Abstract
Background
RHD is an autoimmune disease that arises following infection by S. pyogenes and imposes a heavy burden on public health.
Material/Methods
We detected 11 selected miRNAs expressed in the cardiac tissues of 11 RHD patients and 11 controls. By employing dual-luciferase assay and Western blot, we identified the relationship between TLR2 and miR-101 and miR-101. We used ELISA to test the concentration of TNF-α, IL-1β, and IL-6.
Results
In cardiac tissue of RHD patients, miR-101 was significantly down-regulated (p=0.011). Ectopically expressed miR-101 repressed the luciferase activity by 27% through targeting TLR2 3′UTR. Combined with the results of Western blot, we confirmed that TLR2 is a direct target gene of miR-101. miR-101 knock-down is related to over-stimulated immune response in PGN-activated THP-1 cells. We detected a significantly higher concentration of TNF-α (p=0.0017), IL-1β (p=0.015), and IL-6 (p=0.014) in serum samples. TLR2 had a higher expression in patients in the protein level rather than the mRNA level, indicating that post-transcriptional regulation factors play a crucial role in regulating TLR2 expression.
Conclusions
The present study confirmed that miR-101 targets TLR2 3′UTR and represses TLR2 expression. This work also found an association between down-regulated miR-101 and rheumatic heart disease.
MeSH Keywords: Autoimmune Diseases, MicroRNAs, Rheumatic Heart Disease, Toll-Like Receptor 2
Background
Rheumatic heart disease (RHD), the primarily autoimmune sequelae of acute rheumatic fever (ARF), is an autoimmune disease that arises following infection by S. pyogenes in people ages 3–19 years. The disease remains a major cause of cardiovascular disability in the young, and imposes a heavy burden on public health in the developing world. The incidence of ARF in some developing countries exceeds 50 per 100 000 children [1]. Worldwide incidence of RHD is over 15 million, with 233 000 deaths annually [2]. RHD, the most serious complication, occurs in 30–45% of RF patients and leads to chronic valvular lesions. The pathogenesis of RF/RHD is complex and autoimmune reactions triggered by pathogen infection are thought to be the main factor. Genetic factors that predispose a person to the development of autoimmune reactions were also considered to be related to RHD.
In recent years, much attention has been paid to Toll-like receptors (TLR) and their roles in autoimmune diseases [3,4]. TLRs are a class of proteins that play a key role in the innate immune system. TLRs are a type of pattern recognition receptor (PRR); they recognize molecules that are broadly shared by pathogens but distinguishable from host molecules, collectively referred to as pathogen-associated molecular patterns (PAMPs). Currently, 11 mammalian TLRs have been identified, each of which responds to a specific class of PAMP [5]. TLR2 has also been designated as CD282, which mainly responds to bacterial infection. TLR2 can be activated by a variety of microbial components, including lipoproteins, peptidoglycan, lipoteichoic acid, and, especially, antigens of Gram-positive bacteria, including streptococci [6].
MicroRNA (miRNA) is a short non-coding RNAs that suppress the expression through targeting the 3′ untranslated regions (UTRs) of messenger RNAs (mRNAs). According to the predicted results of bioinformatics, there may be more than 60% of protein coding genes with the expression regulated by miRNA. In the present study, we detected 11 selected miRNAs expression in clinical samples to investigate the relations between miRNAs and RHD pathogenesis.
Material and Methods
Sample collection
We enrolled 11 RHD patients and 11 paired controls in this research. The inclusion criteria for the experimental group were: (i) every patient diagnosed with rheumatic mitral valve insufficiency with or without mitral stenosis, and scheduled for mitral valve replacement; (ii) normal preoperative erythrocyte sedimentation rate and anti-streptolysin O to eliminate rheumatism in active stage; (iii) all patients in New York Heart Association (NYHA) functional class II–III; and (iv) no other complications (patients with acute heart failure were excluded). The inclusion criteria of the control group were: (i) every patient diagnosed with mitral valve prolapse because of mitral chordae tendineae fracture and mitral insufficiency, and scheduled for mitral valve replacement. The other conditions are the same as criteria iii–v of the experimental group. RHD cases and their controls were well matched based on the following details: (i) same sex, (ii) difference of age <5 years old, (iii) difference of left ventricular ejection fraction (EF) <5%, (iv) difference of left ventricular end-diastolic diameter (LVEDD) <10% of the larger of the 2; and (v) other physiological indexes. Left ventricular papillary muscle was transferred from resected mitral valve to physiological saline and liquid nitrogen and then moved to −80°C refrigerator for storage. Four male and 7 female pairs totally matched according to the matching principle. The characteristics of pairing groups are presented in Table 1. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University, and informed consent was obtained from all subjects.
