Abstract
Purpose.
To investigate macular perfusion in healthy Chinese individuals and examine its dependence on age and sex.
Methods.
Healthy adult Chinese individuals were recruited. Macular perfusion was measured by spectral-domain optical coherence tomography (OCT) using the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm. The parafoveal flow index and vessel area density as well as the area of the foveal capillary-free zone (CFZ) were quantified.
Results.
A total of 76 eyes in 45 subjects were included (20 males and 25 females, mean age 36 ± 11 years). The mean parafoveal flow index was 0.099 ± 0.013; the mean vessel area density was 0.891 ± 0.073; and the mean CFZ area was 0.474 ± 0.172 mm2. All three parameters were significantly correlated with age (flow index: P = 0.00; vessel area density: P = 0.00; CFZ area: P = 0.02). The flow index and vessel area density decreased annually by 0.6% and 0.4%, respectively, and CFZ area increased by 1.48% annually. The CFZ area was larger in females than in males, while all three parameters seemed to change more rapidly with age in males than in females.
Conclusions.
In healthy Chinese eyes, macular perfusion decreased with increasing age, and decreased more rapidly in males than in females. The application of OCT angiograms may provide a useful approach for monitoring macular perfusion, although caution must be exercised with regard to age- and sex-related variations.
Keywords: optical coherence tomography (OCT) angiogram, split-spectrum amplitude-decorrelation angiography (SSADA) algorithm, macular perfusion, capillary-free zone
In this study, high-quality OCT angiograms of the macula were acquired using a commercially available OCT system. The flow index and vessel density at the parafoveal area and the CFZ area were found to be age and sex dependent.
The retinal vascular system, especially the part that perfuses the macular area, is essential to normal visual function.1 Numerous factors (e.g., pathology, trauma) can cause severe and irreversible visual damage to the macula.1,2 Ophthalmologists have used a variety of different methods to observe the vasculature in the macular area, including fundus camera3 and fundus fluorescein angiography (FFA).4 While these methods provide important clinical information, their use in clinical settings is limited by their invasive nature or low resolution. Moreover, the monitoring of early and subtle changes in the macular capillary system in clinical settings has proven challenging. Thus, an effective and noninvasive method for monitoring macular perfusion would provide not only a means for early detection of changes related to various pathologies, leading in turn to early interventions, but also a more thorough understanding of the pathophysiology of macular vascular disease.
Recently, development of a new technique known as split-spectrum amplitude-decorrelation angiography (SSADA) has promised new insights for understanding ocular perfusion. The technique, based on optical coherence tomography (OCT), is able to rapidly and accurately quantify retinal and disc blood flow in a noninvasive manner.5,6 Previous research has demonstrated that OCT angiography (angio-OCT) with SSADA offers results with high intravisit repeatability and intervisit reproducibility.7,8 However, an understanding of the status in normal eyes is necessary before its application in clinical settings. Moreover, some previous studies have found that the thicknesses of the retinal nerve fiber layer (RNFL), the macula, and the choroid vary with both age and sex.9–13 Thus, we here report on macular perfusion in normal volunteer subjects to explore the potential effects of age and sex on macular retinal functions.
Methods
Subjects
Normal volunteers were enrolled from April to June 2014. All underwent a complete ophthalmologic examination, which included the following components: determination of best-corrected visual acuity (BCVA); slit-lamp biomicroscopy; refraction measurement using autorefraction and refinement by an experienced optometrist; calculation of the spherical equivalence (SE) using the spherical diopter (D) plus one-half of the cylindrical dioptric power for later analysis; a dilated fundus examination using a three-mirror contact lens; and intraocular pressure (IOP) measurement using noncontact tonometer measurements. Heart rate and blood pressure of subjects were measured at the time of OCT imaging. The mean arterial pressure (MAP) was calculated as the diastolic blood pressure plus one-third the difference between the diastolic and the systolic blood pressure. The ocular perfusion pressure (OPP) was determined by subtracting the IOP from the 2/3 MAP. The medical and family histories of the patients were collected. The inclusion criteria were a BCVA of 16/20 or better, and SE between +1 and −3 D. The exclusion criteria were a prior history of ocular surgery or trauma; BCVA < 16/20; IOP > 21 mm Hg; a family history of glaucoma in a first-degree relative; signs of myopic degeneration or a pathological form of myopia; and other ophthalmic diseases or the presence of any systemic disease that might affect blood flow, such as diabetes mellitus or hypertension. The research was approved by the Institutional Review Board of the Eye and ENT Hospital of Fudan University, and conformed to the tenets of the Declaration of Helsinki. An informed consent form was signed by all subjects.
