23, 24). Robust biofilm formation can be detected either visually, due to a wrinkly colony phenotype (22), or using fluorescent transcriptional reporters, such as a B. subtilis strain containing PtapA-yfp (a construct in which the promoter for the tasA gene, PtapA, drives the production of yellow fluorescent protein [YFP]) (19). We recently used this PtapA-yfp B. subtilis strain to identify numerous soil organisms (predominantly other Bacillus species) that induced biofilm gene expression in B. subtilis (5). Thus, cellular differentiation in B. subtilis can be affected by both intraspecific and interspecific signaling cues (5, 25, 26).
We hypothesized that other soil microbes might secrete metabolites that would inhibit rather that stimulate cellular differentiation in B. subtilis. We explored this possibility by conducting a coculture screen analogous to that described above but using a B. subtilis reporter strain that fluoresces when the tapA promoter is repressed. In this way we identified two pseudomonads that inhibited biofilm gene expression in B. subtilis. Using bioassay-guided fractionation, we identified 2,4-diacetylphloroglucinol (DAPG) as the candidate active molecule produced by P. protegens (27, 28). B. subtilis colonies grown adjacent to P. protegens colonies producing DAPG had altered colony morphologies relative to B. subtilis colonies grown adjacent to a mutant strain of P. protegens unable to produce DAPG (phlD strain) (29). Using a liquid pellicle assay and flow cytometry, we show that subinhibitory concentrations of purified DAPG delays tapA gene expression in B. subtilis. We further show that subinhibitory concentrations of DAPG lead to a reduction in biomass (as detected by crystal violet [CV] staining of biofilms) and a reduction in spores formed in liquid culture. Thus, we have identified an interspecies interaction that inhibits biofilm formation and sporulation in B. subtilis and characterized the ability of the antibiotic DAPG to act as an interspecific signaling molecule that inhibits bacterial differentiation even at subinhibitory concentrations.
MATERIALS AND METHODS
Strains, media, and culture conditions.
The B. subtilis strain was NCIB3610 from our laboratory collection, except where strain PY79 was used as noted; Pseudomonas protegens Pf-5 (previously Pseudomonas fluorescens [30]) and Pseudomonas protegens Pf-5 phlD were from Joyce Loper (U.S. Department of Agriculture). For routine growth, cells were grown on Luria-Bertani (LB)–Lennox medium (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl per liter). For biofilm and sporulation assays, cells were grown in either MSgg broth or on solid (1.5% Bacto agar) MSgg medium (5 mM potassium phosphate [pH 7], 100 mM morpholinepropanesulfonic acid [MOPS; pH 7], 2 mM MgCl2, 700 μM CaCl2, 50 μM MnCl2, 50 μM FeCl3, 1 μM ZnCl2, 2 μM thiamine, 0.5% glycerol, 0.5% glutamate). All plates were routinely incubated at 30°C, and all broth cultures were shaken at 28°C. The following antibiotics were used (final concentrations): macrolide, lincosamide, and streptogramin (MLS; 1 μg ml−1 erythromycin and 25 μg ml−1 lincomycin); spectinomycin (100 μg ml−1); and chloramphenicol (5 μg ml−1).
Soil screen.
Seven independent soil samples were collected within a 1-mile radius of each other near Squam Lake, NH, during July 2013. The soil samples were from forest areas relatively unperturbed by foot traffic. One to two centimeters of the topmost layer of soil was scraped away before the sample was collected with a metal spatula sterilized with 70% ethanol. Soil samples were sieved to remove large debris and roots and then resuspended in sterile 0.85% NaCl solution via vigorous mixing. Glycerol was added to a final concentration of 20%, and 200-μl aliquots were stored at −80°C. For coculture plates, 100 μl of a thawed soil aliquot and 50 μl of the B. subtilis PtapA-PROR strain (where PROR stands for promoter, repressor, operator, and reporter) that had been resuspended in LB medium to an optical density at 600 nm (OD600) of ∼5 × 10−4 were mixed and spread on a 0.075× MSgg agar plate. Plates were incubated at 30°C for 16 to 20 h before being examined using a Stereo Discovery V8 dissecting scope equipped with a 0.63× Achromat S objective lens and a Lumen Dynamics XCite 120 fluorescence lamp. Fluorescence images were obtained using a 1,000-ms exposure with Zen software (Zeiss); brightness and contrast were linearly adjusted using Adobe Photoshop CS6. Bacteria of interest were physically isolated from the coculture plates and retested using a microcolony lawn assay as previously described (31).
Microcolony lawn assay.
A total of 150 μl of the B. subtilis PtapA-PROR strain at an OD600 of ∼5 × 10−4 was spread on a 0.075× MSgg agar plate and allowed to dry. Resuspended cells of soil isolates to be retested were then spotted as 1-μl cell suspensions at an OD600 of 0.5. To assay liquid samples, wells were made in the plate using autoclaved drinking straws approximately 6 mm in diameter. Thirty microliters of liquid was added and allowed to dry before plates were incubated at 30°C and examined daily for fluorescence from 24 to 72 h.
Conditioned medium.
P. protegens Pf-5 was grown overnight on LB plates. A single colony was resuspended in 50 ml of MSgg broth. Cultures were shaken at 250 rpm at 28°C for 24 h before being centrifuged at 4,500 rpm at 4°C with a Sorvall Legend ST40R (Thermo Scientific) for 30 min. The supernatant was filter sterilized with a 0.22-μm-pore-size Steriflip filter (Millipore) and stored at −20°C until use.
HPLC.
Initial fractionations of the conditioned medium were carried out using 6-ml C18 Sep-Pak columns (Waters) prepared as recommended by the manufacturer. Thirty milliliters of culture supernatant (cell-free conditioned medium) was loaded onto the column, washed, and then eluted using a methanol step gradient (10 ml each of 0%, 20%, 40%, 60%, 80%, and 100% methanol). For high-pressure liquid chromatography (HPLC), 500 ml of conditioned medium and 9 g of Amberlite XAD16N (Sigma-Aldrich) were stirred together at 300 rpm for 2 h at room temperature before being packed into a RediSep Rf preparative chromatography column, rinsed with 50 ml of H2O, and eluted using 50 ml of methanol. The eluant was collected, concentrated using a rotovap to ∼5 ml, and injected onto a reverse-phase HPLC column (Shimadzu Prominence with a 5-ml loop). A gradient between 45 and 60% acetonitrile was run, and fractions and UV data were collected. Solvent was removed from fractions of interest using a SpeedVac.
