Abstract
Aims: Urothelial carcinoma (UC) is the most common tumor involving upper urinary tract (UTUC) and urinary bladder (UBUC) whose molecular survival determinants remains obscured. By computerizing a public transcriptomic database of UBUCs (GSE32894), we identified cell division cycle associated 5 (CDCA5) as the most significantly upregulated gene among those associated with G1-S transition of the mitotic cell cycle (GO:0000082). We therefore analyzed the clinicoptaological significance of CDCA5 expression in our well-characterized UC cohort. Methods and results: Quantigene assay was used to detect CDCA5 transcript levels in 36 UTUCs and 30 UBUCs. We used immunohistochemistry evaluated by H-scores to determine CDCA5 protein expression in 295 UBUCs and 340 UTUCs, respectively. CDCA5 expression was further correlated with clinicopathological features and disease-specific survival (DSS) and metastasis-free survival (MeFS). For both groups of UCs, increments of CDCA5 transcript levels were associated with higher pT status, CDCA5 protein overexpression was also significantly associated with advanced pT status, nodal metastasis, high histological grade, vascular invasion, and frequent mitoses. CDCA5 overexpression was predictive for worse DSS and MeFS in univariate and multivariate analysis. Conclusions: CDCA5 overexpression is associated with advanced clinical features of UC, suggesting its potential value as a prognostic biomarker and a novel therapeutic target.
Keywords: CDCA5, cell division cycle associated 5, urinary bladder, upper tract, urothelial carcinoma
Introduction
Urothelial carcinoma (UC) is the most common histological type of urinary tract malignancy. It may occur anywhere along the urinary tract, from the upper urinary tract (UT; including renal pelvis and ureter) to the urinary bladder (UB). Bladder cancer is ranked the ninth most common cancer and the thirteenth most common cause of cancer mortality among both sexes in the world [GLOBOCAN 2012 http://globocan.iarc.fr]. In contrast, upper tract urothelial carcinoma (UTUC) is uncommon and accounts for only 5% to 10% of all urothelial carcinomas [1]. Etiologically, all UCs—arising from both anatomical locations—are caused by common carcinogens, such as cigarette smoking and occupational exposure to aromatic amines, such as benzidine and β-naphtylanine, etc [2-4]. Nevertheless, certain populations are more susceptible to UTUC than urothelial carcinomas of the urinary bladder (UBUC), e.g., Balkans endemic nephropathy [5,6], Chinese herb nephropathy [6,7], and analgesic nephropathy [8]. In spite of more common promoter hypermethylation in UTUC than UBUC, as revealed by a previous study [9], gene expression profiling of UCs from both anatomical sites shows they are akin to each other [10]. Although UTUCs tend to have higher stage and grade than UBUCs, there is no difference in tumor behavior with respect to location after controlling for the effects of grade and stage [11]. This imply that carcinogenesis in UC occurring anywhere in the urinary tract may share a common molecular pathway.
Alteration in cell cycle control is one of the most important events in carcinogenesis, especially at the checkpoint pathways [12]. There are three main checkpoints, one at the G1-S transition and two at S-phase and G2-M transition [13]. They maintain genomic integrity. Mutations in checkpoint pathways usually permit the survival or the continued growth of cells with genomic abnormalities, thereby enhancing the opportunity for malignant transformation. Of these, the G1-S transition is known to be the restriction point beyond which a cell is committed to dividing [14]. Deregulation in the G1-S transition of the mitotic cell cycle is commonly encountered in oncogenesis, however, the genes associated with this phenomenon have not been systemically evaluated in UC. By performing data mining on documented transcript expression profiles in the Gene Expression Omnibus (GEO, National Center Biotechnology information (NCBI), Bethesda, Maryland, USA) with a special focus on the G1-S transition of the mitotic cell cycle (GO:0000082), we identified that the transcription of the cell division cycle associated 5 (CDCA5) gene was most significantly upregulated from early tumor development and associated stepwise with tumor progression, suggesting it plays a role in tumorigenesis and its progression.
The CDCA5 gene encodes CDCA5 protein (A.K.A. Sororin), a master regulator of sister chromatid cohesion and separation [15]. CDCA5 (Sororin) maintains sister chromatid cohesion by stabilizing the cohesion complex [16]. It ensures accurate chromosome partitioning in both meiotic and mitotic cells, and plays an important role in DNA repair. In one study, CDCA5 was overexpressed in the majority of non-small cell and small cell lung cancers at both mRNA and protein levels [17]. In the same study, positive immunostaining for CDCA5 in 262 non-small cell lung cancer samples was significantly associated with poor prognosis [17]. However, to our best knowledge, no prior report has evaluated CDCA5 expression in UC. We therefore aimed to comprehensively analyze CDCA5 expression and its association with clinicopathological factors and survival in our well-characterized cohort of UC patients.
Materials and methods
Data mining on the GEO to identify overexpressed transcripts in UCs
We carried out data mining on the GEO (National Center Biotechnology Information). We identified one data set, GSE32894 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32894) analyzing transurethral resection specimens from 308 patients with UBUC using Affymetrix U133 Plus 2.0 Array. Statistical software Nexus Expression 3 (BioDiscovery, EI Segundo, California, USA) was used to analyze all probe sets without preselection or filtering. We performed supervised comparative analysis to examine the statistical significance of differentially expressed transcripts on the basis of primary tumor (pT) status and the development of metastatic events. Transcriptomes of high-stage (pT2-pT4) UCs with developed metastases and low-stage (pTa-pT1) UCs devoid of metastasis were used to perform functional profiles, focusing on those related to the G1-S transition of the mitotic cell cycle (GO:0000082). Further survival analysis was performed by dichotomizing all cases into high-expression and low-expression clusters, in order to computerize the prognostic impact of selected genes.