OCT Data Acquisition and Processing
Optical coherence tomography angiography scans were obtained by the spectral-domain system RTVue-XR Avanti (software version 2.0.5.39; Optovue, Inc., Fremont, CA, USA). This system has an A-scan rate of 70,000 scan per second, using a light source centered on 840 nm and a bandwidth of 45 nm. Both eyes of each participant were examined and scanned within the same visit. Three dimensional (3D) OCT angiography scans were acquired over 3- × 3-mm regions by using 5 repeated B-scans at 216 raster positions, each B-scan consisting of 216 A-scans. With a B-scan frame rate of 270 frames per second, each scan can be acquired in ∼3.5 seconds. Four volumetric raster scans, including two horizontal priority (x-fast) and two vertical priority (y-fast), were obtained consecutively. The best x-fast and y-fast scans were processed by the SSADA algorithm,5 and motion artifact was removed by 3D orthogonal registration and merging of two scans. An en face retinal angiogram was created by projecting the flow signal internal to retinal pigment epithelium. All this processing can be achieved using the software included (version 2.0.5.39).
Macular Perfusion Measurement
In order to quantify macular circulation, en face retinal angiograms were output and processed by use of ImageJ software (Image J2x 2.1.4.6 ud4; Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). The parafoveal flow index and the parafoveal vessel area density were calculated as previously described.7,8 In our study, the parafoveal region was defined as an annulus with an outer diameter of 2.86 mm and inner diameter of 1 mm (Supplementary Fig. S1). Briefly, the parafoveal flow index was defined as the average decorrelation value, given by:
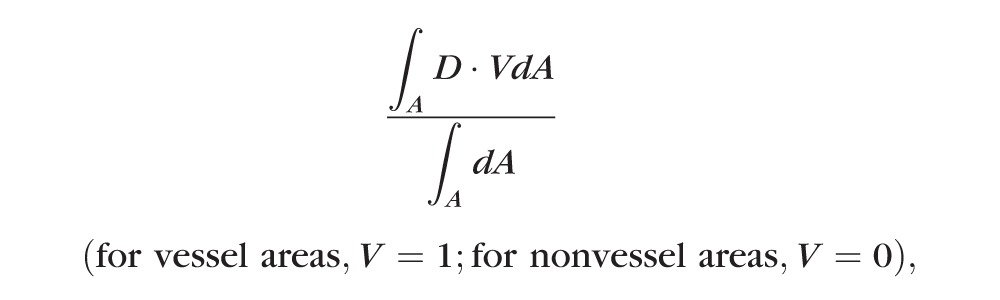 |
where A is the parafoveal area and D is the decorrelation value acquired by the SSADA algorithm.
The vessel area density was defined as the proportion of the total area occupied by vessels, calculated as:
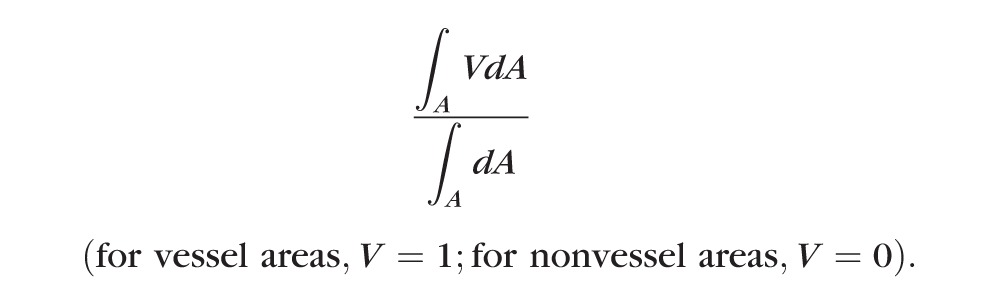 |
The threshold decorrelation value used to judge the value of V as 0 or 1 was set at 0.08, which is two standard deviations above the mean decorrelation value in the noise region, that is, the central foveal capillary-free zone (CFZ).
Capillary-Free Zone Measurements
The CFZ was outlined and measured in images magnified six times (using ImageJ software) (Supplementary Fig. S2).
