Abstract
Purpose.
Lipofuscin (LF) and melanolipofuscin (MLF) of the retinal pigment epithelium (RPE) are the principal sources of autofluorescence (AF) signals in clinical fundus–AF imaging. Few details about the subcellular distribution of AF organelles in AMD are available. We describe the impact of aging and AMD on RPE morphology revealed by the distribution of AF LF/MLF granules and actin cytoskeleton in human tissues.
Methods.
Thirty-five RPE-Bruch's membrane flatmounts from 35 donors were prepared (postmortem: ≤4 hours). Ex vivo fundus examination at the time of accession revealed either absence of chorioretinal pathologies (10 tissues; mean age: 83.0 ± 2.6 years) or stages of AMD (25 tissues; 85.0 ± 5.8 years): early AMD, geographic atrophy, and late exudative AMD. Retinal pigment epithelium cytoskeleton was labeled with AlexaFluor647-Phalloidin. Tissues were imaged on a spinning-disk fluorescence microscope and a high-resolution structured illumination microscope.
Results.
Age-related macular degeneration impacts individual RPE cells by (1) lipofuscin redistribution by (i) degranulation (granule-by-granule loss) and/or (ii) aggregation and apparent shedding into the extracellular space; (2) enlarged RPE cell area and conversion from convex to irregular and sometimes concave polygons; and (3) cytoskeleton derangement including separations and breaks around subretinal deposits, thickening, and stress fibers.
Conclusions.
We report an extensive and systematic en face analysis of LF/MLF-AF in AMD eyes. Redistribution and loss of AF granules are among the earliest AMD changes and could reduce fundus AF signal attributable to RPE at these locations. Data can enhance the interpretation of clinical fundus–AF and provide a basis for future quantitative studies.
Keywords: AMD, lipofuscin, melanolipofuscin, autofluorescence, granule
This is the first description of possible mechanisms for decreasing autofluorescence (AF) both in aging and AMD: degranulation of RPE cells. It shows that redistribution and loss of AF granules are among the earliest subcellular changes in AMD.
Clinical fundus–autofluorescence (AF) has become an indispensable tool in the diagnosis and management of many chorioretinal diseases, especially AMD.1–3 It is a good indicator of retinal pigment epithelium (RPE) health because hypo- and/or hyperfluorescent areas in fundus-AF are often associated with AMD-related extracellular deposits (sub-RPE drusen; subretinal drusenoid deposits)4,5 or lesions directly affecting the RPE (atrophy).6,7 While drusen and subretinal drusenoid deposits become clinically visible at 30 μm,8,9 changes in RPE cells are smaller than that and may not be revealed by current ophthalmoscopic imaging techniques.
Of note, compared with the voluminous studies on clinical fundus–AF, there are only few studies known to us that specifically focused on the cellular and subcellular basis of AF in AMD.10,11 Of these, only one provided high-resolution tissue photodocumentation10 to serve as visualization targets for clinical AF imaging in the way that photoreceptor histology influenced the development of adaptive optics scanning laser ophthalmoscopy.12–15
Closing this knowledge gap would help clinicians and scientists further understand clinical fundus–AF from its origin (i.e., the accumulation and distribution of AF granules in healthy and diseased RPE cells). This topic is of renewed interest due to the arrival of quantitative AF,16–18 an imaging technique that will standardize AF signal across clinic populations. Further, our recent work in the context of prior literature establishes that RPE cell numbers are stable in aging, implying that decreased fundus AF after 70 years19 cannot be attributed to RPE cell loss.10 This finding in turn implicates loss of individual granules, a shift in fluorophore composition relative to the detector sensitivity, and change in cell shape impacting path length of exciting light through fluorophores as potential cell-autonomous mechanisms underlying diminished fundus AF.
Lipofuscin (LF) and melanolipofuscin (MLF) granules of the RPE are the principal subcellular sources of AF signals in clinical fundus AF imaging.20 In this hypothesis-generating histologic survey, we report intracellular distribution of LF/MLF granules in RPE cells from an en face (fundus) view in healthy and AMD human RPE-BrM flatmounts. Simultaneously, the filamentous-actin (F-actin) cytoskeleton of the RPE cells was imaged to report changes in shape and size in eyes affected by AMD. Our goal was to provide a resource for the interpretation of clinical fundus-AF by exploring the cellular and subcellular basis of variation in AF. We find evidence for several pathways of LF/MLF redistribution and morphologic readouts of cellular stress.
Methods
Institutional review at the University of Alabama at Birmingham (Birmingham, AL, USA) approved this study, and all procedures adhered to the Tenets of the Declaration of Helsinki.
Thirty-five human chorioretinal tissues from 35 Caucasian donors were preserved in 0.1 M phosphate-buffered paraformaldehyde (≤4.2 hours after death), cryoprotected in glycerol-buffer, and frozen at −80°C until used. Maculopathy status was determined at the time of accession, as follows: The anterior segment and vitreous was removed, and globes were inspected internally under a dissecting microscope with epi-illumination to accentuate drusen and transillumination to accentuate pigmentary change. Stereo color photographs were graded as normal, questionable, or AMD, using the criteria of the Alabama Age-related Maculopathy Grading System (≥1 druse larger than 125 μm in diameter or an area of pigment change 500 μm in diameter).21 Eyes with smaller drusen or smaller areas of pigment change (questionable) were analyzed in the current study so as to include incipient AMD pathology. These eyes were considered early AMD for the purpose of analysis, because they exhibited changes consistent with those in definite AMD eyes. Of all tissues, 28 were also examined after thawing using spectral-domain optical coherence tomography (SD-OCT, Spectralis; Heidelberg Engineering, Heidelberg, Germany) to confirm absence (for healthy) or presence (for AMD) of drusen, abnormal hypo-/hyperautofluorescence, or atrophic areas. During photomicrography and analysis of RPE-BrM flatmounts (below), we checked whether RPE morphologies visible en face were consistent with those seen in cross-sectional histology (digital sections, glossary, and bibliography at (in the public domain) http://projectmacula) and detailed separately.22,23
Retinal pigment epithelial–BrM flatmounts of the macula and near periphery (20 × 20 mm) were prepared and imaged as described.10 Briefly, retina and choroid were removed under a dissection microscope resulting in 20- to 25-μm thick RPE-BrM flatmounts that were then labeled with 647 Alexa Phalloidin (Life Technologies, Grand Island, NY, USA) to bind the F-actin cytoskeleton. Phalloidin labeling permitted semiautomatic counting (to be reported separately) and assisted in defining cell boundaries as the disease progressed. Bright field and fluorescence imaging was performed using a confocal microscope (BX51; Olympus, Tokyo, Japan) with 460- to 490-nm excitation/emission greater than 505 nm for LF/MLF-AF and 635-nm excitation/emission greater than 650 nm for phalloidin. Z-stacks in 0.4-μm steps were taken from apical to basal RPE. For LF/MLF, z-stacks included the first granules in focus on the apical aspect through last granules out of focus on the basal aspect. Exposure times varied between tissues (7.0–15.5 ms/slice). For cytoskeleton, z-stacks included the apical RPE. Images were systematically acquired at predefined locations, as described.10 In AMD RPE-BrM flatmounts, additional images were captured in areas affected by drusen and/or atrophy. Bright-field images were taken to illustrate intracellular melanosome distribution. For all aforementioned imaging modes, final all-in-focus images were created using the microscope's internal software (extended focal imaging tool, cellSens software version 7; Olympus). To further highlight F-actin bands, the background subtraction and edge detection filter (Sobel; Olympus) tools in the same software were applied. Images were also captured from 10-μm thick vertically-oriented cryosections of additional AMD eyes retained from previous studies.24,25 In describing locations we use “cone-dominated” fovea24 (within 500 μm of the foveal center) and the terminology of Polyak26 for perifovea (1.25–2.75 mm from the foveal center) and periphery (10 mm from the foveal center, at the edge of the tissue).
