Abstract
Background. Continuous subcutaneous insulin infusion (CSII) is an established modality for intensive insulin treatment of type 1 diabetes (T1D), but long-term data concerning satisfaction, CSII function use, safety, and efficacy in real-life conditions are scarce. Methods. We analyzed a cohort of adult patients with T1D treated with CSII for more than 1 year in a single diabetes center. We performed a cross-sectional survey in 2010 (tolerance/satisfaction and behavior forms) and a retrospective analysis of medical records (including HbA1c level, hospitalization, and catheter infections). The primary objective was to assess long-term tolerance/satisfaction, and secondary objectives were safety and efficacy. Results. There were 295 patients analyzed. After a median duration of CSII use of 5 years, overall satisfaction was high for about 90% of patients. Mean CSII-related discomfort scores were low for work, recreation, and sleep and moderate for sport and sexual activity (2.5 ± 1.9, 2.6 ± 1.8, 2.6 ± 2.1, 3.4 ± 2.3, and 4.0 ± 2.9 of 10, respectively). Despite a high level of diabetes education, only one third of patients were using advanced CSII functions. During long-term follow-up, the safety of CSII treatment was good; the hospitalization rate was 0.18 patients/year, and catheter infections were scarce. The HbA1c level dropped about −0.5% independently from CSII duration (P < .05). Conclusions. In this adult cohort, satisfaction and tolerance, together with safety, of CSII were maintained at long-term follow up. The sole basic functions of CSII were currently used by patients. A 0.5% decrease in the HbA1c level was maintained during the study period.
Keywords: continuous subcutaneous insulin infusion, HbA1c, insulin, quality of life, type 1 diabetes
Continuous subcutaneous insulin infusion (CSII) is an established modality for treating type 1 diabetes (T1D) when the HbA1c level persistently remains above 7.5% despite the intensification of health care by a multidisciplinary team and optimization of the patient’s education.1 When compared to multiple daily injections (MDI), CSII allows an equal or lower HbA1c level with fewer mild and severe cases of hypoglycemia.2-5 Most studies on CSII utilization in T1D were prospective and were conducted from several weeks’ to 2 years’ duration.2 Few retrospective studies have questioned the long-term benefit of CSII utilization, and long-term evaluations of satisfaction and quality of life are scarce.6-13 The present cross-sectional survey and retrospective analysis of a cohort of patients with T1D treated by CSII gives the opportunity to evaluate the long-term tolerance and satisfaction of the device and the therapeutic behavior of CSII users in real-life conditions. Safety and efficacy issues are also under the scope of this study.
Methods
This single-center retrospective study and trans-sectional survey was conducted by the endocrinology department from a French university hospital. The study enrolled patients with T1D using CSII for more than 1 year from January 1999 (year of reimbursement of CSII treatment by the French National Health System) until December 2008. Insulin pumps used in our center during this period of time were Medtronic 508, 511, 512, and 515 (Medtronic, Minneapolis, Minnesota, USA); Roche H-Tron, D-Tron, Accu-Chek Spirit, and Accu-Chek Combo (Roche Diagnostics, Meylan, France); and Animas 1100, 1200, and 2020 (Animas, West Chester, Pennsylvania, USA). Patients were excluded if they had no initial HbA1c level at CSII initiation, if CSII use was transitory, if the diagnosis of T1D was not firmly established, and if the insulin used in CSII was not lispro (Lilly, Indianapolis, Indiana, USA), aspart (Novo Nordisk, Bagsvaerd, Denmark), or glulisine (Sanofi, Paris, France). According to standards of care, CSII treatment was initiated during a 5-day in-hospital education program. In addition, some patients had a specific education session on the management of flexible insulin therapy (FIT).
Baseline data at CSII initiation were collected through the hospital medical information system including sex, age, weight, diabetes duration, initial HbA1c level, and indication of CSII treatment. Follow-up data were also collected: CSII treatment duration, withdrawal indication when applicable, frequency of visits at the reference center, frequency and causes of hospitalization (including severe hypoglycemia and ketoacidosis), and catheter infections since CSII initiation.