Table 1.
Information on patients and controls.
| Patients with RHD (n=11) | Control subjects (n=11) | P value | |
|---|---|---|---|
| Age (years) | 40.82±9.94 | 41.73±8.66 | 0.32 |
| Sex (M/F) | 4/7 | 4/7 | 1 |
| EF | 0.60±0.044 | 0.59±0.039 | 0.14 |
| LVEDD (mm) | 53.36±7.28 | 54.73±6.84 | 0.16 |
| Mitral valve stenosis (Y/N) | 9/2 | 0/11 | <0.001 |
| SBP (mmHg) | 120.27±9.96 | 122.64±10.40 | 0.66 |
| BDP (mmHg) | 74.45±6.00 | 76.10±5.60 | 0.42 |
| BMI (kg/m2) | 21.44±2.05 | 21.65±1.60 | 0.82 |
Data are presented as the mean value ±SD. EF – ejection fraction; LVEDD – left ventricular end-diastolic diameter; SBP – systolic blood pressure; DBP – diastolic blood pressure; BMI – body mass index.
Cell culture
A549 and HEK293T cells were cultured in Dulbecco’s Modified Eagle Medium (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum, 100 IU/ml penicillin, and 10 mg/mL streptomycin. The THP1 cell line was maintained in RPMI 1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cells were maintained at 37°C and 5% CO2.
For analysis of THP-1 monocyte response to microbial ligand in vitro, log-phase cells were seeded at 5×105 cells/ml in a 24-well plate. Cells were stimulated with different doses of PGN (derived from Staphylococcus aureus) (0.01–1 μg/ml) (Sigma-Aldrich) for 24 h. The cell culture supernatants were collected for cytokines detection.
RT-qPCR
Quantitative RT-PCR analysis was used to detect the relative expression of selected miRNAs. Total RNA was extracted from tissue samples using Trizol Reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. miRNA expression level was determined by TaqMan miRNA RT Real-Time PCR. Single-stranded cDNA was synthesized by using TaqMan MicroRNA specific reverse transcript primer (Applied Biosystems, Foster City, CA, USA) and then amplified by using miRNA specific primer and TaqMan MGB probes (Applied Biosystems, Foster City, CA, USA). The endogenous U6 snRNA was used for data normalization. All measurements were performed in triplicate.
Dual luciferase assay
The full length of 828bp ERBB2 3′UTR was cloned into pmirGLO plasmid (Promega, Madison, WI, USA) following the firefly luciferase coding sequence to generate reporter vector. For dual luciferase reporter assays, HEK293T cells were seeded in 48-well plates. MiR-101 mimic or inhibitor was co-transfected with luciferase reporter vector by using lipofectamine 2000 (Invitrogen, Carlsbad, CA USA). Cells were harvested at 48 h post-transfection and the luciferase activity was measured by using the Dual-Luciferase Assay system (Promega, Madison, WI, USA). Each treatment was performed in triplicate in 3 independent experiments.
Western blotting
Whole-cell protein extracts were boiled in SDS/β-mercaptoethanol sample buffer, separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and then transferred to PVDF membranes (Amersham Pharmacia Biotech, St. Albans, Herts, UK) by electrophoretic transfer. The specific proteins were detected by specific antibodies. Rabbit anti-TLR2 monoclonal antibody (Abcam, Cambridge, MA, USA) and mouse anti-β-actin monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) were both commercially obtained. The specific protein-antibody complex was detected by using horseradish peroxidase conjugated rabbit anti-mouse or goat anti-rabbit IgG. The β-actin signal was used as a loading control.
ELISA
Cytokine concentrations in cell culture supernatants and serum samples were measured by ELISA using cytokine kits (Abnova) following the manufacturers’ instructions.
Statistical analysis
All the results were analyzed by using SPSS 16 software package and the t test was used. The findings were considered to be significant at a P<0.05.