Patients and tumor specimens
This study was approved by the institutional review board (IRB10302015) of Chi Mei Medical Center. Informed consent has been obtained for those enrolled into BioBank. For imunohistochemical study and statistical analysis, we retrieved urothelial carcinoma cases from the archives of Chi Mei Medical Center between 1996 and 2004. A total of 635 consecutively treated well-characterized urothelial carcinomas, not otherwise specified, were enrolled including 340 tumors originating from the upper urinary tract and 295 arising from the urinary bladder. Other histological variants were excluded. All patients were treated initially by surgical intervention with curative intent. As a rule, patients with urinary bladder urothalial carcinoma (UBUC) with pT3 or pT4 tumors or with nodal involvement received cisplatin-based adjuvant chemotherapy. However, only 29 of 106 pT3 or pT4 and nodal positive patients with upper tract urothelial carcinoma (UTUC) received cisplatin-based adjuvant chemotherapy. The criteria for clinicopathological evaluation were essentially identical to those in our previous works [18]. Two pathologists (IWC & CFL) re-evaluated hematoxylin-eosin sections of all cases. By testing a series of cutoff values, the high mitotic activity was defined as the mitotic rate no less than 10 high power fields which showed most prognostic relevance. For the quantification of CDCA5 transcript levels, fresh tissue from an independent cohort of 36 UTUCs and 30 UBUCs were selected and CDCA5 mRNA was detected with ABI StepOnePlus™ System. Of these, 21 and 15 UTUCs were of pTa-pT1 and pT2 to pT4, respectively; and 15 and 15 UBUCs were of pTa-pT1 and pT2 to pT4, respectively.
Transcriptional level of CDCA5 gene
The materials for molecular genetic examination were harvested from macrodissection of fresh tumor tissue. For quantification of CDCA5 mRNA expression, we extracted total RNAs, quantified them, and submitted them for reverse-transcription. Using pre-designed TaqMan assay reagents (Applied Biosystems), we measured mRNA abundance of CDCA5 (Hs01591589_m1) with the ABI StepOnePlus™ System, as previous described [19]. The fold expression of CDCA5 relative to normal urothelium was calculated by comparative Ct method, after normalization to POLR2A (Hs01108291_m1) as the internal control.
Immunohistochemical staining and scoring of CDCA5
Tissue sections underwent standard procedures for deparaffinization, rehydration and antigen retrieval. Subsequently, the sections were incubated with a primary antibody targeting CDCA5 (NBP1-89530, rabbit polyclonal, Novus Biologicals, CO, USA, dilution 1:100) for an hour. Scoring of CDCA5 immunoreactivity was evaluated based on the combination of the percentage and intensity of positively stained tumoral nuclei to generate an H-score, which was calculated using the following equation: H-score = ΣPi (i + 1), where i is the intensity of stained tumor cells (0-3+), and Pi is the percentage of stained tumor cells for each intensity varying from 0% to 100%. This formula produced a score range from 100-400, where 100 equals to 100% of tumor cells being negative and 400 equals 100% of tumor cells strongly stained (3+) [20,21].
Statistical analysis
Statistics were performed using SPSS V.14.0 software (SPSS Inc., Chicago, Illinois, USA). The median H-score of CDCA5 was used as the cut-off to dichotomize the study cohort, separating cases into high expression and low expression groups. The Chi-square test was used to compare CDCA5 expression status and various clinicopathological parameters. The end points analyzed were disease-specific survival (DSS) and metastasis-free survival (MeFS), calculated from the starting date of resection of tumor to the date the event developed. Patients lost to follow-up were censored on the latest follow-up date. Univariate survival analyses were performed using Kaplan-Meier plots and compared by the log-rank test. Those parameters with univariate p < 0.1 were enrolled into multivariate tests performed using Cox proportional hazards regression. For all analyses, we used two-sided tests of significance with p < 0.05 considered as significant.
Results
CDCA5 identified as a significant differentially upregulated transcript implicating dysregulation of the G1-S transition of mitotic cell cycle in UBUC
From the transcriptomic profile of 93 high-stage (T2-T4) and 213 low-stage (Ta-T1) cases of UBUC, six probes covered six transcripts associated with regulation the G1-S transition of the mitotic cell cycle (GO:0000082) were found (Figure 1). The log2 ratios of one transcript met the selection criteria of > 1.5 increase (p < 0.0001), i.e., CDCA5, with log2 ratio of 0.4639 to 1.5233-fold up-regulation (Table 1). That CDCA5 has not been systemically studied in UCs prompted us to further characterize the endogenous expression levels and clinical significance of CDCA5 protein in UC.
Figure 1.

Analysis of gene expression in urothelial carcinoma from a published transcriptomic dataset (GSE32894). Clustering analysis of genes focusing on the G1-S transition of the mitotic cell cycle (GO:0000082) revealed CDCA5 is the most significantly up-regulated gene associated with increments of pT status. Tissue specimens from tumors with different pT statuses are indicated on top of the heatmap, and expression levels of up-regulated and down-regulated genes are represented as a spectrum of brightness or red and green, respectively. Those unaltered in mRNA transcriptional level are coded black.
Table 1.
