Abstract
Cytochrome P450 1B1 (CYP1B1) expression increases in multi-potential mesenchymal stromal cells C3H10T1/2 during adipogenesis, which parallel with PPARγ, a critical transcriptional factor in adipogenic process. To assess the role of CYP1B1 in fatty acid metabolism, adult C57BL/6J wild-type and CYP1B1 deficiency mice were fed with high fat diets (HFD) for 6 weeks. CYP1B1 deficiency attenuated HFD-induced obesity when compared with their wild type counterparts, and improve glucose tolerance. The reduction in body weight gain and white adipose tissue in CYP1B1 deficient mice exhibited coordinate decreases in fatty acid synthesis (PPARγ, CD36, FAS, SCD-1) and increases in fatty acid oxidation (UCP-2, CPT-1a) when compared with wild type ones. Lower hepatocyte TG contents were consistent with hepatic Oil-Red-O staining in the CYP1B1 deficiency mice. AMPK, a nutrient sensors for energy homeostasis, was activated in both fat pad and liver by CYP1B1 deficiency. However, in vitro system, knock down CYP1B1 in C3H10T1/2 cells does not abolish adipogenesis induced by adipogenic agents IDM (Insulin, Dexamethasone, Methylisobutylxanthine). Our in vivo and in vitro findings of CYP1B1 deficiency in fat metabolism suggest a complex regulation network between CYP1B1 and energy homeostasis.
Keywords: CYP1B1, high fat diet (HFD), diet induced obesity (DIO), fat metabolism, glucose intolerance
Introduction
Obesity is a major public health concerns and the leading nutritional disorder in both developed and developing countries [1]. Obesity not only gives rise to various secondary conditions, such as osteoarthritis, gallstones, obstructive sleep apnea, hypoventilation syndrome, and infertility [2], more important, is an independent risk factor for a wide arrange of chronic diseases including hypertension, type 2 diabetes, myocardial infarction, and certain cancers [3].
The rapid rise in the incidence of obesity in the world has prompted researchers to look for new strategies. Although the cause for fat mass expansion is clear---more calories are consumed than the body burns, and the excessive calories are stored as fat. However, the exact mechanism still remains to be elucidated. Compelling scientific evidence indicates that, apart from the input from lifestyle and environment factors, genetic factors make a large contribution to obesity development. Over the past decade, a variety of transgenic and knockout mouse models made substantial progress in identifying the genetic contribution to the complex mechanisms regulating energy balance [4]. Furthermore, a number of human genes have also been identified that are associated with obesity related disorders [5].
The rate at which the body turns food into energy and the body burns up calories (metabolic rate) is under tight control and genetic factors play an essential role in such energy balance. A genetic predisposition to weight gain was confirmed by twin and adoption studies----the majority of adoptees followed a pattern of weight gain that more closely resembled that of their birth parents than their adoptive parents [6,7].
CYP1B1 is a member of the cytochrome P450 enzyme family 1, subfamily B, polypeptide 1, and constitutively expresses in various tissues including fat, mammary gland, prostate, heart, kidney, brain and eyes [8-12]. Its role in various cancers including ovarian, lung, prostate, esophageal tract, etc., has been well studied [13]. Mutations in CYP1B1 cause primary congenital glaucoma [14]. CYP1B1 disruption in mice ameliorates Ang II independent and genetic models of hypertension [15,16]. CYP1B1 is also expressed in endothelial cells and has been implicated in response to shear stress and oxidative stress [11,12] However, there is very limited information on the implications of CYP1B1 in fat metabolism.
The process of fat pad weight gain involves increased adipogenesis, a process in which a multi-potent mesenchymal stem cell is differentiated into a mature adipocyte. Transcription factor peroxisome proliferator activated receptor gamma (PPARγ) not only is crucial regulator for adipogenesis, but is also required for maintenance of the differentiated state. Moreover, PPARγ is a master transcription factor in modulating the downstream target genes expression.
CYP1B1 is produced by human white adipose tissue (WAT) [8] and its expression increases upon adipogenic stimulation [17], in parallel with PPARγ, suggesting that CYP1B1 could be of importance in energy homeostasis by affecting fat metabolism. CYP1B1 catalyzes a variety of foreign and endogenous lipophilic compounds including fat soluble vitamins, steroid hormones and polyunsaturated fatty acid products [18], it also interacts potently with many plant flavonoids that are present in the human diet [19]. Currently, over the counter remedies for overweight/obesity based on dietary supplements are very popular, suggesting that herb medicine in the future, may serve as supplement in obese treatment and prevention.
Therefore, the present study was performed to examine the effects of CYP1B1 in diet induced obesity (DIO) mice model and to elucidate the possible molecular changes responsible for such effects.
