Abstract
The eradication of malaria will only be possible if effective, well-tolerated medicines kill hypnozoites in vivax and ovale malaria, and thus prevent relapses in patients. Despite progress in the 8-aminoquinoline series, with tafenoquine in Phase III showing clear benefits over primaquine, the drug discovery challenge to identify hypnozoiticidal or hypnozoite-activating compounds has been hampered by the dearth of biological tools and assays, which in turn has been limited by the immense scientific and logistical challenges associated with accessing relevant human tissue and sporozoites. This review summarises the existing drug discovery series and approaches concerning the goal to block relapse.
Keywords: Plasmodium vivax, Radical cure, Hypnozoite, Strategy, Drug discovery assays
Introduction
The microscopic discovery of the Plasmodial protozoal parasite responsible for the signs and symptoms of malaria has been attributed to Dr. Alphonse Laveran, a French physician working in Algeria. His discovery was made in 1880 from astute microscopic observations of pigment-containing protozoa in fresh blood without the assistance of special stains.1
The first taxonomic description of the pathogen responsible for, what has been for a long time incorrectly called ‘benign tertian malaria’, has been attributed to Grassi and Feletti.2 The parasite was initially termed Haemamoeba vivax, but the nomenclature was later revised to Plasmodium vivax (P. vivax). P. vivax is one of five plasmodial species responsible for human malaria.
Later, it was inferred by case observation that there was a latent form of P. vivax, which could give rise to a recurrence of disease many months later without reinfection.3 Over the ensuing decades, many hypotheses were promulgated to understand the mechanism of relapse. It was ultimately concluded that the etiologic agent responsible for episodic relapse was exoerythrocytic in nature, isolated to hepatic cells, and following the primary erythrocytic infection.
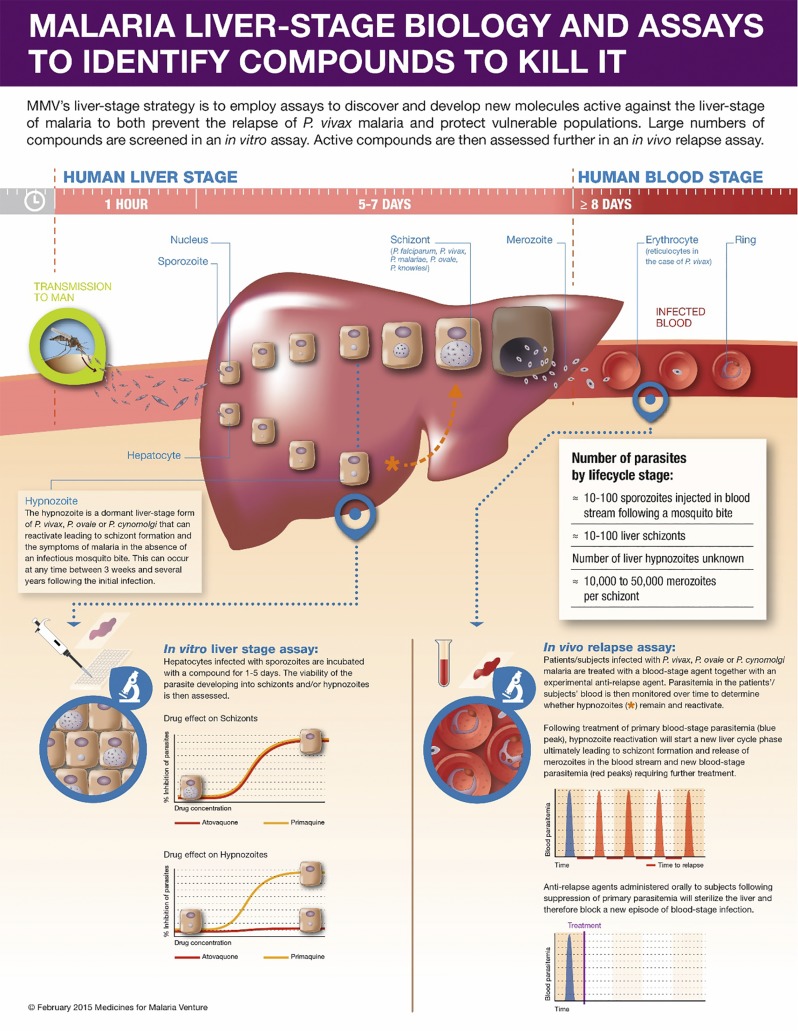
The life cycle of vivax malaria is, for the most part, similar to that of the other species of malaria (Fig. 1). When an infected mosquito takes a blood meal from a human being, sporozoites, present in the salivary glands of the mosquito, are inoculated into capillaries of the upper dermis from where they will reach the portal circulation. Subsequently, they are circulated to the liver where they invade hepatic cells. After a brief cycle of asexual replication of the parasite, the hepatocytes – which can no longer contain the growing, dividing parasite – rupture, releasing 10 – 40 000 merozoites into the general circulation. At that point, the merozoites encounter erythrocytes and are transported intracellularly, where a new cycle of asexual replication and growth begins.4
Figure 1.
Liver stage life cycle of Plasmodium spp. A proportion of vivax sporozoites differentiate to a hypnozoite form that ultimately reactivates and proliferates leading to a blood-stage relapse.
When the single parasite is in the erythrocyte, it is termed an early trophozoite. The trophozoite grows and then begins to asexually replicate, a phenomenon known as schizogony. When schizonts are sufficiently mature, the erythrocytes containing schizonts rupture, releasing merozoites with a subsequent increase in circulating malaria parasites.
After a certain stage, some parasites will transform into gametocytes, both male and female. These are then taken up by mosquitoes during a blood meal and will transform into male and female gametes. The union of male and female gametes together will form diploid zygotes, which in turn become ookinetes. These ookinetes migrate to the midgut of the insect, pass through the gut wall and form the oocysts in the haemolymph. Meiotic division of the oocysts occurs leading to maturation and rupture to release sporozoites, which then migrates to the salivary glands of the female Anopheles mosquito ready to continue the cycle of transmission back to man.5
The main difference between the life cycle of P. vivax and Plasmodium falciparum (P. falciparum) is the development of latent forms – known as hypnozoites – in the liver.6 The hypnozoites are not eradicated by standard antimalarials and can awaken days to months to years after the last bout of clinical malaria, in the absence of a mosquito bite, unless drugs specifically targetting the hypnozoite are administered.
Clinical manifestations of P. vivax infection include high fevers, chills and rigours, nausea, vomiting and diarrhoea. The manifestations are a consequence in part of ruptured erythrocytes and the cytokine response to the sudden release of parasites, haemoglobin and erythrocyte membranes. Untreated and over time, the paroxysms of fever and chills can occur every 48 hours due to synchronicity of the parasite intraerythrocytic growth. Paroxysms can also occur daily. Tertian fever (paroxysm every third day) is not pathognomonic for P. vivax but also occurs with Plasmodium ovale (P. ovale).7–11
P. vivax, despite relatively low parasitaemia, results in severe symptoms and high burden of morbidity and associated mortality. Anaemia can be profound and the spleen can enlarge markedly as it filters remnants and disposes of ruptured erythrocytes. Anaemia carries its own morbidity and mortality, and enlarged spleens can rupture either spontaneously or secondary to blunt trauma since the enlarged spleen is more fragile. Therefore, the clinical course of P. vivax malaria can be anything but benign and needs to be considered equal to P. falciparum malaria regarding financial investment and priorities.12–14
Although P. vivax has been shown to tolerate temperate regions, its distribution is nevertheless widespread and is present in southeast Asia and Oceania, South and Central America, the Indian subcontinent, eastern Mediterranean and parts of southern Europe, and Africa. Although P. falciparum is more prevalent in Africa than P. vivax, in the other regions of the world populations are, in general, equally likely to be exposed to both species.15 African populations who are Duffy blood group-negative have shown resistance to erythrocyte P. vivax infection demonstrating the importance of the Duffy antigen receptor for invasion of vivax sporozoites. Recent findings in Madagascar, however, have demonstrated now that Duffy-negative and Duffy-positive populations of diverse ethnic backgrounds can both sustain a vivax infection.16
Acute infections of P. vivax have traditionally responded well to a treatment course of chloroquine for the systemic erythrocytic infection. However, in the Indonesian archipelago, sensitivity of the parasite to chloroquine appears to be diminished; therefore, chloroquine is no longer used as a standard treatment. Chloroquine-resistant P. vivax also appears to be expanding its territory in SE Asia.17 For these reasons, treatment in this region as well as Solomon Islands, Vanuatu and now Cambodia is shifting more towards artemisinin combination therapies (ACTs). The advantage of ACTs is the circumvention of chloroquine resistance and the ability to treat falciparum malaria, should a patient have a mixed Plasmodium spp infection.
