Abstract
The aims were to investigate predictors of insulin initiation in new users of metformin or sulfonylureas in primary care practices, in particular, its association with decreased renal function. Data from 9103 new metformin and 1120 sulfonylurea users with normal baseline glomerular filtration rate (eGFR) >90 ml/min/1.73 m2 from 1072 practices were retrospectively analyzed (Disease Analyzer Germany: 01/2003-06/2012). Cox regression models and propensity score matching was used to adjust for confounders (age, sex, practice characteristics, comorbidity). Insulin treatment was started in 394 (4.3%) metformin and in 162 (14.5%) sulfonylurea users within 6 years (P < .001). Kaplan-Meier curves (propensity score matched patients) showed that the metformin group was at a lower risk of insulin initiation compared to sulfonylurea users throughout the study period. A substantial eGFR decline (category: 15-<30 ml/min/1.73 m2) was significantly associated with a higher likelihood to have insulin initiated (adjusted hazard ratio [HR]: 2.39; 95% CI: 1.09-5.23) in metformin but not in sulfonylurea (HR: 0.45; 95% CI: 0.16-1.30) users. New users of sulfonylurea monotherapy in primary care practices in Germany were about 3-fold more likely to start insulin therapy than those with metformin. Kidney function decline was associated with earlier insulin initiation in metformin but not in sulfonylurea users.
Keywords: insulin initiation, type 2 diabetes, kidney function, metformin, sulfonylurea
National and international guidelines provide algorithms for a sequential treatment with antidiabetic agents in type 2 diabetes.1 The first-line oral antidiabetic drug is usually metformin, which mainly improves insulin sensitivity. However, in many patients metformin is insufficient to reach the target for glycemic control (HbA1c).1 Since decades sulfonylureas have been used as a second line treatment option. In numerous patients, physicians may even use sulfonylureas as first-line drugs. In particular, in older patients beta cell function is impaired. Of note, prescription use of sulfonylureas increases with higher age whereas metformin use declines.2,3 Observational studies have indicated that treatment failure and subsequent need for insulin therapy may be higher in sulfonylurea than in metformin users.2
The onset of insulin therapy in type 2 diabetes may also be influenced by other factors. One aspect which has not been previously investigated in observational studies is progressive kidney decline. Current national and international guidelines contraindicate the use of metformin in patients with a low glomerular filtration rate (GFR).4 Metformin is eliminated renally, and an increased risk of lactic acidosis has been found in metformin treatment in renal failure.1 However, whereas in Germany metformin use in patients with a GFR lower than 60 ml/min/1.73 m2 is not allowed,4 guidelines in the United Kingdom and other European countries are less strict allowing use of metformin down to a GFR of 30-45 ml/min/1.73 m2.1,5,6
Sulfonylureas (glibenclamide) are also partially eliminated in urine.1 In the presence of renal impairment, glibenclamide-induced hypoglycemic events may be severe and long-lasting. Therefore, guidelines state that glibenclamide should be used with caution in mild renal impairment (GFR 60-90 ml/min) and should be avoided in more severe stages.1
The aims of the current retrospective database study are to examine (1) insulin initiation in type 2 diabetes patients with onset of metformin or sulfonylurea treatment and (2) the association of kidney function decline with switching from oral antidiabetic drugs to insulin therapy in both groups.
Patients and Methods
Disease Analyzer Database
The Disease Analyzer database (IMS HEALTH) assembles drug prescriptions, diagnoses, and basic medical and demographic data directly obtained from the practice computer system of general practitioners throughout Germany.7 Diagnoses (ICD-10), prescriptions (Anatomical Therapeutic Chemical [ATC] Classification System), and the quality of reported data were continuously monitored by IMS based on a number of quality criteria (eg, completeness of documentation, linkage of diagnoses, and prescriptions).
Study Population
Overall, the database included 1072 general practices continuously reporting to IMS HEALTH during the study period (January 2003-December 2012). First, all type 2 diabetes patients with a first-line prescription (index date) of either metformin (ATC: A10J) or sulfonylurea (ATC: A10H) (n = 39 997) were selected. Further inclusion criteria were continuous treatment in the same practice (≥1 visit during the 12 months before index date and ≥1 visit during at least 12 months after index date) and age at index date above 40 years. Patients with prescriptions of DPP4 inhibitors, GLP-1 agonists, glitazones, or glinides during the study period were excluded. Furthermore, patients with diagnosed renal or liver insufficiency before index date were excluded, leaving a sample of 20 446 patients. Finally, only patients with ≥1 estimated eGFR 0-183 days before and at least one eGFR after index date were included (n = 10 223).