LC-MS.
Liquid chromatography-mass spectrometry (LC-MS) data for the HPLC fractions were acquired on an Agilent 6520 Accurate-Mass quadrupole time-of-flight (Q-TOF) mass spectrometer with an electrospray ionization (ESI) source in positive ion mode. The drying gas temperature was held at 300°C, and the fragmentor voltage was 175 V. Compounds were separated using a reverse-phase Kinetex column with a gradient of solvents. Acetonitrile with 0.1% formic acid was run isocratically for 2 min at 2%, followed by a gradient from 2 to 95% over 13 min, and then held at 95% for 2 min against water with 0.1% formic acid.
NMR.
Spectra for 1H and 13C nuclear magnetic resonance (NMR) were recorded at room temperature (RT) with a Bruker Avance III instrument (600 MHz and 150 MHz, respectively).
Colony coculture.
Bacillus subtilis was spotted at a 0.5-cm distance from either Pseudomonas protegens Pf-5 or Pseudomonas protegens Pf-5 phlD onto an MSgg plate; 1 μl of cell suspension at an OD600 of 0.5 was used for both bacteria. Plates were incubated at 30°C for coculture growth. Images were captured at 24, 48, or 72 h using a Stereo Discovery V8 dissecting scope equipped with a 0.63× Achromat S objective lens and an AxioCam with Zen software (Zeiss). Brightness and contrast of images were linearly adjusted using Photoshop CS6.
MIC determinations.
B. subtilis was grown to an OD600 of 1.0 in 3 ml of LB broth at 37°C. Ten microliters of liquid culture was diluted into 1 ml of MSgg broth in 24-well plates. Ten microliters of DAPG (various concentrations) in 100% methanol was added to a given well. Plates were sealed with breathable Aeraseals (Excel Scientific) to retain moisture and prevent cross-contamination of cells and shaken at 30°C at 300 rpm (a speed sufficient to disrupt pellicle formation). At each time point, three OD600 readings per well were measured using a Tecan plate reader and averaged. The average of two biological replicates is shown; bars are standard deviations.
Pellicle growth and flow cytometry analysis.
B. subtilis was grown to an OD600 of ∼1.0 in LB medium at 37°C. Twenty microliters of this culture was added to 1 ml of 1× MSgg in a 24-well plate (Falcon). To a given well, DAPG (or the equivalent volume of methanol) was added to a final concentration of 1.12 μM. Plates were covered with an Aeraseal and grown without agitation at 30°C for 18 or 38 h. Images were then captured using a Stereo Discovery V8 dissecting scope equipped with a 0.63× Achromat S objective lens and an AxioCam with Zen software (Zeiss). For flow cytometry analysis, pellicles were harvested as described by Vlamakis et al. (17). Briefly, cells were fixed in 4% paraformaldehyde, washed in phosphate-buffered saline (PBS), resuspended in GTE buffer (20 mM Tris, pH 8, 50 mM glucose, 10 mM EDTA), and stored at 4°C until use. Samples were sonicated and passed through a 38-μm-pore-size filter before being diluted in PBS and measured on a BD LSR II flow cytometer (BD Biosciences) operating a solid-state laser at 488 nm. For each sample, 50,000 events were measured. Data were captured using FACSDiva software (BD Biosciences) and analyzed using FlowJo, version 10.0.7r2. The percentages shown were calculated by taking the percentage of the cell populations with fluorescence greater than the level of the wild-type control strain for each of four biological replicates and averaging them. Significance was evaluated using a Student's t test.
Crystal violet biofilm quantification.
Crystal violet staining of biofilms was performed essentially as described by Branda et al. (23). Briefly, B. subtilis strain PY79 was grown to an OD600 of 1.0 in 3 ml of LB broth at 37°C. The liquid culture was diluted 500-fold in MSgg broth containing 200 μM NaCl, 50 μg/ml tryptophan, and 50 μg/ml phenylalanine. DAPG in 100% methanol was added to a final concentration of 1.12 μM. Three hundred microliters of the two dilutions was added to six borosilicate glass tubes each and incubated at 37°C for 40 h without agitation. After 40 h, 500 μl of 1% crystal violet was added to each tube, and tubes were left to sit for 15 min. Crystal violet was removed from the tubes, and each tube was gently rinsed three times with 1 ml of double-distilled H2O (ddH2O). Tubes were dried overnight at RT. To quantify biofilm, 1 ml of 33% acetic acid was added. After 10 min, the sample was diluted 5-fold, and OD570 measurements were taken. For each biological replicate, the values from the six technical replicates were averaged, and the values of the DAPG-treated samples were normalized to the value of the methanol control. The averaged fold change is shown for three biological replicates; they were analyzed for significance using a Mann-Whitney U test.
Spore counts.
B. subtilis was grown to an OD600 of 1.0 in 3 ml of LB broth at 37°C. Ten microliters of liquid culture was diluted into 50 ml of MSgg broth and grown with shaking at 250 rpm at 28°C. At each time point, 1 ml of culture was taken and sonicated at setting 3 with a 550 Sonic Dismembrator (Fisher Scientific). Half of the sample was reserved, and the other half was heated at 80°C for 30 min. Both unheated and heated samples were serially diluted in LB broth and plated on LB plates for colony counting after overnight growth at 30°C. Significance was calculated using a Student's t test.
RESULTS
Construction of a reporter strain to detect inhibition of cellular differentiation.
To discover microbes that secrete biofilm-inhibiting compounds, we designed a B. subtilis reporter strain called PtapA-PROR (for promoter, repressor, operator, and reporter) that produces YFP (yellow fluorescent protein) when the promoter for the tapA operation (PtapA) is repressed. The PtapA-PROR strain contains the following: (i) PtapA driving the production of the cI protein from Enterobacter phage λ (λcI) and (ii) the λ promoter (λPhypercIo2) containing the λcI operator binding sites driving expression of the yfp gene (see Fig. S1 in the supplemental material) (32). Thus, when PtapA-PROR is plated onto biofilm-inducing medium (MSgg), the PtapA promoter is active, λcI is produced, and yfp expression is turned off. This results in a nonfluorescent B. subtilis colony. When PtapA-PROR is plated onto biofilm-inhibiting medium (LB), the PtapA promoter is repressed, no λcI protein is produced, and the λPhypercIo2 promoter drives production of YFP. This strain thus allows us to detect the inhibition of biofilm gene expression in B. subtilis colonies by other microbes as an activation of fluorescence when colonies are grown on MSgg.