Summary of differentially expressed genes associated with Transition of G1-S transition of mitotic cell cycle and showed stepwise alterations during cancer progression in the transcriptome of urothelial carcinoma of urinary bladder
| Probe | Comparing T2-4 to Ta | Comparing T1 to Ta | Comparing T2-4 to T1 | Gene symbol | Biological process | Molecular function | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| log ratio | p-value | log ratio | p-value | log ratio | p-value | ||||
| ILMN_1683450 | 1.5233 | < 0.0001 | 1.0594 | < 0.0001 | 0.4639 | < 0.0001 | CDCA5 | G1/S transition of mitotic cell cycle, cell cycle, cell division, mitosis, mitotic chromosome condensation, mitotic metaphase plate congression | chromatin binding, protein binding |
| ILMN_1666305 | 0.8475 | < 0.0001 | 0.5026 | < 0.0001 | 0.3449 | 0.0011 | CDKN3 | G1/S transition of mitotic cell cycle, cell cycle, cell cycle arrest, dephosphorylation, negative regulation of cell proliferation, regulation of cyclin-dependent protein kinase activity | Hydrolase activity, protein binding, protein serine/threonine phosphatase activity, protein tyrosine phosphatase activity, protein tyrosine/serine/threonine phosphatase activity |
| ILMN_1711894 | 0.5142 | < 0.0001 | 0.2972 | 0.0198 | 0.217 | 0.0389 | MYB | G1/S transition of mitotic cell cycle, calcium ion transport, regulation of transcription; DNA-dependent | DNA binding, protein binding, transcription activator activity, transcription factor activity |
| ILMN_1748883 | 0.455 | < 0.0001 | 0.1736 | 0.0038 | 0.2815 | < 0.0001 | CDKN2D | DNA synthesis during DNA repair, G1/S transition of mitotic cell cycle, anti-apoptosis, autophagic cell death, cell cycle arrest, negative regulation of caspase activity, negative regulation of cell cycle, negative regulation of cell growth, negative regulation of cell proliferation, negative regulation of phosphorylation, regulation of cyclin-dependent protein kinase activity, response to UV, response to retinoic acid, response to vitamin D | Cyclin-dependent protein kinase inhibitor activity, protein kinase binding |
| ILMN_1766169 | 0.8965 | < 0.0001 | 0.1708 | 0.0173 | 0.7258 | < 0.0001 | BCAT1 | G1/S transition of mitotic cell cycle, branched chain family amino acid biosynthetic process, cell proliferation, metabolic process | Branched-chain-amino-acid transaminase activity, transferase activity |
| ILMN_1784602 | -0.6946 | < 0.0001 | -0.3761 | 0.0017 | -0.3185 | 0.0073 | CDKN1A | G1/S transition of mitotic cell cycle, G2/M transition of mitotic cell cycle, cell cycle arrest, cellular response to extracellular stimulus, goid 43071, induction of apoptosis by intracellular signals, negative regulation of apoptosis, negative regulation of cell growth, negative regulation of cell proliferation, negative regulation of cyclin-dependent protein kinase activity, negative regulation of phosphorylation, positive regulation of B cell proliferation, positive regulation of fibroblast proliferation, response to DNA damage stimulus, response to UV | Cyclin binding, cyclin-dependent protein kinase inhibitor activity, metal ion binding, protein kinase inhibitor activity, zinc ion binding |
CDCA5 mRNA transcriptional levels are higher in UBUC and UTUC with advanced pT stage
In the tested 30 UBUCs and 36 UTUCs, CDCA5 transcriptional level was significantly higher in tumors with higher pT status (p = 0.008 and p = 0.021, respectively), suggesting it plays a role in tumor progression (Figure 2).
Figure 2.
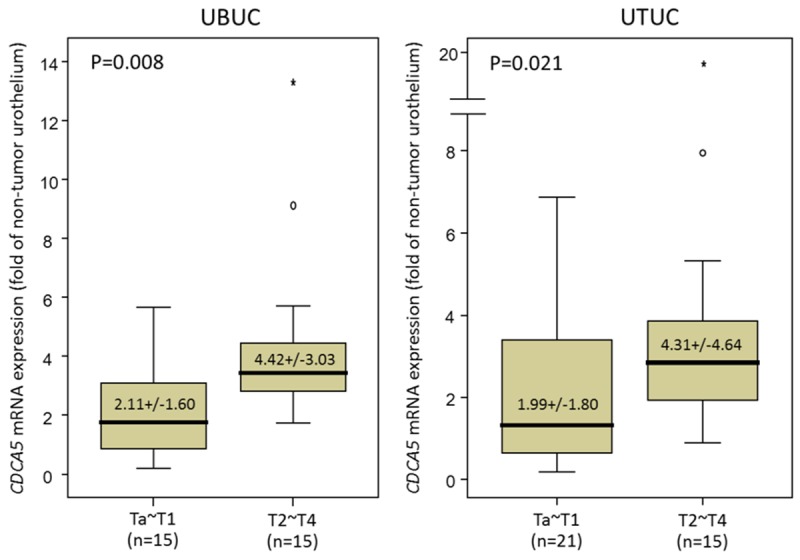
Quantitative real-time PCR (qPCR) analysis showed that CDCA5 mRNA expression was significantly increased in both UBUCs (left panel) and UTUCs (right panel) with advanced primary pT status. (p = 0.008 and p = 0.021, respectively).
Clinicopathological findings regarding UTUC
The clinicopathological characteristics of the UTUC patients are listed in Table 2. No sex predilection was noted. The patients’ age at diagnosis ranged from 34 to 87 years (median, 68 years). Sixty-two (18.2%) cases had multiple foci of tumors; of these, 49 (14.4%) cases involved both the renal pelvis and ureter. The majority of cases (n = 284, 83.5%) were of high histological grade. Advanced pT stages (pT2-T4) were seen in 159 (46.8%) of cases. About half (n = 167, 49.1%) of the cases showed frequent mitosis. Vascular invasion and perineurial invasion were observed in 106 cases (31.2%) and 19 cases (5.9%), respectively. Nodal metastasis was detected in 28 (8.2%) patients.
Table 2.