Pros and Cons of Current Relapse Prevention
For the last approximately 95 years, 8-aminoquinoline drugs have been known to be effective against the exoerythrocytic form of P. vivax, thereby preventing relapse following treatment with an effective blood schizonticide. Primaquine phosphate is currently the only marketed monotherapy drug for this indication. Typically, primaquine is administered concurrently with chloroquine at a dose of 15 mg base per day for 14 days (210 mg total dose). In some regions such as in the Indonesia archipelago, higher doses of primaquine (30 mg base for 14 days; 420 mg total dose) are administered to prevent relapse.18,19
Although shorter treatment regimens have been tested in clinical trials, it would appear that total treatment dose corresponds with overall efficacy.20 Since higher daily doses to achieve the effective total dose may be associated with more subjective side effects, shorter treatment regimens with higher daily doses have therefore not been widely used or recommended. On the other hand, patient compliance with a 14-day regimen of drug to prevent relapse may diminish the overall effectiveness of the standard regimen despite the demonstration of reasonable efficacy in clinical trials.20
Gastrointestinal discomfort and side effects are associated with the oral administration of primaquine, especially at the higher doses. There is evidence to indicate that gastrointestinal discomfort can be mitigated by administration with food.21–26
Primaquine metabolism is complex and appears to be via enzymes such as the cytochrome P450s and monoamine oxidase, and involves reactive intermediates that have defied attempts to quantitate in plasma. The reactive metabolites are thought to reduce glutathione, rendering erythrocytes with low activity in glucose-6-phosphate dehydrogenase (G6PD) more susceptible to oxidative damage to haemoglobin, and possibly through metabolite-haemoglobin adduct formation.27,28 Downstream effects include Heinz body formation and ultimately erythrocyte destruction.29 There are many genetic variants of G6PD deficiency worldwide. Depending on the quantitative interaction between G6PD enzyme activity and glutathione concentration, red blood cells (RBCs) are able to deal with drug-induced oxidative stress to varying degrees. Since primaquine at doses that prevent relapse may variably lead to clinically relevant reductions in erythrocytes in individuals who are G6PD deficient, the development of novel point of care G6PD tests that can be reliably used in malaria field conditions will allow the testing of patients whose G6PD status is unknown. This will enable an evaluation of patient levels of G6PD activity prior to being given a full treatment course to prevent relapse as recommended by the World Health Organisation (http://www.who.int/malaria/areas/global_technical_strategy/draft-gts-english.pdf?ua = 1).30,31
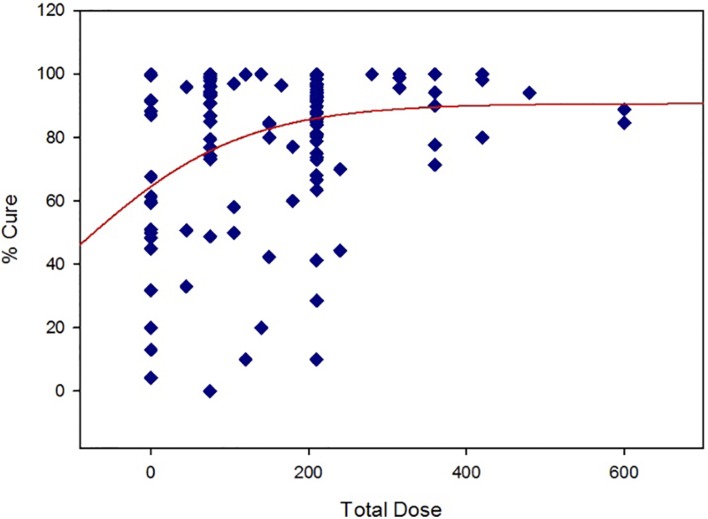
A recent publication regarding primaquine radical cure of P. vivax has compiled data from multiple studies reported beginning in the 1950s.20 The rationale for the critical review was the apparent lack of consensus over the optimal dosing regimen for relapse prevention. Since the compiled data were published as supplementary files, a scatter plot of total dose (primaquine base mg/day × treatment length in days) versus % cure has been generated for studies in which primaquine was given with chloroquine. A simple Emax curve (SigmaPlot, Systat Software, ver. 11, San Jose, CA, USA) was fit to the data for illustration purposes (Fig. 2).
Figure 2.
Total dose of primaquine versus % cure.
From this representation of the data, there is a wide range of ‘spontaneous’ cures when no primaquine is given. Although there is a fair amount of scatter of response at lower total doses, the upper asymptote of the Emax model begins to plateau at the total dose associated with 15 mg/day for 14 days. The scatter around that dose is still fairly wide, with tighter responses around the higher doses. The model also predicts that no dosing regimen overall consistently gives a complete cure, although the probability is greater with the higher total doses tested. Although patient compliance may account for this phenomenon to some degree, pharmacogenetic polymorphisms may also account for this variability.
In a recent experimental P. vivax challenge study to assess the efficacy of a novel vaccine in development, volunteers were treated with rescue chloroquine and primaquine when they became patently parasitemic.32 Unexpectedly, several volunteers relapsed despite adequate treatment with primaquine. Subsequent investigations indicated that CYP2D6 polymorphisms may have been responsible for the lack of expected efficacy. That primaquine may indeed be a prodrug requiring metabolic activation has been further corroborated in animal studies utilising CYP2D knockout mice infected with Plasmodium berghei (P. berghei).33 Further laboratory assays with CYP2D6 isoenzymes have confirmed that primaquine metabolic conversion to hydroxylated metabolites is stereospecific and a function of CYP2D6 enzyme activity.34 Whether the anti-hypnozoite effects of the active metabolite(s) can be separated from the haemolytic effects is an area of active investigation.35,36
If CYP2D6 is necessary for metabolic activation, then efficacy (% cure) is subject to genetic polymorphisms. Individuals who are poor metabolizers may still be at risk for relapse. Similarly, individuals who are intermediate metabolizers may require a high dose of primaquine to overcome the decreased metabolic conversion of parent to active metabolite(s). The consequences of ultra-metabolizer status is unknown but should be considered in assessments of both efficacy and safety. Since CYP2D6 metabolizer status varies significantly by ethnic group, it is probable that regional differences in CYP2D6 polymorphisms may account, in part, for regional differences in dose–response, though full genotypic and phenotypic analyses are required to fully understand the situation as well as understanding the contribution of other enzymes to the active metabolites.
Next-Generation 8-Aminoquinolines
Primaquine's activity on hypnozoites and resulting effects on blocking relapse have led international teams to deliver improved back-up compounds that overcome primaquine's liabilities – 14 days of dosing and the risk of anaemia in G6PD deficient patients.
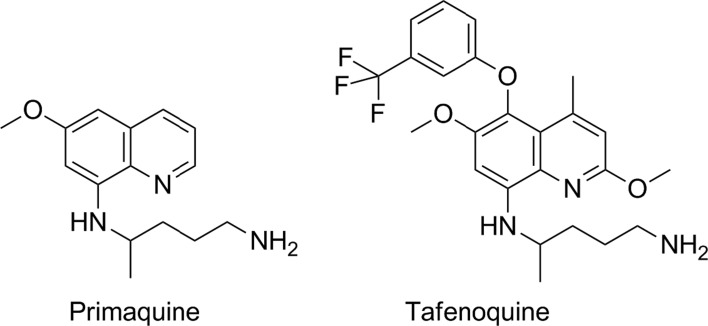
The second-generation 8-aminoquinoline is tafenoquine, also known as WR238605 or etaquine (Fig. 3). Tafenoquine is an 8-aminoquinoline but critically has a 5-substituted phenol ether. This functional group is inert to metabolism itself and protects the 5-position of the quinoline core from Phase I oxidation.37 Interestingly, since the 5-hydroxy and related metabolic products of primaquine are thought to be responsible for efficacy, it is important to note that no such direct reactive substructures can be formed with tafenoquine (given the 5-phenylether), and the metabolic stability of the compound is dramatically improved over primaquine. In human beings, tafenoquine demonstrates an elimination half-life of 14 days and in a Phase II anti-relapse clinical study showed equal or better anti-relapse efficacy from a single 300 mg dose than 14 days of 15 mg primaquine.38 The compound is in Phase III clinical trials with GSK and Medicines for Malaria Ventures (MMV) and remains the next-generation 8-aminoquinoline, overcoming all compliance issues with a single-dose therapy. Finally, the pharmacokinetic profile of tafenoquine is such that a future triple combination with other potential single-dose combinations currently in clinical studies could potentially yield the first single exposure radical cure and prophylaxis or SERCAP.39
Figure 3.
The structures of leading 8-aminoquinolines: primaquine, tafenoquine.
Researchers at Walter Reed Army Institute of Research (WRAIR) were able to discover tafenoquine – a compound with substantial improvements in pharmacokinetics and efficacy over primaquine21 – however, despite the structural change that blocks 5-hydroxylation and an inability to form potential quinone imines analogous to primaquine, tafenoquine, like primaquine, has a risk of haemolytic anaemia in certain low active G6PD patients. This means that it is likely to be used in concert with a diagnostic test to screen for patient G6PD deficiency. Clearly, given this risk, the drug is likely to be contraindicated in pregnancy because of a possible intravascular haemolytic risk in the mother and the foetus.40
Despite the low metabolic turnover of tafenoquine and an inability to form a 5, 8-para iminoquinone, the burden of evidence is that a tafenoquine metabolite is responsible for efficacy. First, activity in in vitro parasite assays in which metabolism is absent, demonstrates moderate to low potency of tafenoquine (and other 8-aminoquinolines) both in terms of asexual blood as well as liver stages (such as P. berghei or Plasmodium yoelii (P. yoelii) infected HepG2 cells).41 However, when tafenoquine is tested in cell assays with Phase I metabolism, such as the in vitro Plasmodium cynomolgi (P. cynomolgi) infected primary rhesus hepatocyte assay, the compound, like primaquine, is active.42 Second, excellent evidence for activity through metabolism, at least in P. berghei-infected mice on liver schizonts, has been demonstrated elegantly by a group from WRAIR in which, similar to primaquine, tafenoquine was tested in a mouse CYP2D knockout model CYP.43,44 Tafenoquine showed excellent efficacy in the wild type but not in the knockout and the sensitivity of the drug was rescued, almost totally, with high-dose tafenoquine in the knockout/knock-in model. Consequently, it is clear that metabolites of tafenoquine are responsible for prophylaxis in the mice and, if this relates to anti-relapse efficacy in human beings, there is a potential that CYP2D or other CYP polymorphisms in patient populations might affect efficacy outcomes. Clinical studies would be required to assess this.
Tafenoquine is reported to undergo biliary excretion and C-oxidation on the 8-amino alkyl chain.45 Furthermore, whilst amino-phenolic metabolites have been reported, the overall metabolic position remains unclear.37 Assuming that an iminoquinone is necessary for activity, a possible mechanistic route that could explain this could involve O-dealkylation of the 6- and 2-methoxy groups and oxidation to give an extended highly reactive intermediate, though this is unproven.46 Either way, the clinical data showing long half-life and duration of efficacy coupled with the suggestion of active metabolites implies that a small amount of an active component is generated in vivo and is released over time from the long-lasting parent (akin to a depot formulation). Such an intermediate would be reactive and short-lived as well as toxic to the parasites.
Understanding the features necessary for efficacy in the 8-aminoquinolines is the first step; however, the ultimate goal is to also understand the features necessary for the haemolytic risk in G6PD deficient patients too and thus determine whether a new 8-aminoquinoline can be synthesised bearing sufficient efficacy without haematological toxicity.
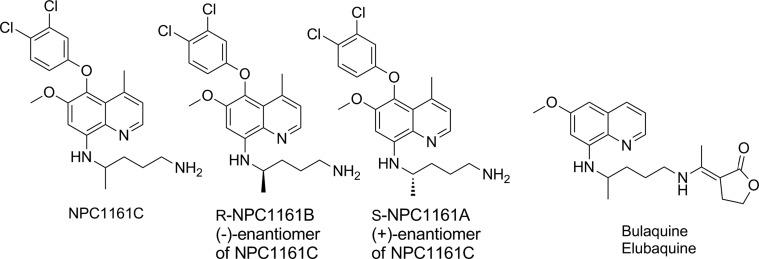
The most direct programme to improve on primaquine led to the delivery of bulaquine – an exo-butenolide enamine analogue of primaquine.47 The compound has undergone clinical studies and is marketed in India, however, additional PK and efficacy studies are required to demonstrate superior safety in G6PD deficient patients48; from a medicinal chemistry perspective, bulaquine is likely to be a prodrug of primaquine. NPC-1161B is a next-generation 8-aminoquinoline, similar to the tafenoquine core that has been profiled extensively in the hope of separating toxicity from efficacy (Fig. 4).49 Structurally, the molecule retains the metabolic block at C-5 but removal of the 2-OMe group (when compared with tafenoquine). Furthermore, studies on the enantiomers (in line with studies on the enantiomers of other 8-aminoquinolines) have suggested further improvements by using a single stereoisomer at the 8-position. As such NPC-1161B, the ( − ) or R enantiomer shows improved efficacy to tafenoquine in most in vitro cell assays as well as in the P. berghei sporozoite prophylaxis model.50 The metabolism of NPC-1161B is poorly understood and whilst, without oxidation through enzymes such as aldehyde oxidase, similar metabolites to tafenoquine cannot obviously be formed, more data are required to fully understand what species are present and responsible for efficacy. Full data for primaquine, tafenoquine and NPC-1161B are shown in Table 1.