Age-related macular degeneration affected RPE cells were also imaged using high-resolution structured illumination microscopy (HR-SIM), a multicolor microscopy technique that surpasses twice Abbe's resolution limit.27 In this mode of microscopy a grid pattern is superimposed on the specimen, while images are simultaneously captured. This periodic light pattern allows down-modulation of high-frequency sample information that is normally not transferred by a standard wide-field microscope by converting this information to a Moiré pattern that makes it accessible for detection by HR-SIM. Postimaging processing requires specific algorithms to extract high-resolution information and to reconstruct images, resulting in significantly better resolution (≈110-nm lateral) compared with wide-field or confocal microscopy. We recently showed that HR-SIM is a suitable tool for examination of individual LF granules.28 High-resolution SIM imaging was performed on an ELYRA-S.1 system (Zeiss, Jena, Germany). Excitation was at wavelengths of 488, 561, and 642 nm using grating periods of 28 (for 488 nm) and 34 μm (for 561 and 642 nm) and a ×63, numerical aperture 1.40 plan apochromat oil immersion objective. Depending on excitation wavelength, approximately 100 serial sections of 90- to 110-nm axial spacing each with 15 raw images (5 grating positions × 3 rotations) were acquired. Images were exposed for 100 to 150 ms using an EMCCD camera iXon 885 (Andor Technology Ltd., Belfast, UK) cooled to −63°C. Reconstructions of raw image sequences were performed using the instrument software (ZEN 2010; Zeiss). Locations for HR-SIM imaging (in normal and AMD flatmounts) do not necessarily correspond to locations for confocal AF imaging. For HR-SIM AMD flatmount imaging, RPE cells at different stages of degranulation were chosen randomly.
Results
Study results are based on a survey of RPE cells at 1729 locations in 25 AMD eyes (85.0 ± 5.8 years, mean ± SD) and 782 locations in 10 age-matched controls (83.0 ± 2.6 years).
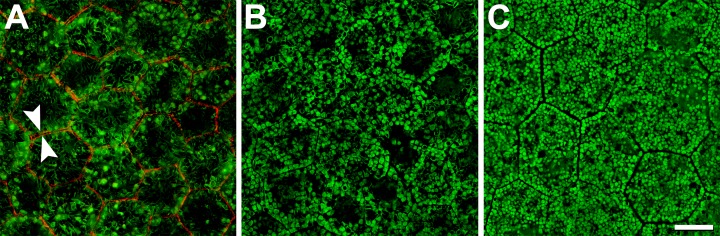
Normal RPE cells in older adults are filled with AF-LF and -MLF granules, revealed by HR-SIM. Figure 1A shows homogeneously AF granules (LF) as well as granules with elongated hypo-AF cores surrounded by AF shells (MLF). At 460- to 490-nm excitation, melanosomes are minimally AF and are thus visible in dark relief against the cushion of other organelles. Space-filling nuclei also reduce AF signal within cells, a phenomenon that is especially prominent in small foveal RPE cells (Fig. 1A). High-resolution SIM images reveal highly ordered packing of granules, which are similarly sized and evenly distributed throughout the cytoplasm. Fovea and perifovea26 show fewer and larger LF granules, and many more MLF granules and light blocking melanosomes, compared with periphery (compare Figs. 1A–C). Each RPE cell is delimited by a F-actin containing and evenly labeled cytoskeleton of uniform thickness that runs in a straight line along each side, meeting at sharp vertices (arrows in Fig. 1A). Cytoskeletons of adjacent cells form parallel lines like railroad tracks.
Figure 1.
Normal RPE granule distribution and F-actin cytoskeleton. High-resolution structured illumination microscopy reveals the intracellular distribution of autofluorescent LF and MLF granules. Melanosomes (minimally AF and not visible) and nonfluorescent cell nuclei lead to dark areas within the cells ([A] fovea; [B] perifovea). With increasing distance from the fovea (out to 10-mm eccentricity), cells contain fewer light-blocking melanosomes and more LF/MLF granules (C). The normal RPE cytoskeleton consists of a circumferential F-actin bundle (red in [A]), which follows the cell's polygonal shape. Adjacent cells have parallel cytoskeletons like railroad tracks (arrowheads in [A]). The F-actin band runs at the apical third of the RPE cell. Retinal pigment epithelial cells can be barrel-shaped89 and individual cell bodies can thus bulge into adjacent cells basolaterally. From an en face view, granules from one cell might seem to extend into adjacent cells. Donor: 83 years, female. F-actin labeled with AlexaFluor647-Phalloidin. Scale bar: 10 μm.
We observed striking intracellular granule redistribution and loss in aging and in AMD, with different patterns in the two conditions. Cells tended to exhibit reorganization of their granules in one of two ways: degranulation (granule-by-granule loss) and/or aggregation.
Degranulation is observable in both aged normal and AMD eyes (Fig. 2). Cells redistribute their granules (Fig. 2A), leading to granule-free zones in the cytoplasm (Figs. 2B, 2C). With ongoing redistribution, very few AF granules are visible (Fig. 2D). Yet the F-actin cytoskeleton is intact, indicating that these cells are still present. An additional explanation for reduced RPE-AF in healthy and AMD eyes is that some cells densely pack with melanosomes (Figs. 2E, 2F) with a normal cytoskeleton (not shown). Although it seems unlikely that these cells degranulated of LF/MLF then filled with melanosomes, it is possible that LF granules are still present, and AF is completely blocked by melanosomes.
Figure 2.