Study outcome was the first insulin prescription recorded in the database after index date. Therefore, no insulin prescriptions prior to index date and within 90 days after index date were allowed to avoid misclassification of prescriptions already present at baseline. GFR was estimated using the Modification of Diet in Renal Disease formula (eGFR = 186 × (serum creatinine mg/dl)– 1.154 × (age)– 0.203 (*0.742 when female).4
Macrovascular complications were determined based on primary care diagnoses (ICD-10 codes) for coronary heart disease (I20, I24, I25), myocardial infarction (I21, I22, I23, I25.2), stroke (I63, I64, G45), peripheral vascular disease (E11.5, E14.5, I73.9), and congestive heart failure (I50). Microvascular complications included retinopathy (E11.3, E14.2) and neuropathy (E11.4, E14.4). Lipid disorders, hypertension, and depression were assessed as potential confounders. Furthermore, a revised version of the Charlson Comorbidity Index (CCI) was used as generic marker of comorbidity.8 Demographic data included age, sex, health insurance (private/statutory), type of primary care (diabetologist/general practitioner), and practice region (urban/rural; East/West Germany). Data on HbA1c, fasting glucose measurements, and body mass index, which were only available in a subgroup, were also analyzed.
Statistical Analysis
Differences in characteristics of patients with metformin or sulfonlyurea were assessed using t tests, Wilcoxon tests, or chi-square tests. The analyses of insulin-free period were carried out using Kaplan-Meier curves and log-rank tests. To control for confounding, one-to-one matching was carried out based on a propensity score that was constructed as the conditional probability of insulin initiation as a function of age, sex, type of health insurance, and type of primary care treatment.
A multivariate Cox regression model was fitted with insulin initiation as dependent variable separately for metformin and sulfonylurea users. eGFR after index date was classified into the following groups: >90, 60-<90, 30-<60, 15-<30 ml/min/1.73 m2, respectively.4 Because of very few numbers, no further category <15 ml/min/1.73 m2 was established. Furthermore, baseline eGFR, potential confounders (age, sex, diabetologist care, private health insurance, practice in East Germany), Charlson Comorbidity Score, hypertension, lipid disorders, depression, and cotherapy with ACE inhibitors, diuretics, and statins were included as independent variables. In separate models, baseline HbA1c and body mass index were included, which were only available in a subsample. Two-sided tests were used and a P value < .05 was considered as statistically significant. All analyses were carried out using SAS 9.3 (SAS Institute, Cary, USA).
Results
There were 9103 and 1120 patients with first-time prescriptions of metformin or sulfonlyureas in the practices, respectively (Table 1). The average follow-up observation period (years) after insulin initiation was comparable for the groups (mean (SD): metformin: 3.2 (1.6); sulfonylurea: 3.7 (1.8) years). There was no significant difference in the time from first recorded diabetes diagnosis in the practice until onset of medical therapy in both groups (Table 1).
Table 1.
Baseline Characteristics of Primary Care Patients With First-Line Prescriptions of Metformin or Sulfonylureas: IMS HEALTH Disease Analyzer, Germany.
| Variable | Metformin | Sulfonylurea |
|---|---|---|
| n | 9103 | 1120 |
| Age (years) | 65.0 (10.6)* | 71.3 (10.6)* |
| Time from first diabetes diagnosis in practice (years) | 1.3 (2.4) | 1.1 (2.1) |
| Males (%) | 50.7* | 47.3* |
| Private health insurance (%) | 5.4 | 4.9 |
| Diabetologist treatment (%) | 2.6 | 2.4 |
| Region (West Germany) (%) | 79.6* | 75.3* |
| Urban residencya (%) | 29.4 | 27.9 |
| eGFR (ml/min/1.73 m2) | 81.1 (18.8)* | 72.0 (21.0)* |
| HbA1c (%)c | 7.3 (1.4)* | 7.6 (1.6)* |
| BMI (kg/m2)d | 31.6 (5.2)* | 29.4 (5.1)* |
| Diagnosed comorbidity (%) | ||
| Coronary heart disease | 28.7* | 37.3* |
| Myocardial infarction | 7.4* | 11.0* |
| Stroke | 7.1* | 10.7* |
| Peripheral vascular disease | 7.2* | 12.9* |
| Congestive heart failure | 13.1* | 24.1* |
| Hypertension | 81.8 | 82.9 |
| Hyperlipidemia | 55.4* | 50.5 |
| Retinopathy | 0.9 | 1.4 |
| Neuropathy | 4.1 | 4.6 |
| Depression | 23.6 | 23.7 |
| Charlson comorbidity score | 1.3 (0.9)* | 1.5 (1.2)* |
| Cotherapy (%) | ||
| ACE inhibitors | 65.6* | 70.8* |
| Diuretics | 44.9* | 61.4* |
| Statins | 52.8 | 51.9 |
Data are means (SD) or proportions (%). ID, index date of new prescriptions of metformin or SU.