The cross-sectional survey was conducted from January until December 2010 by telephone, email, or conventional mail to collect actual data including the recent HbA1c level (<3 months), weight, insulin daily dose, and basal/bolus ratio. Patients were also asked about their knowledge and behavior concerning diabetes: prior education to FIT, use of advanced CSII options (bolus wizard, temporary basal rate), frequency of self-monitoring blood glucose (SMBG), and length of seasonal switch to MDI. Also, CSII satisfaction or discomfort evaluation forms were collected. Discomfort with CSII was evaluated using an analogic score graded from 1 (no discomfort) to 10 (maximal discomfort) for different situations (work, recreation, sleep, sport, and sexual activity).
Version 9.1.3 of SAS software (SAS Institute Inc, Cary, North Carolina, USA) was used to perform statistical analyses. Diabetes and CSII duration are expressed as the median (interquartile range [IQR]). Other quantitative variables are expressed as the mean ± standard deviation. The HbA1c variation from baseline was analyzed using the paired Student t test. Comparisons between HbA1c levels according to CSII duration and between discomfort scores were performed using a Student t test. The relationship between initial characteristics and outcome measures was analyzed with logistic regression and Poisson regression for binary outcomes and count data, respectively. Additionally, correlations between quantitative parameters were assessed using the Pearson correlation coefficient. A P value <.05 was considered to denote significance.
Results
Among 423 patients treated with CSII for at least 1 year, 295 met inclusion criteria (CSII initiation after 1999 and available initial HbA1c level). Baseline characteristics of this population are described in Table 1. Indications of CSII treatment were “above target” HbA1c level (50.7%), brittle diabetes (11.6%), patient’s request (9.4%), pregnancy (9.1%), recurrent hypoglycemia (5.4%), dawn phenomenon (3.3%), and miscellaneous (10.5%). Only 39 patients (13.2%) discontinued CSII treatment during the study period (16 for unknown reasons, 11 for side effects, 6 for personal decisions, 6 for professional mobility). Among the remaining 256 patients, 219 completed the survey in 2010, including 199 patients with available recent HbA1c levels (flowchart in Figure 1).
Table 1.
Baseline and Follow-up Characteristics of the Population.
| Overall population (N = 295) | |
|---|---|
| Baseline characteristics | |
| Female sex, n (%) | 156 (52.9) |
| Age at CSII initiation, y | 33.9 ± 10.9 |
| Diabetes duration, median (interquartile range), y | 15 (10-26) |
| Weight, kg | 68.2 ± 9.9 |
| HbA1c level, % | 8.2 ± 1.6 |
| Follow-up characteristics in 2010 | |
| Age, y | 39.2 ± 13.4 |
| CSII duration, median (interquartile range), y | 5 (3-8) |
| Weight, kg | 71.6 ± 14.3 |
| HbA1c level, % | 7.6 ± 1.0 |
| SMBG frequency per day | 5.2 ± 2.3 |
| Total daily insulin use, U/kg/d | 0.62 ± 0.19 |
| Basal insulin/total insulin ratio, % | 49.7 ± 14.6 |
| Functional insulin therapy education, % | 62.0 |
| Temporary basal rate user, % | 43.4 |
| Bolus wizard user, % | 23.8 |
| ≥1-mo/y seasonal switch for MDI, % | 10.6 |
| Discomfort scores | |
| Recreation | 2.6 ± 1.8 |
| Work | 2.5 ± 1.9 |
| Sleep | 2.6 ± 2.1 |
| Sport | 3.4 ± 2.3 |
| Sexual activity | 4.0 ± 2.9 |
Values are expressed as mean ± standard deviation unless otherwise indicated. CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; SMBG, self-monitoring blood glucose.
Figure 1.
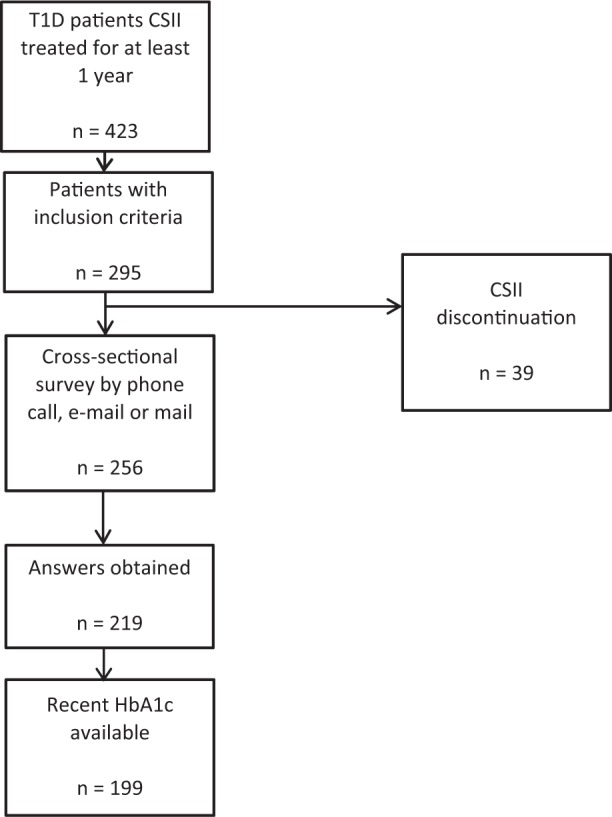
Flowchart.