Correlations between CDCA5 Expression and other important clinicopathological parameters in urothelial carcinomas
| Parameter | Category | Upper urinary tract urothelial carcinoma | Urinary bladder urothelial carcinoma | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Case no. | CDCA5 expression | p-value | Case no. | CDCA5 expression | p-value | ||||
|
|
|
||||||||
| Low | High | Low | High | ||||||
| Gender | Male | 158 | 74 | 84 | 0.277 | 216 | 104 | 112 | 0.339 |
| Female | 182 | 96 | 86 | 79 | 43 | 36 | |||
| Age (years) | < 65 | 138 | 73 | 65 | 0.377 | 121 | 62 | 59 | 0.686 |
| ≥ 65 | 202 | 97 | 105 | 174 | 85 | 89 | |||
| Tumor location | Renal pelvis | 141 | 60 | 81 | 0.055 | - | - | - | - |
| Ureter | 150 | 85 | 65 | - | - | - | - | ||
| Renal pelvis & ureter | 49 | 25 | 24 | - | - | - | - | ||
| Multifocality | Single | 278 | 140 | 138 | 0.779 | - | - | - | - |
| Multifocal | 62 | 30 | 32 | - | - | - | - | ||
| Primary tumor (T) | Ta | 89 | 78 | 11 | < 0.001* | 84 | 62 | 22 | < 0.001* |
| T1 | 92 | 45 | 47 | 88 | 46 | 42 | |||
| T2-T4 | 159 | 47 | 112 | 123 | 39 | 84 | |||
| Nodal metastasis | Negative (N0) | 312 | 165 | 147 | < 0.001* | 266 | 138 | 128 | 0.033* |
| Positive (N1-N3) | 28 | 5 | 23 | 29 | 9 | 20 | |||
| Histological grade | Low grade | 56 | 44 | 12 | < 0.001* | 56 | 41 | 15 | < 0.001* |
| High grade | 284 | 126 | 158 | 239 | 106 | 133 | |||
| Vascular invasion | Absent | 234 | 142 | 92 | < 0.001* | 246 | 129 | 117 | 0.045* |
| Present | 106 | 28 | 78 | 49 | 18 | 31 | |||
| Perineural invasion | Absent | 321 | 167 | 154 | 0.002* | 275 | 141 | 134 | 0.066 |
| Present | 19 | 3 | 16 | 20 | 6 | 14 | |||
| Mitotic rate (per 10 high power fields) | < 10 | 173 | 101 | 72 | 0.002* | 139 | 85 | 54 | < 0.001* |
| ≥ 10 | 167 | 69 | 98 | 156 | 62 | 94 | |||
Statistically significant.
Clinicopathological findings regarding UBUC
The majority of UBUC patients were male (n = 216, 73.2%) and were older than 60 years (n = 214, 72.5%). As shown in Table 2, most (n = 239, 81%) were of high histological grade and 123 (41.7%) of them were in advanced stages (pT2-T4) at diagnosis. High mitotic activity (≥ 10 per high power fields) was found in 156 cases (52.9%). Lymph node metastasis was observed in 23.6% of patients (n = 29). In addition, vascular invasion and perineurial invasion had been observed in 49 cases (16.6%) and 20 cases (6.8%), respectively.
Correlations between immunoactivity of CDCA5 and parameters in UTUC and UBUC
CDCA5 showed variable nuclear expression in UC of both sites. The medium value of H-score as the cut-off was 210 and 215 for UTUC and UBUC, respectively. After dichotomizing tumors into low and high CDCA5 expression, as Table 2 demonstrates, increased CDCA5 expression in urothelial carcinoma of both sites was significantly associated with increment of pT status (Figure 3, p < 0.001), lymph node metastasis (UTUC, p < 0.001; UBUC, p = 0.033), higher histological grade (p < 0.001, both), vascular invasion (UTUC, p < 0.001; UBUC, p = 0.045) and frequent mitosis (UTUC, p = 0.002; UBUC, p < 0.001). Increased CDCA5 expression was statistically significantly (p = 0.002) associated with perineurial invasion in UTUC only.
Figure 3.
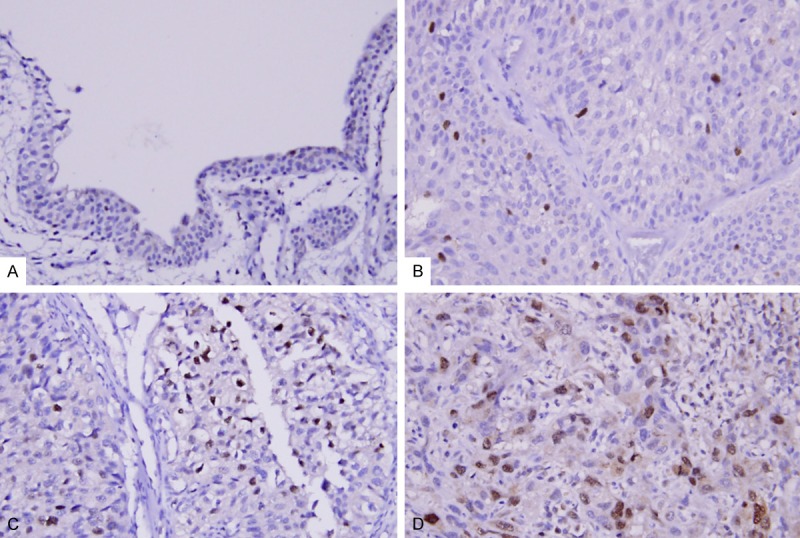
CDCA5 immunostaining on representative sections of non-tumor urothelium (A), non-invasive urothelial carcinoma of low grade (B) and high grade (C) and high-grade infiltrating urothelial carcinoma (D), respectively, exhibits stepwise increased CDCA5 expression.