Figure 4.
Structures of NPC1161C and enantiomers B and C, and bulaquine.
Table 1. Key in vitro and in vivo data for primaquine, tafenoquine and NPC-1161B.
| Compound | Pf EC50 NF54 (nM)/Py or Pb HepG2 (nM)41,51,52 | Pc rhesus hepatocytes (hypnozoites/schizonts)% inhibition at 10 μM42 | Pb in vivo prophylaxis mouse model ED100 (mg/kg) based on dosing day − 1, 0 and 144 | Pc in vivo radical cure rhesus model – oral dose giving radical cure in combination with chloroquine53 |
| Primaquine | 1191/9000 (Pb) | >90%/>90% | 20 mg/kg | 7 doses primaquine q.d. p.o. 0.6 mg/kg and 10 mg/kg chloroquine |
| Tafenoquine | 631/30 000 (Pb) | 20–70%/>90% | 3 mg/kg | 3 doses tafenoquine q.d. p.o. 0.6 mg/kg and 16 mg/kg chloroquine |
| NPC-1161B | 419/720 (Py) | >90%/>90% | 1 mg/kg | No published data |
Pf: P. falciparum; Pv: P. vivax; Pb: P. berghei.
Despite the promise associated with NPC-1161B, whether or not it can be developed further will depend on its differentiation regarding the risk of anaemia in G6PD deficient patients when compared to primaquine or tafenoquine.
Many other 8-aminoquinolines have been synthesised and tested, however, without robust validation in in vitro liver stage assays and data to confirm a reduced risk of anaemia in G6PD deficient patients, the task of conducting a logical optimisation project is severe. If assays are delivered that easily allow for a demonstration and separation of efficacy from toxicity then perhaps future 8-aminoquinoline radical curative agents will be forthcoming.
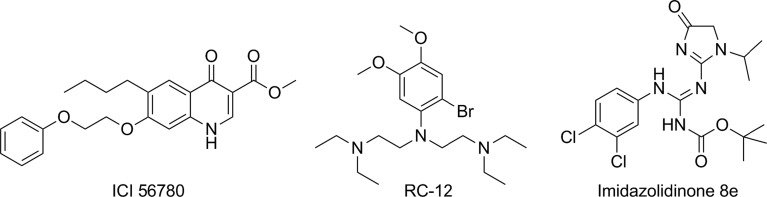
Very few other chemotypes have been reported to have activity on hypnozoites in the literature. Schmidt54 and Puri and Dutta55 have published data showing that the quinolones ICI 56780 (also known as WR197236) and WR194905 are active in the in vivo P. cynomolgi rhesus model. Whilst these compounds do have liver schizonticidal activity, no evidence of activity on hypnozoites in vitro has been published yet and the interpretation of the radical curative activity is confounded by the potent asexual liver and blood-stage activity of the series such that the relapse inhibition could conceivably be a result of posttreatment prophylaxis from an intramuscular dose of compound and leaching out from a ‘depot’ over time. The physical properties of such quinolones also present a major development challenge, so it is unlikely that such series will be developed further unless definitive anti-hypnozoite activity is determined along with acceptable drug-like solubility and oral bioavailability.
A second chemical class with modest anti-relapse activity in the P. cynomolgi rhesus model is RC-12.56 RC-12 is an electron rich ring with amine side chains; interestingly, an overlay with primaquine (Fig. 5) is apparent and putative metabolites of RC-12 can theoretically form iminoquinones. RC-12 was progressed to human studies and failed to show efficacy for prophylaxis or radical cure; this could be, in part because of the weaker activity, but also because of differential metabolism between primates and human beings – such that the active metabolites released in rhesus were inaccessible in human being – further data would be required to support this hypothesis.57
Figure 5.
Structures of quinolones, RC-12 and imidazolidinone – 8e.
A third series are the imidazolidinone derivatives from WRAIR.58 This series was identified from in vivo screening and demonstrated activity in the P. cynomolgi rhesus radical curative model. On oral dosing of 7 × 50 mg/kg, compound 8e from the paper (Fig. 5) demonstrated curative activity in one animal and a delay of relapse of 28 days in a second. The nature of the molecule delivering killing (e.g. authors postulate oxidised and cyclised possibilities) and the ease with which high oral bioavailability can be achieved are factors that will need clarification before a clinical candidate is likely to be forthcoming.
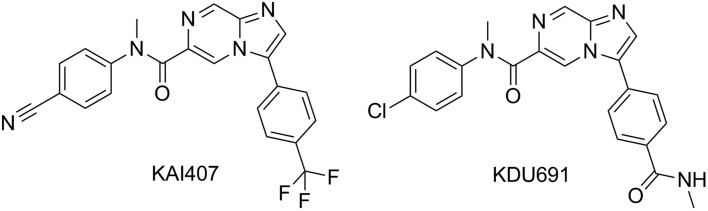
Recently, with the generation of the in vitro P. cynomolgi assay where sporozoites infect primary rhesus hepatocytes, considerable screening has been performed to find new series.59 Apart from confirming the activity of known positive controls (as shown above), a novel mechanistic class has been identified – Plasmodium PI4K inhibitors. Researches from Novartis Institute for Tropical Disease in collaboration with the Genomics Institute of the Novartis Foundation, Biomedical Primate Research Centre, Swiss Tropical and Public Health Institute and MMV have identified an imidazopyrazine series, exemplified by KAI407 and KDU691, that selectively inhibits Plasmodium PI4K and shows inhibition of not only liver schizonts, in the P. cynomolgiin vitro assay, but also inhibition of the development of hypnozoite (Fig. 6).42,60 This is a major breakthrough and is the first group of compounds to show potential for true prophylaxis of malaria species, including those that form hypnozoites, since primaquine and tafenoquine. Careful studies with the frontrunners, KAI407 and KDU691, on the mature hypnozoites will be necessary to confirm whether these have hypnozoiticidal potential and the ability to block relapse. Indeed, the current format of the P. cynomolgi assay, where compounds are added within the testing plate at the same time as sporozoites,42,60 screen for compounds acting on the development of sporozoites into either schizonts or hypnozoites (chemo-preventive) and not for compounds active on already formed hypnozoites. This will be an important point that will need to be carefully addressed in the development of new in vitro liver stage assays since preventing the formation of hypnozoites is not the same as killing an established hypnozoite. Indeed, there is evidence reviewed elsewhere that killing the youngest hepatic stage parasites requires only a single 30 mg dose of primaquine given within 48 hours of sporozoite inoculation, whereas 210–420 mg primaquine is required to kill an established hypnozoite.61,62
Figure 6.
Structures of KAI407 and optimised KDU691.
The paucity of available treatments to cure and kill all P. vivax parasites infecting patients can be explained by two main factors: (1) the complex biology of the parasite compared to the other Plasmodium strains, and in particular its ability to form liver dormant forms, called hypnozoites, that can be re-activated weeks or years later leading to a new episode of malaria in the absence of a mosquito bite; and (2) the lack of industrialised and low cost drug discovery in vitro assays that recapitulate this biology and which would allow for the screening of large chemical libraries directly on the human parasite, especially hypnozoites, to deliver compounds that block relapse (TCP3a as defined in Refs. 39,46).