In 2010, the median duration of CSII treatment was 5 years (IQR, 3-8 years). Overall satisfaction of the CSII device was high for 93.1% of patients. We found 94.9% of patients promoting CSII use and 87.7% disapproving CSII removal. Discomfort related to CSII use in everyday life situations was low, with a mean discomfort score of 2.5 ± 1.9 of 10 for work, 2.6 ± 1.8 for recreation, and 2.6 ± 2.1 for sleep. Discomfort was significantly higher for sport and sexual activity (3.4 ± 2.3 and 4.0 ± 2.9, respectively; P < .001) (Figure 2). We found a negative correlation between discomfort scores for sport and age (R = −0.21; P < .01). Despite a high level of diabetes education (62% of patients were trained to FIT), only few patients were using advanced CSII functions (23.8% bolus wizard and 43.4% temporary basal rate). We found a negative correlation between the use of temporary basal rate and baseline HbA1c level (–24% temporary basal rate use for every 1% increase in HbA1c level; P < .05). Only 10.6% declared switching seasonally to MDI more than 1 mo/y. Survey responders claimed performing SMBG 5.2 ± 2.3 times/day.
Figure 2.
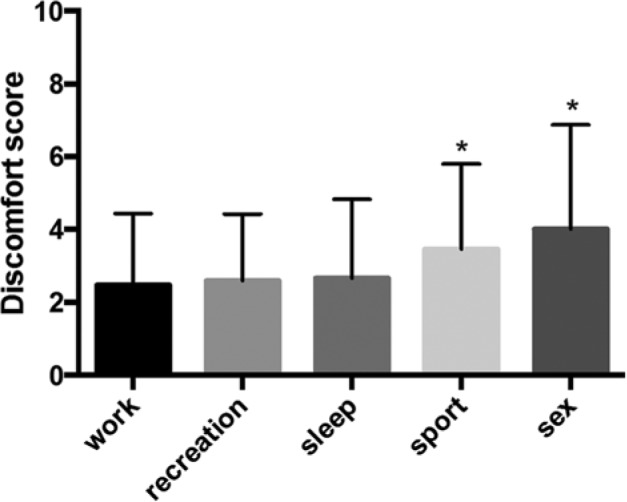
Discomfort with continuous subcutaneous insulin infusion, evaluated using an analogic score graded from 1 (no discomfort) to 10 (maximal discomfort) for different situations (work, recreation, sleep, sport, and sexual activity). *P < .001 (compared to scores for sleep, recreation, and work).
Hospitalizations (excluding CSII initiation) during the study period were scarce: none in 54.2%, <1 hospitalization/year in 41.7%, and ≥1 hospitalizations/year in 4.1% of patients. Overall, the hospitalization rate was 0.18 patients/year, including 0.01 and 0.02 patients/year for severe hypoglycemia and ketoacidosis, respectively. Catheter infections were scarce: 92.9% of patients never had any infection, 5.1% (15 patients) had only 1 episode, and 2.0% (6 patients) had several catheter infections (2-8 episodes) since the initiation of CSII treatment. None of these infections required a hospitalization stay. A negative correlation was observed between catheter infection incidence and age (R = −0.16; P < .05) and a positive correlation between catheter infection incidence and HbA1c level (R = 0.26; P < .001).
At CSII initiation, the mean HbA1c level was 8.2% ± 1.6%. In 2010, during the cross-sectional survey, the mean HbA1c level was 7.6% ± 1.0%, corresponding to a 0.5% ± 1.3% decrease from baseline (P < .001). At that time, HbA1c level was negatively correlated with the claimed frequency of SMBG performed per day (R = −0.23; P < .01). At follow-up, the decrease in the HbA1c level from baseline remained stable, ranging between −0.4% to −0.5% (P < .05) for CSII durations of 1 to 3, 4 to 6, and ≥7 years (Figure 3).