Survival analysis for UTUC
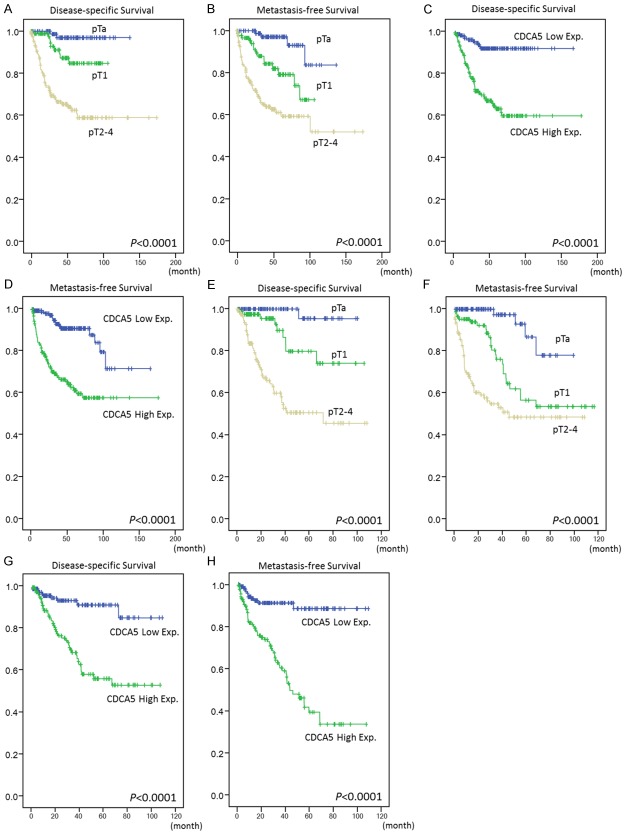
The univariate and multivariate analyses of associations between clinical outcomes and various clinicopathological parameters in UTUC cases are demonstrated in Table 3. Multivariate analysis showed that poor DSS was significantly associated with multifocality (p = 0.005), nodal metastasis (p < 0.001), high histological grade (p = 0.043), and perineurial invasion (p = 0.001). Similar results were also seen with MeFS. In UTUC, pT stage (Figure 4A, 4B) and vascular invasion were significantly associated with worse DSS and MeFS in univariate (p < 0.0001) but not in multivariate analyses. UTUC tumor location correlated with poor patient DSS in univariate analysis only (p = 0.0079).
Table 3.
Univariate log-rank and multivariate analyses for Disease-specific and Metastasis-free Survivals in Upper urinary tract urothelial carcinoma
| Parameter | Category | Case no. | Disease-specific survival | Metastasis-free survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
|
| ||||||||||||
| No. of event | p-value | R.R. | 95% C.I. | p-value | No. of event | p-value | R.R. | 95% C.I. | p-value | |||
| Gender | Male | 158 | 28 | 0.8286 | - | - | - | 32 | 0.7904 | - | - | - |
| Female | 182 | 33 | - | - | - | 38 | - | - | - | |||
| Age (years) | < 65 | 138 | 26 | 0.9943 | - | - | - | 30 | 0.8470 | - | - | - |
| ≥ 65 | 202 | 35 | - | - | - | 40 | - | - | - | |||
| Tumor side | Right | 177 | 34 | 0.7366 | - | - | - | 38 | 0.3074 | - | - | - |
| Left | 154 | 26 | - | - | - | 32 | - | - | - | |||
| Bilateral | 9 | 1 | - | - | - | 0 | - | - | - | |||
| Tumor location | Renal pelvis | 141 | 24 | 0.0079* | 1 | - | 0.868 | 31 | 0.0659 | 1 | - | 0.907 |
| Ureter | 150 | 22 | 0.814 | 0.438-1.513 | 25 | 0.913 | 0.379-3.763 | |||||
| Renal pelvis & ureter | 49 | 15 | 1.266 | 0.352-4.555 | 14 | 0.799 | 0.326-3.658 | |||||
| Multifocality | Single | 273 | 48 | 0.0026* | 1 | - | 0.005* | 52 | 0.0127* | 1 | - | 0.009* |
| Multifocal | 62 | 18 | 2.985 | 1.381-6.454 | 18 | 2.780 | 1.288-6.003 | |||||
| Primary tumor (T) | Ta | 89 | 2 | < 0.0001* | 1 | - | 0.065 | 4 | < 0.0001* | 1 | - | 0.578 |
| T1 | 92 | 9 | 2.202 | 0.451-10.754 | 15 | 1.796 | 0.560-5.762 | |||||
| T2-T4 | 159 | 50 | 3.679 | 0.774-17.488 | 51 | 1.855 | 0.562-6.124 | |||||
| Nodal metastasis | Negative (N0) | 312 | 42 | < 0.0001* | 1 | - | < 0.001* | 55 | < 0.0001* | 1 | - | 0.001* |
| Positive (N1-N2) | 28 | 19 | 5.087 | 2.733-9.469 | 15 | 2.952 | 1.570-5.549 | |||||
| Histological grade | Low grade | 56 | 4 | 0.0215* | 1 | - | 0.043* | 3 | 0.0027* | 1 | - | 0.023* |
| High grade | 284 | 57 | 3.073 | 1.038-9.096 | 67 | 4.007 | 1.214-13.222 | |||||
| Vascular invasion | Absent | 234 | 24 | < 0.0001* | 1 | - | 0.083 | 26 | < 0.0001* | 1 | - | 0.002* |
| Present | 106 | 37 | 1.684 | 0.934-3.035 | 44 | 2.639 | 1.444-4.823 | |||||
| Perineural invasion | Absent | 321 | 50 | < 0.0001* | 1 | - | 0.001* | 61 | < 0.0001* | 1 | - | 0.015* |
| Present | 19 | 11 | 3.567 | 1.711-7.435 | 9 | 2.579 | 1.200-5.539 | |||||
| Mitotic rate (per 10 high power fields) | < 10 | 173 | 27 | 0.167 | - | - | - | 30 | 0.0823 | 1 | - | 0.285 |
| ≥ 10 | 167 | 34 | - | - | - | 40 | 0.989 | 0.969-1.009 | ||||
| CDCA5 expression | Low | 170 | 10 | < 0.0001* | 1 | - | 0.016* | 15 | < 0.0001* | 1 | - | 0.006* |
| High | 170 | 51 | 2.415 | 1.175-4.966 | 55 | 2.354 | 1.273-4.353 | |||||
Statistically significant.