Material and method
Animal and diets
CYP1B1 knockout mice (KO) on a C57BL/6J background were kindly provided by Frank J. Gonzalez (National Cancer Institute, Bethesda, Maryland, USA). C57BL/6J wild type (WT) mice were purchased from Animal experiment center of Hubei Provincial Academy for Preventive Medicine. Both mouse strains were further backcrossed for at least three generations before being used for experiments. Genotyping was performed by PCR on tail biopsies to distinguish WT and KO mice. The PCR primer sequences were as follows: Neomycin forward: 5’-TTG GGT GGA GAG GCT ATT CGG CTA TGA-3’, Neomycin reverse: 5’-GGC GCG AGC CCC TGA TGC TC-3’ (Amplicon size 460 bp); CYP1B1 forward: 5’-CTG AGT TGG ACC AGG TTG TGG-3’; CYP1B1 reverse: 5’-CAT GGA TTC TAA ACG ACT AGG-3’ (Amplicon size 365 bp). Beginning at 6 weeks of age, KO and WT mice were fed with HFD (60% calories from fat; Silaike, Shanghai) for 6 weeks. Body weight and daily food intake were recorded twice a week. At the time of sacrifice, tissues and blood were collected for analysis. All mice had free access to water and food, and were housed under alternating 12-hour light and dark cycles. All experiments were approved by the Animal Care and Utilization Committee of Wuhan University and were in accordance with institutional guidelines.
Physiological parameters measurement
Blood samples from mice were centrifuged or 15 min at 1500 rpm to collect serum for insulin measurement (mouse insulin ELISA kit, Cayman). For glucose tolerance test (GTT), 8-h-fasted mice were injected intraperitoneally with dextrose (1 g/kg, sigma). Blood samples were drawn at 0, 15, 30, 60, 90 and 120 min and blood glucose were measured by kits (Johnson Onetouch Ultra).
Oil-Red-O (ORO) staining and hepatic TG contents
Liver tissues from HFD-induced KO and WT mice were placed in OCT (VWR Scientific, St. Louis, MO) and rapidly frozen for sections (5 um). Then liver sections were fixed for 1 h in 10% formalin and rinsed three times with ddH2O. Sections were dehydrated for 5 min in 100% isopropanol, stained with filtered Oil Red O working solution (60% Oil Red O stock solution/40% water) for 1 h at room temperature, and then rinsed in ddH2O for 5 min. Then sections were observed with the inverted microscope and captured for images. Triglyceride in Liver and cell lysate were extracted and analyzed according to Schwartz’s method [21].
Immunohistochemistry
Epididymal adipose tissue samples were fixed with 4% PFA, and embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). An H&E-stained adipose image was captured from one slide of each of three animals per group and the relative adipocyte area was determined using NIH ImageJ software.
Real-time RT-PCR
Total RNA was isolated from tissues or cells lysate by Trizol (Invitrogen). RT reactions were performed using standard method (5-min reverse transcriptase inactivation at 95°C, and 40 cycles at 95°C for 15 s, 56°C for 15 s and 72°C for 20 s). cDNA were analyzed by real-time PCR using SYBR Green (Genecopoeia) normalized to reference gene (Cyclophylin or beta-actin based on the amplification efficiency of the primers) on an Applied Biosystems Stepone Plus Real Time PCR system. Gene expression was calculated as 2-ΔΔCt. Primer sequences are listed in Supplement Table 1.
Western blot
Standard immunoblotting procedure was carried out with 100 mg protein per sample from tissues or cells. Briefly, cellular proteins and immune complexes were submitted to SDS-PAGE, transferred to nitrocellulose blotting membranes, and blocked in 5% fat-free milk, and probed with the different primary antibodies according to the manufacturer’s recommendations. The membranes were visualized by chemiluminescence reagents (SuperSignal West Pico, Pierce) and quantitated with ImageJ software. Antibodies were listed in Supplement Table 2.
siRNA and cell culture
CYP1B1 specific siRNA was to knockdown CYP1B1 expression in wild type C3H10T1/2 cells [12]. Briefly, the 19-base specific sequence, its reverse complementary sequence and terminal sequences were synthesized and cloned into pSUPER-retro vector digested with same enzymes (Invitrogen). Cells expressing siRNA with maximum knockdown were used for further analyses. Knockdown sequence were also listed in Supplement Table 1. Pluripotent stem cell C3H10T1/2 (ATCC) was cultured with 10%FBS/DMEM (Gibco) and maintained at 37°C and 5% CO2.
Statistical analyses
All data were expressed as mean ± SEM and statistical comparisons were assessed by 2-tailed student t tests. P < 0.05 was considered significant difference.
Results
CYP1B1 expression increases during adipogenesis, in parallel with alterations in PPARγ expression
We found both CYP1B1 mRNA and protein levels were elevated in C3H10T1/2 cells upon adipogenic differentiation (Figure 1A and 1B), consistent with previous report (17). Adipocyte plays a vital role in energy homeostasis and reserve energy as triglyceride in the animal’s body. To explore the possible role of CYP1B1 in energy metabolism in vivo, we measure CYP1B1 expression levels in different tissues in C57BL/6J mice and found that it constitutively expressed in liver, muscle and white adipose tissue, and the fat pad has the highest level of CYP1B1 expression (Figure 1C).
Figure 1.