The current strategy favoured by MMV, relies principally on the use of surrogate assays, which are already available to the community and brought to a level of miniaturisation and throughput that are compatible with the support of drug discovery programmes targetting malaria eradication.63 Optimism in the outcome of such strategy must nevertheless be tempered due to the obvious caveat that it focuses on assays that involve neither the human parasite nor human host tissue. Compounds pre-selected based on their activity against the asexual blood stage of the parasite or large unbiased chemical libraries can be directly screened on the liver stage using an assay that is based on the development of P. berghei or P. yoelii liver schizonts within a human hepatoma cell line.51 This high-throughput assay, although not directly relevant for finding compounds that will kill hypnozoites, pragmatically demonstrates that a compound can kill liver stage parasites (albeit rodent schizonts) and is used as a filter to narrow-down the number of compounds which will enter the next phase of the screening cascade. It is important to note, however, that all compounds shown so far to kill hypnozoites are also active on the liver developing schizonts and whilst compounds selective for mature hypnozoites might be missed, dual acting compounds such as the current radical cure standard of care, primaquine, will be identified. This reduced panel of compounds is then tested in the next key assay of the screening cascade which uses the surrogate Plasmodium strain phylogenetically most closely related to P. vivax, namely P. cynomolgi (Fig. 7). This Plasmodium species produces both large and small exoerythrocytic forms in the liver of its host (rhesus monkeys) as well as in primary rhesus hepatocytes in vitro. These forms have now been validated as schizonts and hypnozoites, respectively.64,65 The potential of the compounds identified as positives in the rodent liver stage assay as radical cure agents is measured by recording their cidal activity on mature P. cynomolgi hypnozoites. The proof of concept in vivo for the anti-hypnozoiticidal potential of a preclinical molecule is obtained if a molecule demonstrates anti-relapse activity (out to 100 days) in the gold standard preclinical model, the P. cynomolgi-infected rhesus model.66 The ultimate validation will finally take place in human clinical trials after a full preclinical safety and pharmacokinetic package has been obtained. The key radical cure human models in this case are a Phase II clinical study in patients with confirmed P. vivax monoinfection and with a primary endpoint measured as the percentage of relapse-free efficacy at 6 months post initial dose similar to that used in the tafenoquine DETECTIVE trial38 and/or a Phase II ‘out of transmission’ model where Indonesian soldiers who have spent up to 12 months in P. vivax endemic areas (Papua) are returned to their malaria-free base (Java) and given new anti-relapse agents and followed-up over the course of 12 months.67 It is important to note that because all radical cure agents available on the market or in late phase development bear some risks of haemolysis in G6PD deficient patients, every preclinical candidate will therefore be evaluated for its safety profile in the recently developed and validated human (hu) RBCs-SCID mouse model using blood from volunteers with low active G6PD.68
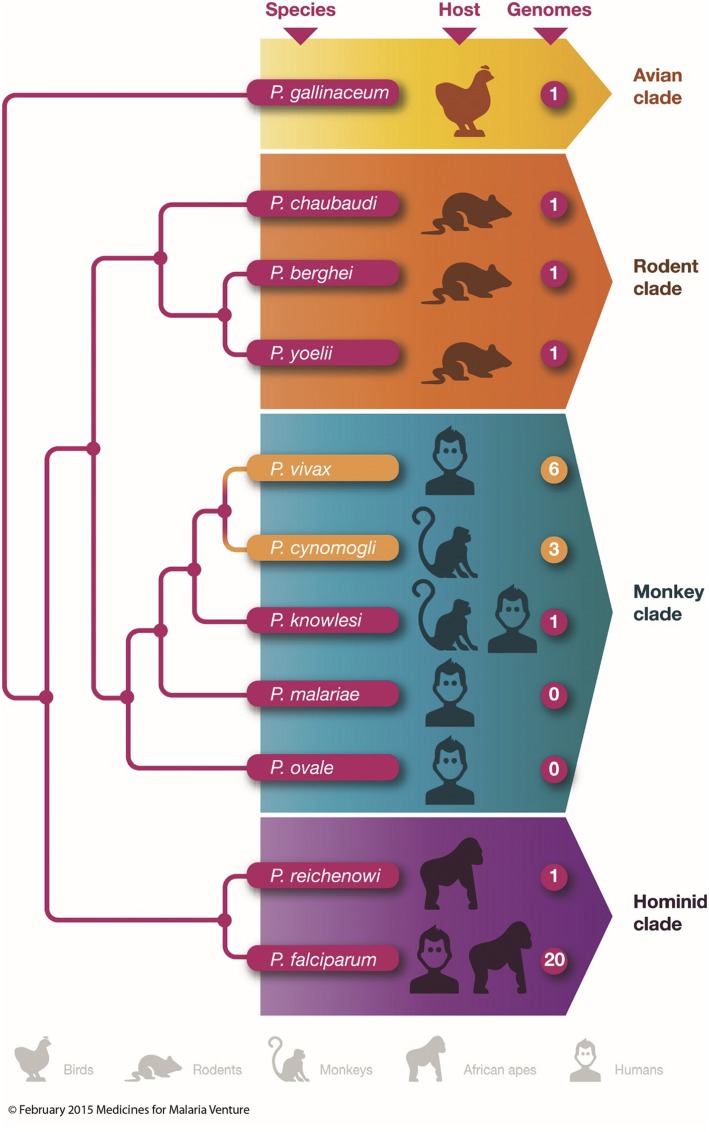
Figure 7.
Phylogenetic tree of Plasmodium spp causing malaria in different species adapted from Hall.72 Branches are not to scale but represent how closely related each species is with each other. The shorter the branch, the closer the genome of each Plasmodium spp. Each plasmodia species is associated with its natural vertebrate host and the number of assembled whole-genome sequences that are publically available, either in public databases or through genome centres is also labelled as described by Hall.
While the current screening cascade is not optimal as it uses many surrogate assays, it has the advantage that it can be used immediately and can also serve to identify compounds with potential for chemoprophylaxis (TCP4 as defined in Ref. 39). TCP4 compounds bearing an extremely well-tolerated profile will be highly desirable to protect vulnerable populations such as pregnant women, children and field workers in remote and high transmission areas. MMV and The Bill and Melinda Gates Foundation (BMGF) are currently collaborating to find solutions to the key issues currently standing in the way of a robust, affordable and validated high-throughput drug discovery assay to discover novel anti-relapse reagents in the same manner as phenotypic asexual blood-stage (ABS) assays have been used to identify new ABS inhibitors.69,70 Establishing such liver stage in vitro assays will also permit the identification of compounds, which could re-activate hypnozoites into schizonts.71 Re-activated hypnozoites could then be eliminated using schizonticides that are currently available. This approach could represent an alternative to hypnozoiticidal compounds and is a justifiable drug discovery strategy. Compounds with such characteristics have recently been identified by Dembélé et al. using their P. cynomolgi assay.65
The major road blocks for the development of high throughput in vitro P. vivax assays are: (i) stable and infectable hepatocyte cell lines that are permissive to P. vivax, or alternatively, access to a reliable source of primary human hepatocytes, (ii) sustainable access to a large amount of viable sporozoites with high infectivity, (iii) specific biomarkers of hypnozoites to enable identification and quantification in the host cells, and (iv) anti-relapse agents with desirable pharmacological profiles that do not require metabolism for their activity in order to act as positive controls to validate new in vitro P. vivax assays. In addition, the assay needs to be low cost so as to enable large-scale screening.
Strategy to overcome identified challenges
Infectable hepatocytes
Several previous studies have demonstrated that P. vivax sporozoites are permissive to both primary human hepatocytes as well as hepatoma cell lines (such as HepG2 and HC-04 cells) resulting in the full development of P. vivax liver stages and the observation of large developing schizonts and small non-developing hypnozoites in vitro.73–76 This plethora of in vitro studies is encouraging; however, several issues remain to be solved. Despite the availability of freshly isolated and cryopreserved primary hepatocytes from a variety of sources, they are in limited supply, do not proliferate in vitro and are highly variable in their ability to support infection of P. vivax sporozoites with a very low infection rate observed on average (∼0.1%). A human hepatocyte cell line (HC-04) that allows for complete P. vivax liver stage development has also been reported, but despite the advantages of solving the supply and variability issues associated with primary hepatocytes, the rate of infection still remains low: 0.1%.75 Furthermore, maintenance of hepatocytes in culture for 5–21 days, which would be needed to observe complete liver stage development and potential hypnozoite reactivation, is difficult as culture conditions are often not commensurate with extended periods of time due to overgrowth and loss of the monolayer. Major progress in this area has been accomplished in recent months by two groups each of them using different approaches. The first group uses a microscale human liver platform composed of cryopreserved, micropatterned cocultures (MPCC) of human primary hepatocytes surrounded by supportive stromal cells.76 The MPCC platform can maintain individual patient hepatocytes between 4 and 6 weeks and has demonstrated the ability to support the liver stages of P. falciparum and P. vivax parasites in a medium-throughput format (96-well plate assay). This offers promise on the way to a high-throughput assay amenable for drug screening. While the ability to maintain long-term culture of primary hepatocytes represents a major improvement in the search for the ideal P. vivax assay, this assay still uses primary hepatocytes and therefore the caveats associated with this, such as limited cell availability, lot-to-lot variability issues, use of a co-culture that increases the potential technical difficulty in performing in vitro culture and a rate of infection that still remains low (∼0.1%). An alternative system that is currently being assessed for its potential as a source of good infectable hepatocytes is based on the design of a microfluidic bilayer device (MBD) featuring two microfluidic channels separated by a polydimethylsiloxane (PDMS) membrane.77 Interestingly, the authors describe that static culture of a small number of either primary hepatocytes or human hepatoma cell lines (HepG2, HC-04 and FRF™-KO mouse-expanded human hepatocytes i.e. FHH) in this MBD setting is sufficient to maintain their phenotype for up to 3 weeks post-seeding, without the need for complex perfusion or co-culture. This simple system shows promise and if permissive to P. vivax infection, could represent an additional viable approach. Indeed, while having metabolising active cells such as primary hepatocytes is key for the activity of positive controls such as primaquine, this might be a major obstacle in the identification of primary hits as screening compounds are often metabolically unstable and are rapidly turned over by CYP-P450 enzymes present in primary hepatocytes. Therefore, a screen reliant on metabolically active primary hepatocytes carries the risk of generating a large number of false negatives. It is therefore important to continue developing new in vitro assays in which liver metabolism is substantially reduced such as with hepatoma cell lines (HC-04, HepG2 and FHH), where such risks will be limited. Some limitations, regarding the MBD system described earlier, will however have to be addressed before it can be deployed as a drug discovery assay. So far, the device only uses eight chambers, and the feasibility of producing a cheap and ready to use 96 or ideally 384-well device will be the major hurdle for such an assay to be used as a screening tool.
Source of sporozoites
From a practical point of view, access to a reliable and homogenous source of sporozoites in quantity sufficient to produce the biomass necessary for P. vivax drug screening assay constrains high-throughput cellular screening against hypnozoites. The major challenge is that sporozoites are produced in the salivary gland of mosquitoes fed on infected blood; however, there is currently no continuous in vitro blood-stage culture system for P. vivax, which could generate the number of clonal blood-stage parasites, including the key sexual gametes necessary for mosquito infection. In the absence of P. vivaxin vitro blood-stage culture, the community relies on harvesting sporozoites from mosquitoes directly fed on infected patient blood collected from the field. Despite this being laborious, there are groups in endemic countries that have access to large numbers of P. vivax infected patients and therefore blood from which sporozoites can be obtained for low to medium screening (see below). P. vivax cannot be maintained in vitro under the same conditions developed to cultivate P. falciparum.78 In addition, P. vivax parasites preferentially invade reticulocytes, which are difficult to obtain in a sufficient amount and concentration to establish long-term cultures (1–2% of circulating RBCs). After a century of research and despite the use of several different methods, only short-term cultures ( < 1 month) have been achieved so far, maintaining infection for a few P. vivax schizogony cycles only (for review, see Ref. 79). The preference for younger erythrocytes makes establishing continuous blood-stage culture of P. vivax particularly challenging as a continuous and reliable source of reticulocytes is required. Working with reticulocytes is technically difficult and labour intensive. Although it is theoretically possible to use whole adult human blood as a potential source, the concentration of reticulocytes is really low,79–82 and the short lifespan of young erythrocytes circulating in the peripheral blood (24 hours) makes it unreliable as a continuous source of cells for P. vivax culture. Three major sources of reticulocytes have been used thus far. As summarised by Udomsangpetch et al.,83 two sources of reticulocytes have been utilised in the past: hemochromatotic blood that can contain up to 7% reticulocytes84 and umbilical cord blood (UCB) collected at birth, which contains 1–5% reticulocytes.85 Both techniques have yielded promising results with cultivation periods ranging from continuous culture, but with low parasitaemia, with reticulocytes from hemochromatotic blood to 30–40 days with reticulocytes obtained from UCB. However, one major challenge is that these cultures require a constant re-supply of fresh reticulocytes and therefore access to large amounts of either cord blood or haemochromatosis patients is needed. This also introduces donor to donor variability in experimentation. A third source of reticulocytes for P. vivax culture is haematopoietic stem cells (HSCs) isolated from UCB or from embryonic sources (ESC). One advantage of HSCs is that the asynchronous maturation of the erythroid cells enables continuous production of fresh reticulocytes that have already been shown as suitable target cells for P. vivax invasion.86–89 Finally, latest recent report from Van et al.88 shows that CD34+-enriched populations of UCB produced the highest amount of reticulocytes, and these cells can be invaded by P. vivax. Van et al. also provide proof that P. vivax parasites have a preference for invading immature reticulocytes, which reduces further the panel of blood cells available for infection and therefore increases the technical difficulty of P. vivax blood-stage cultures. The cost, the technical difficulty, labour intensity and for some countries the need for special authorisations could make methods that rely on HSC-derived reticulocytes less accessible. In this regard, the results obtained by Noulin et al.87 showing that it is possible to cryopreserve P. vivax infected HSC-derived reticulocytes introduces the possibility of creating stocks of reticulocytes to use as target cells for the establishment of an in vitro culture of P. vivax. Some recent work focuses on the development of cell lines, either from erythroleukemia (for review, see Ref. 90) or immortalised HSC (for review, see Ref. 91) that may mature more reproducibly in vitro and generate reticulocytes. It is, however, still early days and despite interesting progress no major breakthroughs have been published.