Repeatability and Reproducibility
Repeatabilities of the flow index, vessel area density, and CFZ area measurements were calculated from two sets taken from each eye during a single visit by a single operator; 15 eyes were included. For CFZ area measurements, intraobserver repeatability and interobserver reproducibility were evaluated on all 76 images by two observers, who each measured the same scan from each eye twice. Intraclass correlation (ICC) and Bland–Altman plots were used to assess repeatability and reproducibility.
Statistical Analyses
Statistical analyses were performed using SPSS, version 20.0 (SPSS, Inc., Chicago, IL, USA) and MedCalc, version 11.4 (MedCalc Software, Ostend, Belgium). Linear mixed model was performed to determine the effects of IOP, SE, OPP, heart rate (HR), sex, and age on the flow index, vessel area density, and CFZ area, as well as the difference of flow index, vessel area density, and CFZ area between sexes. Student's t-tests were used to compare the difference of age, IOP, SE, OPP, and HR between sexes and the difference of flow index, vessel area density, and CFZ area between left and right eyes. Intraclass correlation and Bland–Altman plots were used to assess repeatability and reproducibility (ICC values of 0.81–1.00 indicate almost perfect agreement between repeated measurements; values less than 0.40 indicate poor to fair agreement). The Bland–Altman analysis tested for proportional biases between repeated measurements. For all tests, values of P < 0.05 were considered statistically significant.
Results
A total of 76 eyes from 45 normal Chinese subjects were included in the study. Demographically, the sample (summarized in Supplementary Table S1) included 20 males (35 eyes) and 25 females (41 eyes); the mean age was 36 ± 11 years (range, 24–59 years). Examination results yielded the following: mean IOP, 14.6 ± 2.5 mm Hg (range, 9.9–21 mm Hg); mean SE, −1.2 ± 1 D (range, −3 to 1 D); mean flow index for the parafoveal area, 0.099 ± 0.013 (range, 0.071–0.127), mean vessel area density, 0.891 ± 0.073 (range, 0.691–0.997); and mean CFZ area, 0.474 ± 0.172 mm2 (range, 0.128–0.976 mm2).
The mean ICC between two measurements from 15 eyes was 0.910 for vessel area density, 0.925 for the flow index, and 0.901 for CFZ area; a Bland–Altman plot showed good reliability (Supplementary Figs. S3A–C). The mean ICC for measurement of CFZ area was 0.989 and 0.971 for intraobserver repeatability and interobserver reproducibility, respectively. The Bland–Altman analysis (Supplementary Figs. S3D, S3E) also showed good intra- and interobserver reliability.
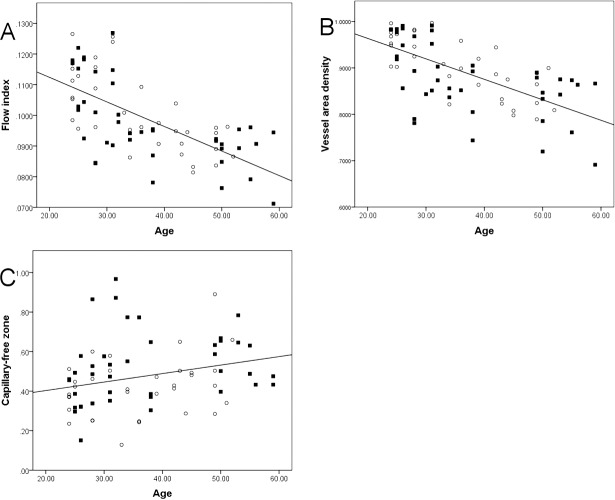
Values of the flow index and vessel area density at the parafoveal area were similar in male and female subjects and also in left and right eyes. A significant correlation between the age of subjects and both the flow index and vessel area density (Table 1) was found, but not between IOP, SE, or other measures. Both the flow index and the vessel area density decreased with increasing age (Figs. A, B), showing average annual reductions of 0.6% and 0.4% (Supplementary Table S2), respectively. The decreases in both the flow index and vessel area density with age were greater in male than in female subjects. The average annual reduction in the flow index was 0.68% in males (slope, −0.000915/year) and 0.58% in females (slope, −0.000722/year) (Supplementary Table S2); the average annual reduction in vessel area density was 0.46% in males (slope, −0.005041/year) and 0.38% in females (slope, −0.003932/year) (Supplementary Table S2).