Figure 3.
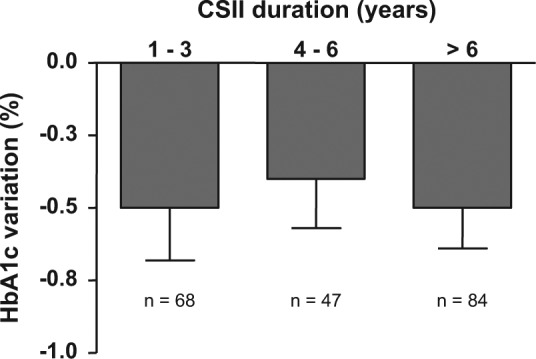
HbA1c variation from baseline to recent value (during the survey) according to continuous subcutaneous insulin infusion treatment duration (mean ± standard error of the mean). HbA1c level changes from baseline are significant in all groups (P < .05). No significant difference between groups.
Discussion
In this study, we described the characteristics, behavior, and satisfaction of a cohort of patients with T1D treated with CSII for several years. A few prospective trials have questioned the potential benefits of CSII versus MDI in adult patients with T1D, including quality of life, but these trials rarely exceeded 2 years.2-5 Recent retrospective studies have reported the long-term efficacy and safety of CSII in adult and younger patients, but quality of life and satisfaction were not questioned in these studies.11-13 The design of our study included data collection on CSII utilization and satisfaction. We observed that patients with CSII had a high level of satisfaction several years after CSII initiation and were prone to encourage their peers to move to pump therapy. Similarly, a recent study demonstrated that peer support is most efficient for shifting patients with diabetes to multiple injection therapy.14
We also showed that discomfort scores with CSII in everyday life situations were low except for sport and sexual activity. Discomfort during sport was higher for younger patients. This finding should prompt the clinician to enhance education about physical activity management in younger patients treated with CSII. Quality of life in patients with T1D treated with CSII was investigated in few studies. Using the insulin delivery system rating questionnaire, 2 studies have found similar results showing low discomfort with CSII.15,16 However, Riveline et al17 found that 10% of patients alleged discomfort with CSII during sexual activity because of constraint of the catheter. In a multivariate analysis, these patients had higher HbA1c levels and more frequent cases of mild hypoglycemia.17 In contrast, we did not find any correlation between the discomfort score and glucose control.
There is a paucity of published data on the rate of use of advanced functions of CSII, such as bolus wizard and temporary basal rate, in real-life settings. However, the percentage of bolus wizard users is 50% to 100% in some reports.18,19 In our study, these functions were used by few patients (23.8% for bolus wizard and 43.4% for temporary basal rate) and even less when they had poor glycemic control. This is surprising given the young age of patients at CSII initiation (34 years) and the high level of diabetes education to FIT (60.3% of patients). This discrepancy with published data can be explained by the lack of systematic training to these advanced functions in our standard training sessions. Advanced CSII parameters were discussed in advanced sessions not offered to all patients. Several authors found an improvement in fasting glucose, postprandial glucose, and HbA1c levels when using the bolus wizard function.20,21 The bolus wizard calculator is considered to be an easy, time-saving, and safe option that does not increase the risk of severe hypoglycemia.21,22 The use of bolus calculators for FIT dramatically decreases errors in insulin dose calculation.23 The use of other pump options such as the temporary basal rate also provides an improvement in HbA1c levels.24
Seasonal switch to MDI concerned only 10% of our cohort. These switches were usually of 1-month duration and did not exceed 2 months, therefore explaining the lack of glucose control impairment associated with seasonal switch.
In our cohort, the mean SMBG frequency was high (5.2 ± 2.3 times/day), reflecting the high proportion of patients performing FIT. In our study that was previously published,25 the frequency of SMBG per day negatively correlated with the HbA1c level. Previous reports described that the HbA1c level decreases by 0.2% for each additional SMBG check performed per day, up to 5 times/day.25
In the present study, CSII efficacy and safety could not be analyzed due to the retrospective design, lack of a control group, and missing data. Nevertheless, results are consistent with those of previous meta-analyses of prospective studies, which found a reduction in HbA1c levels of −0.2% to −0.7% with CSII in comparison to MDI.2,3,5 In retrospective studies, CSII use in adults with T1D decreased HbA1c levels by −0.5% to −1.3% when baseline HbA1c levels ranged between 7.6% to 9.6%, respectively.6-10 However, recent retrospective analyses highlighted a progressive increase in HbA1c levels after a 5- to 7-year follow-up with a residual HbA1c improvement from baseline of only -0.2%.11-13 In contrast, our finding is a sustained −0.5% decrease in HbA1c levels with pump therapy after 7 years of utilization, perhaps explained by a high degree of educational support for FIT at the moment of CSII initiation.