Coculture screen to identify soil microbes that inhibit biofilm gene expression in B. subtilis.
Armed with the PtapA-PROR strain, we used it in a modification of a coculture screen designed to identify interspecies interactions (5, 31). We grew B. subtilis PtapA-PROR in mixed coculture with diluted soil on 0.075× MSgg agar plates and examined the plates for fluorescence after 16 to 20 h of growth. MSgg agar was used to stimulate biofilm formation (22), and the 0.075×-diluted concentration was used to reduce colony size and facilitate our screening. We screened approximately 1.9 × 106 soil colonies from seven soil samples before an instance of fluorescence induction was observed (Fig. 1A). We isolated bacteria from the nonfluorescent colony closest to the fluorescent B. subtilis colonies and retested their ability to induce fluorescence in PtapA-PROR B. subtilis using a microcolony lawn assay.
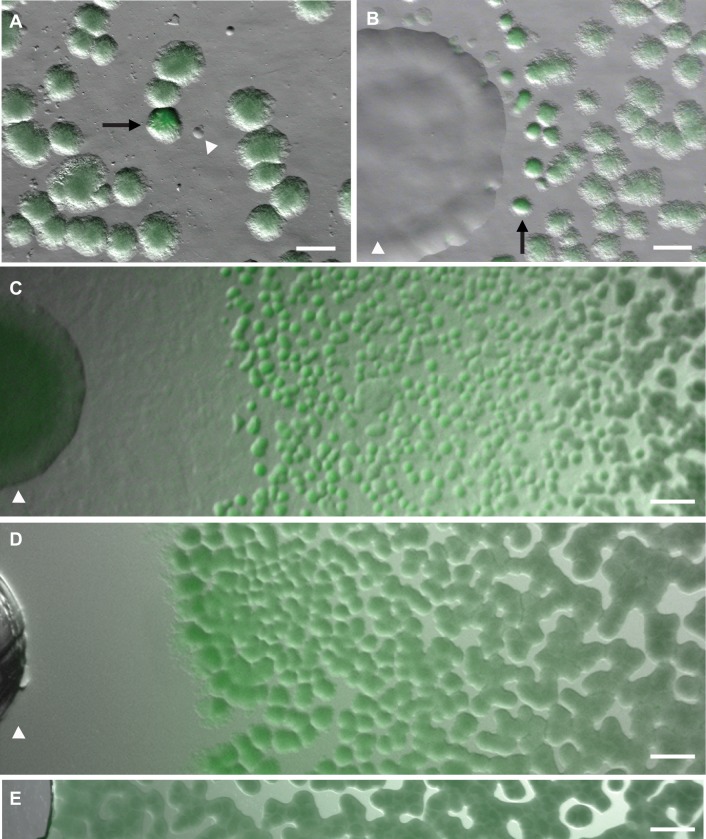
FIG 1.
Coculture reveals that P. putida and P. protegens inhibit biofilm formation in B. subtilis. (A) A coculture screen of B. subtilis PtapA-PROR mixed with soil on the biofilm-inducing agar medium MSgg. A soil colony (arrowhead) inhibited biofilm gene expression in the nearby B. subtilis colony (arrow), as indicated by the increased fluorescence of the B. subtilis colony (1 day's growth). Scale bar, 500 μm. (B) This P. putida soil isolate (arrowhead) was retested in a microcolony lawn assay, where it inhibited biofilm formation in B. subtilis PtapA-PROR colonies (arrow), as indicated by the increased fluorescence and smoother morphology of these colonies (1 day's growth). Scale bar, 1 mm. (C) P. protegens Pf-5 (arrowhead) inhibited biofilm gene expression in a microcolony lawn of PtapA-PROR B. subtilis, resulting in a large zone of fluorescing B. subtilis colonies (3 days' growth). Scale bar, 1 mm. (D) The conditioned medium from P. protegens (placed into a well created in the agar; arrowhead) inhibited biofilm gene expression in PtapA-PROR B. subtilis (3 days' growth). Scale bar, 1 mm. (E) The PtapA-PROR B. subtilis lawn adjacent to a well containing MSgg medium as a control displays similar background fluorescence across the entire image (3 days' growth). Scale bar, 1 mm.
The microcolony lawn assay involves placing PtapA-PROR B. subtilis cells onto a 0.075× MSgg plate at a concentration that results in a lawn of small colonies and then spotting a suspension of the cells being tested onto it for coculture growth. In addition to inhibiting the growth of B. subtilis, this soil isolate also induced fluorescence in the B. subtilis colonies closest to it, indicating that it was secreting a compound that inhibited biofilm gene expression (Fig. 1B). Consistent with this interpretation, the fluorescent B. subtilis colonies displayed smoother colony morphology than B. subtilis colonies more distant from the isolate (Fig. 1B).
Pseudomonas putida and Pseudomonas protegens inhibit biofilm gene expression in B. subtilis.
We sequenced the 16S rRNA gene of this isolate and identified it as a Pseudomonas putida strain. Inspired by previous work (where the ability to induce biofilm gene expression in B. subtilis was conserved broadly within the Bacillus genus [5]), we tested other Pseudomonas species for their ability to activate fluorescence in PtapA-PROR B. subtilis. Pseudomonas syringae was unable to activate PtapA-PROR fluorescence; however, Pseudomonas protegens Pf-5 (previously Pseudomonas fluorescens [30]) elicited fluorescence in PtapA-PROR B. subtilis (Fig. 1C).
Identification of 2,4-diacetylphloroglucinol as the compound inhibiting biofilm gene expression in B. subtilis.