Table 1.
Linear Mixed Model Analysis of the Relationships Between Age, IOP, SE, HR, and OPP (Independent Variables) and Parafoveal Flow Index, Parafoveal Vessel Area Density, and Area of the CFZ (Dependent Variables)
Figure.
Correlations between age and the parafoveal flow index, parafoveal vessel area density, and area of the capillary-free zone (CFZ) in normal subjects. Parafoveal flow index (A) and vessel area density (B) were negatively correlated with age, while CFZ area (C) was positively correlated.
The CFZ area was larger in females than in males (male mean, 0.42 mm2; female mean, 0.52 mm2; P = 0.012; Table 2); however, no difference was found between left and right eyes. A significant correlation between age and CFZ area was found, but not between IOP, SE, or other measures and CFZ area. The CFZ area increased with increasing age (P = 0.02; Table 1; Fig. C), showing an average annual increase of 1.48%. The increase in the CFZ area with age was greater in males than in females (males, 2.04%; females, 1.28%; Supplementary Table S2).
Table 2.
Comparison of Results by Sex Analyzed by Linear Mixed Model

Discussion
We examined the perfusion of the macular area in normal Chinese subjects using an OCT angiogram, based on measurements of the parafoveal flow index and vessel area density, as well as CFZ area. Results showed a significant negative correlation between age and parafoveal flow index and vessel area density. Also, the flow index and vessel area density decreased with age more rapidly in male than in female subjects. The CFZ area was 0.474 mm2; the area was larger in females than in males, was positively correlated with age, and was found to enlarge more quickly with age in males than in females.
Metabolic levels in retinal tissues are very high, and studies have shown that oxygen consumption by these tissues (measured per tissue weight) is the highest of that by any tissue in the body.14 Retinal vasculature is not only regulated by angioactive factors, such as angiotensin,15 nitric oxide (NO),16 and others,17 but is also affected by local and systemic factors such as IOP,18 blood pressure,19 and diabetes.20 Many of these factors, if they exceed certain levels, can lead to abnormalities in the vascular system and cause secondary functional as well as structural damage to the retina.21,22 Some of this damage may involve the macula and may cause severe visual impairment.22,23 As a result, the study of retinal perfusion is essential not only to acquire baseline information on physiological variations among normal subjects, but also for early diagnosis and monitoring of macular vascular disease.
Various techniques have been used to study retinal perfusion, including laser Doppler flowmetry,24 indocyanine green angiography,25 and laser speckle imaging.26 However, some of these are limited by their nonquantitative or invasive nature while others are limited to measurements of blood flow in large vessels located within or around the optic disc. In many macular vascular diseases, such as in diabetic retinopathy,22 macular telangiectasia,27 Leber's miliary aneurysms,28 and cystoid macular edema after cataract extraction,29 the pathologies develop primarily in capillaries and small arteries. To date, techniques for quantitative assessment of microcirculation in the retina, especially in the macular area, have been inadequate. Using the blue-field entoptic technique, Grunwald et al.30 were able to quantify microcirculation in healthy volunteers; however, this technique, which tests microcirculation by asking subjects to match the velocities and densities of computer-simulated particles displayed on a screen with those of entoptically observed leukocytes, requires reasonably good vision and so is not suitable for clinical use in patients with visual impairments. Using SSADA, which was first described by Jia and et al.5 in 2012, and high-speed OCT, it is now possible to quantify perfusion of the disc as well as of the macula. Wei et al.7 reported that the technique was able to measure macular flow in normal subjects with excellent repeatability (1.3% intravisit and 2.1% intervisit coefficients of variation, respectively). In this study, in addition to measurements of the parafoveal flow index and vessel area density, we also measured the CFZ area in the fovea. Laatikainen and Larinkari4 successfully measured the diameter of the foveal CFZ using FFA. However, the FFA approach is limited on account of its invasive nature and the presence of adverse reactions in subjects,31 especially in consecutive clinical observations or follow-up. In this study, the CFZ was documented by OCT using a noninvasive procedure with good repeatability. As a result, OCT angiography and the parameters it provides may be a good choice for studies of macular vascular disease.