Concerning safety issues, we found an incidence of 0.01 hospitalizations/patient-year for severe hypoglycemia, which is particularly low. Such a low rate of hypoglycemia was also observed in a prospective study showing a dramatic 4.2-fold reduction in severe hypoglycemia when switching from MDI to CSII, with the highest reduction occurring in older patients and in those with the highest incidence of hypoglycemia at baseline.3 In retrospective studies, a 74% to 96% reduction in the rate of severe hypoglycemia was observed with CSII compared to MDI.6,8 We also found a very low rate of hospitalization for diabetic ketoacidosis (DKA). The incidence of DKA with CSII utilization is controversial, with an increase in studies performed before 1993 and conflicting results in subsequent studies.26 The occurrence of DKA with CSII relates to environmental and educational issues, as shown in 2 recent studies in which DKA frequency was reduced in children with good family care and in adults trained to FIT.27,28 Concerning catheter issues, a low rate of infections was observed in accordance with previous studies, but they were more frequent in young patients with poor metabolic control who were prone to change their catheters at intervals greater than 3 days.29
Our study presents several limitations including the lack of a comparative group of MDI, existence of missing data, lack of a baseline satisfaction/tolerance questionnaire, lack of an evaluation in patients who withdrew their CSII device (but only 13.2% of patients withdrew), and approximate incidence of severe hypoglycemia and DKA indirectly determined by the frequency of hospitalization. However, the strengths of our study are its large sample population, its long mean length of follow-up, and its real-life conditions.
Conclusions
We show in this retrospective study a high level of satisfaction in long-term CSII users, claiming minimal discomfort. Safety and efficacy were maintained for several years of pump utilization, despite the minimal use of advanced pump functions.
Footnotes
Abbreviations: CSII, continuous subcutaneous insulin infusion; DKA, diabetic ketoacidosis; FIT, flexible insulin therapy; MDI, multiple daily injections; SMBG, self-monitoring blood glucose; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Statistical analysis was partially funded by Medtronic France.
References
- 1. Lassmann-Vague V, Clavel S, Guerci B, et al. When to treat a diabetic patient using an external insulin pump: expert consensus. Société Francophone du Diabète (ex ALFEDIAM) 2009. Diabetes Metab. 2010;36:79-85. [DOI] [PubMed] [Google Scholar]
- 2. Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51:941-951. [DOI] [PubMed] [Google Scholar]
- 3. Pickup JC, Sutton AJ. Severe hypoglycemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765-774. [DOI] [PubMed] [Google Scholar]
- 4. Pickup JC, Renard E. Long-acting insulin analogs versus insulin pump therapy for the treatment of type 1 and type 2 diabetes. Diabetes Care. 2008;31:S140-S145. [DOI] [PubMed] [Google Scholar]
- 5. Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodrigues AS, Reid HA, Ismail K, Amiel SA. Indications and efficacy of continuous subcutaneous insulin infusion (CSII) therapy in type 1 diabetes mellitus: a clinical audit in a specialist service. Diabet Med. 2005;22:842-849. [DOI] [PubMed] [Google Scholar]
- 7. Riveline J-P, Jollois F-X, Messaoudi N, et al. Insulin-pump use in everyday practice: data from an exhaustive regional registry in France. Diabetes Metab. 2008;34:132-139. [DOI] [PubMed] [Google Scholar]
- 8. Rudolph JW, Hirsch IB. Assessment of therapy with continuous subcutaneous insulin infusion in an academic diabetic clinic. Endocr Pract. 2002;8:401-405. [DOI] [PubMed] [Google Scholar]
- 9. Norgaard K. A nationwide study of continuous subcutaneous insulin infusion (CSII) in Denmark. Diabet Med. 2003;20:307-311. [DOI] [PubMed] [Google Scholar]