>100 000 inhabitants.
eGFR: estimated glomerular filtration rate (MDRD formula).
Recorded values: MET n = 7658, SU: n = 947.
Recorded values: MET n = 3377, SU: n = 339.
P < .05 metformin vs sulfonylurea group
The metformin group was younger and comprised more males. Furthermore, practice residency was more often in West Germany (P < .05). There were no significant differences for privately insured patients, diabetologist care, and rural/urban practice residency between both groups (P > .05). In subgroup analyses, the average recorded BMI at baseline was higher in metformin than in sulfonylurea users (Table 1). Furthermore, baseline eGFR was significantly higher in patients with metformin treatment. Mean HbA1c was somewhat lower in patients with metformin (P < .001).
Although hyperlipidemia was more often diagnosed in patients with metformin and no difference was found for hypertension, macrovascular diseases were significantly more often observed in sulfonylurea users (Table 1). In particular, significant differences were found for baseline prevalence of diagnosed coronary heart disease, previous myocardial infarction, stroke, peripheral vascular disease, and congestive heart failure between the 2 groups. Depression was diagnosed in almost one-quarter in both groups (no difference). No significant differences between the 2 groups were also observed with respect to baseline microvascular complications (retinopathy, neuropathy). Overall, the sulfonylurea cohort had considerably more concomitant diseases than the metformin users, which resulted in a higher CCI (P < .001). Comedication with ACE inhibitors and diuretics was frequent in both groups, which was higher among sulfonylurea users (P < .001) (statins: no difference).
In both groups there was a comparable decline in renal function during the study period. The average absolute decline of eGFR was ∑10.3 (SD: 8.9) ml/min/1.73 m2 and −10.9 (SD: 9.9) ml/min/1.73 m2 in metformin and sulfonylurea users (p = 0.567), respectively.
Kaplan-Meier survival analyses
The cumulative incidence of insulin treatment was 4.3% for the metformin group and 14.5% for the patients receiving sulfonylureas over the study period of 6 years. The corresponding figures for the propensity score matched pairs (n = 1117) were 5.6% (metformin) and 14.5% (sulfonylureas), respectively. Figure 1 presents Kaplan-Meier curves for insulin prescriptions for the 2 matched groups. The metformin users were at a decreased risk of insulin initiation, which started already during the first year after index date and persisted over the whole study period. The insulin-free survival curves showed a significant difference between the 2 groups (log-rank test: P < .0001).
Figure 1.
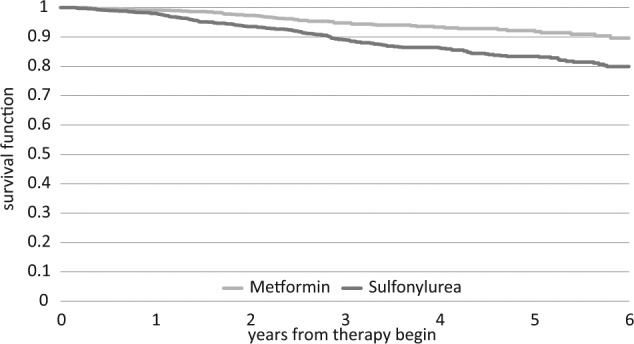
Kaplan-Meier curves for initiation of insulin therapy in type 2 diabetes patients using metformin or sulfonylureas over 6 years. Propensity score matching for age, sex, diabetologist treatment, private health insurance (metformin: n = 1117; SU: n = 1117). Log-rank test: P < .001.