In the present study, using SSADA we successfully measured macular perfusion: parafoveal flow index, and vessel area density, as well as CFZ area, in normal Chinese subjects. As both a previous study4 and our results showed a great variation in CFZ area, to avoid the impact of this variation, we measured only the flow index and the vessel area density at the parafoveal area (the annulus area between the inner 1-mm and outer 2.86-mm-diameter circles). All measurements and derived parameters were found to be correlated with age. The decrease in total retinal flow and increase in CFZ area with increasing age have been reported by Grunwald et al.30 and Laatikainen and Larinkari4 In addition, Emeterio et al.32 reported that the thickness and amount of blood flow in the choroid, which essentially consists of layers of vessels of different sizes and which provides metabolic support for the retinal pigment epithelium (RPE) and the outer retina, were significantly and negatively correlated with age. Our findings are in agreement with theirs. As to the reason, Vandewalle et al.33 speculated that these changes in perfusion were the result of tissue loss and a corresponding reduction in oxygen and nutrient demand, thus resulting in a reduction in blood supply. Previously Sung et al.10 and Girkin et al.11 reported that macular thickness significantly decreased with increasing age; our finding of macular perfusion partially supports the theory of Vandewalle et al.33
Our results show that the parafoveal flow index and vessel area density decrease with increasing age at a rate of 0.6% and 0.4% per year, respectively. Sung et al.10 measured the thickness of the RNFL around the optic nerve head and reported that the thickness decreases with increasing age at a rate of 0.255 μm per year. As the overall average thickness of the RNFL was 100.8 ± 10.5 μm, the loss represents a decrease of approximately 0.25% per year. On the other hand, Leenders et al.34 reported that blood flow in the brain decreases at a rate of approximately 0.50% per year, which is comparable to our findings regarding blood flow in the parafoveal area.
Using FFA, Mansour et al.35 and Bresnick et al.36 found that the CFZ area was 0.35 to 0.41 mm2 in normal eyes; these values are slightly smaller than those obtained in this study, which might be explained in the following way. The SSADA measurements are based on measurements of changes in reflections and backscattering of light. Previous studies have determined that not all capillaries are open at the same time.37 Thus, some of the capillaries around the CFZ might be closed during OCT scanning, which takes approximately 1 to 2 seconds (the fluorescence in the capillary persists for approximately 10 minutes),38 and this may account for the difference between the results of the different studies.
The reduction in macular perfusion might account for other findings in older individuals. Reductions in perfusion might lead to relative ischemia in the fovea and might contribute to the etiology of macular entities that are triggered by ischemia. Previously, Ding et al.13 reported that the subfoveal choroid thickness is significantly reduced in people older than 60 years; also, Ito et al.39 reported that changes in choroidal circulation are more prominent in individuals older than 50 years. In the present study, a linear decrease in the parafoveal flow index and vessel area density, as well as an enlargement of the CFZ area, was found in the macula in younger individuals. Thus, while age-related changes in choroid might be involved in the etiology of age-related macular degeneration (ARMD), found mostly in elder individuals,40 the decreasing retinal perfusion observed in younger individuals may be related to the development of macular entities that are more common in slightly younger people, such as type 1 macular telangiectasia, for which the mean age at presentation is 40 years.41
Our results showed that while the parafoveal flow index and vessel area density were not related to sex, the CFZ was larger in females than in males; this might be related to the thinner fovea found in females.42,43 In addition, in females the larger CFZ area was accompanied by a reduction in the decrease of the flow index, vessel area density, and enlargement of CFZ with age; it seems that the reduction in the rate of decrease in females effectively compensates for the large CFZ, such that flow in the macula is maintained. The mechanisms behind these patterns require further study.
The present study was a cross-sectional study involving a limited number of cases and including only Chinese subjects. Differences in retinal structure in subjects with different racial backgrounds have been reported11,44,45; thus, our results must be verified by other multicenter-based studies with larger numbers of cases from different racial backgrounds. In addition, the relationship between the change in macular perfusion and the function and structure of the macula should be examined further. Also, measurements of SSADA depend on the beam parameters of the OCT system, the scan pattern, the angiography algorithm used, and the threshold flow value to detect vessels. Therefore care must be taken to compare results even from the same system, especially given that the scan pattern and processing algorithms software are rapidly changing.
In this study, high-quality OCT angiograms of the macula were acquired using a commercially available OCT system. The flow index and vessel area density at the parafoveal area and CFZ area were found to be age and sex dependent. Thus, our results indicate that research on macular perfusion should take into account the possibility of age- and sex-related variations.