Several groups are now exploring the increasing availability of humanised mice (for review, see Refs. 92,93) that produce human reticulocytes from engrafted stem cells or are injected with human red blood cells as models, for the maintenance, propagation and transmission of P. vivax isolates. The notion is that mice will be used as a ‘factory’ for the regular production of P. vivax gametocytes for standard membrane feeding assays (SMFA) or direct feeding assays to produce sporozoites. One promising model that has recently been published is the MI(S)TRG mice model (immunodeficient Rag2-/-Il2rg-/- mice), in which human versions of four genes encoding cytokines important for innate immune cell development and erythropoiesis are knocked in to their respective mouse loci (human M-CSF, human IL-3, human GM-CSF, human TPO and human SIRP1α).94,95 Those mice demonstrate a high efficiency of human haematopoietic engraftment (e.g. human cells almost completely replace mouse cells in the bone marrow) and support multiple lineages of human haematopoiesis including reticulocytes. While this system shows promise, one of the limitations is that survival of human RBCs in the peripheral circulation is suboptimal, as they are engulfed by mouse phagocytes. This problem will need to be addressed in the future by genetic strategies to support the development and survival of human RBC population in the periphery. If this is achieved, the next step will be to infect MI(S)TRG mice with P. vivax and examine their potential to develop a full asexual blood-stage infection with the production of gametocytes. Another promising humanised mice model described by Vaughan et al.96 uses immunocompromised and furamyl acetoacetate hydrolase-deficient mouse (FRG mouse97,98) backcrossed to the non-obese diabetic (NOD) background, which are then repopulated with human hepatocytes and human RBCs. These mice demonstrated full liver stage infection to blood-stage infection for P. falciparum. The team is currently working on reproducing this using P. vivax and, if successful, the model could provide unique opportunities for in vivo studies of P. vivax and the discovery of new drugs targetting schizonts and hypnozoites (less costly and less demanding in terms of quantity of compound needed compared with primate models). If the mice demonstrate presence of gametocytes in the peripheral blood, then the model would also provide another potential source to feed mosquitoes and later on produce P. vivax sporozoites. Finally, nonhuman primates have also been shown to be reliable models for human malaria, including for P. vivax. Indeed, a large number of P. vivax isolates has been adapted to grow in primate species such as Aotus and Saimiri monkeys (for review, see Ref. 99). These strains were shown to develop liver as well as blood stages producing circulating gametocytes that might be used for sporozoite production in mosquitoes. Several factors will need to be considered when using such models. There are only a few primate centres available worldwide for conducting malaria research using Aotus and Saimiri monkeys and fewer centres have expertise in mosquito feeding and cell culture, further narrowing down the potential institutions that might be used for this work. Another challenge with the Aotus and Saimiri model is that the monkeys become immune to Plasmodium and can therefore no longer be used repeatedly for subsequent infections, highlighting the fact that a large number of monkeys might be needed to support the regular production of sporozoites required for a high-throughput P. vivax liver stage in vitro assay. The associated cost and ethical approvals will also be challenging.
Interestingly, all the above strategies currently rely on generating P. vivax sporozoites at the same research site or close to research sites where hepatocyte tissue is cultured and new in vitro liver stage platforms for P. vivax have been developed. Another option is to transfer the in vitro culture assays directly to sites where sporozoites are generated from blood obtained from patients infected with P. vivax. The latter approach requires access to a regular and reliable source of infected patient blood, an insectary, and tools and technical capacity for membrane feeding of mosquitoes and their dissection to isolate P. vivax sporozoites. All these critical components need to reside in the same country, ideally in close geographic proximity, and the team responsible for liver stage assays will need to have the experience of managing scale up of sporozoite production for use in high-throughput screens. There are few scientific groups capable of gathering all such assets in the same region. For example, an insectary has been established in Iquitos, Peru, in collaboration with the University of Cayetano (Peru) and the University of California, San Diego (USA), and has successfully established for the first time in South America, an An. darlingi colony derived from wild-caught mosquitoes obtained in the northeastern Peruvian Amazon region of Iquitos that was confirmed for its P. vivax vectorial capacity.100 The feasibility of infecting in vitro liver hepatocyte culture with the sporozoites produced weekly in their insectary is currently being tested. One major challenge will be to continue improving sporozoite yields per mosquito, which is currently suboptimal (1000–6000 sporozoites per mosquito), in order to provide the necessary sporozoite biomass for a high-throughput screening assay. India also represents a country of interest, as there is the possibility to access large amounts of P. vivax infected patients each year (up to 90% of the population is at risk and >7% suffering from the disease in certain area of the country, Ref: http://worldmalariareport.org/andhttp://www.map.ox.ac.uk/). It is possible that all the infrastructure necessary for the regular generation of sporozoites might be established in India as several groups have experience and expertise in malaria.101 Moreover, the presence of pharmaceutical drug discovery expertise in the country is of interest as an industrial partner might facilitate assay setup, validation and screening. Thailand, where P. vivax sporozoite production is already in place102–105 and the number of vivax malaria cases is high (up to 52% of the population is at risk and >7% suffering from the disease in certain area of the country, Ref: http://worldmalariareport.org and http://www.map.ox.ac.uk/), is also a potential country for future drug screening campaigns. The advantage of running high-throughput screens in countries such as India and Thailand is that it taps into local expertise, promotes capacity building in endemic countries and provides access to local P. vivax strains and, thus, a better insight into the regional biology of the parasite. The downside of this strategy is that it relies on collection of field isolates with all the caveats associated to this: inter patient variability in infectiousness to mosquitoes, heterogeneity of P. vivax strains from batch to batch, considerable logistics involved in finding patients and the need of highly skilled technical staff for drawing blood and performing experimentation.
A major advance in access to large amount of P. vivax sporozoites would be the development of a reliable and effective method of sporozoite cryopreservation, building on the established work of Sanaria Inc. at Rockville, MD, USA.106,107 To date, the work recently published with cryopreserved versus fresh sporozoites using the MPCC platform76 has demonstrated that a wide range of infection rates were observed between cryopreserved batches and that fresh sporozoites achieved infection rates that were 7- to 13-fold higher than cryopreserved sporozoites.76 Moreover, only 25% of the cryopreserved sporozoites demonstrated good gliding motility and when added to 10 000 patterned hepatocytes, resulted in only a 3% positive capacity to traverse cells. This relatively low percentage of viable sporozoites means that large numbers of fresh sporozoites per vial will need to be cryopreserved. It is important to note that, despite the low number of viable sporozoites, the sporozoites that infect hepatocytes lead to full parasite development and maturity resulting in ultimate release of infective merozoites.74 This underscores the fact that cryopreservation is achievable, but work remains to be done to improve cryopreservation efficiency before such methods can be used to support a P. vivax liver stage drug screening platform. A path currently followed by MMV and BMGF is to link experts in malaria with expert cryobiologists with experience in cryopreservation of mammalian cells and tissues. Low temperature banking has played a pivotal role, first for gametes and later for embryos and immature germ cells, and the development of optimised cryopreservation techniques in this field has taken more than 50 years.108,109 There is information and expertise from the area of cryobiology, in particular the recent progress in sperm (another mobile and fragile cellular organism) and oocyte cryopreservation (for review, see Refs. 110,111) that will likely benefit the malaria community.
Identification of hypnozoites biomarkers
To date, no markers for hypnozoites have been identified, and the current techniques for detection of hypnozoites rely on parasite surface protein staining [for example using anti-circumsporozoite protein (CSP) monoclonal antibodies that are commercially available73,75,76,112,113 and distinctive morphology of the small exoerythrocytic forms]. Pharmacological validation using primaquine and tafenoquine, the only drugs that have been shown to block P. vivax relapse in man, are often used to confirm the presence of hypnozoites in P. vivax culture systems and animal models. However, in vitro primaquine and tafenoquine require metabolic activation to release the liver stage active components and so are poor positive controls in non-metabolising cell cultures. The identification of specific markers for hypnozoites is key and the use of global approaches such as metabolomics114,115 and transcriptomics,116 which have already been used for other purposes in the field of malaria will be helpful. Further, the development of transgenic fluorescent P. cynomolgi59 and P. vivax parasites stably expressing fluorescent markers with the possibility to identify and isolate the different forms using imaging or fluorescence-activated cell sorting (FACS) will allow the generation of pure populations of hypnozoites. The method will aid the development of metabolomic and transcriptomic approaches and will undoubtedly lead to the identification of novel liver stage targets for drug discovery efforts and biomarkers of dormant liver forms. While the community waits for hypnozoite biomarkers to become available, the use of strains such as the long-latency Korean strain of P. vivax isolate, which mainly forms hypnozoites in the liver,74 might be desirable in phenotypic screens seeking a radical cure agent on a naturally produced pure population of hypnozoites.