Results
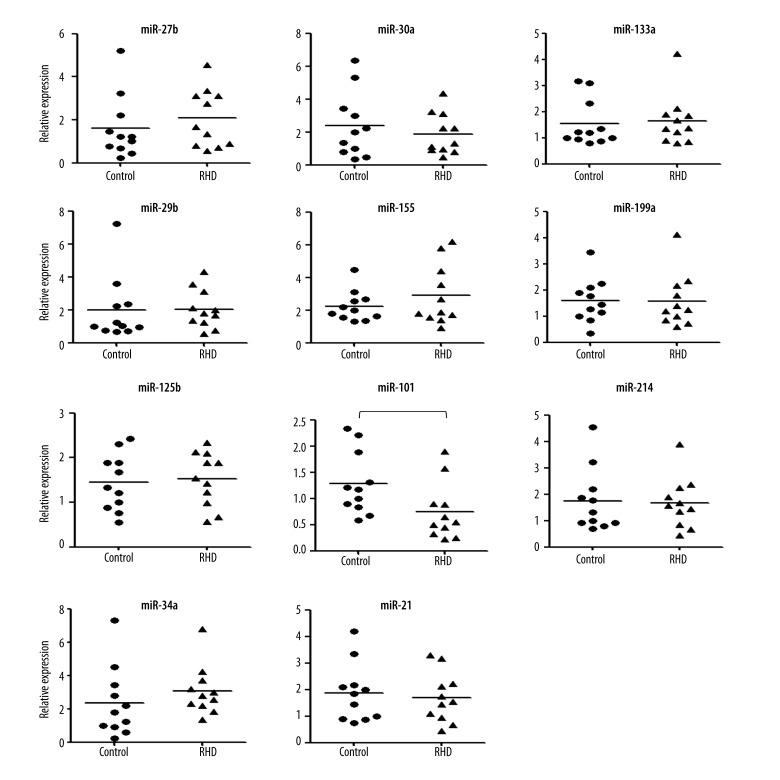
We collected left ventricular papillary muscle samples from RHD patients and detected the expression of 11 candidate miRNAs by real-time RT-PCR. As shown in Figure 1, the expression of miR-101 was significantly down-regulated in the RHD patients, suggesting a role of miR-101 in RHD pathogenesis.
Figure 1.
Detecting the expression of 11 selected miRNA in cardiac tissue samples. Total RNA was extracted from tissue samples using Trizol reagent following the manufacturer’s instructions. The expression level of candidate miRNAs was detected by TaqMan miRNA RT Real-Time PCR. Single-stranded cDNA was synthesized by using the TaqMan MicroRNA Reverse Transcription kit and then amplified by using TaqMan Universal PCR Master Mix together with miRNA-specific TaqMan MGB probes. The U6 snRNA was used for normalization. Each sample in each group was measured in triplicate and the experiment was repeated at least 3 times for the detection of miRNAs. The t test was used to analyze the results and P<0.05 was considered statistically significant. * P<0.05.
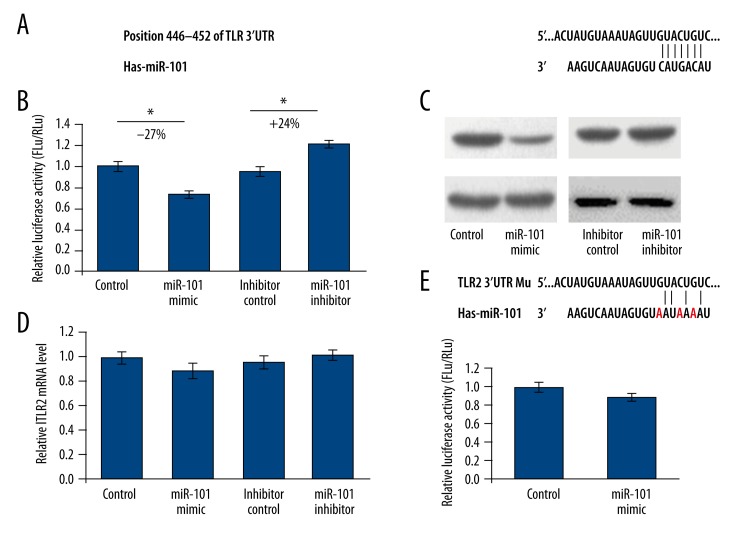
To further investigate the biological function of miR-101, we first predicted the feasible targets of miR-101 by using an online bioinformatics tool, TargetScan (http://www.targetscan.org). To our surprise, TLR2, an important sensor for pathogen infection, is a predicted target gene of miR-101(Figure 2A). To understand whether the expression of TLR2 was impressed by miR-101, full lengths of 828 bp 3′-UTR from TLR2 were cloned into the downstream of the firefly luciferase gene in pmirGLO Vector to generate pmirGLO-TLR2 reporter vector. HEK293T cells were co-transfected with pmirGLO-TLR2 and miR-101 mimics or inhibitor. At 48 h after transfection, the cells were lysed and luciferase activity was detected. As shown in Figure 2B, the luciferase activity was significantly suppressed by the ectopically expressed miR-101 by about 27% (P<0.05) when compared with the miRNA control. Furthermore, the luciferase activity was up-regulated by the miR-101 inhibitor by about 24% (P<0.05) compared with the anti-miR control. These results indicate that miR-101 targets the 3′-UTR of TLR2, leading to the change in firefly luciferase translation.