- 10. Matejko B, Cyganek K, Katra B, et al. Insulin pump therapy is equally effective and safe in elderly and young type 1 diabetes patients. Rev Diabet Stud. 2011;8:254-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlsson BM, Attvall S, Clements M, et al. Insulin pump-long-term effects on glycemic control: an observational study at 10 diabetes clinics in Sweden. Diabetes Technol Ther. 2013;15:302-307. [DOI] [PubMed] [Google Scholar]
- 12. Cohen ND, Hong ES, Van Drie C, Balkau B, Shaw J. Long-term metabolic effects of continuous subcutaneous insulin infusion therapy in type 1 diabetes. Diabetes Technol Ther. 2013;15:544-549. [DOI] [PubMed] [Google Scholar]
- 13. Mameli C, Scaramuzza AE, Ho J, Cardona-Hernandez R, Suarez-Ortega L, Zuccotti GV. A 7-year follow-up retrospective, international, multicenter study of insulin pump therapy in children and adolescents with type 1 diabetes. Acta Diabetol. 2014;51:205-210. [DOI] [PubMed] [Google Scholar]
- 14. Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153:507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peyrot M, Rubin RR. Validity and reliability of an instrument for assessing health-related quality of life and treatment preferences: the insulin delivery system rating questionnaire. Diabetes Care. 2005;28:53-58. [DOI] [PubMed] [Google Scholar]
- 16. Barnard KD, Skinner TC. Cross-sectional study into quality of life issues surrounding insulin pump use in type 1 diabetes. Pract Diab Int. 2008;25:194-200. [Google Scholar]
- 17. Riveline JP, Franc S, Biedzinski M, et al. Sexual activity in diabetic patients treated by continuous subcutaneous insulin infusion therapy. Diabetes Metab. 2010;36:229-233. [DOI] [PubMed] [Google Scholar]
- 18. Matejko B, Grzanka M, Kieć-Wilk B, Małecki MT, Klupa T. Clinical factors affecting the perception of hypoglycemia in type 1 diabetes patients treated with personal insulin pumps. Ann Agric Environ Med. 2013;20:152-154. [PubMed] [Google Scholar]
- 19. Cukierman-Yaffe T, Konvalina N, Cohen O. Key elements for successful intensive insulin pump therapy in individuals with type 1 diabetes. Diabetes Res Clin Pract. 2011;92:69-73. [DOI] [PubMed] [Google Scholar]
- 20. Klupa T, Benbenek-Klupa T, Malecki M, Szalecki M, Sieradzki J. Clinical usefulness of a bolus calculator in maintening normoglycemia in active professional patients with type 1 diabetes treated with continuous subcutaneous insulin infusion. J Int Med Res. 2008;36:1112-1116. [DOI] [PubMed] [Google Scholar]
- 21. Gross TM, Kayne D, King A, Rother C, Juth S. A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin pump therapy. Diabetes Technol Therap. 2003;3:365-369. [DOI] [PubMed] [Google Scholar]
- 22. Zisser H, Wagner R, Pleus S, et al. Clinical performance of three bolus calculators in subjects with type 1 diabetes mellitus: a head to head to head comparison. Diabetes Technol Ther. 2010;12:955-961. [DOI] [PubMed] [Google Scholar]
- 23. Colin I, Paris I. Glucose meters with built-in automated bolus calculator: gadget or real value for insulin-treated diabetic patients? Diabetes Ther. 2013;4:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson J, McFann K, Chase HP. Factors affecting improved glycaemic control in youth using insulin pumps. Diabet Med. 2010;27:1174-1177. [DOI] [PubMed] [Google Scholar]
- 25. Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, Holl R; DPV-Wiss-Initiative. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:11-17. [DOI] [PubMed] [Google Scholar]
- 26. Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26:1079-1087. [DOI] [PubMed] [Google Scholar]
- 27. Hanas R, Ludvigsson J. Hypoglycemia and ketoacidosis with insulin pump therapy in children and adolescents. Pediatr Diabetes. 2006;7:32-38. [DOI] [PubMed] [Google Scholar]
- 28. Sämann A, Mühlhauser I, Bender R, Hunger-Dathe W, Kloos C, Müller UA. Flexible insulin therapy in adults with type 1 diabetes and high risk for severe hypoglycemia and diabetic keto-acidosis. Diabetes Care. 2006;29:2196-2199. [DOI] [PubMed] [Google Scholar]
- 29. Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol. 2010;4:976-982. [DOI] [PMC free article] [PubMed] [Google Scholar]