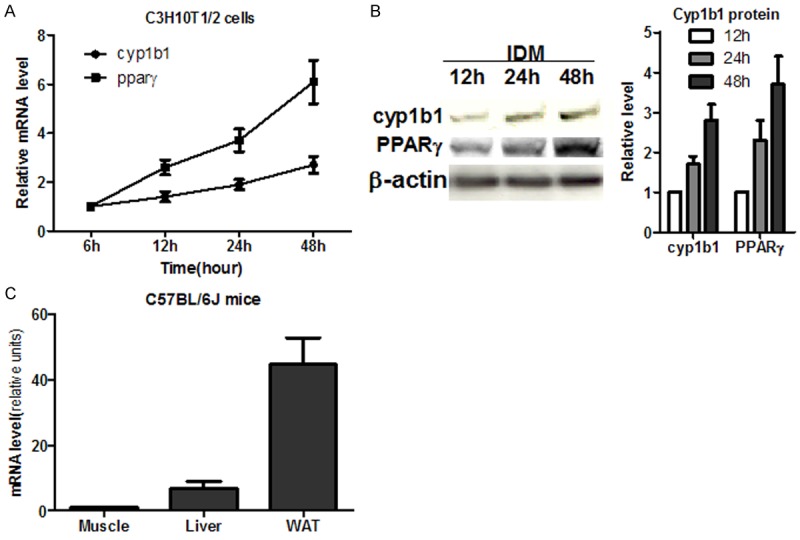
Cyp1b1 expression during adipogenesis. A. CYP1B1 mRNA expression in C3H10T1/2 cell during adipogenesis (n = 3); B. CYP1B1 and PPARγ protein expression in C3H10T1/2 cells upon adipogenic differentiation (n = 3); C. CYP1B1 mRNA expression in C57BL/6J male mice (n = 3). WAT: white adipose tissue.
CYP1B1 deficiency attenuates high fat diet induced obesity in adult C57BL/6J mice
To evaluate the potential effects of CYP1B1 on body weight regulation and energy metabolism, 6-week-old wild type C57BL/6J and CYP1B1 deficient mice were fed with high fat diet (HFD) for 6 weeks. As shown in Figure 2A, at first 4 weeks, CYP1B1 deficiency did not markedly affected body weight gain, however, after 5 weeks, CYP1B1 knock out led to significant decrease in body weight in HFD feeding mice. Significant reduction of body weight gain and epididymal fat pad weight in CYP1B1 deficient mice was observed when compared with wild type ones (Figure 2B). CYP1B1 deficient mice also had reduced adipose size (Figure 3A). However, no difference in Liver weight was observed between both groups. To determine whether the difference in body weight gain and adiposity in both groups after HFD feeding was the result of calorie intake, we recorded the food consumption and there were no differences between two groups (Figure 2C), suggesting different adiposity in these animals were not associated with calorie consumption.
Figure 2.
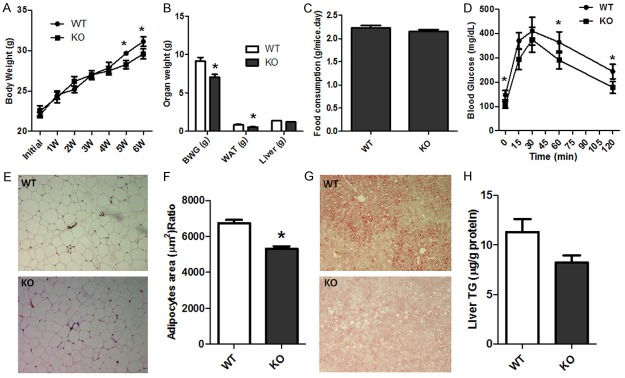
CYP1B1 deficiency attenuated mice body weight gain and adiposity induced by high fat diet feeding (n = 6-7). A. Mice body weight; B. Body weight gain, white adipose tissue and liver weight; C. Food consumption; D. Glucose tolerance test (GTT); E. H&E of Epididymal fat pad; F. Adipose size; G. Liver Oil-Red-O; H. Liver TG content. WT: wild type mice; KO: CYP1B1 deficient mice; W: week; BWG: body weight gain; WAT: white adipose tissue; TG: Triglyceride.
Figure 3.
