Abstract
Background: Immune thrombocytopenia (ITP) is an acquired and autoimmune disease of adults and children characterized by decreased platelet production. CXC chemokine ligand-13 (CXCL13) participates in multiple immunological responses. However, it is still unknown the relationship between CXCL13 and ITP. Methods: Plasma CXCL13 was detected in ITP (n = 30) children. CD4+ T cells was isolated from peripheral blood mononuclear cells (PBMCs) from healthy volunteers. Treated CD4+ T cells with dexamethasone and/or miR-125-5p mimic/inhibitor, to observe the regulation of CXCL13. Results: Compared with controls, ITP children had elevated plasma CXCL13, the concentration of which was reduced after treatment. In vitro, dexamethasone decreased CXCL13 level in in dose- dependent and in time-dependent manner. MiR-125-5p mimic decreased CXCL13 level and miR-125-5p inhibitor increased CXCL13 level in CD4+ T cells. CXCL13 was implied to be target gene of miR-125-5p. MiR-125-5p inhibitor also canceled dexamethasone induced decrease of CXCL13. Conclusion: CXCL13 is the target gene of miR-125-5p, which is possibly involved in the pathological process of ITP.
Keywords: Immune thrombocytopenia, chemokine, lymphocytes, miR-125-5p
Introduction
Immune thrombocytopenia (ITP) is an acquired and autoimmune disease of adults and children characterized by decreased platelet production. Childhood ITP is a large percentage of total ITP population [1]. Most ITP children recover within 6-12 months, and meta-analysis data shows that 20-25% children with peripheral blood platelet count < 100 × 109/L lasting for more than12 months will develop chronic disease [2]. Even so, digestive and urinary tracts bleeding, intracranial hemorrhage and subconjunctival hemorrhages are often accompanied with decreased platelet level and threaten the lives of patients [3,4]. The pathogenesis of ITP remains not fully understood. However, most scholars consider humoral immune abnormalities leading to antiplatelet autoantibodies produced by B lymphocytes, which cause platelet damage, is the main reason for thrombocytopenia [5]. In addition, Tregs are decreased in number or exhibit defective suppressive functions in patients with ITP, which play critical roles in the maintenance of peripheral immune tolerance [6]. Early studies reported that platelet-reactive CD4+ T cells in ITP patients had an activity of promoting antiplatelet autoantibody response and were involved in the production of pathogenic antiplatelet autoantibodies in patients with ITP [7].
CXC chemokine ligand-13 (CXCL13) is a small cytokine belonging to the CXC chemokine family, mainly secreted by secondary lymphoid tissue, lymph gland and serum follicular dendritic cells [8]. The primary functions of CXC chemokine family are chemoattraction and activation of leukocytes in multiple immunological responses [9]. Chemokine receptor-ligand pair CXC chemokine receptor-5 (CXCR5) and CXCL13 is a key component in immune system [10,11]. CXCL13 is required for B1 cell homing, natural antibody production and body cavity immunity [12]. In addition, it has been reported that CXCL13 plays a key role in recruitment of B cells and T-cell subsets in pathological conditions, and is considered to be a therapeutic target in various immune diseases [13,14]. Research shows that CXCL13 level is elevated in ITP patients [15]. However, the relationship between CXCL13 and ITP is still unknown, and the regulation of CXCL13 in ITP remains to be further explored. In present study, plasma levels of CXCL13 in ITP children were determined and underlying mechanism was investigated in vitro.
Materials and methods
Collection of patient samples
The human study was approved by Ethics Committee of Soochow University Affiliated Children’s Hospital. We performed a retrospective review of children diagnosed with ITP in Soochow University Affiliated Children’s Hospital (from January 2009 to December 2012). The medical records of all ITP children were collected: (1) general information: age and gender; (2) clinical data: platelet count, hemoglobin concentration, severity of bleeding and type of ITP at last presentation of ITP. Severe hemorrhage was defined according to the diagnostic criteria of Bolton-Maggs and Moon’s bleeding assessment tool [16]. IVIG, corticosteroids, splenectomy, immune therapy, and chemotherapy were included in the treatment of ITP.