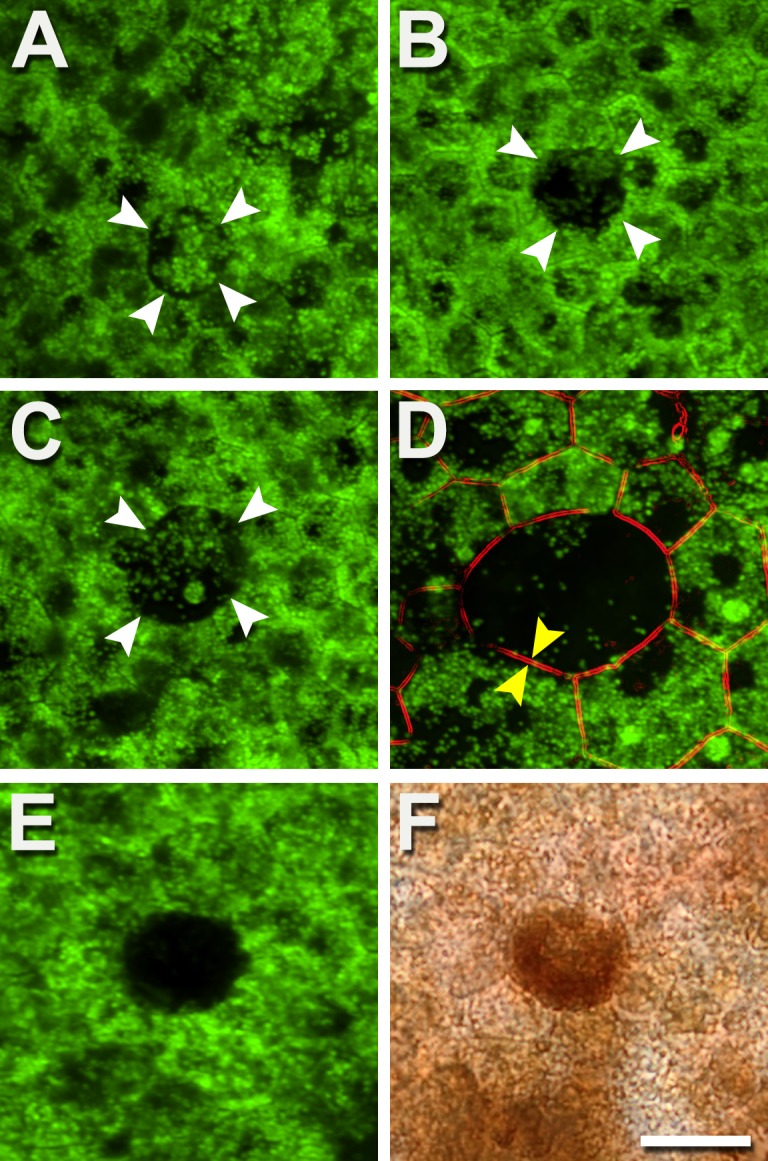
Autofluorescent granules disappear individually, reducing AF. Degranulation results in a diminished or absent AF signal from affected cells, yet the cells are still present. (A) The central cell (white arrowheads) is circular rather than polygonal and has redistributed granules. (B) The central cell (white arrowheads) is almost devoid of granules. (C) The central cell has a few individual granules and one aggregation (see also Fig. 3). (D) An enlarged cell with a circular profile has an actin cytoskeleton (parallel bands, between yellow arrows) and few granules. (E, F) Epifluorescence and bright-field images of the same area show that decreased AF could also be associated with densely packed melanosomes, in both healthy and AMD eyes. Few AF granules are visible (E). Donors: (A–C) 84 years, male, incipient AMD; (D) 86 years, female, incipient AMD; (E, F) 69 years, male, late exudative AMD. Autofluorescent intensity signals were normalized to a fluorescence reference but were not normalized across panels. Scale bar: 20 μm.
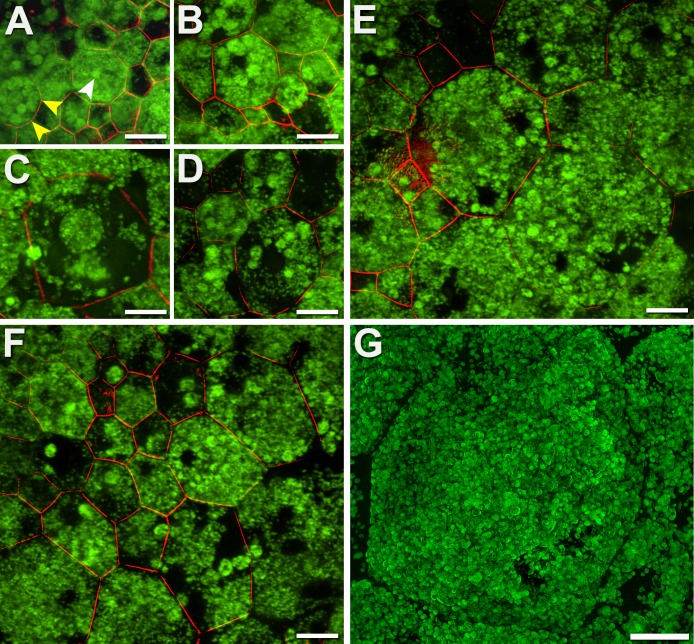
Aggregation is the more dramatic form of LF/MLF redistribution, observable almost exclusively in AMD eyes (Figs. 3A–F). Retinal pigment epithelial cells aggregate granules into packets several micrometers in diameter (median: 5.1 μm, range, 2.5–20.9 μm; analysis of 24 aggregates in 9 RPE cells; Fig. 3A), sometimes filling the whole cell and giving it the appearance of crumbling (Fig. 3E). In most cells, however, the cytoplasm surrounding the aggregates empties of granules. Although AF associated with aggregates can be locally intense, the presence of granule-free areas in the same cells reduces total cellular AF. In late degenerative stages, as aggregates dwindle in number, AF signal diminishes even further (Figs. 3C, 3D, 3F). Degranulation can occur contemporaneously with aggregation (Figs. 3E, 3F). Remarkably, the cytoskeleton of cells undergoing LF/MLF loss as well as their neighbors seems intact, suggesting the persistence of epithelial properties.
Figure 3.
Autofluorescent granules aggregate into packets and disappear, reducing AF. (A–F) Retinal pigment epithelial cells of AMD eyes sequester LF/MLF granules into dense packets (white arrowhead), in association with a rounding of the cytoskeleton (yellow arrowheads [A]). In late stages, packets of LF/MLF granules are reduced in number diminishing total AF signal further (C–F). Aggregation proceeds concurrently with degranulation (D). (G) Massive enlarged cell, possibly healthy, with hundreds of AF granules and not yet undergoing degranulation. A histologic cross-section of granule aggregations is shown in Supplementary Figure S3. Donors: (A, B, D–F) 83 years, female, incipient AMD; (C, G) 81 years, male, late nonexudative AMD. (A–F) Confocal microscopy, (G) HR-SIM. F-actin labeled with AlexaFluor647-Phalloidin. Scale bars: 20 μm.
The normally precise packing geometry of RPE becomes highly variable in AMD, and within that context, some cells enlarge excessively (Fig. 3G). Cells can grow to 4 to 5× the typical 15-μm size (in unaffected perifovea, Supplementary Table 5 in Ach et al.10). Enlarged cells can contain hundreds of regularly packed LF granules, while the cytoskeleton also remains intact (not shown). Thus, despite the remarkable appearance, it would be difficult to say that these giant cells are not healthy.
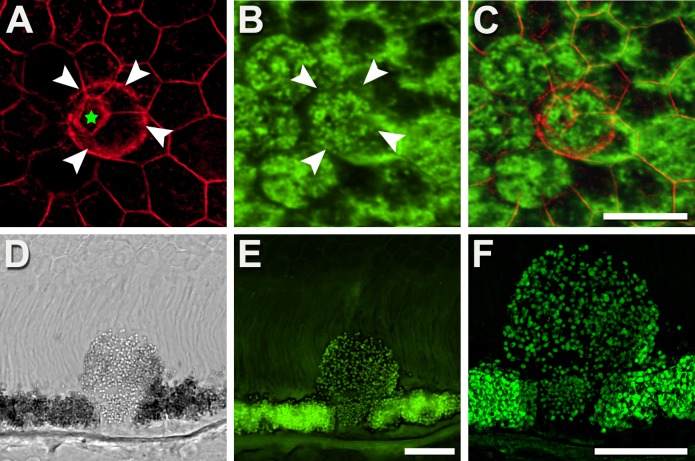
Some RPE cells are crowded out of the monolayer, a phenomenon observed in healthy and AMD-affected eyes (Fig. 4). These mushroom-like cells have stems in the RPE mosaic, delimited by cytoskeletons of neighboring cells, and caps protruding into the subretinal space, delimited by their own encircling cytoskeletons (Fig. 4A). As mushroom cells were found in eyes with attached retinas (Fig. 4), the subretinally extruded material cannot be considered secondary to postmortem retinal detachment. These subretinal portions also contain LF/MLF, yet no melanosomes are visible. The additional volume in the cap reduces intracellular LF/MLF granule concentration but because en face AF imaging uses projection images, total AF signal from this cell would resemble those around it (Figs. 4B, 4C). Throughout, there were no obvious holes in the mosaic.