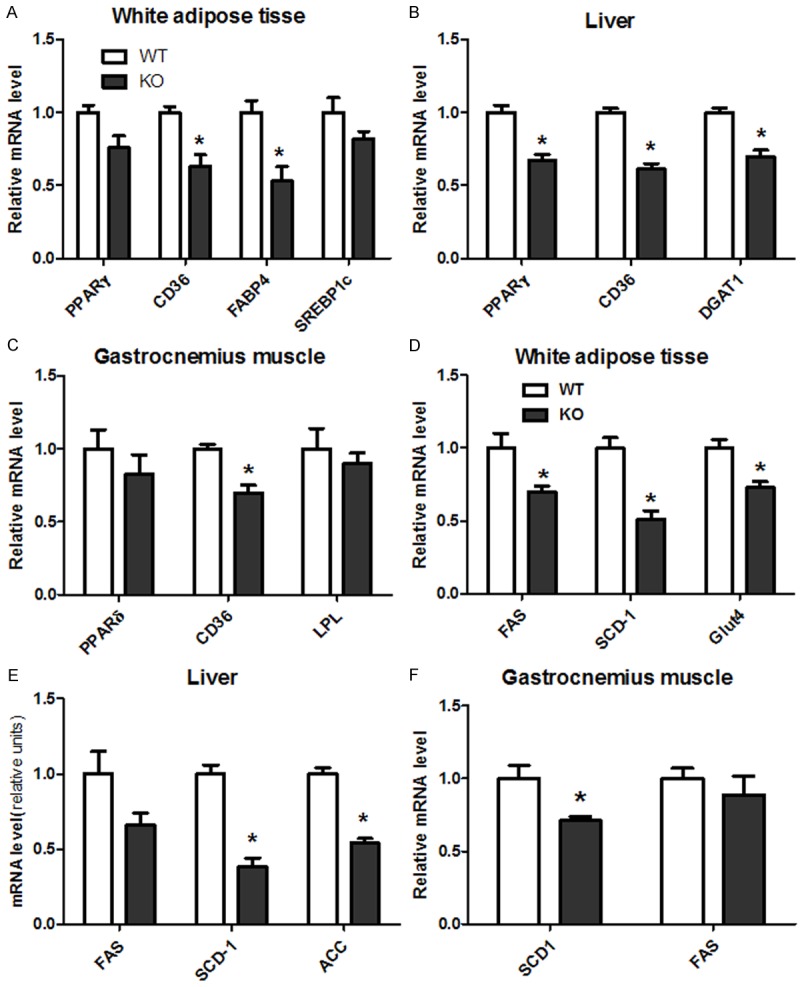
CYP1B1 deficiency suppressed genes expression involved in fatty acid uptake and synthesis in white adipose tissue, Liver and skeletal muscle in HFD feeding mice. A. Genes of fatty acid uptake in white adipose tissue; B. Genes of fatty acid uptake in liver; C. Genes of fatty acid uptake in gastrocnemius muscle; D. Genes of fatty acid synthesis in white adipose tissue; E. Genes of fatty acid synthesis in liver; F. Genes of fatty acid synthesis in gastrocnemius muscle. PPARγ: Peroxisome proliferative activated receptor γ; CD36: Fatty acid translocase (FAT); DGAT1: Diglyceride acyltransferase 1; FAS: Fatty acid synthase; SCD-1: Stearoyl-CoA desaturase 1; ACC: Acetyl-CoA carboxylase; Glut4: Glucose transporter 4; PPARδ: Peroxisome proliferative activated receptor δ; LPL: Lipoprotein lipase; PGC1α: Peroxisome proliferative activated receptor γ, coactivator 1 α.
CYP1B1 deficiency improve insulin sensitivity in HFD feeding
To assess the effects of the absence of CYP1B1 on insulin sensitivity, we measured fasting blood glucose and insulin concentration and performed glucose tolerance test. There was significant decreased in glucose and insulin levels in CYP1B1 deficient mice when compared to wild types (Table 1). Lower glucose and insulin levels indicating mice with CYP1B1 deletion have a better glucose homeostasis when challenging with HFD. Following the administration of glucose, animals with absence of CYP1B1 displayed improved glucose intolerance (Figure 2D). However, the attenuated effect of CYP1B1 deficiency on serum triglyceride (TG) levels was not significant (Table 1).
Table 1.
Physiological parameters
| WT (n = 4) | KO (n = 4) | p value | |
|---|---|---|---|
| Blood glucose (mg/dL) | 142 ± 19 | 107 ± 14 | 0.028 |
| Serum insulin (ng/ml) | 21.2 ± 1.7 | 14.4 ± 2.1 | 0.045 |
| Serum TG (ug/g) | 1.1 ± 0.2 | 0.87 ± 0.1 | 0.084 |
CYP1B1 deficiency alters fat metabolism in liver and white adipose tissue, but not in muscle
The fact that lower adiposity without decreasing food intake suggests CYP1B1 mediates decreased energy retention and/or increased energy expenditure. To gain insight into molecular mechanisms for energy balance in CYP1B1 deficiency mice, we measured mRNA/protein levels of genes involved in fatty acid synthesis and fatty acid oxidation in white adipose tissue, liver and skeletal muscle.
In epididymal fat pad, CYP1B1 deficiency significantly suppressed mRNA levels of PPARγ, CD36, FABP4, FAS, SCD-1 and Glut4 (Figure 3A and 3D), when compared with wild type mice. Similarly, PPARγ, CD36, DGAT1, FAS, SCD-1 and ACC (Figure 3B and 3E) expressions in liver were also inhibited by CYP1B1 deficiency, although FAS did not reach significant level. In contrast, CYP1B1 deficiency exhibited less effects on genes involved in fatty acid synthesis in muscle, only CD36 and SCD-1 mRNA expression (Figure 3C and 3F) were markedly reduced, relative to the respective tissue of HFD wild type mice.