5 ml peripheral venous blood was collected by sterile syringes and then transferred to heparin sodium treated centrifugal tube from ITP children before and after treatment, and plasma was obtained by centrifuging at 3500 rpm for 10 min then stored at -80°C before biomarkers detection.
Detection of protein CXCL13 by ELISA
CXCL13 protein levels of plasma or cells were detected by ELISA kit (Shanghai Jining biological technology co., LTD, China) according to the manufacturer’s instructions.
Cell culture
CD4+ T cells were isolated from peripheral blood mononuclear cells (PBMCs) from healthy volunteers. As previously described [17], monocytes were depleted by adhesion for 30 min and CD4+ T cells were isolated by negative selection with magnetic beads using Automacs (Miltenyi Biotec, Germany) according to the manufacturer’s instructions. CD4+ T Cells were incubated in complete RPMI 1640 medium containing 5% pooled human serum for 3 days. Inoculated cells to 24 well plates to culture. Treated cells with different concentrations of dexamethasone (Sigma, USA).
Quantitative PCR
The total RNA of CD4+ T cells was isolated with TRIzol (Invitrogen, USA) and concentrated by isopropanol precipitation method. 2 μg RNA per sample was reverse transcript to cDNA by M-MuLV Reverse Transcriptase (Promega, USA). The relative expression of CXCL13 mRNA and miR-125-5p was quantified by TaqMan Gene Expression Assays (Applied Biosystems, USA). β-actin and U6 were respectively served as internal control genes.
Down-regulation and over-expression of miR-125-5p in cells
The expression of miR-125-5p in cells was regulated by transfected with mimic, inhibitor and negative control of miR-125-5p (Ribobio Co., Ltd. China). The Lipofectamine 2000 reagent (Invitrogen, USA) was used to conduct cell transfection according to the manufacturer’s instructions.
3’UTR luciferase assay
The 3’UTR luciferase assay was performed as previously described [18]. In brief, 3’UTR of CXCL13 containing putative miR-125a-5p binding sites were amplified by RT-PCR and then cloned into pMIR-REPORTTM vectors (Ribobio Co., Ltd. China). The constructs were co-transfected with pre-MIR-125a-5p or a negative control into 3T3 cells using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Transfected cells for 48 h, the relative luciferase activity was determined using the Dual-Luciferase Reporter Assay System Kit (Promega, USA).
Statistical analysis
Statistical analysis was conducted with SPSS18.0 software. Enumeration data was expressed as number (percentage). Measurement data was expressed as mean ± SD. The univariate correlations of plasma CXCL13 levels with disease activity parameters were analyzed by Spearman method. The difference between two groups was determined by independent-samples T test. P value less than 0.05 was considered statistically significant.
Results
Demographic, clinical, and laboratory data of children
There were 30 children diagnosed with ITP in this study (Table 1). The ratio of male to female was 1:1.3. The mean age of children diagnosed with ITP was 5.4 ± 3.7 years. Of the 30 patients, 56.7% had none or mild hemorrhage, 26.7% ITP children had moderate hemorrhage and 16.7% suffered with severe hemorrhage. 8 (26.7) subjects had a platelet count < 10 × 109/L and 3 (10.0) had a hemoglobin concentration < 90 g/L at the time of hemorrhage. Of the 30 patients, 20 patients were diagnosed with acute ITP and 10 patients were chronic ITP.
Table 1.