Cox regression analysis
The multivariate hazard ratios of the Cox regression models (backward selection) for metformin and sulfonylurea users are shown in Tables 2 and 3. These data show that a large decline in kidney function (eGFR 15-<30) was strongly associated with insulin initiation in the metformin group, whereas slighter GFR deteriorations were not related to onset of insulin therapy. Furthermore, male sex, diabetologist care, history of stroke, and prescriptions of diuretics and statins were associated with a higher likelihood to start insulin therapy in the metformin group. The age group 60-80 years (reference: ≤60 years) and patients with diagnosed hyperlipidemia had a lower risk of insulin initiation.
Table 2.
Association of Patient Characteristics With Insulin Initiation in Metformin Users: Multivariate Cox Regression Analyses (Backward Selection).
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age (56-80 years)a | 0.57 | 0.46-0.72 | <.0001 |
| Age (>80 years)a | 0.99 | 0.66-1.47 | .9395 |
| Male sex | 1.67 | 1.36-2.10 | <.0001 |
| eGFR (60-<90)b | 0.94 | 0.73-1.22 | .6438 |
| eGFR (30-<60)b | 1.12 | 0.77-1.63 | .5466 |
| eGFR (15-<30)b | 2.39 | 1.09-5.23 | .0300 |
| eGFR (baseline) | 1.00 | 0.99-1.01 | .5319 |
| Diabetologist care | 1.94 | 1.14-3.32 | .0153 |
| Hyperlipidemia | 0.65 | 0.51-0.82 | .0003 |
| Stroke | 1.51 | 1.09-2.11 | .0135 |
| Hypertension | 0.85 | 0.67-1.11 | .2259 |
| Diuretics | 2.38 | 1.90-2.98 | <.0001 |
| Statins | 1.35 | 1.07-1.71 | .0133 |
Reference: age ≤ 60 years.
Reference: eGFR ≥ 90 ml/min/1.73 m2.
Table 3.
Association of Patient Characteristics With Insulin Initiation in Sulfonylurea Users: Multivariate Cox Regression Analyses (Backward Selection).
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age (56-80 years)a | 0.76 | 0.48-1.21 | .2406 |
| Age (>80 years)a | 0.98 | 0.55-1.74 | .9442 |
| Male sex | 1.02 | 0.74-1.41 | .8973 |
| eGFR (60-<90)b | 0.62 | 0.39-0.99 | .0464 |
| eGFR (30-<60)b | 0.79 | 0.45-1.36 | .3883 |
| eGFR (15-<30)b | 0.45 | 0.16-1.30 | .1410 |
| eGFR (baseline) | 0.99 | 0.98-1.01 | .5146 |
| Heart insufficiency | 1.52 | 1.07-2.16 | .0198 |
| Hypertension | 0.89 | 0.58-1.38 | .6126 |
| Diuretics | 2.27 | 1.49-3.46 | .0001 |
Reference: age ≤ 60 years.
Reference: eGFR ≥ 90 ml/min/1.73 m2.
In contrast to metformin, a GFR decline was not related to insulin initiation in sulfonylurea users (Table 3). There was a borderline significance with a lower insulin therapy risk for eGFR values between 60-<90 ml/min/1.73 m2 (P = .046). Furthermore, a diagnosed congestive heart failure and prescriptions of diuretics were related to an increased risk of insulin treatment. Both prescriptions of thiazides and other diuretics were related to an increased risk of insulin therapy (data not shown).
In subgroup analyses, we further adjusted for baseline glycemic control (HbA1c) and body mass index. The significantly increased risk for insulin therapy in metformin users related to eGFR (15-<30) persisted in these models (data not shown). No association between eGFR and insulin initiation was found in the sulfonylurea group. In both patient cohorts, baseline HbA1c (%) but not BMI was an independent predictor for onset of insulin treatment (metformin: HR: 1.42; 95% CI: 1.30-1.54; sulfonylureas: 1.37: 1.17-1.61).
Discussion
About 3-fold as many type 2 diabetes patients started insulin therapy within 6 years after receiving a first-time prescription of sulfonylureas compared to metformin in primary care practices in Germany. As a novel finding, the present study indicates that a substantial eGFR decline is a significant predictor of initiating insulin therapy in metformin, but not in sulfonylurea users in general practices.