In an effort to identify the biofilm-inhibiting compound(s) produced by these pseudomonads, we grew liquid cultures of P. putida and P. protegens and isolated their conditioned (cell-free) media. We tested the ability of these conditioned media to activate fluorescence in B. subtilis PtapA-PROR using a microcolony lawn assay in which we placed the conditioned medium into a well cored out of the agar in place of a bacterial colony. The conditioned medium from P. protegens strongly induced fluorescence in the PtapA-PROR B. subtilis strain (Fig. 1D). For unclear reasons, we were unable to obtain conditioned medium from P. putida (grown in either liquid or solid culture) that maintained activity. We therefore focused our efforts on the biofilm-inhibiting compound produced by P. protegens.
We applied the P. protegens conditioned medium to a solid-phase C18 column and separated it into fractions using a 20% step gradient (progressively running 0%, 20%, 40%, 60%, 80%, or 100% methanol over the column). Based on the activity of the resulting six fractions, which we tested in the microcolony lawn assay, the active compound was eluted during the 60% methanol step (i.e., eluted within the 40 to 60% methanol range). This fraction also retained antibiotic activity. The conditioned medium was then further separated using HPLC, which led to seven peaks that possessed strong UV signals. Only one of the fractions associated with these peaks specifically activated PtapA-PROR fluorescence in the microcolony lawn assay (see Fig. S2 in the supplemental material).
NMR of the bioactive HPLC fraction resulted in a distinct 1H spectrum that indicated a highly pure, small compound (see Fig. S3 in the supplemental material). In combination with a 13C NMR spectrum (see Fig. S3), the compound was identified as 2,4-diacetylphloroglucinol (DAPG) (Fig. 2). DAPG is a broad-spectrum antibiotic whose biosynthesis genes are conserved within many fluorescent pseudomonads (27, 28, 33, 34). The presence of DAPG in the active fraction was confirmed with LC-MS (see Fig. S3). We therefore identified DAPG as a candidate molecule potentially responsible for the ability of P. protegens to inhibit biofilm gene expression in B. subtilis (as monitored by PtapA-PROR fluorescence).
FIG 2.
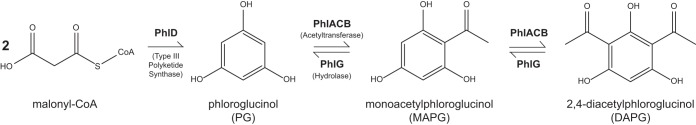
The biosynthesis pathway and genes involved in DAPG production (27, 28). CoA, coenzyme A.
DAPG production by P. protegens affects colony morphology in B. subtilis.
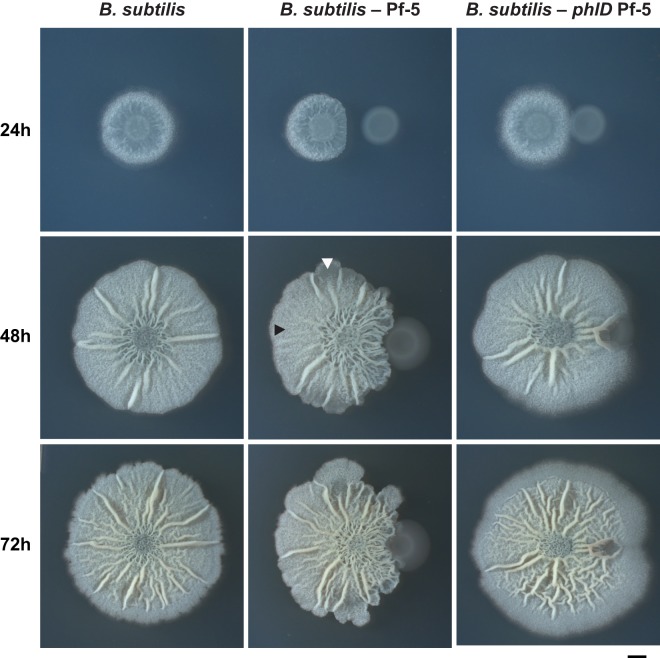
B. subtilis colony morphology and cell differentiation have been well characterized on full-strength (1×) MSgg agar plates (19, 22, 35, 36). We therefore used this as a platform to characterize the effect of P. protegens DAPG production on B. subtilis development. We grew B. subtilis colonies on 1× MSgg and examined how their morphologies changed over time when they were grown in adjacent cocultures with P. protegens (Fig. 3). B. subtilis colonies grown next to wild-type P. protegens exhibited changes in morphology, with altered wrinkling patterns and an apparent decrease in fruiting body formation relative to B. subtilis colonies grown alone (Fig. 3; see also Fig. S4 in the supplemental material). Fruiting bodies are the raised aerial structures that give B. subtilis colonies their overall fuzzy and textured appearance (in addition to the larger macroscopic wrinkles due to biofilm formation) and are the sites of sporulation during B. subtilis development (22). These coculture phenotypes are a direct result of DAPG production by Pf-5 since they were not observed when B. subtilis was grown next to a P. protegens strain unable to produce DAPG due to the deletion of phlD, a major DAPG biosynthesis gene (27, 29, 37) (Fig. 2 and 3).
FIG 3.
DAPG production by P. protegens alters colony morphology in B. subtilis when they are grown in coculture. B. subtilis grown on 1× MSgg agar either alone, next to the DAPG-producing wild-type P. protegens Pf-5, or next to the DAPG-biosynthesis mutant P. protegens Pf-5 phlD after 24, 48, and 72 h. In the cocultures, B. subtilis is shown on the left of each panel. The centers of the initial cell suspensions were placed 0.5 cm away from each other. In the center panel, the black arrowhead points to a region of the colony exhibiting normal fruiting body formation, while the white arrowhead indicates a region where a lack of fruiting bodies leads to smooth colony morphology (see Fig. S4 in the supplemental material). Scale bar, 2 mm.
Determination of the MIC of DAPG on B. subtilis.