Demographic, clinical, and laboratory data of children with ITP
| Variables | No. Patients (%) |
|---|---|
| Age at the first presentation of ITP (y) | |
| < 1 | 6 (20.0) |
| 1-3 | 7 (23.3) |
| 3-7 | 8 (26.7) |
| > 7 | 9 (30.0) |
| Male | 13 (43.3) |
| Hemorrhage severity | |
| None or mild | 17 (56.7) |
| Moderate | 8 (26.7) |
| Severe | 5 (16.7) |
| Platelet count < 10 × 109/L at the time of hemorrhage | 8 (26.7) |
| Hemoglobin concentration < 90 g/L at the time of hemorrhage | 3 (10.0) |
| Type of ITP at last presentation of ITP | |
| Acute | 20 (66.7) |
| Chronic | 10 (33.3) |
Plasma levels of CXCL13 in ITP children
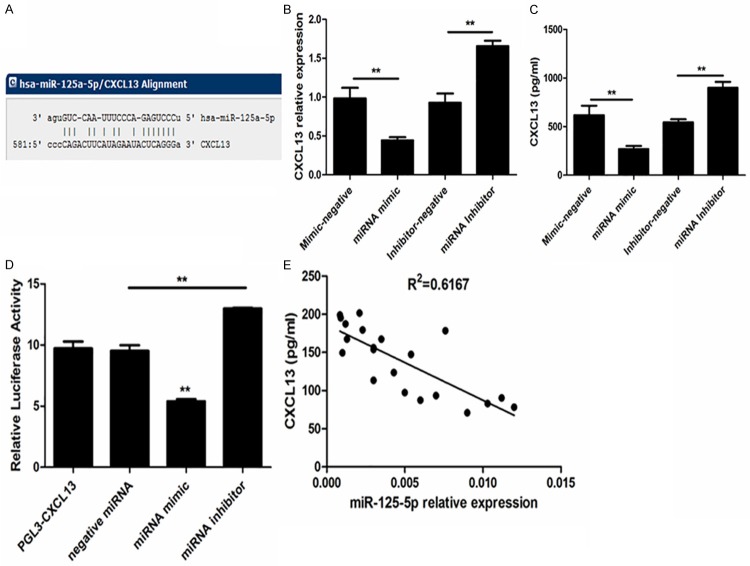
CXCL13 concentration of peripheral blood was measured in ITP children and healthy children. As shown in Figure 1, ITP patients had a significant increase of plasma CXCL13 level compared with controls.
Figure 1.
Plasma levels of CXCL13 in ITP and healthy children. Plasma CXCL13 was determined by ELISA kit. The concentration of CXCL13 was elevated in ITP children; n (CON) = 30; n (ITP) = 30.
Plasma levels of CXCL13 in ITP children before and after treatment
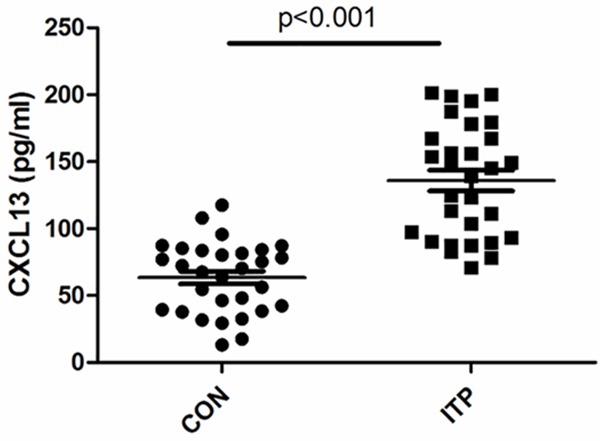
To compare the plasma CXCL13 level between chronic ITP and acute ITP, the difference was analyzed. As shown in Figure 2A, the CXCL concentration of acute ITP was higher than that of chronic ITP (P = 0.0561). In addition, CXCL13 level was significantly decreased in ITP after treatment (Figure 2B), which dropped to control level.
Figure 2.
Plasma levels of CXCL13 in ITP children before and after treatment. Plasma CXCL13 was determined by ELISA kit; n (Chronic) = 10; n (Acute) = 20; n (ITP) = 30; n (Treatment) = 30.
Univariate correlations of plasma CXCL13 levels with disease activity parameters
The correlation of plasma CXCL13 level and disease activity parameters were analyzed. As shown in Table 2, the CXCL13 level had no statistic correlation with age and gender in ITP children before or after treatment. The results showed that plasma CXCL13 level was positively correlated to hemorrhage severity, platelet count and hemoglobin concentration respectively in ITP patients before treatment. However, the correlations were absent after treatment.