Figure 4.
Retinal pigment epithelial cells shaped like mushrooms redistribute LF/MLF granules. (A–C) En face view: Some RPE cells elevate parts of their cytoplasm into the layer of outer segments. This elevated bleb is delineated by its labeled F-actin (white arrowheads) and exceeds the dimensions of the stem delimited by the cytoskeleton of its neighbors (pentagon; green star in [A]). The bleb is filled with autofluorescent LF/MLF granules (E, F) that when viewed from above has AF signal resembling cells around it (B, C). (D–F) Vertical view: A histologic section shows a mushroom-like cell expanding into the layer of outer segments, possibly the initial step of sloughed RPE cells (as described by Zanzottera et al.22). Of note, the mushroom cell originates from a continuous RPE layer; microglia, selecting, and engulfing individual RPE cells out of an otherwise intact RPE seems unlikely. Donors: (A–C) 69 years, male, atrophic AMD; (D–F) 87 years, female, incipient AMD. (A) Fluorescence confocal, F-actin labeled with AlexaFluor647-Phalloidin; (B) fluorescence confocal, 488-nm excitation; (C) image overlay; (D) differential interference contrast mode; (E) fluorescence confocal; (F) fluorescence HR-SIM, both 488-nm excitation. Scale bars: 20 μm.
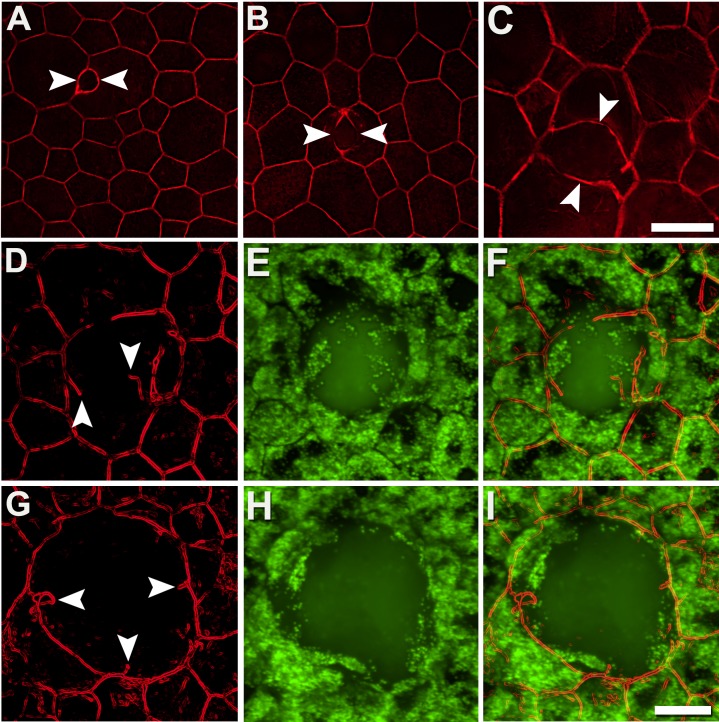
Age-related macular degeneration–specific pathology also affects RPE cytoskeleton. F-actin bundles bend slightly outward, and interior angles of vertices increase, as affected cells round (Figs. 3A, 3D). It also includes separation of the F-actin cytoskeleton of adjacent cells, interruption of individual cell cytoskeletons, and formation of stress fibers (Figs. 5, 6). Separation started with a partial (<50%) dilatation of two adjacent cytoskeletons (Fig. 5A) and continued, as if unzipping, until completely disconnected (Fig. 5C). These changes culminate in cytoskeleton interruptions, with free ends, curls, and loops (Figs. 5D, 5G). The enlargement of RPE cells also leads to the formation of stress fibers crossing each cell in arbitrary directions (Fig. 6). These fibers appear to exert additional forces on the surrounding cytoskeleton, leading to thickening at points of insertion and an overall ragged appearance. Stress-traction exerted appears to create concavities in the previously straight sides of individual cells (Figs. 6B, 6C).
Figure 5.
The cytoskeleton of adjacent RPE cells separates and becomes interrupted. In early stages (A), parallel adjacent cytoskeleton bands are separated partially (≤50%, between white arrowheads). In advanced stages (B, C) greater than 50% cytoskeleton bands are separated. Cytoskeleton interruptions are shown (D–I) with free ends, sometimes furled or pointing in different directions. Interrupted cytoskeleton is often associated with AF sub-RPE deposits ([E, H] 488-nm excitation). Donors: (A, B) 94 years, female, incipient AMD; (C) 81 years, male, geographic atrophy; (D–I) 86 years, female, late exudative AMD. F-actin labeled with AlexaFluor647-Phalloidin. Scale bars: 20 μm.
Figure 6.
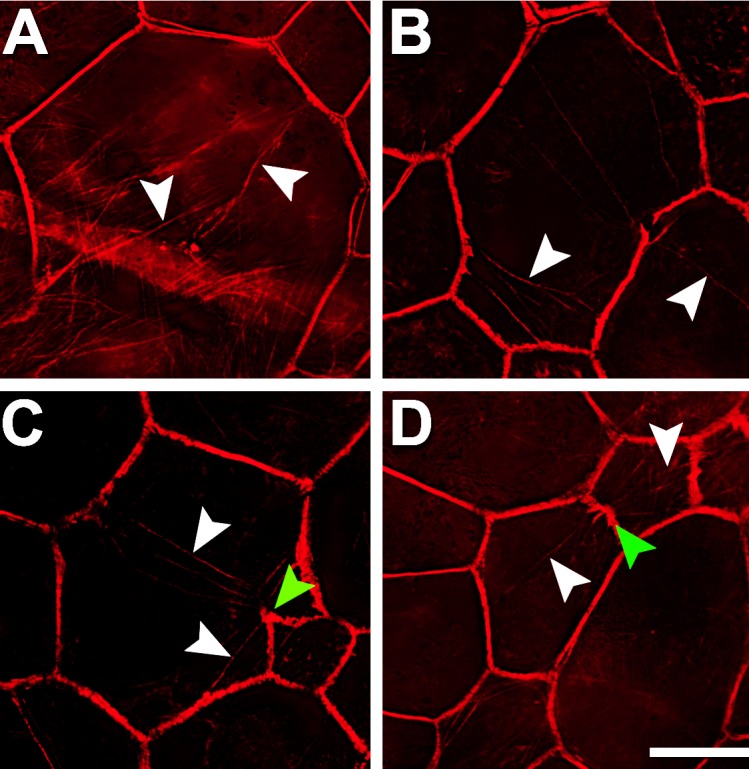
Retinal pigment epithelial cells have stress fibers in AMD-affected eyes. Multiple intracellular stress fibers (white arrowheads) arbitrarily cross RPE cells (A–D), all of which are enlarged. At sites where stress fibers insert, the cytoskeleton appears frayed and thickened ([C, D] green arrowheads). Although RPE cells are found within atrophic areas, only those outside the atrophic area had recognizable actin cytoskeleton and thus stress fibers. Donors: (A, D) 94 years, female, incipient AMD; (B, C) 81 years, male, geographic atrophy. F-actin labeled with AlexaFluor647-Phalloidin. Scale bar: 20 μm.