At the protein level, PPARγ was decreased by 23% (Figure 4A, P = 0.09) and 31% (Figure 4B, P < 0.05) in epididymal adipose tissue and liver, respectively, in CYP1B1 deficiency mice compared with control tissues. These results suggest that CYP1B1 may specifically regulate PPARγ expression in WAT and liver, and that decreased PPARγ expression may account, at least in part, for the decreased energy retention in HFD fed CYP1B1 deficient mice.
Figure 4.
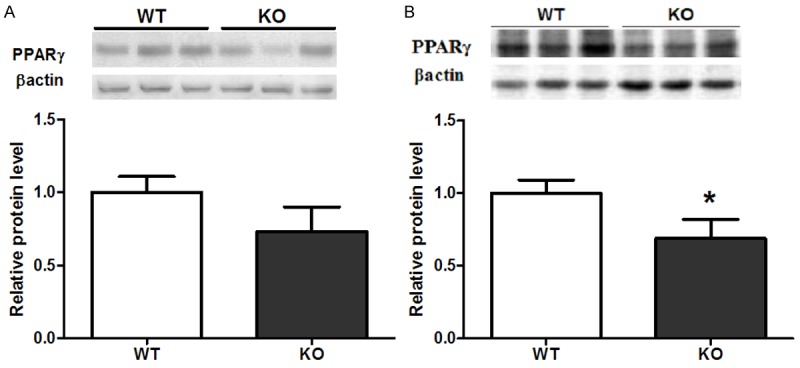
CYP1B1 deficiency inhibited PPARγ expression in both fat pad and liver. A. White adipose tissue; B. Liver.
On the other hand, genes controlling fatty acid β-oxidation were also regulated by CYP1B1. Messenger RNA levels of UCP2, a gene promotes mitochondrial fatty acid oxidation, was markedly increased in both WAT and liver tissues in HFD fed CYP1B1 deficient mice (Figure 5A and 5B). CYP1B1 deficiency also elevated CPT-1a expression (Figure 5B), an essential mitochondrial enzyme in β-oxidation of long chain fatty acids in liver. However, UCP3 mRNA (Figure 5C), a major uncoupling protein in skeletal muscle, were increased slightly (P > 0.05) in gastrocnemius muscle in CYP1B1 deficient mice.
Figure 5.
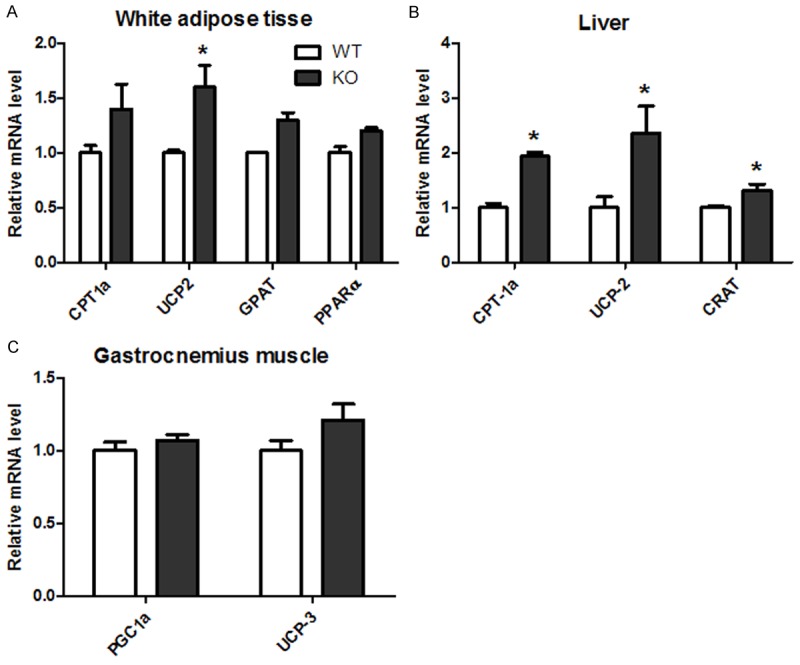
CYP1B1 deficiency increased genes expression involved in fatty acid β-oxidation in white adipose tissue, Liver and muscle in HFD feeding mice. A. Genes of fatty acid β-oxidation in white adipose tissue; B. Genes of fatty acid beta oxidation in liver; C. Genes of fatty acid beta oxidation in gastrocnemius muscle. CPT-1a: Carnitine palmitoyltransferase 1a; UCP-2: uncoupling protein 2; GPAT: Glycerol-3-phosphate acyltransferase; CRAT: Carnitine acetyltransferase; UCP3: uncoupling protein 3; PPARα: Peroxisome proliferative activated receptor α.
CYP1B1 deficiency enhances AMPK phosphorylation in liver and white adipose tissue
AMPK is an important nutrient sensors and plays an important role in cellular and whole body energy homeostasis [21]. Activation of AMKP in liver and skeletal muscle has been demonstrated to inhibit synthesis of fatty acids, hepatic gluconeogenesis while increasing fatty acid oxidation, muscle glucose transport, and calorie intake [22]. AMPKα1 also has been reported to be the dominant isoform in isolated epididymal adipocytes and cultured 3T3-L1 adipocytes [23,24]. Therefore, we detected AMPKα1 signaling in adipose and liver in mice, and found that CYP1B1 deficiency enhance AMPK phosphorylation in both epididymal fat pad and liver after 6 weeks HFD feeding when compared with wild type mice (Figure 6A and 6B).