Conclusion
The malaria elimination and eradication agenda requires the discovery of novel P. vivax anti-relapse medicines as the existing tools are insufficient to the task. The only approved drug that is effective against hypnozoites is primaquine. However, it suffers from several liabilities such as compliance due to long treatment courses (14 days), severe haemolytic toxicity in G6PD deficient patients and contraindication in pregnant women or significant gastrointestinal disturbance limiting its use in the clinic. The only one other drug to kill P. vivax liver stages, tafenoquine, is currently in clinical development and despite major improvement in the dosing regimen, reducing it from 2 weeks to one single-dose administration, its safety profile in particular versus G6PD deficient patients remains similar to primaquine. There remains, therefore, a major need to deliver new and better tolerated medicines to eradicate P. vivax malaria. To date, to the best of our knowledge, there are no institutions or pharmaceutical companies currently screening for hypnozoiticidal chemical entities using a P. vivax liver in vitro stage assay with a direct readout on hypnozoites. It is interesting to note that until recently P. vivax has been the truly neglected parasite species within the global malaria community. This was due, in part, because the burden of disease was still poorly understood but more importantly because this malaria was still, incorrectly, considered as benign by the community. This neglect reflects the number of novel anti-relapse compounds under development. Only two out of 37 regulatory preclinical and clinical development projects in the global antimalarial pipeline are currently specific to P. vivax relapse prevention. This is insufficient, in particular, since the time taken to develop new medicines from discovery to final registration is likely to be greater than 10 years. Therefore, the discovery molecules of today are the new medicines of the mid-2020s at the earliest. Moreover, attrition rates in drug discovery and development will make the challenge even greater as several hundreds of hits will have to be found in order to produce one well-tolerated medicine to treat those populations in need. The focus from the malaria community as well as funding bodies and drug discovery and product development partnerships such as the BMGF or MMV, is now strongly shifting to strengthen the P. vivax pipeline by providing in vitro as well as in vivo assays fit for screening large libraries of chemical compounds and discovering the next generation of anti-hypnozoite agents. As described in this review, emerging good quality and exciting tools have now demonstrated the proof of concept that such robust assays could be developed. It is now time for academic researchers, industrial partners, non-governmental agencies, foundations and funding agencies to team up together for the extra ‘push’ that will allow the scale-up and industrialisation of these assays necessary for the screening of large small molecule libraries and subsequent drug discovery and development. The goal is clear – to discover, develop and deliver the next generation of well-tolerated and efficacious medicines for the treatment of patients suffering from vivax malaria that will, ultimately, enable and contribute to the eradication of malaria.
Acknowledgements
The authors would like to thank Didier Leroy, Stephan Duparc and Tim Wells for helpful discussions and comments on the manuscript. In addition, Pierre Chassany and Elizabeth Poll are thanked for their invaluable contributions to the figure describing the liver stage life cycle and the phylogenetic tree. Finally, the authors wish to thank all the partners of MMV and The Bill and Melinda Gates Foundation who are collaborating to tackle vivax malaria and have contributed to this review by their work.
Disclaimer Statements
Contributors BC is a director and JNB is a vice president and head of the drug discovery group, and both are part of the discovery team at MMV and have long experience in developing drugs both for neglected disease such as malaria as well as many other disease areas. OV is a program officer at the BMGF and has extensive experience in developing assays to support several neglected disease areas such as malaria and Tuberculosis. Finally, DLW is vice president clinical pharmacology at Great Lakes Drug Development / Certara and a consultant for integrated development at BMGF. DLW has several years' experience within the drug discovery industry. All authors contributed equally to the manuscript and will act as guarantors.
Funding The Bill and Melinda Gates Foundation.
Conflicts of interest The authors declare that they have no conflicts of interest.
Ethics approval Not applicable.
References
- 1.Molyneux ME. History of discoveries, malaria. Encycl Malar. 2014:1–10. [Google Scholar]
- 2.Grassi B, Feletti R. Sui parassiti della malaria. Rif Med. 1890;6:62–4. [Google Scholar]
- 3.Thayer W. Lectures on the malarial fevers. New York: Appleton; 1897. [Google Scholar]
- 4.Rodrigues T, Prudêncio M, Moreira R, Mota MM, Lopes F. Targeting the liver stage of malaria parasites: a yet unmet goal. J Med Chem [Internet] 2011;55((3)):995–1012. doi: 10.1021/jm201095h. [cited 2012 Jul 18]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22122518. [DOI] [PubMed] [Google Scholar]
- 5.Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E et al. Gametocyte dynamics and the role of drugs in reducing the transmission potential of Plasmodium vivax. J Infect Dis [Internet] 2013;208((5)):801–812. doi: 10.1093/infdis/jit261. [cited 2013 Aug 26]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3733516&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markus MB. Malaria: origin of the term “Hypnozoite”. J Hist Biol [Internet] 2010 doi: 10.1007/s10739-010-9239-3. [cited 2011 Nov 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20665090. [DOI] [PubMed] [Google Scholar]
- 7.Mueller Y, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale – the “bashful” malaria parasites. Trends Parasitol. 2007;23((6)):278–83. doi: 10.1016/j.pt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microbiol Rev. 2005;18((3)):570. doi: 10.1128/CMR.18.3.570-581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird JK. Malaria caused by Plasmodium vivax: recurrent, difficult to treat, disabling, and threatening to life – averting the infectious bite preempts these hazards. Pathog Glob Health [Internet] 2013;107:475–479. doi: 10.1179/2047772413Z.000000000179. Available from: http://www.scopus.com/inward/record.url?eid = 2-s2.0-84892713548&partnerID = 40&md5 = 0b41d85f92844b820c1ed375d88dc214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ et al. The anaemia of Plasmodium vivax malaria. Malar J [Internet] 2012;11((1)):135. doi: 10.1186/1475-2875-11-135. [cited 2012 May 8]. Available from: http://www.malariajournal.com/content/11/1/135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis [Internet] Adv Parasitol. doi: 10.1016/B978-0-12-397900-1.00003-7. Elsevier; 2012. Available from: http://dx.doi.org/10.1016/B978-0-12-397900-1.00003-7. [DOI] [PubMed] [Google Scholar]
- 12.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg [Internet] 2007;77((6 Suppl)):79–87. [PMC free article] [PubMed] [Google Scholar]
- 13.Battle KE, Gething PW, Elyazar IRF, Moyes CL, Sinka ME, Howes RE et al. The global public health significance of Plasmodium vivax [Internet] Adv Parasitol. doi: 10.1016/B978-0-12-397900-1.00001-3. Elsevier; 2012. [cited 2013 Feb 22]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3548406&tool = pmcentrez&rendertype = abstract. [DOI] [PubMed] [Google Scholar]
- 14.Baird JK. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev [Internet] 2013;26((1)):36–57. doi: 10.1128/CMR.00074-12. [cited 2013 May 22]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23297258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64((1–2 Suppl)):97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 16.Ménard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A. 2010;107((13)):5967–71. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis [Internet] 2014;3099((14)):1–10. doi: 10.1016/S1473-3099(14)70855-2. Price et al. Open Access article distributed under the terms of CC-BY; [cited 2014 Sep 9]. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1473309914708552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebringer A, Heathcote G, Baker J, Waller M, Shanks GD, Edstein MD. Evaluation of the safety and tolerability of a short higher-dose primaquine regimen for presumptive anti-relapse therapy in healthy subjects. Trans R Soc Trop Med Hyg. 2011;105:568–73. doi: 10.1016/j.trstmh.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Guidelines for the treatment of malaria [Internet] 2nd edn. WHO; 2010. p. 197. Available from: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf?ua = 1. [PubMed] [Google Scholar]
- 20.John GK, Douglas NM, von Seidlein L, Nosten F, Baird KJ, White NJ et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J [Internet] 2012;11((1)):280. doi: 10.1186/1475-2875-11-280. [cited 2012 Aug 20]. Available from: http://www.malariajournal.com/content/11/1/280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. Am J Trop Med Hyg [Internet] 1998;58((5)):645–9. doi: 10.4269/ajtmh.1998.58.645. [DOI] [PubMed] [Google Scholar]
- 22.Fryauff DJ, Baird JK, Basri H, Sumawinata I, Purnomo P, Richie TL et al. Randomised placebo-controlled trial of primaquine for prophylaxis of falciparum and vivax malaria. Lancet. 1995;346:1190–3. doi: 10.1016/s0140-6736(95)92898-7. [DOI] [PubMed] [Google Scholar]
- 23.Baird JK, Fryauff DJ, Basri H, Bangs MJ, Subianto B, Wiady I et al. Primaquine for prophylaxis against malaria among nonimmune transmigrants in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1995;52:479–84. doi: 10.4269/ajtmh.1995.52.479. [DOI] [PubMed] [Google Scholar]
- 24.Weiss WR, Oloo AJ, Johnson A, Koech D, Hoffman SL. Daily primaquine is effective for prophylaxis against falciparum malaria in Kenya: comparison with mefloquine, doxycycline, and chloroquine plus proguanil. J Infect Dis. 1995;171((6)):1569–75. doi: 10.1093/infdis/171.6.1569. [DOI] [PubMed] [Google Scholar]
- 25.Clayman CB, Arnold J, Hockwald RS, Yount EH, Edgecomb JH, Alving AS. Toxicity of primaquine in caucasians. J Am Med Assoc. 1952;149((17)):1563–8. doi: 10.1001/jama.1952.72930340022010b. [DOI] [PubMed] [Google Scholar]
- 26.Shanks GD, Kain KC, Keystone JS. Malaria chemoprophylaxis in the age of drug resistance. II. Drugs that may be available in the future. Clin Infect Dis. 2001;33:381–5. doi: 10.1086/321866. [DOI] [PubMed] [Google Scholar]
- 27.Ganesan S, Tekwani BL, Sahu R, Tripathi LM, Walker LA. Cytochrome P(450)-dependent toxic effects of primaquine on human erythrocytes. Toxicol Appl Pharmacol [Internet] doi: 10.1016/j.taap.2009.07.012. Elsevier B.V.; 2009 [cited 2011 Jun 21];241(1):14–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19616568. [DOI] [PubMed] [Google Scholar]
- 28.Salvidio E, Pannacciulli I, Tizianello A, Ajmar F. Nature of hemolytic crises and the fate of G6PD deficient, drug-damaged erythrocytes in Sardinians. N Engl J Med. 1967;276:1339–44. doi: 10.1056/NEJM196706152762402. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher KA, Barton PF, Kelly JA. Studies on the mechanisms of oxidation in the erythrocyte by metabolites of primaquine. Biochem Pharmacol. 1988;37:2683–90. doi: 10.1016/0006-2952(88)90263-8. [DOI] [PubMed] [Google Scholar]