Table 2.
Univariate Correlations of plasma CXCL13 levels with disease activity parameters
| Before Treatment, rho (P) | After Treatment, rho (P) | |
|---|---|---|
| Age | 0.032 (0.871) | 0.029 (0.916) |
| Gender | 0.043 (0.932) | 0.048 (0.908) |
| Hemorrhage severity | 0.345 (0.021) | 0.137 (0.318) |
| Platelet count | 0.404 (0.002) | 0.142 (0.412) |
| Hemoglobin concentration | 0.298 (0.040) | 0.083 (0.245) |
CXCL13 decreased in CD4+ T cells treated with dexamethasone
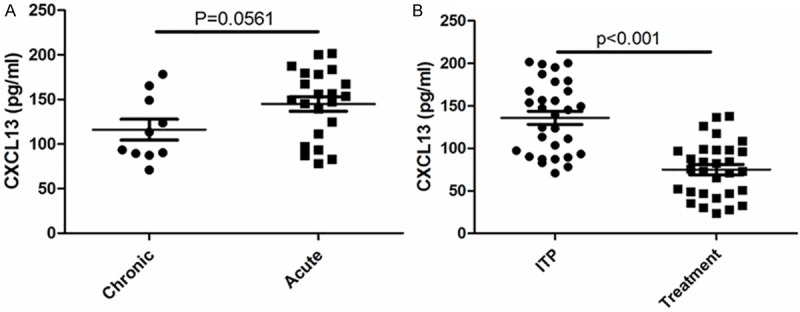
To further investigate the relationship between CXCL13 and ITP and its mechanism, in vitro experiments were performed. Dexamethasone was used to treat peripheral blood CD4+ T lymphocytes from healthy human in different concentrations and times of treatment. As presented, the CXCL level of CD4+ T lymphocytes was decreased in dose- dependent (Figure 3A) and time-dependent manner (Figure 3B).
Figure 3.
CXCL13 decreased in CD4+ T cells treated with dexamethasone. CD4+ T cells was isolated from peripheral blood of healthy donors; different concentration of dexamethasone was treated cells for 24 h; 10 μM dexamethasone was used to treat the cells for different times; all experiments were repeated three times; *p < 0.05 vs control; **p < 0.01 vs control.
MiR-125-5p was associated with plasma CXCL13 in ITP
CXCL13 was predicted to be a putative target of miR-125-5p by the miRNA-Gene-network analysis and target prediction programs. The alignment was shown in Figure 4A. Transfected CD4+ T lymphocytes with mimic or inhibitor of miR-125-5p and incubated for 24h, the quantity of CXCL13 was detected by real-time PCR. The data showed that miR-125-5p mimic significantly down-regulated CXCL13 expression and miR-125-5p inhibitor markedly up-regulated CXCL13 level in CD4+ T lymphocytes (Figure 4B). Incubated cells for 48h after transfection, and the protein secretion of CXCL13 were quantified. Similar to the mRNA expression, CXCL13 protein secretion was decreased in miR-125-5p mimic and increased in miR-125-5p inhibitor (Figure 4C). Luciferase activity assay presented that miR-125-5p targeted to 3’UTR of CXCL13 (Figure 4D). The miR-125-5p expression of ITP patients was also quantified in peripheral blood mononuclear cells (PBMCs). As shown in Figure 4E, plasma CXCL13 was negatively correlated with PBMC miR-125-5p expression in ITP patients.
Figure 4.
MiR-125-5p was associated with plasma CXCL13 in ITP. CXCL13 was predicted to be a putative target of miR-125-5p by the miRNA-Gene-network analysis and target prediction programs (A); Transfected with CD4+ T cells with mimic, inhibitor or negative control of miR-125-5p, the relative CXCL13 mRNA was detected by real-time PCR (B), CXCL13 concentration was determined by ELISA (C), and the relative luciferase activity was measured by Dual-Luciferase Reporter Assay System Kit (D); univariate correlation analysis between plasma CXCL13 level and miR-125-5p relative expression was performed in ITP patients (E); **P < 0.01.