Figure 6.
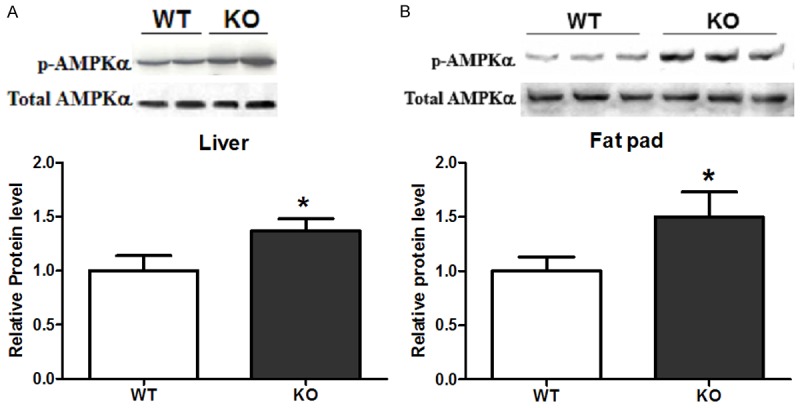
CYP1B1 deficiency enhanced AMPK Signaling in fat and liver tissue after 6 weeks HFD feeding. A. White adipose tissue; B. Liver. AMPK: AMP activated protein kinase.
Knock down CYP1B1 expression in C3H10T1/2 cells does not abolish adipogenesis upon hormone cocktail induction
To explore the molecular mechanism of CYP1B1 in lipogenesis and lipolysis in in vitro system, CYP1B1 levels were knocked down by transfecting multi-potential cells C3H10T1/2 with a CYP1B1 specific siRNA. CYP1B1 expression was confirmed by western blotting (Figure 7A). However, adipogenic process induce by IDM cocktail was only slightly inhibited by CYP1B1 knock-down. Figure 7B and 7C showed the adipogenesis and TG contents in C3H10T1/2 cells after 8 days differentiation. The reduced mRNA and protein levels of the critical adipogenic transcription factor PPARγ (Figure 7D and 7E) also did not reach significant levels by CYP1B1 knockdown. These results suggest a complex regulation network between CYP1B1 and fat metabolism.
Figure 7.
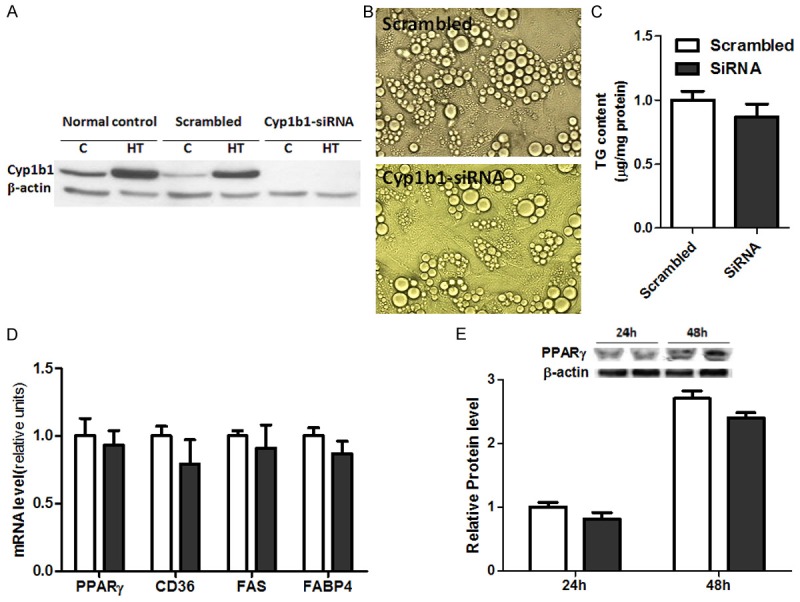
Knock down CYP1B1 in C3H10T1/2 cells by siRNA failed to suppress adipogenesis. A. Western blot confirmed CYP1B1 silence in C3H10T1/2 cells by siRNA; B. C3H10T1/2 cells after 8 days differentiation under IDM condition; C. TG contents in C3H10T1/2 cells after 8 day differentiation; D. Gene expression involved in adipogenesis; E. PPARγ protein expression.
Discussion
Our findings demonstrated an important role of CYP1B1 in energy metabolism that modulates diet induced obesity and insulin sensitivity in adult mice. We showed that CYP1B1 constitutively expressed in fat pad, liver and muscle of C57BL/6J mice. Overall deletion of CYP1B1 in mice exhibited extensive impacts on genes expression of those tissue, especially in fat pad and liver, and in paralleled with lower adiposity and improved glucose intolerance in CYP1B1 deficient mice (KO) after 6 weeks HFD feeding. Compared with wild type (WT) mice, such metabolic modifications occurred in CYP1B1 deficient ones without change in caloric intake, suggesting a switch in energy utilization.