Supplementary Material
Acknowledgments
Supported in part by research grants from the National Major Scientific Equipment program (2012YQ120080) and the Shanghai Committee of Science and Technology (Grant 13430710500); National Institutes of Health (NIH) R01 EY023285 (DH, YJ); NIH 8ULI TR000128 (DH, YJ); NIH R01 EY024544 (DH, YJ); DP3 DK104397 (YJ, DH); and Research to Prevent Blindness (DH, YJ).
Disclosure: J. Yu, None; C. Jiang, None; X. Wang, None; L. Zhu, None; R. Gu, None; H. Xu, None; Y. Jia, Optovue, Inc. (F), P; D. Huang, Optovue, Inc. (F, I), P; X. Sun, None
References
- 1. Crawford TN,, Alfaro DR,, Kerrison JB,, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009; 5: 8–13. [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa S,, Oishi A,, Ogino K,, Makiyama Y,, Kurimoto M,, Yoshimura N. Association of retinal vessel attenuation with visual function in eyes with retinitis pigmentosa. Clin Ophthalmol. 2014; 8: 1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sinthanayothin C,, Boyce JF,, Cook HL,, Williamson TH. Automated localisation of the optic disc, fovea, and retinal blood vessels from digital colour fundus images. Br J Ophthalmol. 1999; 83: 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laatikainen L,, Larinkari J. Capillary-free area of the fovea with advancing age. Invest Ophthalmol Vis Sci. 1977; 16: 1154–1157. [PubMed] [Google Scholar]
- 5. Jia Y,, Tan O,, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012; 20: 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia Y,, Wei E,, Wang X,, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014; 121: 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei E,, Jia Y,, Tan O, et al. Parafoveal retinal vascular response to pattern visual stimulation assessed with OCT angiography. PLoS One. 2013; 8: e81343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X,, Jia Y. Spain R, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol. 2014; 98: 1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim MC,, Hoh ST,, Foster PJ,, et al. Use of optical coherence tomography to assess variations in macular retinal thickness in myopia. Invest Ophthalmol Vis Sci. 2005; 46: 974–978. [DOI] [PubMed] [Google Scholar]
- 10. Sung KR,, Wollstein G,, Bilonick RA, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009; 116: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Girkin CA,, McGwin GJ,, Sinai MJ,, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011; 118: 2403–2408. [DOI] [PubMed] [Google Scholar]
- 12. Fujiwara A,, Shiragami C,, Shirakata Y,, Manabe S,, Izumibata S,, Shiraga F. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn J Ophthalmol. 2012; 56: 230–235. [DOI] [PubMed] [Google Scholar]
- 13. Ding X,, Li J,, Zeng J, et al. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011; 52: 9555–9560. [DOI] [PubMed] [Google Scholar]
- 14. Yu DY,, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001; 20: 175–208. [DOI] [PubMed] [Google Scholar]
- 15. Schonfelder U,, Hofer A,, Paul M,, Funk RH. In situ observation of living pericytes in rat retinal capillaries. Microvasc Res. 1998; 56: 22–29. [DOI] [PubMed] [Google Scholar]
- 16. Nagaoka T,, Sakamoto T,, Mori F,, Sato E,, Yoshida A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Invest Ophthalmol Vis Sci. 2002; 43: 3037–3044. [PubMed] [Google Scholar]
- 17. Kur J,, Newman EA,, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012; 31: 377–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alagoz G,, Gurel K,, Bayer A,, Serin D,, Celebi S,, Kukner S. A comparative study of bimatoprost and travoprost: effect on intraocular pressure and ocular circulation in newly diagnosed glaucoma patients. Ophthalmologica. 2008; 222: 88–95. [DOI] [PubMed] [Google Scholar]
- 19. Boltz A,, Told R,, Napora KJ, et al. Optic nerve head blood flow autoregulation during changes in arterial blood pressure in healthy young subjects. PLoS One. 2013; 8: e82351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pemp B,, Schmetterer L. Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 2008; 43: 295–301. [DOI] [PubMed] [Google Scholar]
- 21. Tso MO,, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982; 89: 1132–1145. [DOI] [PubMed] [Google Scholar]