Because it was impossible to know the concentration of DAPG present in this colony assay (both due to unknown levels of DAPG production by Pf-5 as well as to its diffusion through the agar), we decided to use liquid assays instead to further characterize the effects of this compound on B. subtilis. This would allow us to ensure the exposure of B. subtilis to a consistent concentration of DAPG. We also wanted to distinguish whether the observed colony phenotypes were due to the killing activity of DAPG or to another effect independent of its antibiotic activity. To test this, we determined the MIC of DAPG on B. subtilis by measuring the growth of B. subtilis in liquid shaken cultures containing various concentrations of DAPG (see Fig. S5 in the supplemental material). These growth curves showed that a concentration of 8.92 μM DAPG had a dramatic negative impact on growth, a concentration of 4.46 μM DAPG caused a moderate delay in growth that was overcome with time, and a concentration of 2.28 μM DAPG had no impact on the growth rate of B. subtilis (see Fig. S5). We elected to be conservative and use 1.12 μM DAPG for most of our liquid assays. This is 2-fold less than the concentration at which any growth inhibition was observed in B. subtilis (2.28 μM) and ensured that any effects we observed were not due to an inhibitory effect of DAPG on B. subtilis growth.
Subinhibitory levels of purified DAPG inhibit biofilm gene expression in B. subtilis.
Based on our PtapA-PROR results and the impact that P. protegens had on B. subtilis colony morphology (Fig. 3), it appeared that DAPG affected cellular differentiation in B. subtilis. We focused on the B. subtilis phenotypes controlled by the master regulator Spo0A: motility, biofilm formation, and sporulation (14). We grew strains of B. subtilis containing individual fluorescent transcriptional reporters (Phag-yfp [motility], PtapA-yfp [biofilm formation], and PsspB-yfp [sporulation]) (19) so that we could monitor the proportion of cells expressing genes specific for each of these cell types. We allowed these strains to form pellicles in standing liquid cultures in either the presence or absence of 1.12 μM DAPG, harvested the cells from the pellicles at 18 h, and fixed them for flow cytometry to analyze their fluorescence at the single-cell level. This allowed us to determine the effect of subinhibitory concentrations of DAPG on the differentiation of B. subtilis into these different cell types during pellicle formation.
Our flow cytometry data indicated that there were no differences in motility gene expression levels between the treatments, and this time point was too early to detect any sporulating cells (Fig. 4). However, pellicles grown with 1.12 μM DAPG had, on average, a 50% reduction in PtapA gene transcription (Fig. 4), indicating that subinhibitory concentrations of DAPG inhibited biofilm gene transcription. Note that these data indicate that many B. subtilis cells are in alternative cell states not captured by these three Spo0A-specific reporters (e.g., protease producers, competent cells, swarming cells, etc.) that may also have effects on biofilm formation but are not being directly monitored in this experiment (14, 38, 39).
FIG 4.
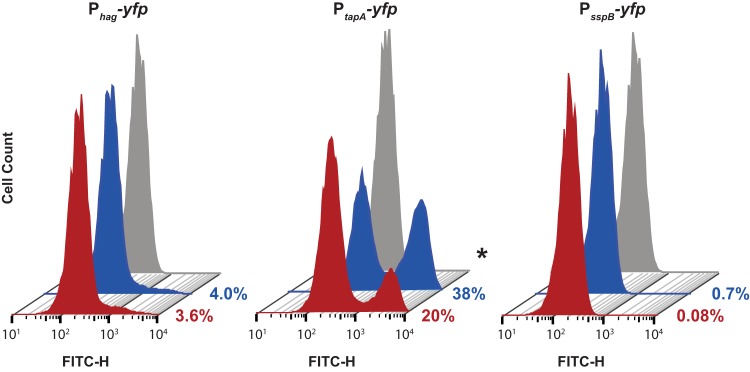
Flow cytometry analysis of B. subtilis cells from pellicles containing cell-type-specific reporters. B. subtilis strains containing the reporters for motility (Phag-yfp), biofilm formation (PtapA-yfp), and sporulation (PsspB-yfp) were grown as pellicles for 18 h in 1× MSgg before being resuspended, fixed, and analyzed by flow cytometry. Representative histograms of the fluorescein isothiocyanate (FITC)-H positive (fluorescent) cells are shown; 50,000 cells were analyzed for each histogram. Pellicles were either grown in the presence of subinhibitory concentrations of DAPG (red) or in an equivalent volume of methanol as a control (blue); the gray histograms are from wild-type control cells containing no reporter construct (and thus represent the background signal of nonfluorescent cells). The percentages of fluorescent cells in each sample (an average obtained from four independent experiments) are indicated to the right of the histograms. Significance was evaluated using a Student's t test with unequal variance. *, P < 0.05.
Subinhibitory levels of purified DAPG inhibit biofilm formation in B. subtilis.
To test whether DAPG's impact on biofilm gene transcription correlated with an effect on biofilm formation itself, we allowed B. subtilis cells to continue growing as pellicles for 38 h. This resulted in the formation of morphological wrinkles characteristically associated with biofilm formation (22) (Fig. 5A). These wrinkles were greatly diminished in the B. subtilis cultures exposed to subinhibitory concentrations of DAPG (Fig. 5A). While this pellicle assay allows a visualization of the biofilm morphology, it is not well suited to biomass quantification due to the fact that the pellicles form almost exclusively on the air-liquid interface. We therefore adopted an assay from Branda et al. in which they showed that the related B. subtilis strain PY79 (40) forms biofilms on borosilicate surfaces, which can be quantified using crystal violet (CV) staining (23). Cultures were grown for 40 h with or without 1.12 μM DAPG and stained with 1% CV (Fig. 5B), and then the CV stain was solubilized and quantified. After values were normalized to those of the methanol-treated control, we observed an approximately 20% reduction between the untreated and DAPG-treated samples (Fig. 5C).
FIG 5.