Figure 2.
MiR-101 suppresses TLR2 expression by targeting 3′UTR. (A) The predicted interaction between miR-101 and TLR2 3′UTR. (B) HEK293T cells were co-transfected with miRNA control, miR-101 mimic, anti-miR control or miR-101inhibitor and pmir-GLO-TLR2 for dual-luciferase assay. (C) TLR2 protein levels of A549 cells transfected with miR-101 mimic or miR-101inhibitor were detected by Western blot. (D) The TLR2 mRNA level in A549 cells was detected by qRT-PCR. (E) A reporter vector containing 3 nucleotides mutant TLR2 3′UTR was used to confirm the target site of miR-101. The results were analyzed by t test and P<0.05 was considered as statistically significant. * P<0.05, ** P<0.01.
To further identify the binding region of miR-101, a plasmid with mutant seed sequence was constructed (Figure 2E). We used a vector containing a putative miR-101 binding region in the 3′-UTR of TLR2 with 3 mutant nucleotides (designated as pmirGLO-TLR2-mu). The histogram in Figure 2E shows that the enzyme activity was not significantly reduced in cells transfected with miR-control compared with miR-101 mimic (P>0.05). These data indicate that miR-101 may suppress TLR2 expression through targeting the seed sequence at the 3′-UTR of TLR2.
To further examine whether endogenous TLR2 expression is suppressed by miR-101, A549 cells were transfected with miR-101 mimics or inhibitor. TLR2 protein level was detected by Western blot 48 h post-transfection. As shown in Figure 2C, the level of TLR2 protein level was significantly suppressed by miR-101 mimics and up-regulated by miR-101inhibitor compared with corresponding controls in A549 cells. The TLR2 mRNA levels were not significantly changed in these 4 groups (Figure 2D). These results indicate that miR-101 repressed endogenous TLR2 expression by directly suppressing TLR2 mRNA translation.
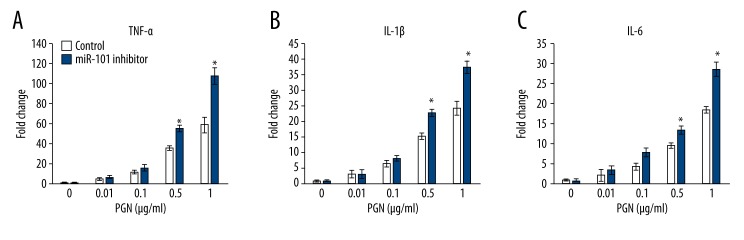
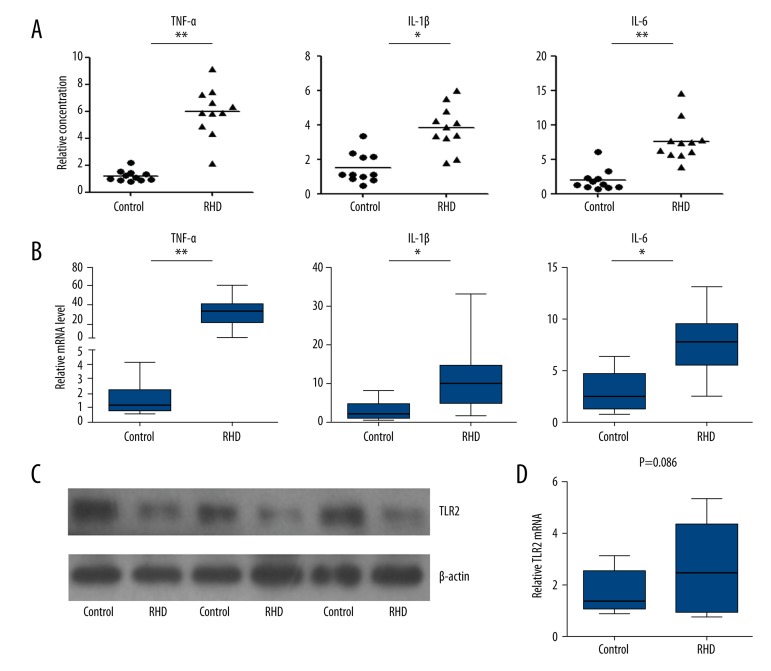
To understand the physiological relevance of induction of miR-101, THP-1 cells were used. THP-1 cells were seeded in a 24-well plate and then transfected with miR-101 inhibitor or corresponding control. At 48 h after transfection, cells were stimulated with various doses of PGN (0.01–1 μg/ml) for 24 h. The cell culture supernatants were collected for cytokines detection. As shown in Figure 3, the concentration of TNF-α, IL-1β, and IL-6 displayed a dose-dependent relationship with PGN. Meanwhile, the miR-101 knockdown cells showed a significantly more radical response when stimulated by 0.5 and 1 μg/ml than the control groups, with higher cytokines concentration. These results are in accordance with the data from clinical samples. As shown in Figure 4A, the serum cytokines (TNF-α, IL-1β, and IL-6) level was significantly higher than the controls, and cardiac tissue samples also have higher level cytokines (Figure 4B). We also detected the TLR2 expression in cardiac tissue samples in both protein and mRNA levels. As shown in Figure 4C, almost all the RHD clinical tissue samples had a higher TLR2 expression in protein levels. However, the mRNA level of TLR2 was not significantly changed in RHD patient samples (Figure 4D).