Figure 4.
Kaplan-Meier plots reveal the prognostic significance of pT status and CDCA5 expression for disease-specific and metastasis-free survival of upper tract urothelial carcinoma (A-D) and urinary bladder urothelial carcinoma (E-H).
Survival analysis for UBUC
As demonstrated in Table 4, both univariate and multivariate analyses revealed advanced pT stage was significantly associated with both dismal DDS and MeFS (both p < 0.0001 for univariate analysis, Figure 4E, 4F; p < 0.001 and p = 0.015 for multivariate analyses, respectively). Perineural invasion and mitotic rate were also significantly associated with poor DDS (p = 0.025 and p = 0.032, respectively). Lymph node metastasis was associated with poor MeFS (p = 0.040) in multivariate analysis, as well. Histological grade and vascular invasion in UBUC are associated with both worse DDS and MeFS in univariate analysis to a statistically significant degree (all p < 0.005), but not in multivariate analysis.
Table 4.
Univariate log-rank and multivariate analyses for disease-specific and metastasis-free survivals in urinary bladder urothelial carcinoma
| Parameter | Category | Case no. | Disease-specific survival | Metastasis-free survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
|
| ||||||||||||
| No. of event | p-value | R.R. | 95% C.I. | p-value | No. of event | p-value | R.R. | 95% C.I. | p-value | |||
| Gender | Male | 216 | 41 | 0.4446 | - | - | - | 60 | 0.2720 | - | - | - |
| Female | 79 | 11 | - | - | - | 16 | - | - | - | |||
| Age (years) | < 65 | 121 | 17 | 0.1136 | - | - | - | 31 | 0.6875 | - | - | - |
| ≥ 65 | 174 | 35 | - | - | - | 45 | - | - | - | |||
| Primary tumor (T) | Ta | 84 | 1 | < 0.0001* | 1 | - | < 0.001* | 4 | < 0.0001* | 1 | - | 0.015* |
| T1 | 88 | 9 | 4.621 | 0.501-42.641 | 23 | 3.798 | 1.105-13.060 | |||||
| T2-T4 | 123 | 42 | 17.283 | 1.978-105.997 | 49 | 4.941 | 1.451-16.828 | |||||
| Nodal metastasis | Negative (N0) | 266 | 41 | 0.0002* | 1 | - | 0.352 | 61 | < 0.0001* | 1 | - | 0.040* |
| Positive (N1-N2) | 29 | 11 | 1.397 | 0.691-2.822 | 15 | 1.903 | 1.030-3.515 | |||||
| Histological grade | Low grade | 56 | 2 | 0.0013* | 1 | - | 0.802 | 5 | 0.0007* | 1 | - | 0.581 |
| High grade | 239 | 50 | 1.214 | 0.266-5.528 | 71 | 1.333 | 0.480-3.702 | |||||
| Vascular invasion | Absent | 246 | 37 | 0.0024* | 1 | - | 0.273 | 54 | 0.0001* | 1 | - | 0.803 |
| Present | 49 | 15 | 0.685 | 0.348-1.348 | 22 | 1.077 | 0.601-1.929 | |||||
| Perineural invasion | Absent | 275 | 44 | 0.0001* | 1 | - | 0.025* | 66 | 0.0007* | 1 | - | 0.117 |
| Present | 20 | 8 | 2.531 | 1.121-5.714 | 10 | 1.780 | 0.866-3.662 | |||||
| Mitotic rate (per 10 high power fields) | < 10 | 139 | 12 | < 0.0001* | 1 | - | 0.032* | 23 | < 0.0001* | 1 | - | 0.077 |
| ≥ 10 | 156 | 40 | 2.100 | 1.067-4.133 | 53 | 1.599 | 0.951-2.688 | |||||
| CDCA5 expression | Low | 147 | 9 | < 0.0001* | 1 | - | 0.016* | 14 | < 0.0001* | 1 | - | < 0.001* |
| High | 148 | 43 | 2.467 | 1.181-5.152 | 62 | 3.030 | 1.657-5.540 | |||||
Statistically significant.
Prognostic significance of CDCA5 expression in UC
As shown in Tables 3, 4, in univariate analysis, patients with either UTUC or UBUC exhibiting high CDCA5 nuclear expression had significantly worse DSS and MeFS (p < 0.0001 for all, Figure 4C, 4D, 4G and 4H). Remarkably, in multivariate analysis, CDCA5 overexpression remained an independent prognosticator predicting dismal DSS and MeFS for all UCs (Tables 3, 4). To further confirm the prognostic significance of CDCA5 expression, we performed subgroup analysis. As shown in Figure S1, in four groups namely UTUC of low- and high-pT status and UBUC of low- and high-pT status, CDCA5 over-exression was still associated with poor DSS and MeFS. Of three of these four groups (UTUC-Low- and high-stage and UBUC-Low stage), the log-rank test revealed statistical significance for both DSS and MeFS (all p < 0.05).