Discussion
This is the largest en face survey of the cellular and subcellular basis of RPE AF from human AMD tissues. We undertook this survey and another of cross-sectional histology,22,23 under the hypothesis that the RPE exhibits a stereotypic set of stress responses that can be defined, quantified, and followed, ultimately in clinical imaging. These cellular morphologies may not be unique to AMD but they can be studied effectively in the large sample of aged and AMD eyes afforded by the donor population. In this report, our data show how the AF loss in geographic atrophy (GA) may begin with the loss of AF granules of individual RPE cells via degranulation and intracellular aggregation of LF/MLF granules. We further hypothesized that the different clinical stages of AMD, as defined on a per eye basis, will differ by the frequencies of the different RPE phenotypes present at each stage; the current data do not address this question. Although motivated by questions raised by clinical imaging, our work subscribes to the philosophy that excellent morphologic description is also prerequisite for accurate localization of molecular mechanisms, to be identified in subsequent studies.
The Abundance of Granules Apparent in High-Resolution Views of RPE-BRM Flatmounts Was Not Previously Conveyed by Other Methods
Lipofuscin/MLF and melanosomes have a common origin in the lysosomal pathway, and how the balance of these granules and their total number are regulated remains to be understood.29,30 The granule mix in RPE changes with age, in the context of an overall increase, with fewer melanosomes, more LF, and more complex granules, especially MLF.31,32 Absolute numbers of granules (LF versus MLF versus melanosomes) within individual cells have yet to be determined using imaging or mathematical modeling33 techniques. Feeney-Burns31 reported 41 LF granules per RPE cell (profiles/profile) in the maculas of 61- to 100-year-old donors, using single section transmission electron microscopy (TEM) images, without conversion to numbers per volume or per cell via stereology or 3-dimensional reconstruction. As disclosed by our flatmounts subjected to HR-SIM, RPE cells have far more than 41 granules per cell. Consistent with early descriptions of lysosomal enzyme activity continuing late in adulthood20 and reports that postnatal RPE can generate melanosomes,29 we hypothesize that RPE LF/MLF granules are dynamic organelles, with measurable rates of formation, maturation, and deletion that combine for optimal balance. An accurate assessment of the total number of RPE granules and proportions of each type, for example, with reconstructions of single cells via 3-dimensional electron microscopy34 or via HR-SIM, would provide important insight into organelle regulation, the potential for renewal over the lifespan, and strengthen the basis of AF imaging. Such studies are planned.
The Intracellular Distribution of Autofluorescent Granules Is Spatially Organized, Implying a Regulated Process
High-resolution SIM images reveal an orderly distribution of LF/MLF granules within RPE cells in normal aged eyes. Granules similar in size and shape, and methodically arranged, give the impression that LF/MLF accumulation is a normal and regulated process and not a stochastic event brought on by disease. Intracellular distribution also depends on retinal location, which provides different LF/MLF/melanosomes ratios and age. Early single-section TEM studies reported differences among macula, equatorial, and peripheral regions in intracellular granule distribution in normal RPE cells but included no31 or minimally characterized35 AMD eyes. Further, only one study of healthy eyes distinguished between RPE serving fovea (cones-only) and perifovea (cones and rods).10 Regular cellular functions require organized structures, of special importance for polarized and geometrically precise cells such as RPE, which are tasked with maintaining accuracy of the sampling array of photoreceptors.
In Both Aging and AMD, RPE Cells Redistribute AF Organelles by Degranulation
The term degranulation was suggested by the finding of still-intact cells containing greatly reduced numbers of AF granules. The difference between aging and disease was that degranulation was the principle means of AF reduction in aging and aggregation was more prominent in AMD. Our methods do not allow us to distinguish whether this phenomenon indicates insufficient granule formation, excessive granule deletion, or other processes suggested by the dynamic model posed above. For example, degranulation might be part of an active control mechanism to avoid overload in cells of constant volume via active extrusion, described for melanosomes,31 or via autophagic renewal. Degranulation via exosome pathways is a possibility, because crystallins and other proteins are released by RPE in this manner.36–39 However, exosomes (30–100 nm) are too small to accommodate native LF/MLF granules (∼1 μm) and are not visible using common light-microscopy techniques. Autofluorescence structures less than 1 μm were reported outside RPE using HR-SIM,40 which are still too large for exosomes.
Redistribution of AF granules by aggregation is a process specific to the examined AMD eyes and may represent classic apoptotic bodies.41,42 Aggregations varied in size, up to 20-μm diameter in our study, are large relative to previously reported autophagic bodies (diameter, 300–900 nm).43 Aggregations are often multiple within one cell. They have been described as intracellular “LF congregations” within RPE cells overlying drusen,44 and they have been illustrated in GA eyes (Figs. 5H, 5I of Rudolf et al.12). We propose that the intracellular granule aggregations seen currently are the forerunners of the material released into basal laminar deposit (BLamD). Based on previous and ongoing histologic studies, nonnucleated granule-containing aggregates are shed basally into the BLamD, which always accompanies cells at this stage of degeneration (a stage previously called 2B).12,22,45 In a separately reported histologic survey of RPE morphology in late AMD, we renamed this cellular phenotype Shedding. Shedding of aggregates is distinct from the apical migration of nucleated RPE cells into the neurosensory retina.22,46 Further, the shed granule aggregates are visible in clinical SD-OCT as hyperreflective spots within a split RPE-BrM band signifying BLamD.22
Our observations can be compared and contrasted with TEM observations of Feeney-Burns and colleagues47 showing shedding of RPE cytoplasm lacking LF into small drusen of eyes without BLamD. These authors speculated that shedding of aliquots of cytoplasm by RPE cells may serve to dispose of “old or damaged membranes and organelles.”48 Alternatively, seminal ultrastructural studies of cells fated for regulated cell death described membrane-bounded inclusions of local cytoplasm contents as apoptotic bodies.42 Given the huge number of LF and MLF granules in aged human RPE, and the association with a cellular phenotype apparently on a death pathway22 leads to the intriguing hypothesis that AF granule aggregates are apoptotic bodies. The triggers for apoptosis remain to be determined and are suggested to involve the inflammasome49 and vascular insufficiency10,50/micronutrient deficiency.