Recently, a retrospective cohort study using a similar database in the United States also found that in 2 matched groups of older patients (≥65 years) about twice as many subjects started insulin therapy within 3 years after receiving their first prescription of sulfonylurea monotherapy compared with metformin therapy.2 Furthermore, initiating treatment with sulfonylureas was related to a shorter time to insulin onset compared to metformin.2 These results are consistent with previous cohort studies from Canada9,10 and Europe.11-13 Most of these studies have not carefully matched for baseline characteristics like the US study and the present study. But the general impression is that sulfonylurea treatment is associated with a higher likelihood for initiating insulin therapy compared to metformin treatment.
In the A Diabetes Outcome Progression Trial (ADOPT),14 a randomized controlled trial to evaluate the durability of glycemic control in patients receiving monotherapy with rosiglitazone, metformin or a sulfonylurea (glyburide), the annual rate of β-cell function decline—as determined by homeostatic model assessment (HOMA)—was greatest in the glyburide group (6.1%), intermediate in the metformin group (3.1%), and least in the rosiglitazone group (2.0%).14 Thus, the increased treatment failure with glyburide was related to an increased beta cell function decline compared to metformin and rosiglitazone.14 Therefore, insulin therapy may be required earlier in these patients.
Impaired kidney function occurs in up to 40% of type 2 diabetes patients.15 Once the eGFR declines below 60 ml/min the oral antidiabetic therapy needs to be rechecked according to German guidelines.4,16 In Germany, prescription use of metformin is contraindicated if the eGFR falls below 60 ml/min.4,16 Other European guidelines are less strict suggesting to stop metformin in patients once eGFR is <45 ml/min.5 The present study suggests that a substantial decline in eGFR may alert primary care physicians in Germany to switch from metformin to insulin therapy. GFR levels <30 ml/min were associated with an adjusted 2-fold higher likelihood of initiating insulin therapy in metformin users.
In contrast, no association between renal failure and start of insulin therapy was found in sulfonylurea (glibenclamide) users. In Germany, sulfonylurea prescriptions are allowed in patients with an eGFR between 30 and 59 ml/min but are contraindicated if renal function further declines.4,16 In the present study, kidney function decline was not significantly related to starting insulin therapy in sulfonylurea users. On the other hand, renal failure is a major risk factor for hypoglycemia. The higher risk of hypoglycemia induced by sulfonylureas is due to accumulation related to lower renal clearance.1,4,5 Thus, further studies are needed to examine if primary care physicians are more alert to the potential side effects (lactate acidosis) of metformin treatment in kidney failure compared to side effects (hypoglycemia) of sulfonylureas.
Several of the other factors that we observed to be associated with initiation of insulin therapy may reflect their associations with insulin sensitivity. As an example, statin use may reflect lipid disorders which are related to insulin resistance. Furthermore, both in metformin and sulfonylurea users, prescription use of diuretics were associated with an increased risk of insulin initiation. In particular, thiazides are known to induce a mild form of glucose intolerance.9 In contrast, a previous study from Canada found that patients taking thiazide diuretics in the year before initiation of oral antidiabetic drugs were less likely to initiate insulin therapy.9
In line with a previous study from Sweden, the age group ≤60 years had a higher risk of insulin treatment compared to older patients (60-80 years).11 Furthermore, baseline HbA1c was a significant predictor of onset of insulin therapy.11 BMI was not associated with insulin therapy in the present study (mostly obese subjects), whereas a lower BMI was related to start of insulin treatment in a database study from Scotland.13 In line with the present study (metformin users), male sex was significantly related to switching to insulin in the Tayside study in the United Kingdom.13 In addition, comorbidity like previous stroke and heart insufficiency were associated with insulin initiation, which most likely reflects a more advanced disease stage. Finally, diabetologist care was association with a higher likelihood to start insulin therapy in the metformin group, which may reflect less resistance to start insulin therapy.
Several limitations of the present study should be mentioned. First, no valid information on diabetes type, prescribed daily doses, and important outcome measures (eg, hypoglycemia) were available in the database. Furthermore, no valid information on diabetes duration was available. Also assessment of comorbidity relied on ICD-codes by primary care physicians only. Second, HbA1c and fasting glucose values were only available for a subgroup at baseline but not during the course of insulin treatment. Finally, the low prevalence for microvascular complications observed in the present study compared to population-based estimates indicated that a substantial number of patients who have such complications were missed.