Discussion
When the cells receive growth-promoting stimuli, the ultimate outcome is the entry of quiescent cells into the cell cycle. The regulation of the cell cycle is extremely important to the cell; because the cell cycle dysregulated by mutations or amplification of related genes leads the cell to grow autonomously, a characteristic of cancers. The cell cycle in eukaryotic cells is regulated by a complex set of molecules, including cyclins, cyclin-dependent kinases (CDKs), CDK inhibitors, as well as retinoblastoma (RB) protein, and p53. Dysregulation of cell cycle control is one of the most important events in carcinogenesis, especially the checkpoint pathways [12]. There are three main checkpoints: the G1-S, S-phase and G2-M checkpoints [13]. The G1-S checkpoint checks for DNA damage; if DNA damage is present, the DNA-repair mechanisms that arrest the cell cycle will be activated. The delay in cell cycle progression gives the cell time to repair its DNA. If the damage is unrepairable, apoptotic pathways will be stimulated. Therefore, the G1-S checkpoint prevents cells with defective DNA from replicating and perpetuating mutations or chromosomal breaks in the cell’s descendants [22]. When the cell suffers from genotoxic insult, the intra-S-phase checkpoint is activated, and then transiently and reversibly inhibits the replication-origin firing and protects the integrity of the stalled replication forks [22]. The G2-M checkpoint monitors the completion of DNA replication and checks the safety of both the initiation of mitosis and the separation of sister chromatids. Defects in the G2-M checkpoint give rise to chromosomal abnormalities [23,24]. The most important of these is the G1-S checkpoint, known as the restriction point, after which the cell is committed to DNA replication and cell division [13]. Dysregulation in the G1-S transition of the mitotic cell cycle occurs commonly in carcinogenesis [25]. Nevertheless, the expressions of these genes have not been systemically evaluated in UC until now. By performing data mining on the published transcript expression profiles of UBUCs (GSE32894) in the GEO, with a special focus on the G1-S transition of the mitotic cell cycle (GO:0000082), we identified the gene, cell division cycle associated 5 (CDCA5), as the most significantly upregulated gene associated stepwise with disease progression.
The CDCA5 gene encodes CDCA5 protein (A.K.A. Sororin). CDCA5 was initially recognized as a substrate of the anaphase-promoting complex [26] and as a master regulator of sister chromatid cohesion [27] in cells. CDCA5 is degraded through anaphase-promoting complex-dependent ubiquitination in the G1 phase, and is required for stabilizing binding of the cohesion complex to sister chromatids [27]. However, there is only one study describing the significance of the activation of CDCA5 in carcinogenesis of the human lung [17]. In that study, the authors identified CDCA5 as an up-regulated gene in the majority of lung carcinomas by analyzing gene expression profiles using cDNA microarray containing 27,648 genes or expressed sequence tags. In a immunohistochemical study of 262 non-small cell lung carcinoma cases, 192 cases (73.3%) were judged as positive and 70 (26.7%) as negative. CDCA5 positivity was also significantly associated by log-rank test (p = 0.0143) with shorter tumor-specific survival periods. Furthermore, multivariate analysis demonstrated that CDCA5 status was an independent prognostic factor for surgically treated lung cancer (p = 0.0244) [17]. They also conducted in vitro MTT cell proliferation assay to evaluate the growth-promoting effect of CDCA5. First, when CDCA5 expression was knocked down by CDCA5-specific siRNAs, the growth of the two lung cancer cell lines, A549 and LC319 both with high CDCA5 expression levels, was significantly suppressed compared with the controls (p < 0.0001). Next, when COS-7 cells, a fibroblast-like cell line derived from monkey kidney tissue, were transfected by plasmids containing full-lengths of the CDCA5 gene, the growth of COS-7—CDCA5 cells was promoted compared with the controls, to a significant degree (p < 0.005) [17]. This study also proved that CDCA5 was phosphorylated by ERK at two phosphorylation sites, Ser79 and Ser209. The authors suggested that transactivation of CDCA5 and its phosphorylation by ERK play a pivotal role in lung carcinogenesis [17].
In the present study, CDCA5 overexpression was associated with advanced pT status, nodal metastasis, high histological grade, vascular invasion and frequent mitoses in both UTUC and UBUC to a statistically significant degree (all p < 0.05). Along with other important clinicopathological parameters, such as pT status, nodal metastasis, and histological grade, CDCA5 overexpression also predicted worse DSS and MeFS in patients with urothelial carcinoma from both anatomical sites, both in univariate and multivariate analyses, with statistical significance (all p < 0.05). When we divided our cohort into four groups: UTUC/UBUC of low- or high-stage, we still found high CDCA5 expression was associated with dismal DSS and MeFS. In addition, in three groups of these—UTUC of low- and high-stage and UBUC of low stage, CDCA5 overexpression predicted poor DSS and MeFS with statistical significance (all p < 0.05). Higher CDCA5 transcriptional level was also significantly associated with higher pT status in both UTUC and UBUC (p = 0.021 and p = 0.008, respectively).
Conclusion
In summary, our study demonstrates that CDCA5 plays a significant role in tumor progression in both UTUC and UBUC. In patients with UCs, CDCA5 over-expression and up-regulation in both protein and mRNA levels are predictive of poor DSS and MeFS. Further investigation to elucidate the biological significance of CDCA5 protein expression in UC is essential for exploring the potential of CDCA5-targeted therapy for UC.
Acknowledgements
This work was supported by grants from E-DA Hospital (EDAHP104014 to I-W Chang), Ministry of Health and Welfare (MOHW104- TDU-B-212–124-003, Health and welfare surcharge of tobacco products to C-F Li and W-J Wu) and Kaohsiung Medical University (KMU-TP103G01, KMU-TP103G00, KMU-TP103G04 & KMU-TP103G05). Author contributions: Conception and design: IW Chang, VC Lin, WJ Wu, CF Li; Development of methodology: CN Huang, CT Hsu, CC Li; Acquisition of data: CF Li; Analysis and interpretation of data: I-W Chang, HL He, CF Li; Writing and/or revision of the manuscript: IW Chang, CF Li; Study supervision: WJ Wu, TF Wu, CF Li.