Cell volume is modulated in response to specific stimuli and conditions.51 Early on, it was believed that intracellular accumulation of RPE LF granules depresses cellular function in part from occupying cellular volume but also by disrupting membranes via a detergent-like action of major bisretinoids.11 It is instructive to place RPE LF/MLF in the context of age-pigments in other postmitotic cells, particularly neurons, because of the RPE's neuroectodermal origin and the extensive pathology literature on aging and neurodegeneration. Several excellent reviews collectively put age-related brain LF in a neutral role, since neurons can accumulate large quantities of granules without exhibiting vulnerability to dysfunction and death.52–56 Also, a comprehensive review by Katz and Robison57 states that each cell type's LF is unique to that cell type and distinguishable from truly pathologic ceroids found in rare inherited diseases. Thus, it is telling that LF-packed RPE cells from normal aged eyes look healthy, with intact cytoskeleton, precise geometry, and absence of swelling, as we previously showed using confocal microscopy.
Even Exceedingly Enlarged Cells (Fig. 3G) Can Have Unremarkable Cytoskeleton and Polygonal Shape
As a consequence of this enlargement, the RPE cell density appears qualitatively reduced; quantitative assessments are the focus of our ongoing analysis. Retinal pigment epithelial cells resembling mushrooms were rare, yet captured by two microscopy modalities in healthy and AMD eyes with attached retinas. By HR-SIM LF/MLF was visible within these cells indicating RPE origin without also revealing melanosomes. Our study cannot clarify whether mushrooming stops at the extrusion of caps or continues until whole cells are extruded into the subretinal space in a process of transdifferentiation. As known from published and separately reported histology, apically sloughed cells are a prominent phenotype in AMD eyes.12,22,45,58–61 Subretinally displaced pigment-bearing cells60,62–64 and exploded granules suggestive of vacuolated or lysed cells60,65,66 have been reported for mechanically diverse mouse models.60,67 The relationships among these histologic variants in humans are also described by Zanzottera et al.,22 while their causative pathways and distribution in early and late AMD remain to be explored through further research. Another point exemplified by the mushrooms is that cells must be viewed both en face and from cross-sectional views for definitive identifications and mechanistic insight. From en face only, the AF of the mushrooms looked like their neighbors.
Retinal pigment epithelial cytoskeleton changes significantly with AMD, exhibiting separations and breaks, becoming thickened and connected to stress fibers, with concomitant conversion of cell bodies from convex to concave polygons. These changes were not observable in normal aging, where we reported a mosaic dominated by five to seven neighbored RPE and a small percentage of convex-polygonal cells with greater than or equal to eight neighbors in younger and older adults.10 An intact cytoskeleton is important for the maintenance of RPE cell's shape and function, and for RPE monolayer polarity. In cell culture and mouse models, stress disequilibrates RPE cells and leads to stereotypic cell responses.68–72 Actin seems to play a key role in cell aging and apoptosis.73 The appearance of stress fibers74 was reported for various cells exposed to mechanical forces,75 chemicals,76 and wounds77 and support the concept of a stereotypic repertoire of RPE stress responses, to multiple stressors.22
In the context of stress fibers also thickened actin bundles were observed.76 The cytoskeleton in the AMD affected cells in our study also showed thickened F-actin bundles, especially at areas where stress fibers insert. Focal adhesion complexes function as the structural basis for geometry sensing and transmit traction forces of the cytoskeleton to the extracellular matrix.78 Separation of the cytoskeleton between two adjacent RPE cells in AMD eyes might result from sub-RPE deposits including drusen and thick BLamD, which together provide an upward force from the basolateral aspect. Cell–cell contacts might suffer and get weak or lost under this permanent pressure. The impetus for cytoskeleton changes was not obvious from the images shown here, but our forthcoming work will examine relationships with underlying Bruch's membrane pathology. Separation, breaks of cytoskeleton, and enlargement of RPE cells have also been described for mouse models with mechanistically diverse retinopathies.79,80 Notably, wild-type mice subjected to Alu RNA-induced RPE degeneration also exhibit splits, loose ends, nonpolygonal, and concave polygonal cells with ZO-1 staining81 very similar to our images of cytoskeleton. These findings indicate that the cytoskeleton correlates well with the distribution of zonula occludens junctions and that derangement does not require the presence of sub-RPE deposits.
Study Results Impact Interpretation of Clinical Fundus Autofluorescence
The early RPE cytoskeleton and LF/MLF granule distribution changes in AMD that we herein describe are currently inaccessible to clinical fundus imaging yet have considerable relevance to how these images are interpreted. Even though the pixel sampling in fundus-AF devices can reach values down to 10 μm/pixel,82 the optical resolution is far less. Assuming correct Nyquist sampling,83 the optical resolution has to be at least 20 μm, and might even be worse due to the restricted pupil and optical aberrations. This resolution is not sufficient to discriminate single cells without the help of adaptive optics.84 Our thorough histologic description, however, will serve as source for interpretation, independent from the imaging method used. For example, Delori et al.19 found that fundus-AF in healthy humans increases with age but decreases after age 70. The authors explained this phenomenon by preretinal screening by aged lens19 in addition to possible patient selection bias and methodical challenges. Loss of RPE cells can be excluded85 because our recent study with previous literature shows stable RPE density with age.10 Our data of LF/MLF redistribution suggest that this signature age-effect can now also be attributed to loss of LF/MLF granules in individual RPE cells. Furthermore, definitive evaluation of en face fundus-AF will employ synergistic information available from cross-sectional SD-OCT, as proposed.12 Independently, we will confirm early reports46 with direct clinicopathologic correlation that granule aggregates within BLamD are visible by SD-OCT.22 So even if the aggregates themselves are below the resolution of current fundus-AF and SD-OCT, an associated sign is visible on SD-OCT.
Our study should be viewed in light of its strengths and limitations. Technical advances included the use of the largest sample of eyes yet investigated for histologic AF in AMD, short postmortem tissues that obviated cell variability in staining and size reported by others,31,86 z-stack projection images to simulate the projection image of fundus AF, and HR-SIM for 110-nm resolution of granules over large tissue areas. Limitations include absence of clinical information regarding eye donors, lack of specific marker studies to investigate mechanisms of subcellular reorganization and cell death, lack of TEM to investigate the nature of proposed intracellular organization, and inability to examine RPE and RPE-derived cells out of the RPE layer, such as intraretinal RPE of high prognostic value for progression.22,87,88 Furthermore, the requisite single-snapshot perspective of our en face histology does not allow simultaneous cross-section observation. Whether aggregations are specifically related to AMD has to be further elucidated.
Despite these limitations, our data enable us to meet the next challenges, which include quantifying en face AF from these flat-mounted tissues, further integrating the en face view and cross-sectional morphology, testing hypotheses about the impact of sub-RPE lipid deposits on RPE health as monitored by granule population and packing geometry, and leveraging the longitudinal view of cellular health available through clinical imaging. Upcoming systematic analysis will also include quantitative information on the frequencies of RPE degranulation and aggregation in eyes at defined stages of aging and AMD.
In summary, this is the first description of possible mechanisms for decreasing AF both in aging and AMD: degranulation of RPE cells. We have shown that redistribution and loss of AF granules are among the earliest subcellular changes in AMD. This is important for deepening the knowledge base of clinical-AF, benchmarking model systems, and for identifying molecular mechanisms for potential points of therapeutic entry.
Supplementary Material
Acknowledgments
We thank the Alabama Eye Bank for timely recovery of donor eyes and donor families for their generosity. This study was initiated at UAB.