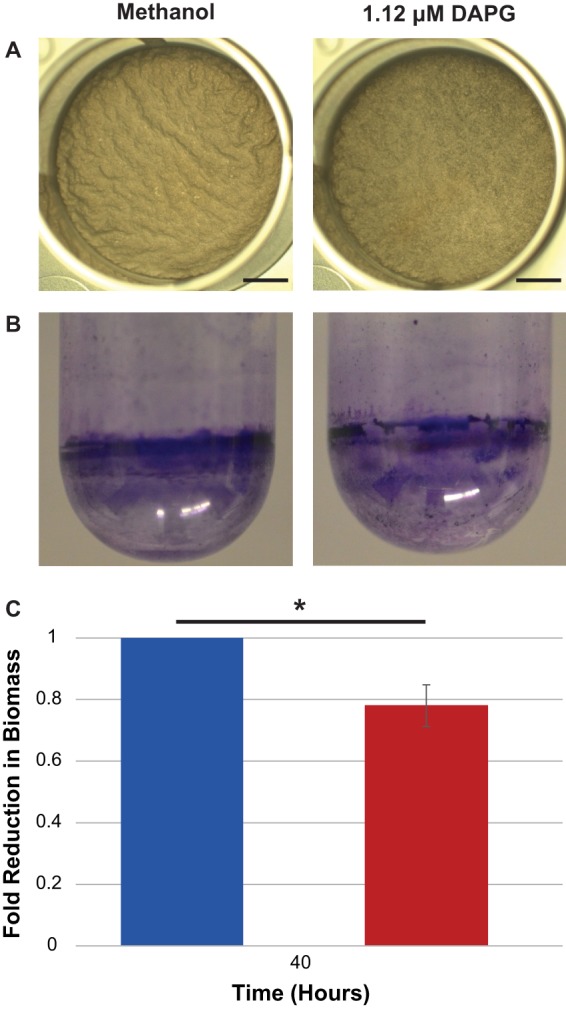
Subinhibitory concentrations of DAPG inhibit biofilm formation in B. subtilis. (A) B. subtilis pellicles grown for 38 h in 1× MSgg in the presence of 1.12 μM DAPG are less wrinkly than control samples treated with methanol. (B) Crystal violet staining of the biofilm formed by B. subtilis strain PY79 on the side of glass culture tubes. (C) Quantification of the crystal violet staining. Values from each of three biological replicates were normalized to the control value and then averaged (error bar is standard deviation). Significance was determined using a Mann-Whitney U test. **, P < 0.05.
Subinhibitory levels of purified DAPG inhibit sporulation in B. subtilis.
Based on the coculture colony morphology of B. subtilis grown with Pf-5 (Fig. 3), as well as the known relationship between the regulation of biofilm formation and sporulation (41), we suspected that DAPG might impact fruiting body formation and thus sporulation in B. subtilis. We therefore performed spore counts on B. subtilis cultures grown in the presence of 2.23 μM DAPG, monitoring both the total number of viable cells (vegetative cells and spores) as well as the total number of viable spores at 24, 30, and 42 h. To ensure that our DAPG-treated cultures were not being inhibited during these experiments, we took OD600 readings of the growth of these cultures to supplement our initial MIC calculations (see Fig. S6 in the supplemental material). Consistent with these OD600 readings, the total number of viable cells was equal between the control and DAPG-treated cultures at all time points (see Fig. S7 in the supplemental material). We calculated the percentage of spores formed for both the untreated and DAPG-treated cultures and normalized these values to those from the untreated control, which allowed us to calculate a fold reduction in the percentage of spores formed when cultures were treated with DAPG (Fig. 6). B. subtilis grown in the presence of 2.23 μM DAPG had a significantly reduced number of spores formed, with almost 10-fold fewer spores than the untreated samples.
FIG 6.
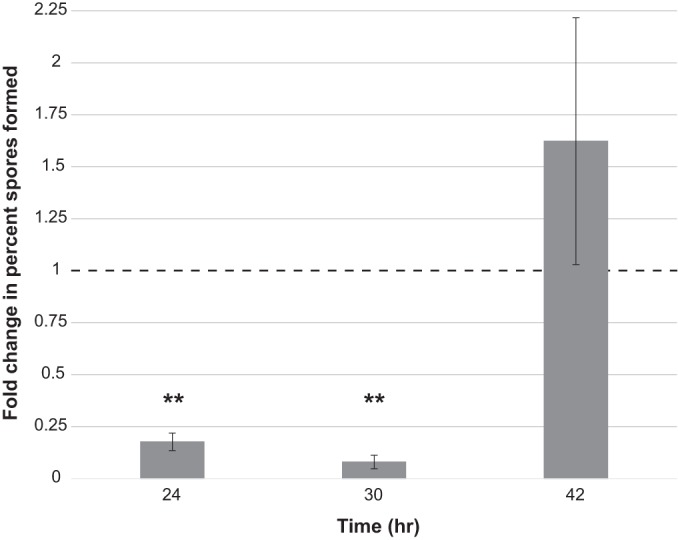
Subinhibitory concentrations of DAPG inhibit spore formation in B. subtilis. B. subtilis liquid shaking cultures were grown with or without 2.23 μM purified DAPG and sampled at 24, 30, and 42 h of growth. Samples were lightly sonicated, divided into heated (80°C for 30 min) and unheated portions, diluted, and plated to count viable spores (heated portion) and all viable cells (unheated portion). The percent spores in each biological replicate was calculated and normalized to the values of the untreated samples for that experiment. The average fold change (in percent spores) for three biological experiments was then calculated for each time point. The dashed line indicates the normalized methanol control value of 1 (indicating equivalent percent spores formed between treated and untreated samples). Error bars represent standard deviations. Significance was calculated using a Student's t test. **, P < 0.005.
DISCUSSION
We have identified an interspecies interaction between P. protegens and B. subtilis that is mediated by subinhibitory concentrations of the antibiotic DAPG. This compound alters B. subtilis colony morphology and is capable of inhibiting its differentiation into both biofilm-producing cells and spores, even at concentrations at which it does not kill. We speculate that the ability of P. protegens to produce DAPG and therefore inhibit biofilm formation and sporulation in B. subtilis might be relevant in their shared natural habitat of the plant root. Biofilm formation is required for B. subtilis to colonize plant roots (42, 43). DAPG could confer a competitive advantage to P. protegens during root colonization in two ways: both by directly inhibiting B. subtilis growth in the areas closest to P. protegens (where the concentrations of DAPG are highest) and by preventing biofilm formation (and therefore colonization [42]) of B. subtilis in the regions beyond those areas (as the concentration of DAPG falls to subinhibitory levels further from P. protegens).
The role of sporulation in the interaction of B. subtilis with plant roots has been less well characterized, making it challenging to speculate on whether inhibiting sporulation could provide P. protegens a competitive advantage. Under conditions when B. subtilis is exposed to rice roots for short periods of time (2 h), transcription of sporulation genes is downregulated (44), whereas B. subtilis cells grown on Arabidopsis roots form spores between 24 and 48 h (42). It could be that sporulation inhibition results in B. subtilis cells remaining susceptible to the killing action of DAPG for longer periods of time, for example, as this compound accumulates in the local environment around P. protegens. Experiments exploring the spatiotemporal dynamics of P. protegens and B. subtilis on plant roots would better reveal how the production of DAPG by P. protegens impacts the colonization and coexistence of these two bacteria when they share this environmental niche.