- 30.Ashley EA, Recht J, White NJ. Primaquine: the risks and the benefits. Malar J. 2014;13:418–24. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recht J, Ashley E, White N. Safety of 8-aminoquinoline antimalarial medicines. Geneva, Switzerland: 2014. World Health Organization. [Google Scholar]
- 32.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF et al. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med [Internet] 2013;369:1381–2. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 33.Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q et al. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J [Internet] 2013;12((1)):212. doi: 10.1186/1475-2875-12-212. [cited 2013 Aug 31]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3689079&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fasinu PS, Tekwani BL, Nanayakkara ND, Avula B, Herath HB, Wang Y-H et al. Enantioselective metabolism of primaquine by human CYP2D6. Malar J [Internet] 2014;13((1)):507. doi: 10.1186/1475-2875-13-507. [cited 2015 Jan 5]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25518709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem [Internet] 2009;44((3)):937–953. doi: 10.1016/j.ejmech.2008.08.011. Elsevier Masson SAS; [cited 2011 Jul 28]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18930565. [DOI] [PubMed] [Google Scholar]
- 36.Saunders D, Vanachayangkul P, Imerbsin R, Khemawoot P, Siripokasupkul R, Tekwani BL et al. Pharmacokinetics and pharmacodynamics of (+)-primaquine and ( − )-primaquine enantiomers in rhesus macaques (Macaca mulatta. Antimicrob Agents Chemother. 2014;58((12)):7283–91. doi: 10.1128/AAC.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crockett M, Kain KC. Tafenoquine: a promising new antimalarial agent. Expert Opin Investig Drugs. 2007;16:705–15. doi: 10.1517/13543784.16.5.705. [DOI] [PubMed] [Google Scholar]
- 38.Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet [Internet]. Elsevier Ltd; 2013 [cited 2013 Dec 19] 6736((9922)):1049–1058. doi: 10.1016/S0140-6736(13)62568-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24360369. [DOI] [PubMed] [Google Scholar]
- 39.Burrows JN, van Huijsduijnen RH, Möhrle JJ, Oeuvray C, Wells TNC, Hooft van Huijsduijnen R et al. Designing the next generation of medicines for malaria control and eradication. Malar J [Internet] 2013;12((187)):187. doi: 10.1186/1475-2875-12-187. [cited 2013 Jun 10]. Available from: http://www.malariajournal.com/content/12/1/187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clyde DF. Clinical problems associated with the use of primaquine as a tissue schizontocidal and gametocytocidal drug. Bull World Health Organ. 1981;59:391–5. [PMC free article] [PubMed] [Google Scholar]
- 41.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EAet al. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites PLoS Med [Internet] 201292)[cited 2012 Mar 16]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3283556&tool = pmcentrez&rendertype = abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeeman A-M, van Amsterdam SM, McNamara CW, Voorberg-van der Wel A, Klooster EJ, van den Berg A et al. KAI407, a potent non-8-aminoquinoline compound that kills Plasmodium cynomolgi early dormant liver stage parasites in vitro. Antimicrob Agents Chemother [Internet] 2014;58((3)):1586–1595. doi: 10.1128/AAC.01927-13. [cited 2014 Jul 14]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3957848&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q et al. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J [Internet] 2013;12((1)):212. doi: 10.1186/1475-2875-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcsisin SR, Sousa JC, Reichard GA, Caridha D, Zeng Q, Roncal N et al. Tafenoquine and NPC-1161B require CYP 2D metabolism for anti-malarial activity: implications for the 8-aminoquinoline class of anti-malarial compounds. Malar J [Internet] 2014;13((1)):2. doi: 10.1186/1475-2875-13-2. [cited 2014 Jan 6]. Available from: http://www.malariajournal.com/content/13/1/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, O'Neil M, Xie L, Caridha D, Zeng Q, Zhang J et al. Assessment of the prophylactic activity and pharmacokinetic profile of oral tafenoquine compared to primaquine for inhibition of liver stage malaria infections. Malar J [Internet] 2014;13((1)):141. doi: 10.1186/1475-2875-13-141. [cited 2014 May 16]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3989846&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells TNC, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol [Internet] 2010;26((3)):145–151. doi: 10.1016/j.pt.2009.12.005. Elsevier Ltd; [cited 2012 Apr 22]. Available from: http://dx.doi.org/10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Puri SK, Dutta GP. Plasmodium cynomolgi: gametocytocidal activity of the anti-malarial compound CDRI 80/53 (elubaquine) in rhesus monkeys. Exp Parasitol [Internet] 2005;111((1)):8–13. doi: 10.1016/j.exppara.2005.05.007. [cited 2011 Dec 22]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16005457. [DOI] [PubMed] [Google Scholar]
- 48.Gogtay NJ, Kamtekar KD, Dalvi SS, Mehta SS, Chogle AR, Aigal U et al. A randomized, parallel study of the safety and efficacy of 45 mg primaquine versus 75 mg bulaquine as gametocytocidal agents in adults with blood schizonticide-responsive uncomplicated falciparum malaria [ISCRTN50134587] BMC Infect Dis [Internet] 2006;6:16. doi: 10.1186/1471-2334-6-16. [cited 2011 Oct 19]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 1389708&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tekwani BL, Walker LA. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr Opin Infect Dis [Internet] 2006;19((6)):623–31. doi: 10.1097/QCO.0b013e328010b848. [DOI] [PubMed] [Google Scholar]
- 50.Succinate Q, Nanayakkara NPD, Ager AL, Bartlett MS, Yardley V, Croft SL et al. Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate. Antimicrob Agents Chemother [Internet] 2008;52((6)):2130–2137. doi: 10.1128/AAC.00645-07. [cited 2011 Aug 4]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 2415774&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meister S, Plouffe DM, Kuhen KL, Bonamy GMC, Wu T, Barnes SW et al. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science. 2011;334:1372–7. doi: 10.1126/science.1211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ploemen IHJ, Prudêncio M, Douradinha BG, Ramesar J, Fonager J, van Gemert G-J et al. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One [Internet] 2009;4((11)):e7881. doi: 10.1371/journal.pone.0007881. [cited 2011 Aug 2]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 2775639&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dow GS, Gettayacamin M, Hansukjariya P, Imerbsin R, Komcharoen S, Sattabongkot J et al. Radical curative efficacy of tafenoquine combination regimens in Plasmodium cynomolgi-infected Rhesus monkeys (Macaca mulatta. Malar J. 2011;10((1)):212. doi: 10.1186/1475-2875-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt LH. Relationships between chemical structures of 8-aminoquinolines and their capacities for radical cure of infections with Plasmodium cynomolgi in rhesus monkeys. Antimicrob Agents Chemother [Internet] 1983;24((5)):615–52. doi: 10.1128/aac.24.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puri SK, Dutta GP. Quinoline esters as potential antimalarial drugs: Plasmodium cynomolgi infections in monkeys. Trans R Soc Trop Med Hyg. 1990;84((6)):759–60. doi: 10.1016/0035-9203(90)90066-n. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt LH, Rossan RN, Fradkin R, Sullivan R, Schulemann W, Kratz L. Antimalarial activities and subacute toxicity of RC-12, a 4-amino-substituted pyrocatechol. Antimicrob Agents Chemother. 1985;28((5)):612–25. doi: 10.1128/aac.28.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clyde DF, McCarthy VC, Miller RM. Inactivity of rc-12 as a causal prophylactic and relapse inhibitor of Plasmodium vivax in man. Trans R Soc Trop Med Hyg. 1974;68((2)):167–8. [Google Scholar]
- 58.Liu X, Wang X, Li Q, Kozar MP, Melendez V, O'Neil MT et al. Synthesis and antimalarial activity of 2-guanidino-4-oxoimidazoline derivatives. J Med Chem [Internet] 2011;54((13)):4523–35. doi: 10.1021/jm200111g. [DOI] [PubMed] [Google Scholar]
- 59.Voorberg-van der Wel A, Zeeman AM, van Amsterdam SM, van den Berg A, Klooster EJ, Iwanaga S et al. Transgenic fluorescent Plasmodium cynomolgi liver stages enable live imaging and purification of Malaria hypnozoite-forms. PLoS One [Internet] 2013;8((1)):e54888. doi: 10.1371/journal.pone.0054888. [cited 2013 May 22]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3554669&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNamara CW, Lee MCS, Lim CS, Lim SH, Roland J, Nagle A et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature [Internet] 2013:4. doi: 10.1038/nature12782. Nature Publishing Group; [cited 2013 Nov 28]. Available from: http://www.nature.com/doifinder/10.1038/nature12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myint HY, Berman J, Walker L, Pybus B, Melendez V, Baird JK et al. Review: improving the therapeutic index of 8-aminoquinolines by the use of drug combinations: review of the literature and proposal for future investigations. Am J Trop Med Hyg. 2011;85((6)):1010–4. doi: 10.4269/ajtmh.2011.11-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg [Internet] 2006;75((3)):402–15. [PubMed] [Google Scholar]
- 63.Leroy D, Campo B, Ding X, Jeremy BN, Cherbuin S. Defining the biology component of the drug discovery strategy for malaria eradication. Trends Parasitol [Internet]. 2014 [cited 2014 Aug 19] 2014:1–13. doi: 10.1016/j.pt.2014.07.004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25131411. [DOI] [PubMed] [Google Scholar]
- 64.Dembele L, Gego A, Zeeman A-M, Franetich J-F, Silvie O, Rametti A et al. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS One [Internet] 2011;6((3)):e18162. doi: 10.1371/journal.pone.0018162. [cited 2011 Jun 21]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3069045&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dembélé L, Franetich J-F, Lorthiois A, Gego A, Zeeman A-M, Kocken CHM et al. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med [Internet] 2014;20((3)):307–312. doi: 10.1038/nm.3461. [cited 2014 Feb 12]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24509527. [DOI] [PubMed] [Google Scholar]
- 66.Deye GA, Gettayacamin M, Hansukjariya P, Im-Erbsin R, Sattabongkot J, Rothstein Y et al. Use of a Rhesus Plasmodium cynomolgi Model to Screen for Anti-Hypnozoite Activity of Pharmaceutical Substances. Am J Trop Med Hyg [Internet] 2012;86((6)):931–935. doi: 10.4269/ajtmh.2012.11-0552. [cited 2012 Jun 12]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22665596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sutanto I, Tjahjono B, Basri H, Taylor WR, Putri FA, Meilia RA et al. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrob Agents Chemother [Internet] 2013;57((3)):1128–1135. doi: 10.1128/AAC.01879-12. [cited 2013 Jun 3]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3591862&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rochford R, Ohrt C, Baresel PC, Campo B, Sampath A, Magill AJ et al. Humanized mouse model of glucose 6-phosphate dehydrogenase deficiency for in vivo assessment of hemolytic toxicity. Proc Natl Acad Sci U S A [Internet] 2013;110((43)):17486–91. doi: 10.1073/pnas.1310402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burrows JN, Chibale K, Wells TNC. The state of the art in anti-malarial drug discovery and development. Curr Top Med Chem [Internet] 2011;11((10)):1226–54. doi: 10.2174/156802611795429194. [DOI] [PubMed] [Google Scholar]