Supported by grants from the German Research Foundation (Bonn, Germany) DFG #AC265/1-1 (TA); National Institutes of Health (Bethesda, MD, USA) Grant R01 EY06109 (CAC); NEI Grant R01 EY015520 (R. Theodore Smith, MD, PhD, NY, USA); Research to Prevent Blindness (New York, NY, USA) (CAC); and the EyeSight Foundation of Alabama (Birmingham, AL, USA) (CAC).
Disclosure: T. Ach, None; E. Tolstik, None; J.D. Messinger, None; A.V. Zarubina, None; R. Heintzmann, None; C.A. Curcio, None
Parts of this paper were presented at ARVO 2014, Orlando, Florida, United States, and at the 112th DOG Congress (German Ophthalmologic Society) 2014, Leipzig, Germany.
References
- 1. Delori FC,, Dorey CK,, Staurenghi G,, Arend O,, Goger DG,, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995; 36: 718–729. [PubMed] [Google Scholar]
- 2. Schmitz-Valckenberg S,, Fleckenstein M,, Scholl HP,, Holz FG. Fundus autofluorescence and progression of age-related macular degeneration. Surv Ophthalmol. 2009; 54: 96–117. [DOI] [PubMed] [Google Scholar]
- 3. von Ruckmann A,, Fitzke FW,, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997; 38: 478–486. [PubMed] [Google Scholar]
- 4. Spaide RF,, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010; 30: 1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delori FC,, Fleckner MR,, Goger DG,, Weiter JJ,, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000; 41: 496–504. [PubMed] [Google Scholar]
- 6. Schmitz-Valckenberg S,, Fleckenstein M,, Gobel AP,, Hohman TC,, Holz FG. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 1–6. [DOI] [PubMed] [Google Scholar]
- 7. Spaide R. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003; 110: 392–399. [DOI] [PubMed] [Google Scholar]
- 8. Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976; 60: 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarks SH,, Arnold JJ,, Killingsworth MC,, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999; 83: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ach T,, Huisingh C,, McGwin G, Jr, et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014; 55: 4832–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wing GL,, Blanchard GC,, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978; 17: 601–607. [PubMed] [Google Scholar]
- 12. Rudolf M,, Vogt SD,, Curcio CA, et al. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013; 120: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curcio CA,, Sloan KR,, Kalina RE,, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990; 292: 497–523. [DOI] [PubMed] [Google Scholar]
- 14. Dubra A,, Sulai Y,, Norris JL, et al. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011; 2: 1864–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorey CK,, Staurenghi G,, Delori F. Lipofuscin in aged and AMD eyes. : Hollyfield J,, Anderson R,, LaVail M. Retinal Degeneration. New York: Springer; 1993: 3–14. [Google Scholar]
- 16. Burke TR,, Duncker T,, Woods RL,, et al. Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci. 2014; 55: 2841–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberg JP,, Duncker T,, Woods RL,, Smith RT,, Sparrow JR,, Delori FC. Quantitative fundus autofluorescence in healthy eyes. Invest Ophthalmol Vis Sci. 2013; 54: 5684–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delori F,, Greenberg JP,, Woods RL, et al. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 2011; 52: 9379–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delori FC,, Goger DG,, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001; 42: 1855–1866. [PubMed] [Google Scholar]
- 20. Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978; 17: 583–600. [PubMed] [Google Scholar]
- 21. Curcio CA,, Medeiros NE,, Millican CL. The Alabama Age-Related Macular Degeneration Grading System for donor eyes. Invest Ophthalmol Vis Sci. 1998; 39: 1085–1096. [PubMed] [Google Scholar]
- 22. Zanzottera EC,, Messinger JD,, Ach T,, Smith RT,, Freund KB,, Curcio CA. The Project MACULA retinal pigment epithelium grading system for histology and optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 3253–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zanzottera EC,, Messinger JD,, Ach T,, Smith RT,, Curcio CA. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015; 56: 3269–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malek G,, Li CM,, Guidry C,, Medeiros NE,, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003; 162: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curcio CA,, Presley JB,, Malek G,, Medeiros NE,, Avery DV,, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005; 81: 731–741. [DOI] [PubMed] [Google Scholar]
- 26. Polyak SL. The Retina: The Anatomy and the Histology of the Retina In Man Ape, and Monkey, Including the Consideration of Visual Functions, the History of Physiological Optics, and the Histological Laboratory Technique. Chicago: University of Chicago; 1941. [Google Scholar]
- 27. Abbe E. Beiträge zur theorie des mikroskops und der mikroskopischen wahrnehmung. Archiv für mikroskopische Anatomie. 1873; 9: 6. [Google Scholar]
- 28. Ach T,, Best G,, Rossberger S,, Heintzmann R,, Cremer C,, Dithmar S. Autofluorescence imaging of human RPE cell granules using structured illumination microscopy. Br J Ophthalmol. 2012; 96: 1141–1144. [DOI] [PubMed] [Google Scholar]
- 29. Schraermeyer U,, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999; 12: 219–236. [DOI] [PubMed] [Google Scholar]
- 30. Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995; 105: 3–7. [DOI] [PubMed] [Google Scholar]
- 31. Feeney-Burns L,, Hilderbrand ES,, Eldridge S. Aging human RPE: morphometric analysis of macular equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984; 25: 195–200. [PubMed] [Google Scholar]
- 32. Gouras P,, Brown K,, Ivert L,, Neuringer M. A novel melano-lysosome in the retinal epithelium of rhesus monkeys. Exp Eye Res. 2011; 93: 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazzitello KI,, Arizmendi CM,, Family F,, Grossniklaus HE. Formation and growth of lipofuscin in the retinal pigment epithelium cells. Phys Rev E Stat Nonlin Soft Matter Phys. 2009; 80: 051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mustafi D,, Kevany BM,, Genoud C, et al. Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration. FASEB J. 2011; 25: 3157–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feher J,, Kovacs I,, Artico M,, Cavallotti C,, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006; 27: 983–993. [DOI] [PubMed] [Google Scholar]
- 36. Lakkaraju A,, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008; 18: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang AL,, Lukas TJ,, Yuan M,, Du N,, Tso MO,, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009; 4: e4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sreekumar PG,, Kannan R,, Kitamura M, et al. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010; 5: e12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gangalum RK,, Atanasov IC,, Zhou ZH,, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011; 286: 3261–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossberger S,, Ach T,, Best G,, Cremer C,, Heintzmann R,, Dithmar S. High-resolution imaging of autofluorescent particles within drusen using structured illumination microscopy. Br J Ophthalmol. 2013; 97: 518–523. [DOI] [PubMed] [Google Scholar]
- 41. Galluzzi L,, JM Bravo-San Pedro,, Vitale I, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015; 22: 58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kerr JF,, Wyllie AH,, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972; 26: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim J,, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000; 69: 303–342. [DOI] [PubMed] [Google Scholar]
- 44. Anderson DH,, Mullins RF,, Hageman GS,, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134: 411–431. [DOI] [PubMed] [Google Scholar]