- 22. Sakata K,, Funatsu H,, Harino S,, Noma H,, Hori S. Relationship between macular microcirculation and progression of diabetic macular edema. Ophthalmology. 2006; 113: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 23. Hayreh SS,, Servais GE,, Virdi PS. Macular lesions in malignant arterial hypertension. Ophthalmologica. 1989; 198: 230–246. [DOI] [PubMed] [Google Scholar]
- 24. Michelson G,, Langhans MJ,, Groh MJ. Clinical investigation of the combination of a scanning laser ophthalmoscope and laser Doppler flowmeter. Ger J Ophthalmol. 1995; 4: 342–349. [PubMed] [Google Scholar]
- 25. Raabe A,, Beck J,, Gerlach R,, Zimmermann M,, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003; 52: 132–139 discussion 139. [DOI] [PubMed] [Google Scholar]
- 26. Cheng H,, Yan Y,, Duong TQ. Temporal statistical analysis of laser speckle images and its application to retinal blood-flow imaging. Opt Express. 2008; 16: 10214–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chin EK,, Kim DY,, Hunter AR, et al. Staging of macular telangiectasia: power-Doppler optical coherence tomography and macular pigment optical density. Invest Ophthalmol Vis Sci. 2013; 54: 4459–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agarwal A. Gass' Atlas of Macular Diseases. 5th ed. Philadelphia: Saunders; 2011: 514–518. [Google Scholar]
- 29. Agarwal A. Gass' Atlas of Macular Diseases. 5th ed. Philadelphia: Saunders; 2011: 501–504. [Google Scholar]
- 30. Grunwald JE,, Piltz J,, Patel N,, Bose S,, Riva CE. Effect of aging on retinal macular microcirculation: a blue field simulation study. Invest Ophthalmol Vis Sci. 1993; 34: 3609–3613. [PubMed] [Google Scholar]
- 31. Kwiterovich KA,, Maguire MG,, Murphy RP, et al. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology. 1991; 98: 1139–1142. [DOI] [PubMed] [Google Scholar]
- 32. Emeterio NO,, Harrison JM,, Muir ER,, et al. Choroidal blood flow decreases with age: an MRI study. Curr Eye Res. 2014; 39: 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vandewalle E,, Abegao PL,, Olafsdottir OB, et al. Oximetry in glaucoma: correlation of metabolic change with structural and functional damage. Acta Ophthalmol. 2014; 92: 105–110. [DOI] [PubMed] [Google Scholar]
- 34. Leenders KL,, Perani D,, Lammertsma AA,, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990; 113 (pt 1): 27–47. [DOI] [PubMed] [Google Scholar]
- 35. Mansour AM,, Schachat A,, Bodiford G,, Haymond R. Foveal avascular zone in diabetes mellitus. Retina. 1993; 13: 125–128. [DOI] [PubMed] [Google Scholar]
- 36. Bresnick GH,, Condit R,, Syrjala S,, Palta M,, Groo A,, Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984; 102: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 37. Honig CR,, Odoroff CL,, Frierson JL. Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol. 1982; 243: H196–H206. [DOI] [PubMed] [Google Scholar]
- 38. Ryan S. Retina. 4th ed. Amsterdam: Elsevier; 2006: 891. [Google Scholar]
- 39. Ito YN,, Mori K,, Young-Duvall J,, Yoneya S. Aging changes of the choroidal dye filling pattern in indocyanine green angiography of normal subjects. Retina. 2001; 21: 237–242. [DOI] [PubMed] [Google Scholar]
- 40. Ardeljan D,, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res. 2013; 37: 68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nowilaty SR,, Al-Shamsi HN,, Al-Khars W. Idiopathic juxtafoveolar retinal telangiectasis: a current review. Middle East Afr J Ophthalmol. 2010; 17: 224–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luo HD,, Gazzard G,, Fong A, et al. Myopia, axial length, and OCT characteristics of the macula in Singaporean children. Invest Ophthalmol Vis Sci. 2006; 47: 2773–2781. [DOI] [PubMed] [Google Scholar]
- 43. Lam DS,, Leung KS,, Mohamed S,, et al. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci. 2007; 48: 376–382. [DOI] [PubMed] [Google Scholar]
- 44. Alasil T,, Wang K,, Keane PA, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013; 22: 532–541. [DOI] [PubMed] [Google Scholar]
- 45. Knight OJ,, Girkin CA,, Budenz DL,, Durbin MK,, Feuer WJ. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012; 130: 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.