Our data indicate that DAPG belongs to the growing list of antibiotics shown to affect bacterial transcription, differentiation, and colony morphology at subinhibitory concentrations (9–12, 44). In contrast to DAPG, most antibiotics that impact biofilm formation at subinhibitory concentrations tend to stimulate its production rather than inhibit it (45, 46). How B. subtilis senses DAPG to delay biofilm formation is still unknown; we have shown that monoacetylphloroglucinol (MAPG), which is the structurally similar precursor to DAPG (Fig. 2), is unable to activate biofilm gene expression in B. subtilis (see Fig. S8 in the supplemental material), indicating a degree of structural specificity for this signaling cue. Interestingly, the dialkylresorcinols, molecules with a single-ring structure similar to that of DAPG, were recently shown to act as interkingdom communication cues controlling virulence in Photorhabdus asymbiotica (47). The dialkylresorcinols are sensed by LuxR “solos” (LuxR receptors with no associated LuxI synthetases) (47). Efforts to identify genes involved in the ability of B. subtilis to detect DAPG might therefore use a candidate approach and examine LuxR-like proteins before searching more broadly using a transposon mutant library screen.
How and whether the mechanisms that DAPG uses to inhibit biofilm formation and sporulation in B. subtilis are linked are still unknown. One possibility is that DAPG possesses two distinct activities acting on these two pathways. If this is the case, DAPG would join decoyinine, a GMP synthetase inhibitor produced by Streptomyces spp. (48, 49), as one of the only interspecies signaling cues known to alter the timing of sporulation in B. subtilis. A simpler explanation might be that the dual impact of DAPG on cell differentiation in B. subtilis is a consequence of the known regulatory connections between biofilm formation and sporulation in B. subtilis. These two processes are regulated by the concentration of Spo0A∼P within a cell: the accumulation of Spo0A∼P leads cells to first produce biofilm matrix before going on to sporulate (19). Thus, if DAPG is affecting B. subtilis biofilm formation by altering the accumulation or degradation of Spo0A∼P (or affecting Spo0A's ability to interact with its downstream targets), it might also disrupt the subsequent differentiation of B. subtilis cells into spores.
We have characterized the ability of DAPG produced by P. protegens to inhibit cell differentiation in B. subtilis. Our original coculture screen also identified another pseudomonad, P. putida, as producing a secreted compound that inhibits biofilm gene expression in B. subtilis. It is unlikely that the activity produced by P. putida is also due to DAPG for the following reasons: (i) P. putida did not kill B. subtilis under our coculture screen conditions; (ii) the biosynthesis genes for DAPG production are exclusively conserved within a small clade within the fluorescent pseudomonads, and sequenced P. putida strains do not possess them (50). In contrast to the results described here (that P. putida and P. protegens inhibit biofilm gene expression in B. subtilis), other soil pseudomonads have been shown to stimulate biofilm formation in B. subtilis (5). These results highlight the diverse activities that the pseudomonads have toward B. subtilis and indicate that additional secreted compounds affecting developmental processes in B. subtilis produced by this group of bacteria remain to be identified.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Roberto Kolter and Ann Hochschild (Harvard Medical School) for their insightful advice and help regarding the construction of the PtapA-PROR strain, Walter Wever for help performing LC-MS and NMR, and Joyce Loper (U.S. Department of Agriculture) for providing the P. protegens Pf-5 and Pf-5 phlD strains.
The University of North Carolina (UNC) Flow Cytometry Core Facility is supported in part by an NCI Center Core Support Grant (P30CA016086) to the UNC Lineberger Comprehensive Cancer Center. E.A.S. and M.J.P. were supported by UNC-CH Start-up Funds. M.J.P. was also funded in part by a Summer Undergraduate Research Fellowship from the Office for Undergraduate Research at the University of North Carolina at Chapel Hill.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02535-14.
REFERENCES
- 1.Fuqua C, Parsek MR, Greenberg EP. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet 35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 2.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.Onaka H, Tabata H, Igarashi Y, Sato Y, Furumai T. 2001. Goadsporin, a chemical substance which promotes secondary metabolism and morphogenesis in streptomycetes. I. Purification and characterization. J Antibiot (Tokyo) 54:1036–1044. doi: 10.7164/antibiotics.54.1036. [DOI] [PubMed] [Google Scholar]
- 4.Willey JM, Gaskell AA. 2011. Morphogenetic signaling molecules of the streptomycetes. Chem Rev 111:174–187. doi: 10.1021/cr1000404. [DOI] [PubMed] [Google Scholar]
- 5.Shank EA, Klepac-Ceraj V, Collado-Torres L, Powers GE, Losick R, Kolter R. 2011. Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proc Natl Acad Sci U S A 108:E1236–E1243. doi: 10.1073/pnas.1103630108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straight PD, Kolter R. 2009. Interspecies chemical communication in bacterial development. Annu Rev Microbiol 63:99–118. doi: 10.1146/annurev.micro.091208.073248. [DOI] [PubMed] [Google Scholar]
- 7.Ryan RP, Dow JM. 2008. Diffusible signals and interspecies communication in bacteria. Microbiology 154:1845–1858. doi: 10.1099/mic.0.2008/017871-0. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Ji J, Li X, Wang J, Li S, Pan G, Fan K, Yang K. 2014. Angucyclines as signals modulate the behaviors of Streptomyces coelicolor. Proc Natl Acad Sci U S A 111:5688–5693. doi: 10.1073/pnas.1324253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 10.Fajardo A, Martinez JL. 2008. Antibiotics as signals that trigger specific bacterial responses. Curr Opin Microbiol 11:161–167. doi: 10.1016/j.mib.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Romero D, Traxler MF, Lopez D, Kolter R. 2011. Antibiotics as signal molecules. Chem Rev 111:5492–5505. doi: 10.1021/cr2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yim G, Wang HH, Davies J. 2007. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci 362:1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleich R, Watrous J, Dorrestein PC, Bowers AA, Shank EA. 23 February 2015. Thiopeptide antibiotics stimulate biofilm formation in Bacillus subtilis. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1414272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez D, Vlamakis H, Kolter R. 2009. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol Rev 33:152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 15.Murray EJ, Kiley TB, Stanley-Wall NR. 2009. A pivotal role for the response regulator DegU in controlling multicellular behaviour. Microbiology 155:1–8. doi: 10.1099/mic.0.023903-0. [DOI] [PubMed] [Google Scholar]