- 70.Wells TNC, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov [Internet] 2009;8((11)):879–891. doi: 10.1038/nrd2972. Nature Publishing Group; [cited 2011 Jun 21]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19834482. [DOI] [PubMed] [Google Scholar]
- 71.Hulden L, Hulden L. Activation of the hypnozoite: a part of Plasmodium vivax life cycle and survival. Malar J [Internet] 2011;10((1)):90. doi: 10.1186/1475-2875-10-90. BioMed Central Ltd; [cited 2011 Sep 10]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3086824&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall N. Genomic insights into the other malaria. Nat Genet [Internet] 2012;44((9)):962–963. doi: 10.1038/ng.2392. Nature Publishing Group; [cited 2013 Aug 8]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22932497. [DOI] [PubMed] [Google Scholar]
- 73.Mazier D, Landau I, Druilhe P, Miltgen F, Guguen-Guillouzo C, Baccam D et al. Cultivation of the liver forms of Plasmodium vivax in human hepatocytes. Nature [Internet] 1984;307((5949)):367–9. doi: 10.1038/307367a0. [DOI] [PubMed] [Google Scholar]
- 74.Hollingdale MR, Collins WE, Campbell CC. In vitro culture of exoerythrocytic parasites of the North Korean strain of Plasmodium vivax in hepatoma cells. Am J Trop Med Hyg [Internet] 1986;35((2)):275–6. doi: 10.4269/ajtmh.1986.35.275. [DOI] [PubMed] [Google Scholar]
- 75.Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg [Internet] 2006;74((5)):708–15. [PubMed] [Google Scholar]
- 76.March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe [Internet] 2013;14((1)):104–115. doi: 10.1016/j.chom.2013.06.005. Elsevier Inc.; [cited 2013 Jul 30]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23870318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maher SP, Crouse RB, Conway AJ, Bannister EC, Adams JH, Kyle DE et al. Microphysical space of a liver sinusoid device enables simplified long-term maintenance of chimeric mouse-expanded human hepatocytes. Biomed Microdevices. 2014;16((5)):727–36. doi: 10.1007/s10544-014-9877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science [Internet] 1976;193((4254)):673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 79.Noulin F, Borlon C, Van Den Abbeele J, D'Alessandro U, Erhart A. 1912–2012: a century of research on Plasmodium vivax in vitro culture. Trends Parasitol [Internet] 2013:1–9. doi: 10.1016/j.pt.2013.03.012. [cited 2013 May 22]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23623759. [DOI] [PubMed] [Google Scholar]
- 80.Larrouy G, Magnaval JF, Moro F. Obtaining intraerythrocytic forms of Plasmodium vivax by in vitro culture. C R Seances Acad Sci III. 1981;292((16)):929–30. [PubMed] [Google Scholar]
- 81.Banerjee T, Sharma SK, Kapoor N, Dwivedi V, Surolia N, Surolia A. Benzothiophene carboxamide derivatives as inhibitors of Plasmodium falciparum enoyl-ACP reductase. IUBMB Life [Internet] 2011;63((12)):1101–1110. doi: 10.1002/iub.553. [cited 2014 May 21]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22006792. [DOI] [PubMed] [Google Scholar]
- 82.Kumar AA, Lim C, Moreno Y, Mace CR, Syed A, Tyne D et al. Enrichment of reticulocytes from whole blood using aqueous multiphase systems of polymers. Am J Hematol. 2015;90((1)):31–6. doi: 10.1002/ajh.23860. [DOI] [PubMed] [Google Scholar]
- 83.Udomsangpetch R, Kaneko O, Chotivanich K, Sattabongkot J. Cultivation of Plasmodium vivax. Trends Parasitol [Internet] 2008;24((2)):85–88. doi: 10.1016/j.pt.2007.09.010. [cited 2011 Jun 27]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18180202. [DOI] [PubMed] [Google Scholar]
- 84.Golenda CF, Li J, Rosenberg R.Continuous in vitro propagation of the malaria parasite Plasmodium vivax Proc Natl Acad Sci U S A. 199794June):6786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Udomsangpetch R, Somsri S, Panichakul T, Chotivanich K, Sirichaisinthop J, Yang Z et al. Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol Int. 2007;56((1)):65–9. doi: 10.1016/j.parint.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Panichakul T, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Cui L, Udomsangpetch R. Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int J Parasitol. 2007;37((14)):1551–7. doi: 10.1016/j.ijpara.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 87.Noulin F, Borlon C, van den Eede P, Boel L, Verfaillie CM, D'Alessandro U et al. Cryopreserved reticulocytes derived from hematopoietic stem cells can be invaded by cryopreserved Plasmodium vivax isolates. PLoS One. 2012;7((7)):1–8. doi: 10.1371/journal.pone.0040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van J, Noulin F, Manesia JK, Rosanas-Urgell A, Erhart A, Abbeele D et al. Hematopoietic stem/progenitor cell sources to generate reticulocytes for Plasmodium vivax culture. PLoS One. 2014;9((11)):e112496. doi: 10.1371/journal.pone.0112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Furuya T, Sá JM, Chitnis CE, Wellems TE, Stedman TT. Reticulocytes from cryopreserved erythroblasts support Plasmodium vivax infection in vitro. Parasitol Int. 2014;63((2)):278–84. doi: 10.1016/j.parint.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zuo Z, Polski J, Kasyan A, Medeiros J. Acute erythroid leukemia. Arch Pathol Lab Med. 2010;139((9)):1261–70. doi: 10.5858/2009-0350-RA.1. [DOI] [PubMed] [Google Scholar]
- 91.Shah S, Huang X, Cheng L. Concise review: stem cell-based approaches to red blood cell production for transfusion. Stem Cells Transl Med [Internet] 2014;3:346–55. doi: 10.5966/sctm.2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ploss A. Mouse models for human infectious diseases. J Immunol Methods. 2014;410:1–2. doi: 10.1016/j.jim.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 93.Kaushansky A, Mikolajczak SA, Vignali M, Kappe SHI.Of men in mice: the success and promise of humanized mouse models for human malaria parasite infections Cell Microbiol. 201416March):602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol [Internet] 2013;31:635–74. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol [Internet] 2014;32:364–72. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N et al. Complete Plasmodium falciparum liver stage development in liver-chimeric mice. J Clin Invest. 2012;122((10)):1–11. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25((8)):903–10. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bissig K-D, Le TT, Woods N-B, Verma IM. Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model. Proc Natl Acad Sci U S A. 2007;104((51)):20507–11. doi: 10.1073/pnas.0710528105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galinski MR, Meyer EVS, Barnwell JW. Plasmodium vivax: modern strategies to study a persistent parasite's life cycle [Internet] Adv Parasitol. 2013 doi: 10.1016/B978-0-12-407826-0.00001-1. Elsevier; [cited 2013 Feb 25]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23384620. [DOI] [PubMed] [Google Scholar]
- 100.Moreno M, Tong C, Guzmán M, Chuquiyauri R, Llanos-Cuentas A, Rodriguez H et al. Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am J Trop Med Hyg. 2014;90((4)):612–6. doi: 10.4269/ajtmh.13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghosh SK, Tiwari S, Raghavendra K, Sathyanarayan TS, Dash AP. Observations on sporozoite detection in naturally infected sibling species of the Anopheles culicifacies complex and variant of Anopheles stephensi in India. J Biosci. 2008;33((3)):333–6. doi: 10.1007/s12038-008-0052-5. [DOI] [PubMed] [Google Scholar]
- 102.Goo Y-K, Seo E-J, Choi Y-K, Shin H-I, Sattabongkot J, Ji S-Y et al. First characterization of Plasmodium vivax liver stage antigen (PvLSA) using synthetic peptides. Parasit Vectors [Internet] 2014;7((1)):64. doi: 10.1186/1756-3305-7-64. Parasites & Vectors; [cited 2014 Nov 27]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3925417&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mathias DK, Jardim JG, Parish LA, Armistead JS, Trinh HV, Kumpitak C et al. Differential roles of an Anopheline midgut GPI-anchored protein in mediating Plasmodium falciparumPlasmodium vivax ookinete invasion. InfectGenet Evol. 2014;28:635–47. doi: 10.1016/j.meegid.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu G, Xia H, Zhou H, Li J, Lu F, Liu Y et al. Susceptibility of Anopheles sinensis to Plasmodium vivax in malarial outbreak areas of central China. Parasit Vectors [Internet] 2013;6((1)):1. doi: 10.1186/1756-3305-6-176. Parasites & Vectors. Available from: Parasites & Vectors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kennedy M, Fishbaugher ME, Vaughan AM, Patrapuvich R, Boonhok R, Yimamnuaychok N et al. A rapid and scalable density gradient purification method for Plasmodium sporozoites. Malar J [Internet] 2012;11((1)):421. doi: 10.1186/1475-2875-11-421. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 3543293&tool = pmcentrez&rendertype = abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Epstein JE, Tewari K, Lyke KE, Sim BKL, Billingsley PF, Laurens MB et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+T cell immunity. Science. 2011;334((6055)):475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 107.Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 108.Gosden RG. Memoir of fertility preservation. Adv Exp Med Biol. 2013;761:85–94. doi: 10.1007/978-1-4614-8214-7_7. [DOI] [PubMed] [Google Scholar]
- 109.Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96((2)):264–8. doi: 10.1016/j.fertnstert.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 110.Holoch P, Wald M. Current options for preservation of fertility in the male. Fertil Steril. 2011;96((2)):286–90. doi: 10.1016/j.fertnstert.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 111.Clark NA, Swain JE. Oocyte cryopreservation: searching for novel improvement strategies. J Assist Reprod Genet. 2013;30:865–75. doi: 10.1007/s10815-013-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hollingdale MR, Collins WE, Campbell CC, Schwartz AL. In vitro culture of two populations (dividing and nondividing) of exoerythrocytic parasites of Plasmodium vivax. Am J Trop Med Hyg. 1985;34((2)):216–22. doi: 10.4269/ajtmh.1985.34.216. [DOI] [PubMed] [Google Scholar]
- 113.Chattopadhyay R, Velmurugan S, Chakiath C, Donkor LA, Milhous W, Barnwell JW et al. Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS One. 2010;5((12)):1–8. doi: 10.1371/journal.pone.0014275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kafsack BFC, Llinás M. Eating at the table of another: metabolomics of host/parasite interactions. Cell Host Microbe. 2010;7((2)):90–9. doi: 10.1016/j.chom.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lakshmanan V, Rhee K, Daily J. Metabolomics and malaria biology. Mol Biochem Parasitol. 2011;175((2)):104–11. doi: 10.1016/j.molbiopara.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fraunholz MJ. Systems biology in malaria research. Trends Parasitol. 2005;21((9)):393–5. doi: 10.1016/j.pt.2005.07.007. [DOI] [PubMed] [Google Scholar]