In conclusion, under real-life conditions, first-time prescription of metformin in type 2 diabetes was associated with lower risk of insulin initiation compared to sulfonylurea monotherapy. Kidney function decline was associated with insulin initiation in metformin but not in sulfonylurea users. Further studies are necessary to investigate if primary care physicians are more alert to the potential side effects of kidney function decline of metformin than of sulfonylurea therapy.
Footnotes
Abbreviations: ACE, angiotensin-converting-enzyme; ATC, anatomical therapeutic chemical classification (EmphRa-Classification); CCI, Charlson Comorbidity Index; GFR, glomerular filtration rate; HR, hazard ratio; ICD, International Classification of Diseases; SD, standard deviation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by an unrestricted grant from Sanofi-Aventis Germany.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55:1577-1596. [DOI] [PubMed] [Google Scholar]
- 2. Fu AZ, Qiu Y, Davies MJ, Engel SS. Initial sulfonylurea use and subsequent insulin therapy in older subjects with type 2 diabetes mellitus. Diabetes Ther. 2012;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Q, Rajagopalan S, Marrett E, Davies MJ, Radican L, Engel SS. Time to treatment initiation with oral antihyperglycaemic therapy in US patients with newly diagnosed type 2 diabetes. Diabetes Obes Metab. 2012;14:149-154. [DOI] [PubMed] [Google Scholar]
- 4. Süfke S, Steinhoff J, Schütt M. Diabetes treatment in patients with chronic kidney disease [in German]. Dtsch Med Wochenschr. 2013;138:1109-1118. [DOI] [PubMed] [Google Scholar]
- 5. Zanchi A, Lehmann R, Philippe J. Antidiabetic drugs and kidney disease—recommendations of the Swiss Society for Endocrinology and Diabetology. Swiss Med Weekly. 2012;142:w13629. [DOI] [PubMed] [Google Scholar]
- 6. Shaw JS, Wilmot RL, Kilpatrick ES. Establishing pragmatic estimated GFR thresholds to guide metformin prescribing. Diabetic Med. 2007;24:1160-1163. [DOI] [PubMed] [Google Scholar]
- 7. Becher H, Kostev K, Schröder-Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47:617-626. [DOI] [PubMed] [Google Scholar]
- 8. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining co-morbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [DOI] [PubMed] [Google Scholar]
- 9. Pérez N, Moisan J, Sirois C, Poirier P, Grégoire JP. Initiation of insulin therapy in elderly patients taking oral antidiabetes drugs. CMAJ. 2009;180:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eurich DT, Simpson SH, Majumdar SR, Johnson JA. Secondary failure rates associated with metformin and sulfonylurea therapy for type 2 diabetes mellitus. Pharmacotherapy. 2005;25:810-816. [DOI] [PubMed] [Google Scholar]
- 11. Ringborg A, Lindgren P, Yin DD, Martinell M, Stålhammar J. Time to insulin treatment and factors associated with insulin prescription in Swedish patients with type 2 diabetes. Diabetes Metab. 2010;36:198-203. [DOI] [PubMed] [Google Scholar]
- 12. Kostev K, Dippel FW. Predictors for the initiation of a basal supported oral therapy (BOT) in type 2 diabetic patients under real-life conditions in Germany. Prim Care Diabetes. 2012;6:329-335. [DOI] [PubMed] [Google Scholar]
- 13. Donnan PT, Steinke DT, Newton RW, Morris AD, DARTS/MEMO Collaboration. Changes in treatment after the start of oral hypoglycaemic therapy in Type 2 diabetes: a population-based study. Diabetic Med. 2002;19:606-610. [DOI] [PubMed] [Google Scholar]
- 14. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [DOI] [PubMed] [Google Scholar]
- 15. Koro CE, Lee BH, Bowlin SJ. Antidiabetic medication use and prevalence of chronic kidney disease among patients with type 2 diabetes mellitus in the United States. Clin Ther. 2009;31:2608-2617. [DOI] [PubMed] [Google Scholar]
- 16. Hasslacher C, Wolf G, Kempe P, Ritz E. Diabetic nephropathy [in German]. Diabetologe. 2010;5:S113-S116. [Google Scholar]