MiR-125-5p involved in the regulation of CXCL13 in T cell
To further confirm whether the CXCL13 expression was regulated by miR-125-5p in treatment of dexamethasone, T lymphocytes were treated with dexamethasone. The miR-125-5p level was increased in time-dependent manner in cells (Figure 5A). In addition, CXCL13 level was decreased by dexamethasone and miR-125-5p inhibitor reversed this effect.
Figure 5.
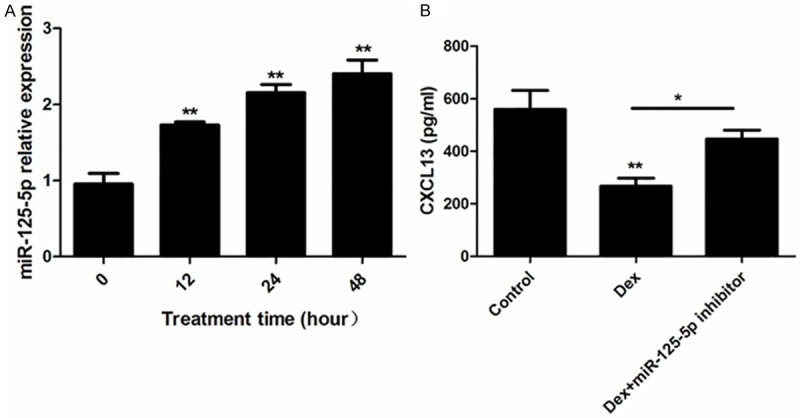
MiR-125-5p involved in the regulation of CXCL13 in CD4+ T cells. Treated CD4+MT cells with 10 μM dexamethasone for different time, the miR-125-5p relative expression was detected by real-time PCR (A); CXCL13 concentration was measured by ELISA kit (B) after dexamethasone treatment with (or without) miR-125-5p inhibitor; *P < 0.05; **P < 0.01 vs control (or the group with 0 h treatment time).
Discussion
Immune thrombocytopenia (ITP) is a common autoimmune disorder. Chemokine CXCL13 is key composition in immunity system. To investigate the relationship between CXCL13 and ITP, we determined the plasma CXCL13 in ITP in vivo first. The major findings showed that plasma CXCL13 was significantly elevated in ITP children. The subjects with acute ITP had higher CXCL13 level than that in chronic ITP. In addition, the patients after treatment had reduced plasma CXCL13 level compared with patients without treatment. Univariate correlation analysis result indicated that plasma CXCL13 level was positively correlated to hemorrhage severity, platelet count and hemoglobin concentration respectively in ITP patients before treatment. Our findings are in line with previous study which found CXCL13 is elevated in ITP patients [15]. All these data implied that CXCL13 was closely related to the development of ITP.
Chemokine CXCL13 is a key factor in establishing adaptive immune response. CXCR5 is the specific receptor for CXCL13. To mediate more effective response to secondary pathogen by memory T cells is a central process of adaptive immunity [19]. The pair of receptor-ligand (CXCL13/ CXCR5) greatly contributes to homing of B-helper cells and a subset of T-helper cells to the lymphoid follicle [20-22]. ITP is an immune-mediated acquired disease. Olsson B et al. revealed that activated T cells were apoptotic resistant in active ITP patients and lead to defective clearance of autoreactive T cells through apoptosis, which might cause a continued immune destruction [23]. CXCL13 may be involved in the pathophysiological procedure of ITP. However, few studies have been emerged to discuss the relationship between CXCL13 and ITP. As showed in our vivo experiments, plasma CXCL13 protein was elevated in ITP patients and positively related (r = 0.345, p = 0.021) to hemorrhage severity.