Abbreviations
- UC
Urothelial carcinoma
- UBUC
Urinary bladder urothelial carcinoma
- UTUC
upper urinary tract urothelial carcinoma
- GEO
Gene Expression Omnibus
- CDCA5
cell division cycle associated 5
- DSS
Disease-specific survival
- MeFS
metastasis-free survival
- CDK
cyclin-dependent kinase
- RB
retinoblastoma
Supporting Information
References
- 1.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester R, Burger M, Cowan N, Böhle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF European Association of Urology. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2011;59:584–594. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin JK, Silverman DT, Hsing AW, Ross RK, Schoenberg JB, Yu MC, Stemhagen A, Lynch CF, Blot WJ, Fraumeni JF Jr. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992;52:254–257. [PubMed] [Google Scholar]
- 3.Pommer W, Bronder E, Klimpel A, Helmert U, Greiser E, Molzahn M. Urothelial cancer at different tumour sites: Role of smoking and habitual intake of analgesics and laxatives. Results of the berlin urothelial cancer study. Nephrol Dial Transplant. 1999;14:2892–2897. doi: 10.1093/ndt/14.12.2892. [DOI] [PubMed] [Google Scholar]
- 4.Shinka T, Miyai M, Sawada Y, Inagaki T, Okawa T. Factors affecting the occurrence of urothelial tumors in dye workers exposed to aromatic amines. Int J Urol. 1995;2:243–248. doi: 10.1111/j.1442-2042.1995.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 5.Dragicevic D, Djokic M, Pekmezovic T, Micic S, Hadzi-Djokic J, Vuksanovic A, Simic T. Survival of patients with transitional cell carcinoma of the ureter and renal pelvis in balkan endemic nephropathy and non-endemic areas of serbia. BJU Int. 2007;99:1357–1362. doi: 10.1111/j.1464-410X.2007.06793.x. [DOI] [PubMed] [Google Scholar]
- 6.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 7.Laing C, Hamour S, Sheaff M, Miller R, Woolfson R. Chinese herbal uropathy and nephropathy. Lancet. 2006;368:338. doi: 10.1016/S0140-6736(06)69079-X. [DOI] [PubMed] [Google Scholar]
- 8.Stewart JH, Hobbs JB, McCredie MR. Morphologic evidence that analgesic-induced kidney pathology contributes to the progression of tumors of the renal pelvis. Cancer. 1999;86:1576–1582. doi: 10.1002/(sici)1097-0142(19991015)86:8<1576::aid-cncr27>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Catto JW, Azzouzi AR, Rehman I, Feeley KM, Cross SS, Amira N, Fromont G, Sibony M, Cussenot O, Meuth M, Hamdy FC. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J. Clin. Oncol. 2005;23:2903–2910. doi: 10.1200/JCO.2005.03.163. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Furge KA, Yang XJ, Teh BT, Hansel DE. Comparative gene expression profiling analysis of urothelial carcinoma of the renal pelvis and bladder. BMC Med Genomics. 2010;3:58. doi: 10.1186/1755-8794-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catto JW, Yates DR, Rehman I, Azzouzi AR, Patterson J, Sibony M, Cussenot O, Hamdy FC. Behavior of urothelial carcinoma with respect to anatomical location. J Urol. 2007;177:1715–1720. doi: 10.1016/j.juro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 13.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 14.Zetterberg A, Larsson O, Wiman KG. What is the restriction point? Curr Opin Cell Biol. 1995;7:835–842. doi: 10.1016/0955-0674(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Pati D. Sororin is a master regulator of sister chromatid cohesion and separation. Cell cycle. 2012;11:2073–2083. doi: 10.4161/cc.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, Pati D. Handcuff for sisters: A new model for sister chromatid cohesion. Cell cycle. 2009;8:399–402. doi: 10.4161/cc.8.3.7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen MH, Koinuma J, Ueda K, Ito T, Tsuchiya E, Nakamura Y, Daigo Y. Phosphorylation and activation of cell division cycle associated 5 by mitogen-activated protein kinase play a crucial role in human lung carcinogenesis. Cancer Res. 2010;70:5337–5347. doi: 10.1158/0008-5472.CAN-09-4372. [DOI] [PubMed] [Google Scholar]
- 18.Huang WW, Huang HY, Liao AC, Shiue YL, Tai HL, Lin CM, Wang YH, Lin CN, Shen KH, Li CF. Primary urothelial carcinoma of the upper tract: Important clinicopathological factors predicting bladder recurrence after surgical resection. Pathol Int. 2009;59:642–649. doi: 10.1111/j.1440-1827.2009.02420.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu LC, Chen LT, Tsai YJ, Lin CM, Lin CY, Tian YF, Sheu MJ, Uen YH, Shiue YL, Wang YH, Yang SJ, Wu WR, Li SH, Iwamuro M, Kobayasshi N, Huang HY, Li CF. Alpha-methylacyl coenzyme a racemase overexpression in gallbladder carcinoma confers an independent prognostic indicator. J Clin Pathol. 2012;65:309–314. doi: 10.1136/jclinpath-2011-200489. [DOI] [PubMed] [Google Scholar]
- 20.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KS Jr. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- 21.McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW, Nicholson RI. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990;50:3545–3550. [PubMed] [Google Scholar]
- 22.Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol. 2001;13:738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Kim ST, Lim DS, Kastan MB. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol Cell Biol. 2002;22:1049–1059. doi: 10.1128/MCB.22.4.1049-1059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 25.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 26.Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18:185–200. doi: 10.1016/j.molcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz J, Watrin E, Lenart P, Mechtler K, Peters JM. Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol. 2007;17:630–636. doi: 10.1016/j.cub.2007.02.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.