- 45. Vogt SD,, Curcio CA,, Wang L, et al. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp Eye Res. 2011; 93: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fleckenstein M,, Charbel Issa P,, Helb HM,, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 4137–4144. [DOI] [PubMed] [Google Scholar]
- 47. Feeney-Burns L,, Gao CL,, Tidwell M. Lysosomal enzyme cytochemistry of human RPE Bruch's membrane and drusen. Invest Ophthalmol Vis Sci. 1987; 28: 1138–1147. [PubMed] [Google Scholar]
- 48. Feeney-Burns L,, Ellersieck MR. Age-related changes in the ultrastructure of Bruch's membrane. Am J Ophthalmol. 1985; 100: 686–697. [DOI] [PubMed] [Google Scholar]
- 49. Tarallo V,, Hirano Y,, Gelfand BD, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012; 149: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Curcio CA,, Owsley C,, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000; 41: 2015–2018. [PubMed] [Google Scholar]
- 51. Hoffmann EK,, Lambert IH,, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009; 89: 193–277. [DOI] [PubMed] [Google Scholar]
- 52. Gray DA,, Woulfe J. Lipofuscin and aging: a matter of toxic waste. Sci Aging Knowledge Environ. 2005; 2005: . [DOI] [PubMed] [Google Scholar]
- 53. Keller JN,, Dimayuga E,, Chen Q,, Thorpe J,, Gee J,, Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004; 36: 2376–2391. [DOI] [PubMed] [Google Scholar]
- 54. Seehafer SS,, Pearce DA. You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol Aging. 2006; 27: 576–588. [DOI] [PubMed] [Google Scholar]
- 55. Double KL,, Dedov VN,, Fedorow H, et al. The comparative biology of neuromelanin and lipofuscin in the human brain. Cell Mol Life Sci. 2008; 65: 1669–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sulzer D,, Mosharov E,, Talloczy Z,, Zucca FA,, Simon JD,, Zecca L. Neuronal pigmented autophagic vacuoles: lipofuscin neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem. 2008; 106: 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Katz ML,, Robison WG. What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch Gerontol Geriatr. 2002; 34: 169–184. [DOI] [PubMed] [Google Scholar]
- 58. Guidry C,, Medeiros NE,, Curcio CA. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002; 43: 267–273. [PubMed] [Google Scholar]
- 59. Hollyfield JG,, Bonilha VL,, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008; 14: 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen M,, Forrester JV,, Xu H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS One. 2011; 6: e22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hu P,, Herrmann R,, Bednar A, et al. Aryl hydrocarbon receptor deficiency causes dysregulated cellular matrix metabolism and age-related macular degeneration-like pathology. Proc Natl Acad Sci U S A. 2013; 110: E4069–E4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hahn P,, Qian Y,, Dentchev T,, et al. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A. 2004; 101: 13850–13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao C,, Yasumura D,, Li X, et al. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest. 2011; 121: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sweigard JH,, Cashman SM,, Kumar-Singh R. Adenovirus-mediated delivery of CD46 attenuates the alternative complement pathway on RPE: implications for age-related macular degeneration. Gene Ther. 2011; 18: 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Malek G,, Johnson LV,, Mace BE, et al. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A. 2005; 102: 11900–11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao Z,, Chen Y,, Wang J,, et al. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011; 6: e19456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ambati J,, Anand A,, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003; 9: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 68. Thanos A,, Morizane Y,, Murakami Y,, et al. Evidence for baseline retinal pigment epithelium pathology in the Trp1-Cre mouse. Am J Pathol. 2012; 180: 1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. He L,, Marioutina M,, Dunaief JL,, Marneros AG. Age- and gene-dosage-dependent cre-induced abnormalities in the retinal pigment epithelium. Am J Pathol. 2014; 184: 1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mao H,, Seo SJ,, Biswal MR, et al. Mitochondrial oxidative stress in the retinal pigment epithelium leads to localized retinal degeneration. Invest Ophthalmol Vis Sci. 2014; 55: 4613–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Imamura Y,, Noda S,, Hashizume K,, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006; 103: 11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen M,, Hombrebueno JR,, Luo C, et al. Age-and light-dependent development of localised retinal atrophy in CCL2−/− CX3CR1GFP/GFP mice. PLoS One. 2013; 8: e61381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gourlay CW,, Ayscough KR. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol. 2005; 6: 583–589. [DOI] [PubMed] [Google Scholar]
- 74. Pellegrin S,, Mellor H. Actin stress fibres. J Cell Sci. 2007; 120: 3491–3499. [DOI] [PubMed] [Google Scholar]
- 75. Tojkander S,, Gateva G,, Lappalainen P. Actin stress fibers – assembly dynamics and biological roles. J Cell Sci. 2012; 125: 1855–1864. [DOI] [PubMed] [Google Scholar]
- 76. Ho T-C,, Yang Y-C,, Cheng H-C,, Wu A-C,, Chen S-L,, Tsao Y-P. Pigment epithelium-derived factor protects retinal pigment epithelium from oxidant-mediated barrier dysfunction. Biochem Biophys Res Commun. 2006; 342: 372–378. [DOI] [PubMed] [Google Scholar]
- 77. Hergott GJ,, Sandig M,, Kalnins VI. Cytoskeletal organization of migrating retinal pigment epithelial cells during wound healing in organ culture. Cell Motil Cytoskeleton. 1989; 13: 83–93. [DOI] [PubMed] [Google Scholar]
- 78. Rape AD,, Guo WH,, Wang YL. The regulation of traction force in relation to cell shape and focal adhesions. Biomaterials. 2011; 32: 2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ding JD,, Johnson LV,, Herrmann R, et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2011; 108: E279–E287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bruban J,, Glotin A-L,, Dinet V,, et al. Amyloid-β(1-42) alters structure and function of retinal pigmented epithelial cells. Aging Cell. 2009; 8: 162–177. [DOI] [PubMed] [Google Scholar]
- 81. Kim Y,, Tarallo V,, Kerur N, et al. DICER1/Alu RNA dysmetabolism induces Caspase-8-mediated cell death in age-related macular degeneration. Proc Natl Acad Sci U S A. 2014; 111: 16082–16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Holz FG,, Schmitz-Valckenberg S,, Spaide RF,, Bird AC. Atlas of Fundus Autofluorescence Imaging. Heidelberg: Springer; 2007. [Google Scholar]
- 83. Heintzmann R. Band-limit and appropriate sampling in microscopy. : Celis JE ed. Cell Biology: A Laboratory Handbook. Waltham, MA: Elsevier Academic Press; 2006: 29–36. [Google Scholar]
- 84. Scoles D,, Sulai YN,, Dubra A. In vivo dark-field imaging of the retinal pigment epithelium cell mosaic. Biomed Opt Express. 2013; 4: 1710–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dorey CK,, Wu G,, Ebenstein D,, Garsd A,, Weiter JJ. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989; 30: 1691–1699. [PubMed] [Google Scholar]
- 86. Burke JM,, Hjelmeland LM. Mosaicism of the retinal pigment epitheliums. Mol Interv. 2005; 5: 9. [DOI] [PubMed] [Google Scholar]
- 87. Folgar FA,, Chow JH,, Farsiu S, et al. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci. 2012; 53: 4626–4633. [DOI] [PubMed] [Google Scholar]
- 88. Leuschen JN,, Schuman SG,, Winter KP,, et al. Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology. 2013; 120: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Anderson M,, Dawson WW,, Gonzalez-Martinez J,, Curcio CA. Drusen and lipid-filled retinal pigment epithelium cells in a rhesus macula. Vet Ophthalmol. 2006; 9: 201–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.