To investigate the mechanism of CXCL13 on ITP development, we performed experiments in vitro. Dexamethasone, a type of steroid medication that often used to treat ITP [24-26], was administrated into peripheral blood CD4+ T lymphocytes from healthy human, and results showed that CXCL13 in CD4+ T lymphocytes was decreased both in dose-dependent and in time-dependent manner. Therefore, in vitro experiments preliminary suggested that CXCL13 regulation participated in immune disorder.
MicroRNA is a small noncoding RNA molecule, taking part in various normal and pathological processes [27-29]. Jernas, M. et al identified 1915 regulated genes and 22 regulated microRNAs that differed between ITP patients and controls by genome-wide expression analyses in T cells. In addition, CXCL13 is demonstrated to be one target gene of miRNAs to regulate ITP [15]. CXCL13 was predicted to be a putative target of miR-125-5p by the miRNA-Gene-network analysis and target prediction programs. To better understand the mechanisms of CXCL13 involved in ITP, we over-expressed or down-regulated the miR-125-5p level in CD4+ T lymphocytes by mimic or inhibitor of miR-125-5p. As expected, over-expression of miR-125-5p decreased the mRNA and protein of CXCL13 while down-regulation of miR-125-5p increased the CXCL13 expression. Luciferase activity assay proved miR-125-5p targeted to 3’UTR of CXCL13. All these results suggested that miR-125-5p played a key role in CXCL13 regulation. In another hand, miR-125-5p expression was time-dependently increased by dexamethasone treatment in CD4+ T lymphocytes. As described above, dexamethasone decreased CXCL13 protein level, and miR-125-5p inhibitor reversed this effect, which further confirmed that chemokine CXCL13 expression was regulated by miR-125-5p. There is evidence that miR-125a acts as an NF-kB inhibitor upon activation of TLR pathway and involves in inhibiting erythroid differentiation in leukemia [30]. Inoue, Y. found miR-125a was expressed in peripheral blood mononuclear cells (PBMCs) and the single nucleotide polymorphisms in precursor-miR-125a was associated with autoimmune thyroid diseases development and prognosis [31]. These previous studies imply that miR-125 is linked to immunological diseases. In present study, we also found miR-125-5p expression of PBMC was negatively correlated with plasma CXCL13 in ITP patients.
In conclusion, plasma CXCL13 level was significantly increased in ITP patients. In vitro experiments confirmed that CXCL13 is the target gene of miR-125-5p, the regulation of CXCL13 by miR-125-5p may be one of mechanisms in development of ITP. Further molecular mechanism of CXCL13 on ITP will be conducted. CXCL13 and miR-125-5p are potential therapeutic targets for ITP.
Disclosure of conflict of interest
None.
References
- 1.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol. 2010;85:174–180. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 2.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, Godeau B, Lechner K, Mazzucconi MG, McMillan R, Sanz MA, Imbach P, Blanchette V, Kühne T, Ruggeri M, George JN. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 3.Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27:495–520. doi: 10.1016/j.hoc.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thota S, Kistangari G, Daw H, Spiro T. Immune thrombocytopenia in adults: an update. Cleve Clin J Med. 2012;79:641–650. doi: 10.3949/ccjm.79a.11027. [DOI] [PubMed] [Google Scholar]