- 16.Fujita M, Gonzalez-Pastor JE, Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol 187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. 2013. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol 11:157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol Microbiol 50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 19.Vlamakis H, Aguilar C, Losick R, Kolter R. 2008. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev 22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi K. 2007. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J Bacteriol 189:4920–4931. doi: 10.1128/JB.00157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita M, Losick R. 2005. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev 19:2236–2244. doi: 10.1101/gad.1335705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A 98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 24.Romero D, Aguilar C, Losick R, Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci U S A 107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez D, Fischbach MA, Chu F, Losick R, Kolter R. 2009. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci U S A 106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oslizlo A, Stefanic P, Dogsa I, Mandic-Mulec I. 2014. Private link between signal and response in Bacillus subtilis quorum sensing. Proc Natl Acad Sci U S A 111:1586–1591. doi: 10.1073/pnas.1316283111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangera MG, Thomashow LS. 1999. Identification and characterization of a gene cluster for synthesis of the polyketide antibiotic 2,4-diacetylphloroglucinol from Pseudomonas fluorescens Q2-87. J Bacteriol 181:3155–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross H, Loper JE. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep 26:1408–1446. doi: 10.1039/b817075b. [DOI] [PubMed] [Google Scholar]
- 29.Kidarsa TA, Goebel NC, Zabriskie TM, Loper JE. 2011. Phloroglucinol mediates cross-talk between the pyoluteorin and 2,4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol Microbiol 81:395–414. doi: 10.1111/j.1365-2958.2011.07697.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramette A, Frapolli M, Fischer-Le Saux M, Gruffaz C, Meyer JM, Defago G, Sutra L, Moenne-Loccoz Y. 2011. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst Appl Microbiol 34:180–188. doi: 10.1016/j.syapm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Shank EA. 2013. Using coculture to detect chemically mediated interspecies interactions. J Vis Exp 80:e50863. doi: 10.3791/50863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochschild A, Lewis M. 2009. The bacteriophage lambda CI protein finds an asymmetric solution. Curr Opin Struct Biol 19:79–86. doi: 10.1016/j.sbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergsma-Vlami M, Prins ME, Staats M, Raaijmakers JM. 2005. Assessment of genotypic diversity of antibiotic-producing pseudomonas species in the rhizosphere by denaturing gradient gel electrophoresis. Appl Environ Microbiol 71:993–1003. doi: 10.1128/AEM.71.2.993-1003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picard C, Di Cello F, Ventura M, Fani R, Guckert A. 2000. Frequency and biodiversity of 2,4-diacetylphloroglucinol-producing bacteria isolated from the maize rhizosphere at different stages of plant growth. Appl Environ Microbiol 66:948–955. doi: 10.1128/AEM.66.3.948-955.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemon KP, Earl AM, Vlamakis HC, Aguilar C, Kolter R. 2008. Biofilm development with an emphasis on Bacillus subtilis. Curr Top Microbiol Immunol 322:1–16. doi: 10.1007/978-3-540-75418-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguilar C, Vlamakis H, Losick R, Kolter R. 2007. Thinking about Bacillus subtilis as a multicellular organism. Curr Opin Microbiol 10:638–643. doi: 10.1016/j.mib.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achkar J, Xian M, Zhao H, Frost JW. 2005. Biosynthesis of phloroglucinol. J Am Chem Soc 127:5332–5333. doi: 10.1021/ja042340g. [DOI] [PubMed] [Google Scholar]
- 38.Verhamme DT, Kiley TB, Stanley-Wall NR. 2007. DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol Microbiol 65:554–568. doi: 10.1111/j.1365-2958.2007.05810.x. [DOI] [PubMed] [Google Scholar]
- 39.Veening JW, Igoshin OA, Eijlander RT, Nijland R, Hamoen LW, Kuipers OP. 2008. Transient heterogeneity in extracellular protease production by Bacillus subtilis. Mol Syst Biol 4:184. doi: 10.1038/msb.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeigler DR, Pragai Z, Rodriguez S, Chevreux B, Muffler A, Albert T, Bai R, Wyss M, Perkins JB. 2008. The origins of 168, W23, and other Bacillus subtilis legacy strains. J Bacteriol 190:6983–6995. doi: 10.1128/JB.00722-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamon MA, Lazazzera BA. 2001. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol 42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- 42.Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. 2013. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci U S A 110:E1621–E1630. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo JH. 2013. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15:848–864. doi: 10.1111/j.1462-2920.2012.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goh EB, Yim G, Tsui W, McClure J, Surette MG, Davies J. 2002. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Natl Acad Sci U S A 99:17025–17030. doi: 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linares JF, Gustafsson I, Baquero F, Martinez JL. 2006. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci U S A 103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 47.Brameyer S, Kresovic D, Bode HB, Heermann R. 2015. Dialkylresorcinols as bacterial signaling molecules. Proc Natl Acad Sci U S A 112:572–577. doi: 10.1073/pnas.1417685112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratnayake-Lecamwasam M, Serror P, Wong KW, Sonenshein AL. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev 15:1093–1103. doi: 10.1101/gad.874201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitani T, Heinze JE, Freese E. 1977. Induction of sporulation in Bacillus subtilis by decoyinine or hadacidin. Biochem Biophys Res Commun 77:1118–1125. doi: 10.1016/S0006-291X(77)80094-6. [DOI] [PubMed] [Google Scholar]
- 50.Moynihan JA, Morrissey JP, Coppoolse ER, Stiekema WJ, O'Gara F, Boyd EF. 2009. Evolutionary history of the phl gene cluster in the plant-associated bacterium Pseudomonas fluorescens. Appl Environ Microbiol 75:2122–2131. doi: 10.1128/AEM.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.