Figure 3.
Low miR-101 level contributes to high cytokines level in PGN-stimulated THP-1 cell culture supernatant. THP-1 cells were seeded at 5×105 cells/ml in a 24-well plate. At 48 h after miR-101 transfection, cells were stimulated with various doses of PGN (0.01–1 μg/ml) for 24 h. The cell culture supernatants were collected and the concentration of TNF-α (A), IL-1β (B), and IL-6 (C) was determined by ELISA. The results were analyzed by t test and P<0.05 was considered as statistically significant. * P<0.05.
Figure 4.
Low miR-101 level is related to over-expressed cytokines and TLR2 in clinical samples. (A) Serum TNF-α, IL-1β, and IL-6 concentration was analyzed by using an ELISA kit. (B) The mRNA level in cardiac tissue samples was detected by qRT-PCR. (C) TLR2 protein in cardiac tissues was detected using Western blot with β-actin as loading control. (D) TLR2 mRNA level in cardiac tissues was detected by using qRT-PCR. The results were analyzed by t test and P<0.05 was considered as statistically significant. * P<0.05, ** P<0.01.
Discussion
RHD is an autoimmune disease that arises following infection by S. pyogenes in young people. NT-proBNP is a well-known biomarker for congenital and acquired heart disease, which is also confirmed to be significantly elevated in children with acute RHD, but many limitations reduced its accuracy [7]. Research indicates that soluble ST2 (suppression of tumorigenicity 2) is a potential early marker for cardiovascular disease, but the relationship between ST2 and RHD is not understood yet [8,9]. Recently, reports indicated that miRNAs participate in the pathogenesis of many kinds of cardiovascular disease. But to date, only the work of 1 group has shown that disturbed miRNAs expression contributes to RHD [10]. In the present study, to investigate the roles of miRNA in the pathogenesis of RHD, we first detected 11 selected miRNAs expression in cardiac tissue samples of RHD patients. We found miR-101 had a significantly down-regulated expression in patients, which may play a role in modulating the immune system by disturbing TLR2 expression. Subsequently, by employing dual-luciferase assay and Western blot, we first identified that TLR2 is a direct target gene of TLR2 and miR-101 knock-down is related to over-stimulated immune response in PGN-activated THP-1 cells. Finally, we detected the concentration and mRNA level of TNF-α, IL-1β, and IL-6 in patient serum samples and found these cytokines levels were significantly higher in patients. Furthermore, the mRNA and protein levels of TLR2 were also detected in cardiac tissue samples. We found that TLR2 had a higher expression in patients, mainly in the protein level rather than the mRNA level, meaning that post-transcriptional regulation factors like miRNA play a crucial role in regulating TLR2 expression.