Due to its mass, muscle tissue has an important role in both energy metabolism and insulin sensitivity. However, CYP1B1 deficiency exerted less influence on genes responsible for lipogenesis and lipolysis in muscle tissue.
Compared to wild type mice, hepatic and fat pad genes expression responsible for fatty acid synthesis and transport are both decrease in KO mice upon 6 weeks HFD feeding. ACC is to provide the malonyl-CoA substrate for the fatty acids synthesis [25]; FAS is to catalyze the synthesis of palmitate from acetyl-CoA and malonyl-CoA into long chain saturated fatty acids; SCD-1 is a key enzyme in the synthesis of monounsaturated fatty acids and hepatic SCD-1 deficiency protects mice from carbohydrate induced adiposity [26]; DGAT catalyzes the formation of triglycerides from diacylglycerol and Acyl-CoA and is essential for the formation of adipose tissue. DGAT knockout mice are lean and resistant to diet-induced obesity [27]. CPT-1 catalyzes the transfer of the acyl group from coenzyme A to carnitine to form palmitoylcarnitine and plays a critical role in the β-oxidation of long chain fatty acids. UCP2 promotes mitochondrial fatty acid oxidation [28]. The suppression of ACC, FAS, SCD-1, DGAT and enhancement of CPT-1, UCP-2 genes expression in CYP1B1 deficient mice are consistent with the reduced fat pad weight, which arises from decreased fatty acid synthesis and increased fatty acid oxidation. In addition, the suppression of PPARγ, a critical transcriptional factor in adipocyte differentiation [29], both in mRNA and protein levels, also makes essential contribution to less fat accumulation in KO mice through decreases in downstream targets, such as CD36/FAT (fatty acid translocase) [30]. Furthermore, the genes responsible for fatty acid synthesis and β-oxidation mediated by the HFD challenge are similar in hepatic and adipose tissues, but more profound in liver although CYP1B1 expression in liver is relatively lower when compared with fat tissue, suggesting other possible regulation by CYP1B1 in energy regulation.
Although decreased TG contents in plasma and liver did not reached statistical levels, reduced fasting blood glucose and insulin levels, alleviated glucose intolerance were markedly observed in CYP1B1 deficient mice when compared with WT mice.
The glucose levels in CYP1B1 KO mice may be regulated by the switch from energy storage as lipid droplets to energy utilization. AMPK activated protein kinase (AMPK) is a major cellular energy sensor and activated by elevated AMP/ATP ratio due to cellular and/or environmental stress, such as energy shortage or higher energy demand [31]. In the liver, AMPK inhibits lipid synthesis and increases lipid oxidation through inhibition of ACC phosphorylation. A similar role of AMPK was observed in adipocytes. Furthermore, AMPK also exhibits inhibitory effects on lipolysis in adipocytes [32]. Therefore, enhanced AMPK activation in liver and fat pad by CYP1B1 deficiency decreased the availability of fatty acids in the plasma, improved insulin sensitization since accumulation of plasma fatty acids will initiate insulin resistance.
However, knockdown CYP1B1 in C3H10T1/2 cells did not effectively prevent adipogenic process raises an important issue. We have several explanation for that: first, mice as a multicellular organism are much more complex than a single cell and there are various tissues that perform coordinated function for energy homeostasis. And genes responsible for lipid metabolism in liver of CYP1B1 deficient mice did have more profound alteration when challenge with HFD. Second, as a monooxygenase, CYP1B1 not only bio-activates a number of exogenous procarcinogens, also metabolizes substrates of endogenous origin including retinol, dietary plant flavanoids [33]. Other tissue in mice might provide such substrates to exert its inhibition on adipogenesis. Third, the suppression of triglyceride synthesis by aryl hydrocarbon receptor (AhR) in mouse embryo fibroblasts (MEFs) and preadipocytes are highly dependent on cell density. AhR loss only elevated TG synthesis in subconfluent cells [34]. AhR is a major transcriptional regulator of CYP1B1, and CYP1B1 expression often correlated with AhR activity. Thererfore, linking ligand metabolism and cell-cell interaction in the interplay between AhR and CYP1B1 might be crucial to understanding the precise mechanisms modulating energy homeostasis.
Conclusion
In conclusion, our results demonstrate that CYP1B1 deficiency prevent adult mice from diet induced obesity and glucose intolerance. Enhanced of AMPK activation might be the signaling mechanism underlying such effects. However, knockdown CYP1B1 expression in C3H10T1/2 cells by siRNA did not statistically suppressed its adipogenesis, suggesting a complex regulation by CYP1B1 in energy homeostasis. Remaining important issues: the precise molecular mechanisms by which CYP1B1 modulates in energy metabolism and whether the effects of CYP1B1 on energy utilization can be translated into a human setting. Considering the worldwide pandemic of obesity, and common used dietary compounds such as flavonoids, such potential has not been investigated in current study and warrants further investigation.
Acknowledgements
This work was supported by National Natural Science Foundation of China (30972463, 81172664) and Open Fund of Hubei Provincial Key Laboratory for Applied Toxicology, Hubei Provincial Academy for Preventive Medicine.