- 5.Chen JF, Yang LH, Chang LX, Feng JJ, Liu JQ. The clinical significance of circulating B cells secreting anti-glycoprotein IIb/IIIa antibody and platelet glycoprotein IIb/IIIa in patients with primary immune thrombocytopenia. Hematology. 2012;17:283–290. doi: 10.1179/1607845412Y.0000000014. [DOI] [PubMed] [Google Scholar]
- 6.Ji X, Zhang L, Peng J, Hou M. T cell immune abnormalities in immune thrombocytopenia. J Hematol Oncol. 2014;7:72–72. doi: 10.1186/s13045-014-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102:1393–1402. doi: 10.1172/JCI4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 9.Schiffer L, Worthmann K, Haller H, Schiffer M. CXCL13 as a new biomarker of Systemic Lupus Erythematodes (SLE) and Lupus Nephritis (LN) - from bench to bedside? Clin Exp Immunol. 2015;179:85–9. doi: 10.1111/cei.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cagigi A, Mowafi F, Phuong Dang LV, Tenner-Racz K, Atlas A, Grutzmeier S, Racz P, Chiodi F, Nilsson A. Altered expression of the receptor-ligand pair CXCR5/CXCL13 in B cells during chronic HIV-1 infection. Blood. 2008;112:4401–4410. doi: 10.1182/blood-2008-02-140426. [DOI] [PubMed] [Google Scholar]
- 11.Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51:364–371. doi: 10.1136/gut.51.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez E, Piccio L, Mikesell RJ, Klawiter EC, Parks BJ, Naismith RT, Cross AH. CXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditions. Mult Scler. 2013;19:1204–1208. doi: 10.1177/1352458512473362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellebjerg F, Bornsen L, Khademi M, Krakauer M, Olsson T, Frederiksen JL, Sorensen PS. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73:2003–2010. doi: 10.1212/WNL.0b013e3181c5b457. [DOI] [PubMed] [Google Scholar]
- 15.Jernas M, Nookaew I, Wadenvik H, Olsson B. MicroRNA regulate immunological pathways in T-cells in immune thrombocytopenia (ITP) Blood. 2013;121:2095–2098. doi: 10.1182/blood-2012-12-471250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolton-Maggs PH, Moon I. Assessment of UK practice for management of acute childhood idiopathic thrombocytopenic purpura against published guidelines. Lancet. 1997;350:620–623. doi: 10.1016/s0140-6736(97)04143-3. [DOI] [PubMed] [Google Scholar]
- 17.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJG, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circulation Research. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 19.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 20.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 21.Fischer L, Korfel A, Pfeiffer S, Kiewe P, Volk HD, Cakiroglu H, Widmann T, Thiel E. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15:5968–5973. doi: 10.1158/1078-0432.CCR-09-0108. [DOI] [PubMed] [Google Scholar]
- 22.Saez de Guinoa J, Barrio L, Mellado M, Carrasco YR. CXCL13/CXCR5 signaling enhances BCR-triggered B-cell activation by shaping cell dynamics. Blood. 2011;118:1560–1569. doi: 10.1182/blood-2011-01-332106. [DOI] [PubMed] [Google Scholar]
- 23.Olsson B, Andersson PO, Jacobsson S, Carlsson L, Wadenvik H. Disturbed apoptosis of T-cells in patients with active idiopathic thrombocytopenic purpura. Thrombosis And Haemostasis. 2005;93:139–144. doi: 10.1160/TH04-06-0385. [DOI] [PubMed] [Google Scholar]
- 24.Din B, Wang X, Shi Y, Li Y. Long-Term Effect of High-Dose Dexamethasone with or without Low-Dose Dexamethasone Maintenance in Untreated Immune Thrombocytopenia. Acta Haematologica. 2015;133:124–128. doi: 10.1159/000362529. [DOI] [PubMed] [Google Scholar]