TLR2 was considered to be activated by streptococci, which is the major pathogen for rheumatic fever, which further contributed to RHD. Although Arg753Gln polymorphism of the TLR2 gene was confirmed to be related to acute rheumatic fever susceptibility in child, 2 research groups have found that there were no relations between this Arg753Gln polymorphism and RHD pathogenesis. This study may provide clues for genetics researchers. Since nucleotides variations can alter mature miRNAs expression or disturb the miRNA-mRNA interaction, mutation sites in the miRNA coding region may contribute to susceptibility to RHD [11–13].
Herein, we showed that miR-101 functions as an immune inhibitor through targeting TLR2 in RHD patients. However, Zhu et al. indicated that miR-101 acted as an immune response activator by suppressing MKP1, which is a negative regulatory factor of the TLR4 pathway [14]. This contradiction may be caused by the complexity of miRNA function pattern and tissue specificity. Since a single miRNA may have hundreds of targeting genes and a single gene may be regulated by tens of miRNAs in at the same time, the function of miR-101 in the immune system needs to be fully studied in the future.
Conclusions
In conclusion, the present study confirmed for the first time that miR-101 targeted TLR2 3′UTR and repressed TLR2 expression. This work also found an association between down-regulated miR-101 and rheumatic heart disease, which sheds light on the mechanism of RHD pathogenesis and may create an opportunity to approach the diagnosis and treatment of RHD.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
Source of support: Departmental sources
References
- 1.Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. 1992;268:2069–73. doi: 10.1001/jama.1992.03490150121036. [DOI] [PubMed] [Google Scholar]
- 2.Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: genetics and pathogenesis. Scan J Immunol. 2007;66:199–207. doi: 10.1111/j.1365-3083.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 3.de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol. 2014;192:4103–11. doi: 10.4049/jimmunol.1301970. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Lee JC, Kim S, et al. Inhibition of autoimmune diabetes by TLR2 tolerance. J Immunol. 2011;187:5211–20. doi: 10.4049/jimmunol.1001388. [DOI] [PubMed] [Google Scholar]
- 5.Underhill DM. Toll-like receptors: networking for success. Eur J Immunol. 2003;33:1767–75. doi: 10.1002/eji.200324037. [DOI] [PubMed] [Google Scholar]
- 6.Moreillon P, Majcherczyk PA. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand J Infect Dis. 2003;35:632–41. doi: 10.1080/00365540310016259. [DOI] [PubMed] [Google Scholar]
- 7.Kotby AA, El-Shahed GS, Elmasry OA, et al. N-Terminal proBNP levels and tissue Doppler echocardiography in acute rheumatic carditis. ISRN Pediat. 2013;2013:970394. doi: 10.1155/2013/970394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccone MM, Cortese F, Gesualdo M, et al. A novel cardiac bio-marker: ST2: a review. Molecules. 2013;18:15314–28. doi: 10.3390/molecules181215314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayes-Genis A, Richards AM, Maisel A, et al. Multimarker testing with ST2 in chronic heart failure. Am J Cardiol. 2015;115(7 Suppl3):76B–80B. doi: 10.1016/j.amjcard.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Zhang Y, Wang N, et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. 2010;122:2378–87. doi: 10.1161/CIRCULATIONAHA.110.958967. [DOI] [PubMed] [Google Scholar]
- 11.Peterlongo P, Caleca L, Cattaneo E, et al. The rs12975333 variant in the miR-125a and breast cancer risk in Germany, Italy, Australia and Spain. J Med Genet. 2011;48:703–4. doi: 10.1136/jmedgenet-2011-100103. [DOI] [PubMed] [Google Scholar]
- 12.Swart M, Dandara C. Genetic variation in the 3′-UTR of CYP1A2, CYP2B6, CYP2D6, CYP3A4, NR1I2, and UGT2B7: potential effects on regulation by microRNA and pharmacogenomics relevance. Front Genet. 2014;5:167. doi: 10.3389/fgene.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Ambrosone CB, DiCioccio RA, et al. A functional polymorphism in the miR-146a gene and age of familial breast/ovarian cancer diagnosis. Carcinogenesis. 2008;29:1963–66. doi: 10.1093/carcin/bgn172. [DOI] [PubMed] [Google Scholar]
- 14.Zhu QY, Liu Q, Chen JX, et al. MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation of MAPKs in macrophages. J Immunol. 2010;185:7435–42. doi: 10.4049/jimmunol.1000798. [DOI] [PubMed] [Google Scholar]