Supporting Information
References
- 1. http://www.who.int/gho/ncd/risk_factors/overweight/en/
- 2.Wilborn C, Beckham J, Campbell B, Harvey T, Galbreath M, Bounty PL, Nassar E, Wismann J, Kreider R. Obesity: prevalence, theories, medical consequences, management, and research directions. J Int Soc Sports Nutr. 2005;2:4–31. doi: 10.1186/1550-2783-2-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skolnik NS, Ryan DH. Pathophysiology, epidemiology, and assessment of obesity in adults. J Fam Pract. 2014;63:S3–S10. [PubMed] [Google Scholar]
- 4.Robinson SW, Dinulescu DM, Cone RD. Genetic models of obesity and energy balance in the mouse. Annu Rev Genet. 2000;34:687–745. doi: 10.1146/annurev.genet.34.1.687. [DOI] [PubMed] [Google Scholar]
- 5.Fawcett KA, Barroso I. The genetics of obesity: FTO leads the way. Trends Genet. 2010;26:266–274. doi: 10.1016/j.tig.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silventoinen K, Rokholm B, Kaprio J, Sørensen TI. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int J Obes (Lond) 2010;34:29–40. doi: 10.1038/ijo.2009.177. [DOI] [PubMed] [Google Scholar]
- 7.Dubois L, Ohm Kyvik K, Girard M, Tatone-Tokuda F, Pérusse D, Hjelmborg J, Skytthe A, Rasmussen F, Wright MJ, Lichtenstein P, Martin NG. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: an internationalstudy of over 12,000 twin pairs. PLoS One. 2012;7:e30153. doi: 10.1371/journal.pone.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellero S, Chakhtoura G, Barreau C, Langouët S, Benelli C, Penicaud L, Beaune P, de Waziers I. Xenobiotic-metabolizing cytochromes p450 in human white adipose tissue: expression and induction. Drug Metab Dispos. 2010;38:679–686. doi: 10.1124/dmd.109.029249. [DOI] [PubMed] [Google Scholar]
- 9. http://www.proteinatlas.org/ENSG00000138061-CYP1B1/tissue.
- 10. http://www.urogene.org/pgdb/gene/107.html.
- 11.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res. 2009;81:669–677. doi: 10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFadyen M, Murray G. Cytochrome P450 1B1: a novel anticancer therapeutic target. Future Oncology. 2005;1:259–263. doi: 10.1517/14796694.1.2.259. [DOI] [PubMed] [Google Scholar]
- 14.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997;6:641–647. doi: 10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- 15.Jennings BL, Anderson LJ, Estes AM, Fang XR, Song CY, Campbell WB, Malik KU. Involvement of cytochrome P-450 1B1 in renal dysfunction, injury, and inflammation associated with angiotensin II-induced hypertension in rats. Am J Physiol Renal Physiol. 2012;302:F408–420. doi: 10.1152/ajprenal.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings BL, Estes AM, Anderson LJ, Fang XR, Yaghini FA, Fan Z, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 gene disruption minimizes deoxycorticosterone acetate-salt-inducedhypertension and associated cardiac dysfunction and renal damage in mice. Hypertension. 2012;60:1510–1516. doi: 10.1161/HYPERTENSIONAHA.112.202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho YC, Zheng W, Yamamoto M, Liu X, Hanlon PR, Jefcoate CR. Differentiation of pluripotent C3H10T1/2 cells rapidly elevates CYP1B1 through a novel process that overcomes a loss of Ah Receptor. Arch Biochem Biophys. 2005;439:139–153. doi: 10.1016/j.abb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Poulos TL. Cytochrome P450: molecular architecture, mechanism, and prospects for rational inhibitor design. Pharm Res. 1988;5:67–75. doi: 10.1023/a:1015920931701. [DOI] [PubMed] [Google Scholar]
- 19.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DM, Wolins NE. A simple and rapid method to assay triacylglycerol in cells and tissues. J Lipid Res. 2007;48:2514–2520. doi: 10.1194/jlr.D700017-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci. 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 23.Salt IP, Connell JM, Gould GW. 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. Diabetes. 2000;49:1649–1656. doi: 10.2337/diabetes.49.10.1649. [DOI] [PubMed] [Google Scholar]
- 24.Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferré P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 25.Tong L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell Mol Life Sci. 2005;62:1784–1803. doi: 10.1007/s00018-005-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV Jr. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 28.Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, Ricquier D, Miroux B, Thompson C. B. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysisderived pyruvate utilization. FASEB J. 2008;22:9–18. doi: 10.1096/fj.07-8945com. [DOI] [PubMed] [Google Scholar]
- 29.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rać ME, Safranow K, Poncyljusz W. Molecular basis of human CD36 gene mutations. Mol Med. 2007;13:288–296. doi: 10.2119/2006-00088.Rac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32:S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 32.Kola B, Grossman AB, Korbonits M. The Role of AMP-Activated Protein Kinase in Obesity. Front Horm Res. 2008;36:198–211. doi: 10.1159/000115366. [DOI] [PubMed] [Google Scholar]
- 33.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 34.Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111:3311–3322. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