- 25.Zaja F, Baccarani M, Mazza P, Bocchia M, Gugliotta L, Zaccaria A, Vianelli N, Defina M, Tieghi A, Amadori S, Campagna S, Ferrara F, Angelucci E, Usala E, Cantoni S, Visani G, Fornaro A, Rizzi R, De Stefano V, Casulli F, Battista ML, Isola M, Soldano F, Gamba E, Fanin R. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115:2755–2762. doi: 10.1182/blood-2009-07-229815. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY, Chan NP, Wong WS, Cheng G. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med. 2003;349:831–836. doi: 10.1056/NEJMoa030254. [DOI] [PubMed] [Google Scholar]
- 27.Khan S, Greco D, Michailidou K, Milne RL, Muranen TA, Heikkinen T, Aaltonen K, Dennis J, Bolla MK, Liu J, Hall P, Irwanto A, Humphreys K, Li J, Czene K, Chang-Claude J, Hein R, Rudolph A, Seibold P, Flesch-Janys D, Fletcher O, Peto J, dos Santos Silva I, Johnson N, Gibson L, Aitken Z, Hopper JL, Tsimiklis H, Bui M, Makalic E, Schmidt DF, Southey MC, Apicella C, Stone J, Waisfisz Q, Meijers-Heijboer H, Adank MA, van der Luijt RB, Meindl A, Schmutzler RK, Müller-Myhsok B, Lichtner P, Turnbull C, Rahman N, Chanock SJ, Hunter DJ, Cox A, Cross SS, Reed MW, Schmidt MK, Broeks A, Van’t Veer LJ, Hogervorst FB, Fasching PA, Schrauder MG, Ekici AB, Beckmann MW, Bojesen SE, Nordestgaard BG, Nielsen SF, Flyger H, Benitez J, Zamora PM, Perez JI, Haiman CA, Henderson BE, Schumacher F, Le Marchand L, Pharoah PD, Dunning AM, Shah M, Luben R, Brown J, Couch FJ, Wang X, Vachon C, Olson JE, Lambrechts D, Moisse M, Paridaens R, Christiaens MR, Guénel P, Truong T, Laurent-Puig P, Mulot C, Marme F, Burwinkel B, Schneeweiss A, Sohn C, Sawyer EJ, Tomlinson I, Kerin MJ, Miller N, Andrulis IL, Knight JA, Tchatchou S, Mulligan AM, Dörk T, Bogdanova NV, Antonenkova NN, Anton-Culver H, Darabi H, Eriksson M, Garcia-Closas M, Figueroa J, Lissowska J, Brinton L, Devilee P, Tollenaar RA, Seynaeve C, van Asperen CJ, Kristensen VN kConFab Investigators; Australian Ovarian Cancer Study Group; Slager S, Toland AE, Ambrosone CB, Yannoukakos D, Lindblom A, Margolin S, Radice P, Peterlongo P, Barile M, Mariani P, Hooning MJ, Martens JW, Collée JM, Jager A, Jakubowska A, Lubinski J, Jaworska-Bieniek K, Durda K, Giles GG, McLean C, Brauch H, Brüning T, Ko YD GENICA Network. Brenner H, Dieffenbach AK, Arndt V, Stegmaier C, Swerdlow A, Ashworth A, Orr N, Jones M, Simard J, Goldberg MS, Labrèche F, Dumont M, Winqvist R, Pylkäs K, Jukkola-Vuorinen A, Grip M, Kataja V, Kosma VM, Hartikainen JM, Mannermaa A, Hamann U, Chenevix-Trench G, Blomqvist C, Aittomäki K, Easton DF, Nevanlinna H. MicroRNA Related Polymorphisms and Breast Cancer Risk. PLoS One. 2014;9:e109973. doi: 10.1371/journal.pone.0109973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: the microRNA perspective. Immunol Rev. 2013;253:112–128. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thum T, Mayr M. Review focus on the role of microRNA in cardiovascular biology and disease. Cardiovasc Res. 2012;93:543–544. doi: 10.1093/cvr/cvs085. [DOI] [PubMed] [Google Scholar]
- 30.Gañán-Gómez I, Wei Y, Yang H, Pierce S, Bueso-Ramos C, Calin G, Boyano-Adánez MDC, García-Manero G. Overexpression of miR-125a in myelodysplastic syndrome CD34+ cells modulates NF-κB activation and enhances erythroid differentiation arrest. PLoS One. 2014;9:e93404. doi: 10.1371/journal.pone.0093404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue Y, Watanabe M, Inoue N, Kagawa T, Shibutani S, Otsu H, Saeki M, Takuse Y, Hidaka Y, Iwatani Y. Associations of single nucleotide polymorphisms in precursor-microRNA (miR)-125a and the expression of mature miR-125a with the development and prognosis of autoimmune thyroid diseases. Clin Exp Immunol. 2014;178:229–235. doi: 10.1111/cei.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]